Abstract
Purpose
Few studies have estimated population prevalence and morbidity of primary immunodeficiency diseases (PIDD). We used administrative healthcare databases to estimate the prevalence of PIDD diagnoses in the United States from 2001 to 2007.
Methods
MarketScan databases compile claims from commercial health insurance plans and Medicaid, recording individual diagnoses for outpatient encounters and hospital stays. We used a cross sectional survey to estimate prevalence of PIDD using related ICD-9 codes (279.0, 279.1, 279.2, 279.8, 279.9, 288.1 and 288.2). Persons with secondary immunodeficiency diagnoses were excluded from analysis.
Results
Between 2001 and 2007, prevalence of any PIDD diagnosis increased from 38.9 to 50.5 per 100,000 among privately insured and from 29.1 to 41.1 per 100,000 among publicly insured persons. B cell defects predominated. Prevalence was more than twice as high among Whites as among Blacks or Hispanics.
Conclusion
In this large database, we found a higher prevalence of diagnosed PIDD than has been reported previously from registries. Increased awareness may have contributed to the increasing prevalence.
Keywords: Primary immunodeficiency, prevalence, epidemiology, genetic diseases, immunology
Introduction
Primary Immunodeficiency diseases (PIDD) are a group of more than 130 distinct disorders caused by genetic defects of the immune system. Most of these disorders are caused by single gene defects, but the variable penetrance of these mutations results in heterogeneous phenotypes which leads to delays in diagnosis. Individuals with PIDD suffer from more frequent and severe infections and are likely to have frequent encounters with healthcare providers prior to a diagnosis of PIDD. Many patients continue to utilize healthcare resources at a higher rate than healthy individuals even after the diagnosis is made and treatment initiated [1].
PIDD are considered “rare” diseases, but most prevalence estimates have been based on selected populations (e.g. specialty clinics, disease registries). Disease registries from several countries suggest a prevalence of 1:8,500 to 1:100,000 persons [2–10]. Analysis of healthcare data from a single county in Minnesota estimated the incidence of PIDD to be 10.3 per 100,000 person-years [11]. Data from a random telephone survey of 10,000 households in the U.S. suggested a prevalence of 1:1200 persons [12].
Previous estimates have been based on disease registries or through reporting of specific PIDD in a clinical population [11, 13–19]. Extrapolating disease prevalence from the two U.S. studies, Bousfiha et al. calculated the estimated expected prevalence of PIDD cases and compared this to reported estimates in disease registries from the U.S., Europe, Asia and Australia. These estimated frequencies were much higher than the reported estimates based on the registry data suggesting both underreporting and ascertainment bias [20]. They concluded that the data obtained from registries does not provide adequate coverage to estimate prevalence or age distribution of PIDD.
In the United States, the development and implementation of newborn screening for severe combined immune deficiency (SCID) has begun to provide data on the birth prevalence of this combined immune deficiency [21–23].
The purpose of this study was to estimate the population prevalence of PIDD in a large national sample of children and adults using a cross sectional survey of data from commercial healthcare claims and Medicaid programs. Data on the number of hospitalizations and length of stay for persons with a PIDD diagnosis were available from the Commercial Claims and Encounters (CCE) for 2001 to 2007 and Multi-State Medicaid (MC) databases for 2001–2005. We also identified the most prevalent comorbidities associated with these hospitalizations. This is the first study to utilize data from a national population based database to estimate PIDD prevalence and hospitalizations.
