Abstract
The epidermal growth factor receptor (EGFR) and a co-receptor denoted HER2/ERBB2, are frequently overexpressed or mutated in solid tumors, such as carcinomas and gliomas. In line with driver roles, cancer drugs intercepting EGFR or HER2 currently outnumber therapies targeting other hubs of signal transduction. To explain the roles for EGFR and HER2 as prime drivers and targets, we take lessons from invertebrates and refer to homeostatic regulation of several mammalian tissues. The model we infer ascribes to the EGFR-HER2 module pivotal functions in rapid clonal expansion of progenitors called transient amplifying cells (TACs). Accordingly, TACs of tumors suffer from replication stress, hence accumulate mutations. In addition, several lines of evidence propose that in response to EGF and related mitogens, TACs might undergo de-differentiation into tissue stem cells, which might enable entry of oncogenic mutations into the stem cell compartment. According to this view, antibodies or kinase inhibitors targeting EGFR-HER2 effectively retard some solid tumors because they arrest mutation-enriched TACs and possibly inhibit their dedifferentiation. Deeper understanding of the EGFR-HER2 module and relations between cancer stem cells and TACs will enhance our ability to control a broad spectrum of human malignancies.
A primer to tissue homeostasis
The numerical maintenance of normal morphology and function, tissue homeostasis, is primarily accomplished by cell turnover. In this highly coordinated process, certain differentiated cells are regularly eliminated and replaced by the expanded progeny of tissue-specific stem cells. 1 The rate of cell turnover varies extensively among organs, averaging 5 days for the intestinal epithelium, 4 weeks for the epidermis, and 6 months for lung epithelium 2, 3. While it is commonly an ongoing process, cell turnover sometimes occurs in intermittent cycles, such as the life-long cycles of growth, involution, and relative quiescence exhibited by hair follicles 4, 5. In addition to its general role in homeostasis, cell turnover contributes to several tissue-specific functions. For instance, adult neurogenesis and cell death seem to be important for learning and memory. 6 Likewise, the mammary gland exhibits cycles of growth and involution, regulated by the ovarian cycle, pregnancy, or weaning. 7 Another tissue, the epidermis, is characterized by a sophisticated mechanism of cell death, cornification, which maintains a physical barrier able to protect the body from environmental insults. 8
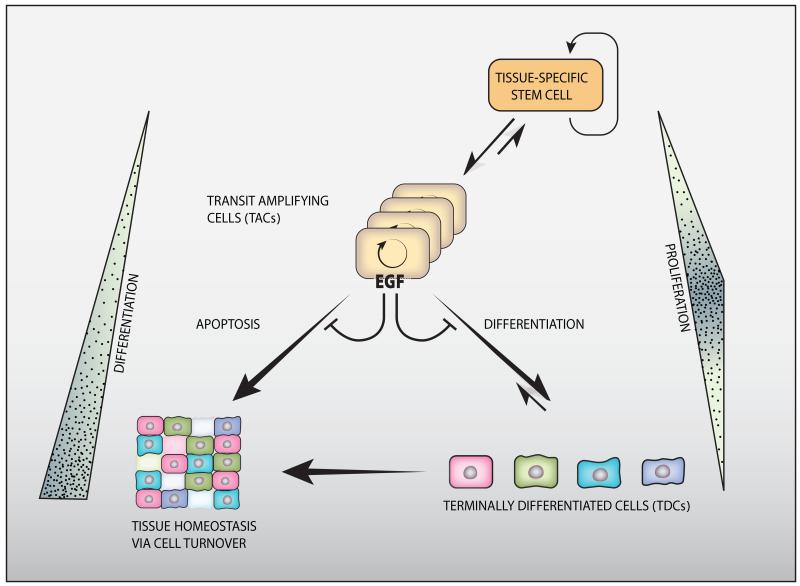
Cell turnover might be considered a three-layered equation (Figure 1). One part of the equation, physiological cell eradication, occurs essentially via programmed death of specific cells (apoptosis), although additional death subroutines may also operate. 9 The next part of the equation is cell replication. While a few tissues, such as liver 10 and the endocrine pancreas 11 appear to be maintained or regenerated by replication of differentiated cells, homeostasis of most organs depends on replication of adult stem cells and their progeny. Adult stem cells are characterized by the ability to self-renew, thereby maintain the stem cell population, and also generate daughter cells able to differentiate into a single, a few (oligopotent), or a large number (multipotent) of cell types. Depending on cell type and organ in question, different strategies are employed for stem cell expansion. The daily production of billions of blood cells is based on a hierarchically organized system, in which the most primitive hematopoietic stem cells are highly quiescent, cycling only once every 145 days, on average. 12 The intestine, another tissue able to produce immense numbers of cells every day, harbors mitotically active stem cells that cycle once every 24 hours. 13 In both tissues, it is the massive expansion of undifferentiated, immediate derivatives of tissue-specific stem cells, denoted transient amplifying cells (TACs), which permits daily production of a colossal number of cells. Newly introduced models of tissue-specific TACs, while conceptually straightforward, come with variations on the theme. In the epidermis, for instance, a population of functionally equivalent cycling progenitors, all located in the basal layer, underlays wound healing. 14 Independently of these differences, the third part of the cellular turnover equation is differentiation, namely: the gradual adoption of a final fate and incorporation into the host organ. One critical, almost universal factor regulating differentiation is exit from the stem cell niche. In the intestine, direct contact to a Paneth cell is necessary and sufficient to maintain the stem cell character, while loss of direct contact to these cells causes stem cells to become TACs. 15
Figure 1. The three-layered organization of cell turnover.
Tissue-specific, adult stem cells are at the apex of the hierarchical three-layered organization. Their asymmetric divisions enable both self-renewal and generation of transit amplifying cells (TACs, or progenitors). TACs undergo several rounds of division (symbolized by a circular arrow), and then differentiate to form the various specialized cells of the tissue. Homeostatic regulation of tissues involves constant apoptosis and renewal, but stem cells are relatively resistant to apoptosis. Note that both stem cells and terminally differentiated cells (TDCs) are characterized by slow rates of mitoses, but the TACs are both short lived and rapidly proliferating. Expansion of the TAC compartment is governed, in some epithelial and neural organs, by the EGFR-HER2 module, which might inhibit both apoptosis and differentiation of TACs’ descendants. Importantly, the process is considered to be unidirectional. However, reversal (dedifferentiation) of TACs and TDC precursors might take place under certain conditions. For example, as discussed in this review, TACs might acquire some features of stemness once the EGFR-HER2 module is active.
Some thirty years ago, Robert Lim and Stephen Hauschka reported that terminal differentiation of mouse myoblasts is accompanied by permanent loss of the epidermal growth factor receptor (EGFR), but myoblasts that are prevented from differentiation bind large amounts of EGF. 16 In the following years, it became clear that timely loss of receptors for specific mitogens is a general regulatory mechanism that prevents continuous replication and allows formation of specialized cell types. In fact, evasion from this regulatory mechanism, propelled by sustained proliferative signaling, is a fundamental capability acquired during the multistep development of tumors. 17 Here we review evidence from invertebrates, rodent models, and tumor specimens, collectively supporting a role for EGFR, and the co-receptor, HER2, in cell turnover, especially in clonal expansion of TACs and their presumed conversion to stem cells in the context of malignancies.