Methods
Databases
Data were derived from the Truven Health MarketScan® 2001–2007 CCE and 2001–2005 MC databases. These data included individual-level, de-identified enrollment data and health insurance claims across the continuum of care (both inpatient and outpatient). The CCE database collects information from employers and health plans across the entire United States (37 contributors in 2001, 138 in 2007) who provide private healthcare coverage for enrollees and their dependents. Information was available for 5,816,905 enrollees in 2001 increasing up to 28,761,500 enrollees in 2007, aged 64 years or younger. Individuals age 65 and older were not included in the analysis since a large proportion of these persons receive their healthcare coverage through Medicare. The MC database included claims for pediatric patients (aged 0–21 years); it included 1,428,884 persons in 2001 increasing to 2,964,706 persons in 2005. (Table 1) The MC database included 6 unidentified states in 2001 and 11 in 2005. Both databases included demographic variables such as age and gender. Only the CCE database includes a variable specifying the geographic region of the United States for each enrollee and only the MC database includes a variable for race. Individual encounters included a unique enrollee identification number with the date of encounter, the site of service (inpatient, outpatient), the diagnosis code, the procedure codes, and information related to claims and payments. For hospital admissions, the length of stay and the diagnosis and procedure codes were recorded.
Table 1.
Demographic characteristics of the Commercial Claims and Encounters (CCE) and Medicaid (MC) databases
| Year | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | |
|---|---|---|---|---|---|---|---|---|
| Total enrollment | CCE | 5,816,905 | 7,656,625 | 7,699,763 | 13,136,933 | 17,560,383 | 16,159,068 | 28,761,500 |
| MC | 1,428,884 | 2,551,574 | 2,715,364 | 2,866,049 | 2,964,706 | – | – | |
| Gender % | CCE Female | 52.0 | 52.4 | 52.6 | 51.8 | 51.8 | 51.5 | 51.3 |
| CCE Male | 48.0 | 47.6 | 47.4 | 48.2 | 48.2 | 48.5 | 48.7 | |
| MC Female | 51.2 | 50.7 | 50.5 | 50.4 | 50.4 | – | – | |
| MC Male | 48.8 | 49.3 | 49.5 | 49.6 | 49.6 | – | – | |
| Age groups CCE (yrs) | 0–21 | 1,827,573 | 2,422,478 | 2,438,885 | 4,223,799 | 5,640,248 | 5,249,125 | 9,461,654 |
| <1 | 102,396 | 146,847 | 151,994 | 261,465 | 363,999 | 331,883 | 599,846 | |
| 1–5 | 329,041 | 460,400 | 465,682 | 823,040 | 1,120,266 | 1,042,244 | 1,879,157 | |
| 6–10 | 390,070 | 516,975 | 517,496 | 910,407 | 1,224,565 | 1,143,177 | 2,072,231 | |
| 11–15 | 446,342 | 583,778 | 590,564 | 1,031,672 | 1,370,225 | 1,274,871 | 2,252,706 | |
| 16–21 | 559,724 | 714,478 | 713,049 | 1,197,215 | 1,561,193 | 1,456,950 | 2,657,714 | |
| 22–34 | 934,999 | 1,300,506 | 1,310,389 | 2,367,229 | 3,252,262 | 2,904,017 | 5,298,314 | |
| 35–44 | 987,971 | 1,281,577 | 1,289,363 | 2,242,456 | 3,027,094 | 2,746,752 | 4,851,016 | |
| 45–54 | 1,157,208 | 1,457,178 | 1,443,143 | 2,398,147 | 3,170,155 | 2,942,310 | 5,190,915 | |
| 55–64 | 909,154 | 1,194,886 | 1,217,983 | 1,905,302 | 2,470,624 | 2,316,864 | 3,959,601 | |
| Age groups MC (yrs) | <1 | 115,569 | 183,977 | 185,844 | 196,110 | 204,352 | – | – |
| 1–5 | 457,139 | 796,288 | 838,013 | 879,144 | 904,336 | – | – | |
| 6–10 | 345,914 | 615,412 | 647,135 | 677,298 | 702,220 | – | – | |
| 11–15 | 283,799 | 539,849 | 588,212 | 622,841 | 635,535 | – | – | |
| 16–21 | 226,463 | 416,048 | 456,160 | 490,656 | 518,263 | – | – |
Inclusion/Exclusion Criteria
Only encounters with a valid unique identification number for each claim in the database were included in our analysis. The CCE and MC databases record outpatient and inpatient encounters, listing 2 ICD-9 codes for each outpatient encounter and up to 15 codes for hospitalizations. The International Classification of Disease, 9th Edition, Clinical Modifications (ICD-9) classifies PIDD according to the predominant immune defect (B cell defects, T cell defects, Combined defects, Neutrophil/Phagocyte defects, and Other nonspecified immune defects). The CCE and MC databases were queried for PIDD using these ICD-9 codes (Table 2). We excluded claims for persons who had any ICD-9 diagnosis code associated with secondary causes of immune deficiency, i.e. malignancies, infection with Human Immunodeficiency Virus (HIV) or treatment with immunosuppressive medications. (Table 2) We also excluded CCE enrollees age 65 years or older, who are covered by Medicare and less likely to participate in commercial insurance plans.
Table 2.
Inclusion and exclusion ICD-9 codes
| Code description | ICD-9 Codes |
|---|---|
| Inclusion | |
| B cell defects (e.g. hypogammaglobulinemia, CVID, XLA) | 279.00–279.09 |
| T cell defects (e.g. DiGeorge syndrome, IPEX) | 279.10–279.19 |
| Combined defects (e.g. SCID) | 279.2 |
| Neutrophil defects (e.g. CGD, LAD, neutropenia) | 288.1, 288.2 |
| Other specified disorders of the immune system (e.g. Complement deficiency, | 279.8, 279.9 |
| Exclusion | |
| HIV | 042–044.9 |
| Malignancies (solid organs) | 140–199 |
| Lymphoid tumors (e.g. multiple myeloma, hodgkins lymphoma) | 200–203.8 |
| Chronic leukemias | 204.1–207.8 |
| Metabolic disorders | 260–273.9 |
| Transplants (kidney, heart, liver) | V42.0–42.9 |
| Comorbidities (hospitalizations) | |
| Infections with specific pathogens | 001–136 |
| Organ specific infections: respiratory | 461–488 |
| Organ specific infections: skin | 680–686 |
| Chronic lung disease | 490–496 |
| Chronic gastrointestinal disease | 555–558 |
| Other comorbidities (fever, weight loss, splenomegaly, lymphadenopathy, bacteremia) | 780.6, 783.2, 783.4, 785.6, 789.2, 790.7 |
Statistical Methods
Data were extracted from MarketScan using Medstat DataProbe (Thomson Medstat, 2006) and analyzed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). Data fields utilized in this analysis included age, gender, region (CCE only), race (MC only), ICD-9 diagnoses, type of encounter (outpatient, inpatient), and length of hospital stay. For CCE, age was stratified in the following groups 0–21, 22–34, 35– 44, 45–54 and 55–64 years. For comparisons of the pediatric populations, we created the following subgroups: <1, 1–5, 6–10, 11–15 and 16–21 years of age.
Administrative prevalence (hereafter referred to as prevalence) for each year (2001–2007 for CCE; 2001–2005 for MC) was the number of persons with any PIDD diagnosis divided by the total enrolled population in a given year. Prevalence per 100,000 persons per year is shown in Table 3 by disease group (B cell defects, T cell defects, neutrophil defects, combined defects and other defects). Diagnosis groups were not mutually exclusive as one person could be counted in more than one group if they had one or more claim(s) with ICD-9 codes from 2 or more different groups. All prevalence estimates were stratified by age, gender, race (MC only) and region (CCE only).
Table 3.