In invertebrates, EGFR primarily expands pools of epidermal and other TACs
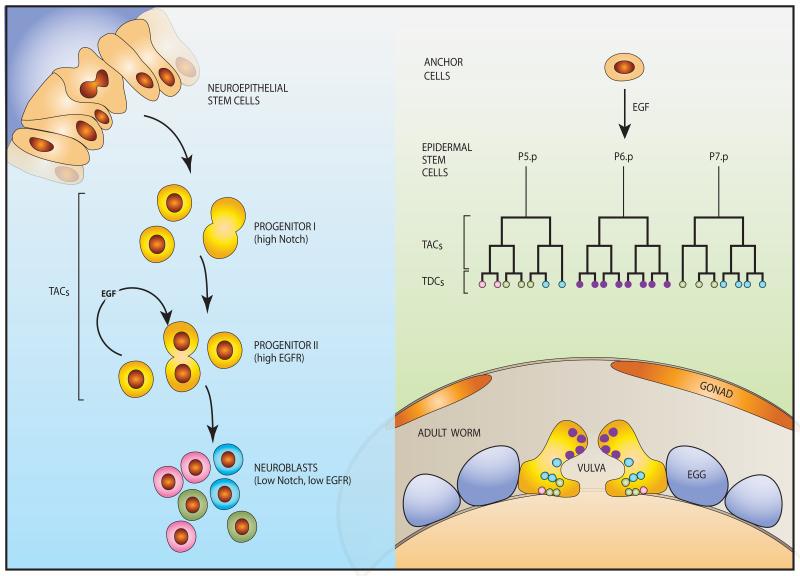
The simplest version of the EGFR module exists in Caenorhabditis elegans, a one millimeter-long worm. The vulva of this hermaphrodite nematode is necessary for egg-laying and for copulation with males, and its induction is one of the best understood morphogenic events 18 (Figure 2, right panel). Vulval cell induction requires LIN-3, an EGF-like factor, and LET-23, an EGFR ortholog, along with components of the RAS-MAP-kinase pathway. Inactivation of this pathway results in the “vulvaless” phenotype, and conversely, hyperactivation results in excessive vulval differentiation (“multivulva” phenotype). During early larval development, a presumed epidermal stem cell generates 11 daughter cells, six of which will form an equivalence group of vulval precursor cells (VPCs), called P3.p through P8.p. Ablation experiments indicated that all six cells of the ventral epidermis have the potential to generate vulva 19. Normally, P6.p receives LIN-3 from the anchor cell in the overlying gonad, and adopts the primary vulval fate while undergoing several cell cycles. A combination of weaker LIN-3 signals and lateral signaling by LIN-12, a Notch-like protein, instructs the neighboring VPCs, P5.p and P7.p, to adopt the secondary vulval fate. This generates the 22 nuclei of the mature vulva. The remaining three VPCs (P3.p, P4.p, and P8.p) divide once and fuse with the surrounding hypodermal syncytium. Importantly, the Hox gene lin-39 is a major determinant of VPCs, which acts by inhibiting fusion and stimulating cell division. In conclusion, the archaic EGFR form, along with Notch, regulates precise expansion of a pool of undifferentiated, yet committed TACs that gradually reach terminal differentiation.
Figure 2. Well-studied developmental processes of invertebrates are controlled by EGFR-driven progenitor cells.
(Right panel) Vulval development in C. elegans is a multi-step process instigated by an inductive signal provided by the gonad-derived anchor cell (AC), in the form of LIN-3, the worm’s form of EGF. The secreted ligand travels a short distance to meet one of six equipotent stem cells, called vulva precursor cells (VPCs; normally P6.p), which express LET-23, the nematode EGFR. Following two divisions of the stimulated VPC and its two neighbors, which might be considered the vulval TACs, 22 differentiated cells are generated and form the vulva proper (lower panel). The other three VPCs form part of the body wall. (Left panel) In the Drosophila optic lobe, symmetrically dividing neuroepithelial stem cells transform into asymmetrically dividing neuroblasts, which later differentiate into many types of neurons. This sequential transformation involves two progenitor classes: type I progenitors are maintained by the Notch pathway, whereas type II progenitors are driven by active EGFRs. Transition to the neuroblast state and terminal differentiation is permitted by downregulation of the Notch and EGFR pathways,
Four EGF-like ligands bind to the insect EGFR molecule, and they regulate an especially broad spectrum of intercellular inductive processes in Drosophila melanogaster. 20 Development of the optic lobe exemplifies the principles of cell turnover (Figure 2, left panel): Reminiscent of nematodes’ vulval development, an insect neuroepithelial stem cell initiates a proneural wave of differentiation in the optic lobe. This is accomplished by an EGFR-driven switch from symmetric to asymmetric stem cell divisions and allows both renewal of the neuroepithelium and differentiation into neuroblasts. Oscillations of Notch activation, and, eventually, simultaneous down-regulation of EGFR and Notch mark completion of the switch and precede a phase of extensive neuroblast proliferation. 21 In this way, EGFR activation controls a phase of neuroblast proliferation and gradual differentiation into at least 63 distinct neuronal subtypes in the optic ganglia of the fly. 22 In summary, in both C. elegans and Drosophila melanogaster EGFR acts upon pre-differentiated progenies of tissue-specific stem cells, to numerically expand the cellular pool available for differentiation. Whether or not EGFR-driven neuroblasts can revert to stem cells remains unknown. It is notable, however, that de-differentiation of germ cell progenitors into functional stem cells has been observed in the ovary of flies. 23
The hardware and software of the mammalian EGFR-HER2 module
Whole genome and single chromosome duplications, which occurred in the course of evolution, expanded the primordial EGFR module, such that 4 receptors (EGFR, HER2/ERBB2, HER3/ERBB3 and HER4/ERBB4) and 11 growth factors exist in mammals. Congruent with duplications, while the receptors’ genes are found in different chromosomes, 4 ligand genes (amphiregulin, epigen, epiregulin and betacellulin) reside in a single cluster located at human chromosome 4 (4q13.3). And whereas EGFR and HER4 are functionally autonomous, meaning that they can bind a growth factor and consequently their intrinsic enzymatic activity undergoes dimerization and stimulation, the other two receptors are non-autonomous: HER2 (also called NEU) cannot bind any known growth factor with high affinity, 24 whereas HER3 is almost devoid of catalytic activity. 25 The key for understanding the logic of this configuration is the process of ligand-induced homo- and heterodimerisation of receptor tyrosine kinases, first described in the context of EGFR and HER2. 26-28 Accordingly, due to a ligand-induced conformational change that projects a dimerization arm, 29 ligand-occupied receptors undergo dimerization, such that the cytoplasmic domains of the dimeric receptor complex are brought together and allow kinase activation, as well as trans-phosphorylation. 30 In addition, the heterodimeric complexes are able to recruit sets of receptor-interacting proteins, to instigate downstream signaling. For example, whereas homodimers of EGFR and HER2 can strongly couple to the RAS-to-ERK pathway, both neuregulin receptors, namely HER3 and HER4, can avidly recruit phosphotidylinositol 3’ kinase, thereby activate the AKT kinase cascade. Heterodimers like the robust HER2-HER3 complex, however, feed both pathways. 31
HER/ERBB signaling might be considered in context of a layered network that gains robustness due to modularity, redundant components and multiple feedback regulatory loops, the function of which is to regain steady state following bursts of activation. 32, 33 Both positive feedback, primarily up-regulation of ligand production, and negative feedback loops contribute to robust function, and they are manipulated in disease states. The majority of feedback regulation involves induction of specific genes, such as genes encoding phosphatases specific to MAP-kinases, and transcriptional repressors, which act as inhibitors of downstream signaling. 34 In addition, an immediate wave of feedback loops, which precedes synthesis of new RNAs and proteins, involves posttranslational protein modifications, such as EGFR phosphorylation at tyrosine-1045, which enables recruitment of an E3 ubiquitin ligase, CBL, and subsequent conjugation of ubiquitin molecules to active receptors. 35, 36 Ubiquitinated receptors are rapidly sorted for intracellular degradation through a stepwise process orchestrated by ubiquitin- and GTP-binding proteins. 37, 38 Unlike EGFR, HER2 largely evades ubiquitination, hence undergoes internalization and recycling, which prolongs signals generated by HER2-containing heterodimers. 39, 40 Another mechanism that enables HER2 to enhance signaling, despite its inability to directly bind a ligand growth factor, entails inhibition of EGF dissociation from HER2-containing heterodimers. For these reasons, such heterodimers are preferred and their signaling might lead to uncontrolled growth. 41, 42
Animal models attribute to EGFR-HER2 critical functions downstream to tissue stem cells, at the level of TACs
In the 1930s, two spontaneous mutant mice displaying an unusually waved hair coat were reported and termed waved-1 and waved-2 (reviewed in 43). Today we know that these classical mutant mouse lines carry point mutations in the genes encoding the EGFR ligand TGF-alpha 44 or the EGFR itself 45, respectively. EGFR can be activated by seven different ligands, which display tissue-specific patterns of expression. 46 Accordingly, in contrast to the multiorgan defects observed in mice lacking EGFR, knockout of individual ligands resulted in either no phenotype or relatively mild, organ-specific defects. For example, TGF-alpha deficiency resulted in hair follicle and eye abnormalities, 47 but different effects on mammary gland development were observed in mice lacking amphiregulin or EGF. 48 Consistent with growth factor-induced expansion of diverse pools of undifferentiated cells, neonatal mice lacking three ligands, amphiregulin, EGF and TGF-alpha, were growth retarded. 49 Growth retardation was shared also by EGFR mutant mice that escaped in utero death; 50-52 surviving animals were smaller but displayed relatively normal tissue architecture and cell type composition, 50-52 suggesting that insufficient proliferation of committed progenitors might be responsible for the general hypoplastic phenotype. In accordance, asymmetric EGFR distribution during mitosis of forebrain progenitors resulted in daughter cells with different proliferative, migratory, and differentiation abilities. 53 These and other lines of evidence (reviewed in 54) indicate that EGFR promotes proliferation of progenitors in many organs (e.g., lung, heart, liver, hair follicles and skin), as well as inhibits terminal cell differentiation (e.g., chondrocytes and osteoblasts).