Prevalence of primary immunodeficiency diseases (PIDD) per 100,000 enrolled persons by database and year
| a. Commercial Claims and Encounters | |||||||
|---|---|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | |
| All PID | 38.9 | 40.8 | 41.2 | 42.0 | 43.2 | 48.4 | 50.5* |
| B cell | 19.7 | 20.4 | 20.5 | 22.3 | 24.2 | 27.1 | 28.1* |
| T cell | 2.9 | 2.8 | 2.5 | 2.6 | 3.0 | 2.7 | 3.4* |
| Combined | 1.6 | 1.7 | 1.8 | 1.6 | 1.9 | 2.1 | 1.9 |
| Neutrophil | 4.6 | 4.6 | 4.7 | 5.4 | 5.2 | 5.8 | 6.9* |
| Other | 11.8 | 12.9 | 13.2 | 11.7 | 10.7 | 12.8 | 12.5 |
| b Medicaid | |||||||
| All PID | 29.1 | 30.8 | 32.8 | 38.3 | 41.1** | ||
| B cell | 15.5 | 13.9 | 13.9 | 18.4 | 18.9** | ||
| T cell | 6.7 | 8.6 | 10.0 | 10.3 | 11.6** | ||
| Combined | 1.5 | 2.0 | 1.9 | 2.2 | 3.8** | ||
| Neutrophil | 3.4 | 3.0 | 3.3 | 3.7 | 3.5 | ||
| Other | 4.0 | 5.4 | 6.1 | 6.4 | 6.1** | ||
Test for trend p<0.0002
Test for trend p<0.0001
The proportion of persons with a hospital admission, the mean number of admissions per person and the mean length of stay were also calculated. The prevalence of comorbidities associated with hospital admissions (pathogen specific infections, respiratory infections, skin infections, chronic lung disease, and other nonspecific diagnoses associated with infections, such as fever, splenomegaly, bacteremia, weight loss) was estimated based on the frequency of these additional ICD-9 codes listed for each hospitalization (Table 2).
Tests for trend were used to evaluate changes in prevalence over time. For univariate analyses, chi square tests were used for categorical variables and nonparametric tests were employed where continuous data were not normally distributed. Linear and logistic regression were used to examine the effect of having a PIDD diagnosis on the number of hospital admissions.
Results
Prevalence of PIDD
The CCE database contained records from 5,816,905 enrollees in 2001 increasing to 28,761,500 enrollees in 2007. The age, gender and regional distribution was similar to the U.S. population based on 2005 U.S. census data (Suppl. Table E1). The MC database contained information from 1,428,884 enrollees in 2001 increasing to 2,964,706 enrollees in 2005. (Table 1) Persons who self-identified as “Black” were over represented in the MC population (34.6–39.2 %) compared to 12.3 % [24].
Population prevalence of any PIDD diagnosis increased 30 % from 38.9 to 50.5 per 100,000 in CCE (p<0.0001) and 41 % from 28.2 to 39 per 100,000 in MC (p<0.0001). When stratified by diagnosis group, B cell defects accounted for most of the primary immunodeficiency diagnoses and also demonstrated the most marked increase in prevalence over time (p<0.0001 for CCE and MC). Both CCE and MC groups saw a significant increase in prevalence of T cell defects (p <0.0002) and Combined immune defects (p<0.01). The prevalence of neutrophil defects increased over time in the CCE population (p<0.0001), but not in the MC population. The prevalence of other defects increased over time in the MC population (p <0.0001) but not in the CCE population (Table 3).
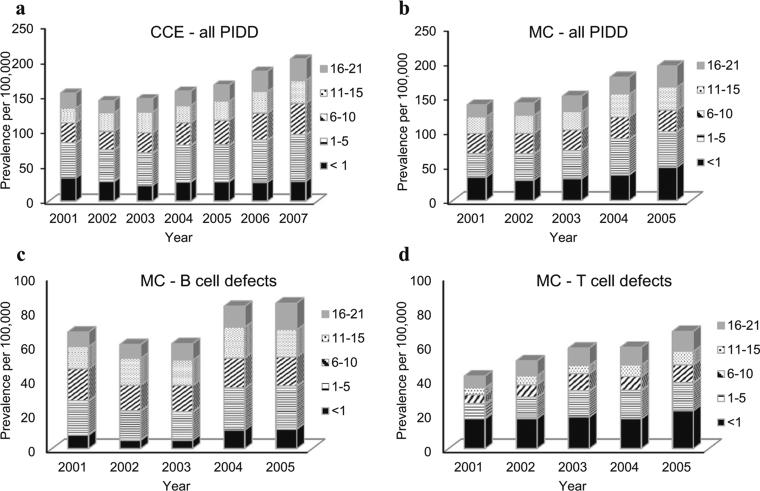
When we stratified the CCE population by age, the older age groups (45–54, 55–64 years) had a higher prevalence for all PIDD diagnoses compared with the population aged 21 and younger. (Suppl. Table E2) In the pediatric population, the average prevalence of any PIDD was highest in the 1–5 years age group (CCE 46 to 67 per 100,000; MC 29 to 42 per 100,000). For B cell defects, the average prevalence was highest in the 1–5 years range (MC 20 to 25 per 100,000) and increased across all years (p<0.0001). In the MC population, the highest prevalence for T cell defects was observed among children <1 year of age (MC 15.6 to 21.5 per 100,000) (Fig. 1).
Fig. 1.
Prevalence of Primary Immunodeficiency diseases by age for: a Commercial Claims and Encounters - pediatric age ranges 0–21; b Medicaid patients for all PIDD; c Medicaid patients for B cell defects, and d for T cell defects
In the CCE population overall, females had a higher prevalence of any PIDD, B cell defects, neutrophil defects, combined immune defects and other immune defects. However, in both the CCE and MC pediatric populations, B and T cell defects were more common in males (results not shown). In the MC population the prevalence of combined immune defects (e.g. SCID) was highest in males (results not shown).
Information on race and ethnicity was available only for the MC database. To assess whether there were any racial disparities in the diagnosis of PIDD, we calculated the prevalence separately for Blacks (B), Whites (W) and Hispanics (H). The following results are based on 2005 data; similar results were found for 2001–2004 (not shown). The prevalence of any PIDD among Whites was more than 2 times higher than among either Blacks or Hispanics (W=47.6 per 100,000, B=19.2, H=22.0). Whites also had higher prevalence than Blacks or Hispanic for B cell defects (25.9 per 100,000 versus 6.8 (B) or 1.3 (H)), T cell (11.0 per 100,000 versus 4.2 (B) or 8.4 (H)), combined immune defects (3.6 versus 2.8 or 1.9) and other defects (7.2 versus 3.7 or 5.2) but not for neutrophil defects.
Associated Morbidity
We used information on number of hospital admissions and length of stay (LOS) to assess PIDD-associated morbidity. In the MC population the proportion of persons with a hospital admission was higher each year from 2001 to 2005 among those with PIDD (range 13.9 to 18.6 %) compared to those without a PIDD diagnosis (range 7.9 to 8.9 %). Overall, persons with PIDD were about twice as likely to be hospitalized, controlling for year [Odds ratio 1.916 to 2.260, p<0.0001]. In the CCE population persons with PIDD were slightly more likely to be hospitalized (OR 1.14, 95 % CI 1.094–1.189, p<0.0001). Having a PIDD diagnosis was also related to the mean number of admissions per person per year for the MC population (1.55 versus 1.16, p<0.0001).