The non-autonomous function of HER2, the ligand-less member of the family, in clonal expansion of specific progenitors is reflected by the phenotypes of animals homozygous for a disrupted HER2 gene. For example, mice defective in HER2, HER4 or neuregulin 1, which binds both HER3 and HER4, die at embryonic day 10.5 due to remarkably similar cardiac defects. 55-57 Apparently, neuregulin 1 is secreted by the endocardium and stimulates adjacent myocytes by binding with a complex comprising HER4 and HER2. This initiates myocyte proliferation and formation of essential finger-like extensions called trabecula. Gene knockout uncovered another early and essential co-receptor function of HER2, namely an ability to assist neuregulin and HER3 in Schwann cell development and in axon myelination: 58-60 due to the absence of Schwann cells in HER2 mutant animals, sensory and motor neurons undergo extensive cell death. In conclusion, the traits of genetically manipulated animals are consistent with an evolutionarily conserved function of HER/ERBB receptors and their ligands, namely accelerating proliferation of subsets of undifferentiated epithelial and other TACs, along with blocking terminal differentiation and enhancing survival of partly differentiated cells.
Roles for EGFR-HER2 in renewal and regeneration of specific tissues
Several tissues exemplify the ability of EGFR to promote regeneration and derive differentiated cells from stem cells. They include liver, 61 lung, 62 and bone 63-66. Furthermore, EGFR signaling supports regeneration of hematopoietic cells after myelosuppressive injury 67: high EGF levels in the bone marrow protect mice after a lethal dose of irradiation, through a mechanism that requires EGFR activation in hematopoietic stem cells and inhibition of apoptosis by repressing PUMA, a proapoptotic protein. Similarly, EGFR is engaged during regeneration of adult tissues. Regeneration is a form of tissue replacement due to pathological insults (e.g., injury), in which adult stem cells respond to loss of particular cell types by activating dedicated division programs. For instance, prominent EGFR immunoreactivity appears after brain injury, in astrocytes adjacent to the lesion. 68 In the same vein, transgenic mice overexpressing EGFR showed accelerated re-myelination and functional recovery following experimental demyelination of the corpus callosum. 69 In the latter model, EGFR enhanced proliferation of progenitors and, at the same time, reduced the number of apoptotic cells. The converse effect was observed in the intestinal mucosa of EGFR-deficient mice subjected to ionizing radiation: compared to normal cells, proliferation capacity was reduced and apoptosis rates were increased in enterocytes lacking EGFR. 70 Below and in Figure 3 we describe two well-characterized examples of homeostatic regulation, which underscore roles for EGFR in amplification of stem cell-derived, early progenitors.
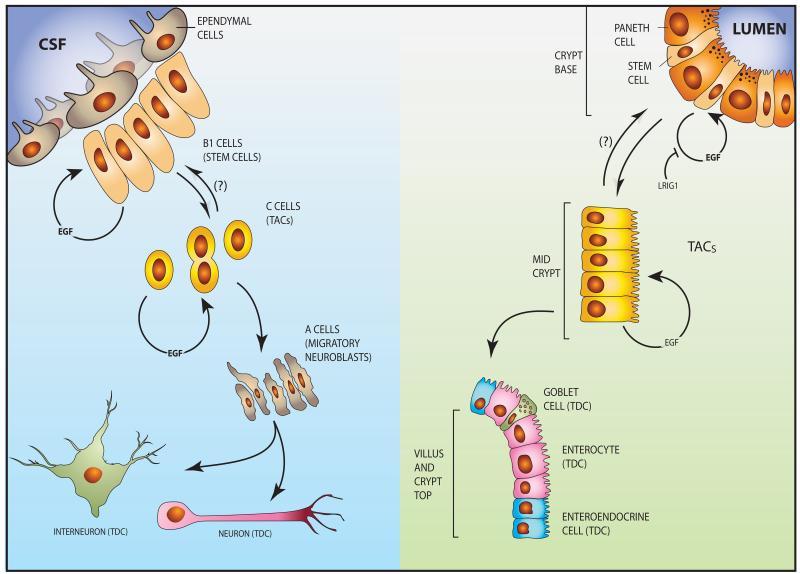
Figure 3. Transit amplifying cells of the intestine and central nervous system of mammals are driven by EGFR-HER2 signals.
(Right panel) The intestinal epithelium is organized into crypt and villus regions, with the stem cells and TACs localized to the crypt. Both quiescent and active stem cells exist in the base of the crypt next to Paneth cells, which provide essential niche signals, including specific EGFR ligands. EGFR is expressed by both SCs and TACs, and its mitogenic function toward SCs is strongly inhibited by LRIG1, a transmembrane molecule that physically binds with both EGFR and HER2. The TACs migrate toward the villus while undergoing EGFR-dependent mitoses followed by differentiation. The differentiation compartment, containing post-mitotic, lineage committed cells, such as goblet cells, enteroendocrine, tuft and Paneth cells, extends from the upper third of the crypt to the villus tip. Note that most differentiated cell populations migrate up the villi, but Paneth cells move downward. A reverse arrow and a question mark show putative dedifferentiation of TACs to SCs. (Left panel) A neural stem cell niche exists in the ventricular-subventricular zone (V-SVZ) in the lateral ventricles of mammalian brains. Ependymal cells line the ventricular surface and project cilia into the cerebrospinal fluid (CSF). The underlying type B1 cells are good candidates for the true adult neural stem cell identity. Type C cells are putative intermediate precursors (TACs) that differentiate to migrating neuroblasts (A cells). The latter divide and migrate out of the niche to form terminally differentiated neurons and interneurons. Note that both B1 (putative SCs) and C cells (TACs) are self-renewable and their mitoses are driven by EGFR signaling.