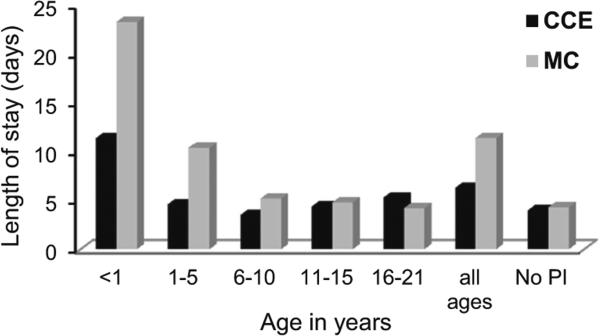
Compared with the enrolled population as a whole, persons with a PIDD had significantly longer hospital stays (mean length of stay [LOS] per year). This was true for the CCE (all ages) [p<0.0001], CCE (0–21 years) and MC (p<0.0001) populations. LOS was significantly longer for children <1 year of age (CCE median=13.6 [range 8.8 to 22.3 days], MC median=21.1 [range 15.0 to 29.8 days]) than for those 1–21 years of age (CCE median=7.9 [6.0 to 9.9 days], p<0.0001; MC mean=9.6 [7.4 to 11.4 days], p<0.0001)]. (Fig. 2 shows mean length of stay for 2005)
Fig. 2.
Mean Length of Stay (in days) in 2005 for Primary Immunodeficiency disease patients, Commercial Claims and Encounters (CCE) and Medicaid (MC)
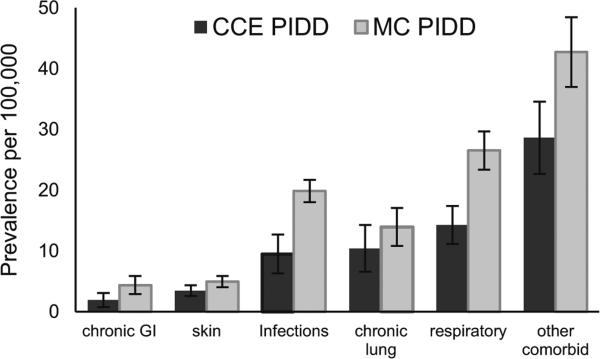
An increase in frequency and severity of infection is the hallmark of PIDD therefore, we examined the prevalence of a variety of comorbid diagnosis groups related to infections and the sequelae of infection associated with hospital admission for persons with any PIDD diagnosis. The ICD-9 diagnosis codes included those for pathogen specific infections, respiratory infections (e.g. pneumonia, sinus infections), skin infections, non-specific diagnoses associated with infection (e.g. fever, splenomegaly, bacteremia, weight loss), and diagnoses associated with sequelae of infection (e.g. chronic lung disease, chronic gastrointestinal disease). For both groups, non-specific infection associated comorbidities, respiratory infections, and pathogen specific infections had the highest prevalence among the comorbid conditions. Chronic lung disease also occurred commonly in both the MC and CCE populations. For each year (2001–2005), the prevalence of all infection related comorbidities was higher in the MC population compared to the CCE population. The mean prevalence of each category of comorbidity over the years 2001–2007 for CCE and 2001–2005 for MC is shown in Fig. 3.
Fig. 3.
Mean prevalence per 100,000 persons with 95 % confidence interval for comorbidities associated with hospitalization for persons with primary immunodeficiency disease for Commercial Claims and Encounters (CCE) and Medicaid (MC)
Discussion
We used a national commercial claims database (CCE) and a sample of Medicaid data (MC) from multiple states to estimate the prevalence and morbidity of PIDD. Unlike previous estimates based on registry reports, our estimates are based on a very large geographically and racially diverse population.
Our prevalence estimates of 5.1:10,000 (CCE) and 3.9:10,000 (MC) for any PIDD are markedly higher than previous estimates from registries and two population surveys, which range from 1:10,000 to 1:100,000. Prevalence increased over time in both CCE and MC. B cell deficiencies accounted for roughly half of the cases, a proportion consistent with previously published reports [9, 13, 25]. Previous studies have suggested that low awareness of PIDD among physicians and the public contributes to delayed diagnosis [26–29]. Public awareness campaigns targeting both physicians and families can change behavior [30]. Campaigns conducted over the past decade to raise awareness of PIDD by educating physicians, other healthcare workers and the public could have contributed to the increase in prevalence seen in our study [18, 29, 31].