Small intestinal stem cells
The intestinal stem cell compartment is one of the best-studied adult stem cell niches in mammals (Figure 3, right panel). This compartment occupies the base of the crypt, whereas the TAC compartment is located at the middle part, and the differentiation compartment, containing post-mitotic, lineage committed cells, extends from the upper third of the crypt to the villus tip. 71 Thus, the crypt is the sole source of cells for intestinal self-renewal; each crypt generating more than 250 new cells per day. A small number of Lgr5+ stem cells, intermingled with Paneth cells, sustain renewal of the entire epithelium. Lgr5+ stem cells replicate once every 24 hours, while their progeny, the TACs, divide even more vigorously (once per 12 hours). Paneth cells are longer-lived, and unlike the TACs, which migrate upward, move downward into the crypt. In addition to secretion of antibacterial peptides, Paneth cells provide essential niche signals. Gene expression profiling of Paneth cells revealed expression of the EGFR ligands TGF-alpha and EGF, among other factors, and EGF appears essential for in vitro growth of organoids derived from Lgr5+ cells. 15 LRIG1 is a non-specific receptor tyrosine kinase inhibitor and its role in the intestinal crypt is still obscure. Nevertheless, the identification of LRIG1+ progenitors within the crypt revealed another level of EGFR-mediated regulation of intestinal homeostasis. LRIG1 inhibits EGFR/ERBB signaling by direct interactions with all HER/ERBB proteins. 72, 73. While the exact position of LRIG1+ cells in the crypt hierarchy and the interdependency of LRIG1+ and Lgr5+ cells remain unclear, two studies recently proved that LRIG1 regulates cell proliferation within the intestinal stem cell niche by tuning EGFR/ERBB signaling. 74, 75 Specifically, loss of LRIG1 resulted in upregulation of EGFR, HER2 and HER3, and, depending on the model, either increased crypt size 74 or led to duodenal adenomas. 75
Independent of these lines of evidence, it has long been known that luminal 76 or subcutaneous administration 77 of EGF exerts trophic actions on the intestine of rodents. Congruently, overexpression of an EGFR ligand, betacellulin, increased proliferation and markedly enlarged the intestinal epithelium. 78 Conversely, as mentioned already, mice with a targeted depletion of EGFR showed a reduction in intestinal crypt size and number, along with reduced cell proliferation. 51, 52 Interestingly, several studies have raised the possibility that early TACs possess plasticity and can act as stem cells. For example, immediate daughters of Lgr5-positive small intestinal stem cells commit to a secretory lineage by expressing Dll1, a ligand of Notch, but they regain stemness after stimulation by WNT factors in vitro or after damage in vivo. 79 Taken together, it is becoming clear that HER/ERBB signaling provides a strong inductive signal for small intestinal SCs and promotes proliferation of functionally plastic TACs.
Neural stem cells
While additional sites seem to exist, the current consensus assumes that neural stem cell niches are limited to two regions of the mammalian brain, the ventricular-subventricular zone (V-SVZ) in the lateral ventricles and the subgranular zone (SGZ) of the hippocampus. 80 In the V-SVZ, the better characterized niche, 81 stem cells correspond to specialized astrocytes (B1 cells), which give rise to intermediate progenitors (C cells), a TAC population that differentiates into neuroblasts (A cells), which divide and migrate out of the niche (Figure 3, left panel). 82 While it was previously known that active EGFRs promote in vitro growth of neural stem cell-derived neurospheres and expands, in vivo, the number of proliferating V-SVZ cells, Doetsch and colleagues were the first to show that the majority of EGFR-positive and EGF-responsive cells in this niche are in fact transit amplifying C cells. 83 By infusing EGF into the lateral ventricles, they demonstrated that exogenous EGF increased proliferation of C cells and simultaneously decreased the number of neuroblasts. Since then, this effect has been confirmed in different experimental settings, for instance during cerebral ischemia. 84 Doetsch and colleagues were also the first who noted that, in addition to C cells, EGFR is expressed by a subset of astrocytes which are, according to a later study, activated stem cells. 85 Interestingly, it was suggested that enhanced EGFR signaling in C cells might suppress Notch signaling, a major B1 cell pathway regulating identity and self-renewal of neural stem cells. 86 In the longer term, EGF stimulation is particularly important for oligodendrogenesis. 87, 88 The EGFR ligand betacellulin was recently shown to similarly stimulate proliferation of V-SVZ progenitor cells. 89 However, in contrast to EGF, BTC infusion increased neurogenesis in vivo, an effect attributable to the ability of this EGFR ligand to additionally activate neuroblasts’ HER4. In summary, similar to their roles in development of the optic lobe of flies, EGFR molecules expressed in the V-SVZ not only mark committed neural stem cells and their direct progeny, the transit amplifying C cells, but also dictate expansion of this class of TACs. As we highlight below, the ability of EGFR-HER2 to expand the pool of neural and other TACs might explain why genetic aberrations frequently activate this signaling module in tumors of brain and other organs.
Mutational and other mechanisms frequently activate the HER/ERBB network in solid tumors
In accordance with the ability of ERBB/HER signaling to expand pools of undifferentiated progenitors, mutations and other aberrations that constitutively stimulate ERBB/HER are frequently detected in cancer. This might create a harmful cycle, because EGF-induced rapid proliferation of TACs not only ensures clonal expansion of mutation-bearing cells, but also accelerates accumulation of additional mutations due to error-prone DNA replication. 90 Genetic alterations of HER/ERBB that have been detected in human cancer include gene amplification, leading to receptor overexpression, activating kinase domain mutations, in-frame deletions, and co-expression of receptors and their ligands. 33 Below we describe the aberrant forms of the receptors and their clinical relevance. For the sake of brevity, this description excludes mutations affecting downstream effectors, such as RAS, BRAF and PI3K.
EGFR aberrations in brain tumors
Genetic amplification, elevated expression, and mutations within the EGFR have been widely implicated in various cancers, including breast, head and neck, prostate and non–small cell lung cancer (NSCLC). Yet, EGFR aberrations are most frequently displayed by high-grade brain tumors of glial origin. 91-93 Amplification of EGFR occurs in 40-60% of primary glioblastoma multiforme (GBM), but lower-grade astrocytomas rarely show similar aberrations 94. In addition, EGFR displays several internal deletions and missense mutations. 95 The most abundant deletion, of exons 2 through 7, is termed EGFRvIII (EGFRΔIII). Although frequently detected in brain tumors (approximately 40% of glioblastomas bear it), this mutation also occurs in various carcinomas. 96-98 Despite inability to bind ligands, EGFRvIII exhibits constitutively high tyrosine phosphorylation, activation of downstream signaling pathways, 99 as well as interactions with MET 100, 101 and regulation of HER2 trafficking 102. Indeed, EGFRvIII molecules are basally dimerized and confer high tumorigenic potential. 103 Importantly, EGFR aberrations in primary GBMs are accompanied by mutations in tumor suppressors (p16Ink4a, p19Arf, and PTEN). Other, less frequent EGFR deletions were identified: carboxyl terminal mutants, collectively termed EGFRvIV, lack either three exons (numbered 25 to 27; EGFRvIVa) or two exons (25 and 26; EGFRvIVb), 98, 104, 105 with the kinase-activating deletion initiating immediately downstream to the enzymatic domain. 106
EGFR aberrations in lung and in other tumors
EGFR kinase domain mutations are almost exclusively found in a fraction of NSCLCs, 107-109 with rare mutations also found in tumors of head and neck, colon, ovary, esophagus, pancreas and breast. 110, 111. The L858R substitution in the activation loop (exon 21), along with short in-frame deletions in the N-terminus of the alpha-C-helix and the preceding loop (exon 19), together account for up to 90% of EGFR mutations of lung cancer. Weakening the hydrophobic interactions between the activation loop and the amino-terminal lobe, these mutations interfere with kinase packing, thus destabilizing the inactive conformation and leading to constitutive catalysis. 112, 113 Correspondingly, the mutant forms are catalytically more active than wild type EGFR, 114, 115 and transgenic mouse models validated that they are sufficient for malignant transformation. 116-119 Another mutation, T790M, is responsible for approximately 50% of cases of acquired resistance to tyrosine kinase inhibitors (TKIs), but affected EGFR molecules remain sensitive to irreversible TKIs. 115, 118, 120-122
HER2 aberrations in tumors