In the CCE population the prevalence of PIDD was highest among 55–64 years old with B cell defects accounting for 50 % in this group. Although PIDD are often considered pediatric diseases, the most common B cell defect, Common Variable Immune Deficiency (CVID), is often diagnosed in adults. The average age at diagnosis of CVID is 28–33 years of age [13, 32]. In contrast, the prevalence of T cell defects in the CCE population was highest among persons 0–21 years of age (4–5 per 100,000); it was even higher in the MC population (6–10 per 100,000), where the prevalence was highest in persons <1 year of age. T cell defects, which include disorders such as DiGeorge syndrome and immune dysregulation polyendocrinopathy enteropathy, X-linked (IPEX), cause severe clinical phenotypes, and are likely to present with symptoms at a young age.
In both the CCE and MC populations, 0–21 years of age, the prevalence of T cell defects and B cell defects was higher in males. In the MC population males had a higher prevalence of combined immune defects and neutrophil defects, as well as all PIDD. X-linked recessive inheritance accounts for about 50 % of cases of SCID in the U.S. [33], and is also the mode of inheritance of several other PIDD, including Bruton's agammaglobulinemia (XLA), X-linked HyperIgM syndrome, Wiskott Aldrich syndrome, IPEX, NFκ β essential modifier (NEMO) and Chronic Granulomatous Disease. These disorders tend to have a severe clinical phenotype with symptoms manifesting in the first year of life.
It is more difficult to explain the higher prevalence of combined immune deficiency (ICD-9 279.2) in persons age 55–64 years in the CCE population (2.1–3.2 per 100,000). ICD-9 279.2 specifies combine immune deficiencies and typically is used for persons with congenital combined immune deficiencies such as SCID or thymic aplasia, not due to DiGeorge syndrome. For the past 30 years combined immune defects (such as SCID) have been successfully treated using bone marrow or hematopoietic cell transplants [34–36]. However, persons over 30 years of age likely would not have benefitted from this advance in treatment. Other studies also identified large groups of adult patients. Among patients hospitalized in New York state between 2000 and 2004, Resnick et al. found that 34 % of patients with a PIDD diagnosis code were>45 years of age. Similar to our findings, 62/143 of hospitalized persons with PIDD had a diagnosis of combined immune deficiency (279.2) [37].
Because ICD-9 codes 279.0, 279.1, and 279.8 used in our analysis are not specific for congenital immune defects, there may be some bias or misclassification. Although we attempted to remove persons with secondary immune deficiency due to HIV and malignancies from the analysis (Table 2), many persons with PIDD develop autoimmune disorders and malignancies and may be treated with drugs causing secondary immune defects. For these reasons, it was not possible to remove all subjects with underlying conditions causing immunosuppression.
The prevalence of any PIDD was more than twice as high in Whites as in Blacks or Hispanics, a pattern observed for all diagnosis groups, except neutrophil defects. Some genetically homogeneous populations have an increased prevalence of specific PIDD (e.g. Artemis SCID in Navajo Indians), but only a few registries have reported prevalence of specific PIDD (e.g. XLA, HyperIgM and CGD) by race [15–17]. In these reports, Whites comprise between 65 and 94 % of the patients. The only population based estimate of PIDD prevalence by race is from Olmstead county Minnesota, where 97 % of cases were in Whites; however this population is very homogeneous (90–95 % White) [11]. A possible explanation for our findings could be underdiagnoses among Black and Hispanic populations as a result of barriers to healthcare, or diagnostic bias. Several studies of PIDD diagnoses in urban clinical settings have reported under-representation of PIDD among minorities [15, 27, 38]. Further study is needed to identify possible impediments to diagnosis. The most accurate way to determine the population prevalence of a disorder is through population-wide screening. Recently published data from states performing newborn screening for Severe Combined Immune Deficiency have observed a birth prevalence of 1:50,000, which is two times higher than previous estimates [39, 40].