Although HER2 is overexpressed in about 20% of NSCLC, gene amplification occurs in only 2% of cases. 123 Similarly rare in NSCLC are in-frame insertions in exon 20 of HER2, which are mutually exclusive with EGFR and KRAS mutations. HER2 overexpression and/or amplification was initially discovered in approximately one third of human breast cancers and this was associated with more aggressive disease, the presence of metastasis, reduced survival and shorter time to relapse. 124, 125 Overall, HER2 gene amplification and/or overexpression at the messenger RNA or protein level occur in approximately 20% of patients with early stage breast cancer 126. Subsequent studies have reported HER2 overexpression and/or amplification in colorectal, salivary gland, bladder, gallbladder, extrahepatic cholangiocarcinomas, cervical, uterine, and testicular tumors. 127 Importantly, up to a fifth of gastric carcinomas and up to a third of gastroesophageal junction adenocarcinomas are positive for HER2 amplification, but HER2 mutations are rare in gastric cancers. 127, 128
Aberrant forms of HER3/ERBB3 and HER4/ERBB4 in solid tumors
Early studies indicated that HER2 requires HER3 to drive breast tumor cell proliferation 129. Moreover, recurrent somatic mutations in HER3 were later identified in approximately 11% of colon and gastric cancers, as well as in hormone-positive breast cancer (4%), ovarian (1%) and NSCLC (1%). 125 The identified mutants were able to transform colonic and breast epithelial cells in a ligand-independent manner. 130 However, in line with the defective catalytic function of HER3, oncogenic activity was dependent on HER2. Notably, the majority of HER3 mutations cluster in the extracellular domain of HER3, but two recurrent kinase domain mutations, S846I and E928G, were identified in human tumors. 131 Unlike other HER-encoding genes, relatively rare mutations, which avoid clustering at specific receptor’s domain, have been identified in HER4. Melanomas present the highest rate of mutations (up to 19%). 132, 133 Yet, considerable fractions of lung, 134 colorectal and gastric tumors also present mutations, and other tumors are characterized by very low frequencies (<1%; e.g., glioma, multiple myeloma, and both prostate and thyroid tumors). Functional analyses performed in vitro, as well as the co-incidence of different mutations and enrichment for non-synonymous mutations, propose that at least a fraction of the identified HER4 mutations act as drivers of malignancy. 132, 135
Aberrant expression of EGF-like growth factors and neuregulins in solid tumors
Based on their studies of tumor viruses, Sporn and Todaro noted that unlike the paracrine (heterotypic) mode of action of EGF-family growth factors in embryogenesis and in wound healing, an autocrine mode, meaning that cells secrete growth factors to which they respond, dominates in solid tumors. 136 Autocrine secretion in tumors is the result of a positive feedback loop acting downstream to activated receptors and stimulating the promoters of several neuregulins and EGF-family ligands. 32, 137 For example, EGFR overexpression and ERK activation in primary brain tumors are often accompanied by increased abundance of the cognate ligands, resulting in self-stimulatory loops and chronic EGFR activation. 138 Additionally, brain tumors display increased abundance of the disintegrin and metalloprotease 12 metalloproteinase (ADAM12), leading to enhanced cleavage of the precursor of the heparin-binding EGF-like growth factor (HB-EGF). 139 Thus, along with prevention of apoptosis, autocrine growth factors of tumors fulfill another important function, namely they potentially expand pools of TAC-like cells harboring oncogenic mutations. For these reasons, high abundance of EGF-family ligands is considered a prognostic factor that predicts aggressive disease course (reviewed in 140). Amphiregulin, a low affinity EGFR ligand, well exemplifies the dual roles of autocrine ligands as TAC-expanding growth factors in both development and cancer. 141 Whereas amphiregulin plays pivotal roles in mammary gland development and in branching morphogenesis, 142 several characteristics associated with advanced cancer have been linked to increased abundance of this ligand. The list includes chronic inflammation, 143 high serum levels of the lysophosphatidic acid (LPA) 144 and expression of a mutant form of p53. 145 Congruently, amphiregulin expression has been associated with worse prognosis of several cancers, including prostate and head and neck tumors. 141 Moreover, high levels of amphiregulin and another ligand of EGFR, epiregulin, predict relatively good responses of colorectal cancer patients to treatment with anti-EGFR antibodies 146.
Clinically approved cancer drugs that directly intercept EGFR-HER2
The current attrition rate of new oncology drugs, which is significantly higher than in other therapeutic areas, 147 is attributable to lack of preclinical models able to recapitulate disease complexity and heterogeneity, and the possibility that targeting the bulk of tumor cells, rather than the small population of stem cells, might underlay low rates of success. 148 Against this backdrop, the high success rate of drugs targeting the EGFR-HER2 module provides a ray of hope. Since the 1998 approval of trastuzumab for the treatment of HER2-positive metastatic breast cancers, 149 several more drugs have been approved, making EGFR-HER2 one of the most effective targets in medical oncology. Importantly, the currently approved drugs are effective on a broad range of carcinomas, and some drugs are active in more than one clinical indication, which is a rare situation in oncology. So far, only two classes of drugs have been approved: (i) monoclonal antibodies (mAbs), either naked or conjugated to a cytotoxic compound, and (ii) tyrosine kinase inhibitors (TKIs), which are either reversible or irreversible (i.e. they covalently bind with the target kinase). In addition, TKIs are either mono-specific or they are designed to inhibit both EGFR and HER2. Notably, while mAbs are absolutely specific to the target receptor, selectivity of TKIs varies considerably. 150 Table 1 lists all clinically approved mAbs and Table 2 lists the currently approved anti-EGFR/HER2 TKIs. Note that these lists leave aside numerous experimental agents, some of which are in advanced clinical trials.
Table 1. Clinically approved monoclonal antibodies specific to EGFR or to HER2.
Listed are the approved antibodies according to the time of first approval.
| Drug Name | Brand Name | Year of Approval |
Main Clinical Indication |
Comments |
|---|---|---|---|---|
| Trastuzumab (Anti-HER2 mAb) | Herceptin | 1998 | Breast cancer (metastatic) | Combined with CT; limited to HER2+ tumors. |
| 2006 | Early breast cancer | Adjuvant setting (after surgery). | ||
| 2010 | Gastric or gastroesophageal junction adenocarcinoma | Combined with CT; limited to HER2+ cases. | ||
| Cetuximab (Anti-EGFR mAb) | Erbitux | 2004 & 2012 | Colorectal cancer (metastatic) | Combined with CT; limited to EGFR-expressing, K-RAS wild type tumors. |
| 2006 & 2009 | Locally or regionally advanced head and neck cancer | Combined with RT or CT. | ||
| Panitumumab (anti-EGFR mAb) | Vectibix | 2006 & 2009 | Metastatic colorectal cancer | Combined with CT; limited to EGFR-expressing, K-RAS wild type tumors. |
| Pertuzumab (anti-HER2 mAb) | Perjeta | 2012 | Breast cancer | Combined with trastuzumab and CT (prior to surgery). |
| Trastuzumab-DM1 (anti-HER2 mAb) | Kadcyla | 2013 | Breast cancer (metastatic) | Single agent; HER2+ patients, who previously received trastuzumab and CT. |
CT, chemotherapy; RT, radiotherapy
Table 2. Clinically approved tyrosine kinase inhibitors (TKIs) specific to EGFR and/or HER2.
Listed are the approved TKIs according to the time of first approval.
| Drug Name | Brand Name | Year of Approval |
Main Clinical Indication |
Comments |
|---|---|---|---|---|
| Erlotinib (Anti-EGFR TKI) | Tarceva | 2004 & 2013 | Lung cancer (NSCLC) | Especially effective on tumors expressing EGFR exon 19 deletions or an exon 21 (L858R) substitution. |
| 2005 | Pancreatic cancer | Combined with CT. | ||
| Gefitinib (Anti-EGFR TKI) | Iressa | 2003 & 2009 (in Europe) | Lung cancer (NSCLC) | |
| Lapatinib (Anti-HER2 TKI) | Tykerb/Tyverb | 2007 & 2010 | Breast cancer | Combined with CT; HER2+ tumors. Also for hormone receptor positive tumors. |
| Afatinib (anti-EGFR TKI) | Gilotrif | 2013 | Lung cancer (NSCLC) | Patients with tumors expressing mutant EGFR (exon 19 deletions or exon 21 mutations). |
CT, chemotherapy.
A scenario linking anti-EGFR/HER2 drugs to TACs and to dedifferentiation
According to a simple scenario, TKIs like lapatinib and antibodies like trastuzumab inhibit expansion of EGFR/HER2-driven TACs, thereby limit tumor growth, as in the case of HER2-positive breast cancer. Several lines of experimental evidence raise an alternative scenario, which relates to dedifferentiation of TACs and models of cancer stem cell (CSC). These latter models assume that only a small fraction of cancer cells can regrow a tumor, while their progeny lack this ability. 151 One line of experimental evidence revealed a correlation in mammary cancer between HER2 amplification and CSC frequency, as assessed by assaying the ALDH-1 marker. 152 Another experimental line showed that trastuzumab not only inhibits proliferation of breast cancer cells in vitro, but this mAb can also reduce several characteristics of stemness. 153 Correspondingly, the emerging model has two features: (i) oncogenic mutations like HER2 amplification affect primarily TACs, rather than CSCs, and (ii) TACs might dedifferentiate, either inducibly or stochastically, to enter the stem cell state. 154 After dealing with the origin of oncogenic mutations, we will discuss below potential roles played by EGFR-HER2 as inducers of dedifferentiation.
For several reasons oncogenic mutations might accumulate faster in TACs, as compared to their accrual in tissue-specific stem cells. Unlike SCs, TACs are short-lived, but they are rapidly dividing, and their numbers exceed by far numbers of stem cells. More frequent mitoses of TACs translate to more abundant DNA damage generated endogenously by errors during DNA replication (termed: replication stress 155). This, according to some calculations, accounts for two thirds of the variation in cancer risk among tissues, although in some tumor types, such as skin and lung cancer, environmental risks might be more dominant. 2 The dedifferentiation scenario is attractive because it means that anti-EGFR/HER2 drugs are endowed with a previously unrecognized feature: the drugs might block entry of mutation-bearing TACs into the long-lived and self-renewing CSC compartment. Hence, it is worthy asking whether there is enough evidence in support of dedifferentiation.
It has long been argued that dedifferentiation is a main source of functional stem cells during tissue repair. For example, differentiating germ cells can revert into functional SCs in ovaries of Drosophila melanogaster, probably as a mechanism that replenishes germ cells after depletion by genotoxic chemicals, radiation or ageing. 23 Regeneration of the heart provides a closer example as it diminishes after the first week of life (in mice), due to downregulation of neuregulin and the co-receptor, HER2. 156 As expected, induction of a constitutively active HER2 in neonatal and adult cardiomyocytes resulted in dedifferentiation and proliferation mediated by ERK and AKT. In the context of tumors, dedifferentiation is likely instigated by oncogenic mutations, since some mutations involve stem cell genes like WNT and Notch, while mutations in proliferation-inducing genes, such as H-RAS, increase TAC reversion to stem cells in model systems. 157 More relevant to this review, growth factors and certain signaling pathways have been implicated as drivers of dedifferentiation. For instance, as discussed previously in this review, neural SCs in the subventricular zone (SVZ) continue to generate new neurons in the adult brain. This is likely due to the action of EGF, which induces conversion of highly mitotic TACs into multipotent neural stem cells. 83 Reprogramming of non-stem mammary cancer cells was similarly induced by transforming growth factor beta 158 or by irradiation, 159 and these events were accompanied by re-expression of specific transcription factors, either OCT4 and KLF4, or ZEB1. Studying yet a third cell lineage, intestinal stem cells, Greten and colleagues identified NF-κB stimulation and inflammation as signaling events that enhance WNT activation and induce dedifferentiation of non-SCs able to acquire tumor-initiating capacity. 160
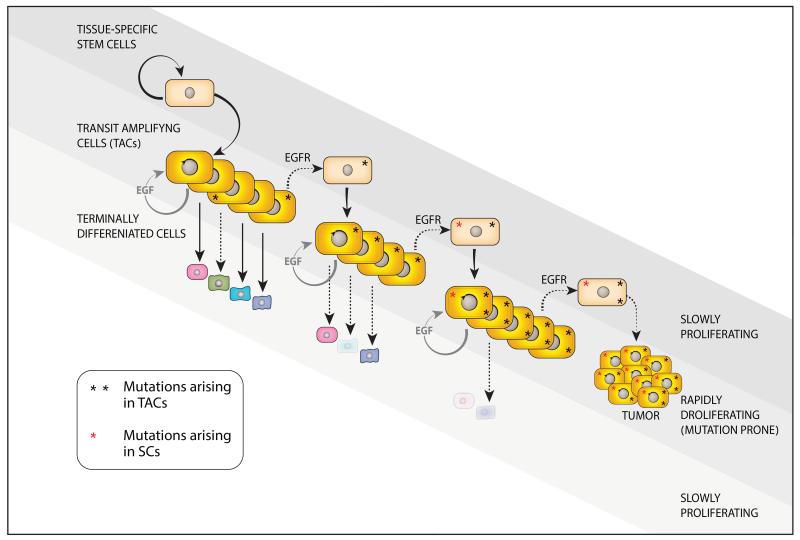
Thus, the emerging roles for the EGFR/HER2 module in dedifferentiation and in acquisition of stem cell characteristics expand their well-characterized actions in mitogenesis, originally described by Carpenter and Cohen some 40 years ago. 161 According to the new hypothesis, the module and the affiliated growth factors not only propel rapid enlargement of the TAC compartment of some regenerating tissues, but they also drive conversion of TACs to a state similar to stem cells (Figure 4). This scenario is especially relevant to solid tumors and multistep accumulation of somatic mutations. Accrual of such mutations might start in the self-renewing compartment of a tissue, 2 or it might affect TACs of epithelial, neural and glial organs, 154 which are often controlled by EGFR-HER2. In case the latter mechanism is correct, dedifferentiation of mutation-bearing TACs would enable cyclic accumulation of oncogenic mutations, along with their clonal fixation. It is therefore logical assuming that agents blocking EGFR or HER2 might arrest some tumors due to their effects on the TAC-to-CSC transition. At present, this mechanism of drug action remains a speculation. However, the importance of this putative action may be exemplified by the dissemination of primary tumor cells. According to the prevailing dogma, clusters of disseminated cancer cells (micro-metastases) will evolve into life-threatening, drug-resistant secondary tumors only if they contain CSCs. The model proposed herein offers an alternative format: dedifferentiation of TACs into CSCs might be driven at sites of metastasis by active forms of the EGFR-HER2 module. Hence, drugs targeting EGFR or HER2 might prevent both expansion of TACs and their dedifferentiation, thereby inhibit disease recurrences. This explanation proposes a mechanistic basis for the high recurrence inhibitory action of trastuzumab when administered in an adjuvant setting (post main treatment), especially within the early time window critical for seeding secondary tumors. 162
Figure 4. The EGFR-HER2 module might propel repeated cycles of TAC dedifferentiation to enable penetration of oncogenic mutations into the stem cell compartment and evolution of more aggressive tumors.
The cellular hierarchy shown in Figure 1 is adopted here. The model presented assumes that active EGFR-HER2 of TACs expands these progenitors and, at the same time, promotes their dedifferentiation to stem cells. Due to their rapid rate of mitoses, the TACs, more than stem cells, suffer more from DNA damage generated endogenously by errors during DNA replication. Accordingly, the combination of frequent mutagenesis and dedifferentiation repeatedly feeds the stem cell compartment with more oncogenic mutations, which gives rise to progressively more aggressive tumors. Environmentally-induced and inborn mutations directly affecting adult SCs likely exacerbate tumor initiation and progression. Because the EGFR-HER2 module controls proliferation and probably also dediffentitaion of TACs, and these progenitors accumulate most mutations, it is predictable that pharmacological interceptors of the module would inhibit mutation accrual by SCs of epithelial and neural organs.
Concluding remarks
While our understanding of tumor cell hierarchies and the multistep genetic process that initiates solid tumors is ever deepening, harnessing this knowledge for better treatment of cancer patients remains unsatisfactory. Because the EGFR-HER2 module is one of the best-explored signaling systems in both vertebrates and invertebrates, and it is also one of the most fertile fields of new cancer drugs, the present review adopted this system in order to contour a model bridging stem cells, tumor progression and opportunities for drug interventions. And although we related here primarily to carcinomas, brain tumors are equally relevant: EGFR is a major driver of glioblastoma multiforme (GBM), 163-165 including critical functions in brain CSCs 166 and, among several growth factors tested, only EGF was essential for maintaining self-renewal of glioma-derived CSCs. 167 The paucity and elusive nature of CSCs pose hurdles that would significantly inhibit attempts to test the proposed model. This is especially difficult because current transgenic technologies simultaneously induce oncogenic mutations in many cells (instead of a single cell) and mimicking the successive mode of mutagenesis is technically difficult. Nevertheless, the expected outcome of validating the model proposed herein, or in fact any model, is huge in terms of our ability to precisely and timely eliminate very small populations of tumor initiating cells and to block the dedifferentiation shunt that likely feeds them anew with oncogenic mutations.
Acknowledgements
We thank Lilach Gilboa and Maik Dahlhoff for insightful discussions. MRS is supported by the German Research Foundation (DFG), the Else-Kröner-Fresenius Stiftung, the Fritz Thyssen Foundation, and the Cicatricial Alopecia Research Foundation. YY is the incumbent of the Harold and Zelda Goldenberg Professorial Chair. Currently, he is Research Professor of the Israel Cancer Research Fund. He would like to acknowledge financial support from the European Research Council, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the family of Mr. Marvin Tanner.
References
- 1.Pellettieri J, Sanchez Alvarado A. Cell turnover and adult tissue homeostasis: from humans to planarians. Annual review of genetics. 2007;41:83–105. doi: 10.1146/annurev.genet.41.110306.130244. [DOI] [PubMed] [Google Scholar]
- 2.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature medicine. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. Journal of cellular physiology. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 7.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nature reviews Molecular cell biology. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 8.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature reviews Molecular cell biology. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 10.Grompe M. Liver stem cells, where art thou? Cell stem cell. 2014;15:257–258. doi: 10.1016/j.stem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 12.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 13.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 14.Doupe DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays: news and reviews in molecular, cellular and developmental biology. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim RW, Hauschka SD. A rapid decrease in epidermal growth factor-binding capacity accompanies the terminal differentiation of mouse myoblasts in vitro. The Journal of cell biology. 1984;98:739–747. doi: 10.1083/jcb.98.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Gupta BP, Hanna-Rose W, Sternberg PW. Morphogenesis of the vulva and the vulval-uterine connection. WormBook. 2012:1–20. doi: 10.1895/wormbook.1.152.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulston JE, White JG. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer R, Shilo BZ. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- 21.Yasugi T, Sugie A, Umetsu D, Tabata T. Coordinated sequential action of EGFR and Notch signaling pathways regulates proneural wave progression in the Drosophila optic lobe. Development. 2010;137:3193–3203. doi: 10.1242/dev.048058. [DOI] [PubMed] [Google Scholar]
- 22.Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Current biology: CB. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 24.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman R, Levy RB, Peles E, Yarden Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry. 1990;29:11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- 27.Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 28.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 29.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Molecular cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A Structural Perspective on the Regulation of the Epidermal Growth Factor Receptor. Annu Rev Biochem. 2015 doi: 10.1146/annurev-biochem-060614-034402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. The EMBO journal. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nature Review Molecular Cell Biology. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- 33.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, Amariglio N, Vaisman N, Segal E, Rechavi G, Alon U, Mills GB, Domany E, Yarden Y. A module of negative feedback regulators defines growth factor signaling. Nature genetics. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- 35.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. The Journal of cell biology. 2010;189:871–883. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Molecular cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 37.Zwang Y, Yarden Y. Systems biology of growth factor-induced receptor endocytosis. Traffic. 2009;10:349–363. doi: 10.1111/j.1600-0854.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 38.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. Journal of cell science. 2012;125:265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 39.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. The EMBO journal. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 40.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. The Journal of biological chemistry. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 41.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Molecular and cellular biology. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. The EMBO journal. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. The American journal of pathology. 2008;173:14–24. doi: 10.2353/ajpath.2008.070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 45.Fowler KJ, Walker F, Alexander W, Hibbs ML, Nice EC, Bohmer RM, Mann GB, Thumwood C, Maglitto R, Danks JA, et al. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci U S A. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider MR. The magnificient seven: epidermal growth factor receptor ligands. Semin Cell Dev Biol. 2014;28 doi: 10.1016/j.semcdb.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 48.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 49.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121:68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 50.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 51.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R, Nature Publishing Group Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 2007;75:770–787. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 55.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 56.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 57.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 58.Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 59.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 60.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 61.Kitade M, Factor VM, Andersen JB, Tomokuni A, Kaji K, Akita H, Holczbauer A, Seo D, Marquardt JU, Conner EA, Lee SB, Lee YH, Thorgeirsson SS. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Genes & development. 2013;27:1706–1717. doi: 10.1101/gad.214601.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 64.Genetos DC, Rao RR, Vidal MA. Betacellulin inhibits osteogenic differentiation and stimulates proliferation through HIF-1alpha. Cell and tissue research. 2010;340:81–89. doi: 10.1007/s00441-010-0929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- 66.Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. Journal of cellular biochemistry. 2011;112:1749–1760. doi: 10.1002/jcb.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, Harris JR, Deoliviera D, Sullivan JM, Chao NJ, Kirsch DG, Chute JP. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature medicine. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieto-Sampedro M, Gomez-Pinilla F, Knauer DJ, Broderick JT. Epidermal growth factor receptor immunoreactivity in rat brain astrocytes. Response to injury. Neuroscience letters. 1988;91:276–282. doi: 10.1016/0304-3940(88)90693-3. [DOI] [PubMed] [Google Scholar]
- 69.Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nature neuroscience. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 70.Iyer R, Thames HD, Tealer JR, Mason KA, Evans SC. Effect of reduced EGFR function on the radiosensitivity and proliferative capacity of mouse jejunal crypt clonogens. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2004;72:283–289. doi: 10.1016/j.radonc.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Clevers H, Batlle E. SnapShot: the intestinal crypt. Cell. 2013;152:1198–1198. e1192. doi: 10.1016/j.cell.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 72.Gur G, Rubin C, Katz M, Amit I, Citri A, Nilsson J, Amariglio N, Henriksson R, Rechavi G, Hedman H, Wides R, Yarden Y. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. The EMBO journal. 2004;23:3270–3281. doi: 10.1038/sj.emboj.7600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laederich MB, Funes-Duran M, Yen L, Ingalla E, Wu X, Carraway KL, 3rd, Sweeney C. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. The Journal of biological chemistry. 2004;279:47050–47056. doi: 10.1074/jbc.M409703200. [DOI] [PubMed] [Google Scholar]
- 74.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature cell biology. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marchbank T, Goodlad RA, Lee CY, Playford RJ. Luminal epidermal growth factor is trophic to the small intestine of parenterally fed rats. Clinical science. 1995;89:117–120. doi: 10.1042/cs0890117. [DOI] [PubMed] [Google Scholar]
- 77.Bashir O, Fitzgerald AJ, Berlanga-Acosta J, Playford RJ, Goodlad RA. Effect of epidermal growth factor administration on intestinal cell proliferation, crypt fission and polyp formation in multiple intestinal neoplasia (Min) mice. Clinical science. 2003;105:323–330. doi: 10.1042/CS20030023. [DOI] [PubMed] [Google Scholar]
- 78.Dahlhoff M, Horst D, Gerhard M, Kolligs FT, Wolf E, Schneider MR. Betacellulin stimulates growth of the mouse intestinal epithelium and increases adenoma multiplicity in Apc+/Min mice. FEBS letters. 2008;582:2911–2915. doi: 10.1016/j.febslet.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 79.van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature cell biology. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell stem cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong CK, Alvarez-Buylla A. SnapShot: adult neurogenesis in the V-SVZ. Neuron. 2014;81:220–220. e221. doi: 10.1016/j.neuron.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci U S A. 2013;110:E1045–1054. doi: 10.1073/pnas.1219563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 84.Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neuroscience letters. 2006;403:63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 85.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Perez O, Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain research reviews. 2011;67:147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galvez-Contreras AY, Quinones-Hinojosa A, Gonzalez-Perez O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Frontiers in cellular neuroscience. 2013;7:258. doi: 10.3389/fncel.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gomez-Gaviro MV, Scott CE, Sesay AK, Matheu A, Booth S, Galichet C, Lovell-Badge R. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci U S A. 2012;109:1317–1322. doi: 10.1073/pnas.1016199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lecona E, Fernandez-Capetillo O. Replication stress and cancer: it takes two to tango. Experimental cell research. 2014;329:26–34. doi: 10.1016/j.yexcr.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification and overexpression of the EGF receptor gene in primary human glioblastomas. J Cell Sci Suppl. 1985;3:161–172. doi: 10.1242/jcs.1985.supplement_3.16. [DOI] [PubMed] [Google Scholar]
- 92.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeuken J, Sijben A, Alenda C, Rijntjes J, Dekkers M, Boots-Sprenger S, McLendon R, Wesseling P. Robust detection of EGFR copy number changes and EGFR variant III: technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009;19:661–671. doi: 10.1111/j.1750-3639.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer research. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 95.Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM, Yoshimoto K, King JC, Nghiemphu P, Yuza Y, Xu Q, Greulich H, Thomas RK, Paez JG, Peck TC, Linhart DJ, Glatt KA, Getz G, Onofrio R, Ziaugra L, Levine RL, Gabriel S, Kawaguchi T, O’Neill K, Khan H, Liau LM, Nelson SF, Rao PN, Mischel P, Pieper RO, Cloughesy T, Leahy DJ, Sellers WR, Sawyers CL, Meyerson M, Mellinghoff IK. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci U S A. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Science signaling. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 100.Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, Furnari FB, White FM. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA, Scott AM, Johns TG. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. doi: 10.1593/neo.09230. 442 p following 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeineldin R, Ning Y, Hudson LG. The Constitutive Activity of Epidermal Growth Factor Receptor vIII Leads to Activation and Differential Trafficking of Wild-type Epidermal Growth Factor Receptor and erbB2. Journal of Histochemistry & Cytochemistry. 2010;58:529–541. doi: 10.1369/jhc.2010.955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer research. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 104.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer research. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 105.Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocrine-related cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- 106.Pines G, Huang PH, Zwang Y, White FM, Yarden Y. EGFRvIV: a previously uncharacterized oncogenic mutant reveals a kinase autoinhibitory mechanism. Oncogene. 2010 doi: 10.1038/onc.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 108.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 109.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 111.Teng YH, Tan WJ, Thike AA, Cheok PY, Tse GM, Wong NS, Yip GW, Bay BH, Tan PH. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast cancer research: BCR. 2011;13:R35. doi: 10.1186/bcr2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR, Meyerson M. Oncogenic transformation by inhibitor-sensitive and - resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nature reviews Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 114.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes & development. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, Chirieac LR, Padera R, Bronson RT, Kim W, Janne PA, Shapiro GI, Tenen D, Johnson BE, Weissleder R, Sharpless NE, Wong KK. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 118.Regales L, Balak MN, Gong Y, Politi K, Sawai A, Le C, Koutcher JA, Solit DB, Rosen N, Zakowski MF, Pao W. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PloS one. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, Liang MC, Perera SA, Zaghlul S, Borgman CL, Kubo S, Takahashi M, Sun Y, Chirieac LR, Padera RF, Lindeman NI, Janne PA, Thomas RK, Meyerson ML, Eck MJ, Engelman JA, Shapiro GI, Wong KK. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwak EL, Jankowski J, Thayer SP, Lauwers GY, Brannigan BW, Harris PL, Okimoto RA, Haserlat SM, Driscoll DR, Ferry D, Muir B, Settleman J, Fuchs CS, Kulke MH, Ryan DP, Clark JW, Sgroi DC, Haber DA, Bell DW. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:4283–4287. doi: 10.1158/1078-0432.CCR-06-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, Sharma SV, Brannigan BW, Mohapatra G, Settleman J, Haber DA. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nature genetics. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 123.Heinmoller P, Gross C, Beyser K, Schmidtgen C, Maass G, Pedrocchi M, Ruschoff J. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:5238–5243. [PubMed] [Google Scholar]
- 124.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 125.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 126.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. The oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 127.Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hechtman JF, Polydorides AD. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med. 2012;136:691–697. doi: 10.5858/arpa.2011-0168-RS. [DOI] [PubMed] [Google Scholar]
- 129.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, Chaudhuri S, Pujara K, Guillory J, Edgar KA, Janakiraman V, Scholz RP, Bowman KK, Lorenzo M, Li H, Wu J, Yuan W, Peters BA, Kan Z, Stinson J, Mak M, Modrusan Z, Eigenbrot C, Firestein R, Stern HM, Rajalingam K, Schaefer G, Merchant MA, Sliwkowski MX, de Sauvage FJ, Seshagiri S. Oncogenic ERBB3 mutations in human cancers. Cancer cell. 2013;23:603–617. doi: 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 131.Jeong EG, Soung YH, Lee JW, Lee SH, Nam SW, Lee JY, Yoo NJ, Lee SH. ERBB3 kinase domain mutations are rare in lung, breast and colon carcinomas. International journal of cancer Journal international du cancer. 2006;119:2986–2987. doi: 10.1002/ijc.22257. [DOI] [PubMed] [Google Scholar]
- 132.Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, Cronin JC, Cruz P, Rosenberg SA, Samuels Y. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nature genetics. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soung YH, Lee JW, Kim SY, Wang YP, Jo KH, Moon SW, Park WS, Nam SW, Lee JY, Yoo NJ, Lee SH. Somatic mutations of the ERBB4 kinase domain in human cancers. International journal of cancer Journal international du cancer. 2006;118:1426–1429. doi: 10.1002/ijc.21507. [DOI] [PubMed] [Google Scholar]
- 134.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kurppa K, Elenius K. Mutated ERBB4: a novel drug target in metastatic melanoma? Pigment Cell Melanoma Res. 2009;22:708–710. doi: 10.1111/j.1755-148X.2009.00635.x. [DOI] [PubMed] [Google Scholar]
- 136.Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 137.Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J, Cold Spring Harbor Lab Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular signalling. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 139.Kodama T, Ikeda E, Okada A, Ohtsuka T, Shimoda M, Shiomi T, Yoshida K, Nakada M, Ohuchi E, Okada Y. ADAM12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin-binding epidermal growth factor. The American journal of pathology. 2004;165:1743–1753. doi: 10.1016/S0002-9440(10)63429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28C:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 142.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsu D, Fukata M, Hernandez YG, Sotolongo JP, Goo T, Maki J, Hayes LA, Ungaro RC, Chen A, Breglio KJ, Xu R, Abreu MT. Toll-like receptor 4 differentially regulates epidermal growth factor-related growth factors in response to intestinal mucosal injury. Laboratory investigation; a journal of technical methods and pathology. 2010;90:1295–1305. doi: 10.1038/labinvest.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panupinthu N, Yu S, Zhang D, Zhang F, Gagea M, Lu Y, Grandis JR, Dunn SE, Lee HY, Mills GB. Self-reinforcing loop of amphiregulin and Y-box binding protein-1 contributes to poor outcomes in ovarian cancer. Oncogene. 2013 doi: 10.1038/onc.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sauer L, Gitenay D, Vo C, Baron VT. Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene. 2010;29:2628–2637. doi: 10.1038/onc.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA, 3rd, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 147.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature reviews Drug discovery. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 148.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nature medicine. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 149.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 150.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nature biotechnology. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 151.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 152.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 154.Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer discovery. 2015;5:22–24. doi: 10.1158/2159-8290.CD-14-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mazouzi A, Velimezi G, Loizou JI. DNA replication stress: causes, resolution and disease. Experimental cell research. 2014;329:85–93. doi: 10.1016/j.yexcr.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 156.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nature cell biology. 2015;17:627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 157.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D’Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem cells. 2012;30:833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 161.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 162.Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22(Suppl 2):S152–155. doi: 10.1016/j.breast.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 163.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes & development. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 164.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 165.Kalman B, Szep E, Garzuly F, Post DE. Epidermal growth factor receptor as a therapeutic target in glioblastoma. Neuromolecular medicine. 2013;15:420–434. doi: 10.1007/s12017-013-8229-y. [DOI] [PubMed] [Google Scholar]
- 166.Mazzoleni S, Politi LS, Pala M, Cominelli M, Franzin A, Sergi Sergi L, Falini A, De Palma M, Bulfone A, Poliani PL, Galli R. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer research. 2010;70:7500–7513. doi: 10.1158/0008-5472.CAN-10-2353. [DOI] [PubMed] [Google Scholar]
- 167.Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. The Journal of biological chemistry. 2008;283:10958–10966. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]