PIDD are chronic diseases and despite therapy with gamma globulin or prophylactic antibiotics, infections often continue to occur. Sequelae of infection, such as chronic lung disease, cause significant morbidity [41]. Our analysis demonstrated a significant increase in hospital admissions and length of stay for PIDD patients compared to the general population. Children <1 year had strikingly longer hospital stays than those 1–21 years of age. This could be explained by the fact that persons with PIDD with the most profound immune defects tend to present to the healthcare system earlier in life. Despite initiation of preventive therapy to reduce infections, persons with PIDD still experience significant infectious complications. We did not analyze the point of care for patients with PIDD, but it has been suggested that care in a specialized center may reduce hospitalizations and reduce health care costs [34].
The most common comorbidities among hospitalized persons with PIDD included non-specific infection associated comorbidities (fevers, splenomegaly, failure to thrive), respiratory infections, pathogen specific infections, and chronic lung disease. In the CCE and MC databases, we could assess the frequency of multiple comorbidities only for hospital encounters, since up to 15 ICD-9 codes were recorded only for inpatient encounters. The higher prevalence of acute infectious comorbidities among the MC population (0–21 years) may reflect the nature of presenting symptoms in younger children as well as their increased exposure to infectious illnesses. Assessment of pathogen specific infections was limited due to incomplete reporting.
Limitations
Our study utilized administrative claims data and without the benefit of medical chart review, we could not confirm PIDD diagnoses. In addition, only 4 digit ICD-9 codes were available for analysis reducing the specificity of disease classification. For example, ICD-9 codes for diagnoses such as hypogammaglobulinemia (279.0), or other specified disorders of the immune system (279.9) do not specify whether these disorders are primary, or secondary. This makes it difficult to distinguish primary from secondary immune deficiencies using diagnostic ICD-9 codes alone. Although we attempted to exclude from analysis persons with secondary immune deficiencies (e.g. HIV infection, malignancies, organ transplants), this information was incomplete and could have resulted in misclassification of cases having a PIDD. Another limitation is that only two ICD-9 codes were reported for each outpatient encounter. In contrast, up to 15 codes were available for inpatient encounters. Thus, there could be under-ascertainment of cases for outpatient encounters if a code for PIDD was not included.
The databases we analyzed included all enrollees in the participating plans across each year. The CCE database does not include all commercial insurance plans and the MC database did not include all 50 states. In both CCE and MC databases persons may enter or be lost from year to year, depending on their insurance eligibility. To the extent that persons previously diagnosed with PIDD but did not have a PIDD-related claim in subsequent years, prevalence of PIDD in those years could be underestimated.
The CCE database's large size (more than 28,000,000 persons in 2007) and its similarity to the U.S. population with regard to gender and age distribution lend strength to our analysis and suggest that our findings could be generalizable to the U.S. population. However, we were not able to adequately assess the prevalence of PIDD in persons>65 years of age since only 0.1 % of the CCE population is in this age group. While gender and age distribution of the MC population mirrors the U.S. population as a whole, Blacks are over-represented. Although this limits the generalizability of the prevalence estimates, it also highlights the disparity in diagnosis of PIDD between Whites, Blacks and Hispanics 0–21 years of age.
Conclusion
This is the largest population-based study of PIDD prevalence to date, comprising almost 10 % of the U.S. population. Our prevalence estimates are markedly higher than those previously reported from registries. We observed increasing prevalence of PIDD during the study period. This trend could be due to increased awareness of PIDD among health care providers or the general population, but other factors such as increased availability of diagnostic testing could also play a role. Additional data is needed to determine if the lower prevalence of PIDD found among Black and Hispanic children on MC also exists in older populations and those with private insurance. Additional efforts should also attempt to elucidate the possible causes of this disparity.
Supplementary Material
Acknowledgments
This research was supported in part by an appointment (RWP) to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. Thanks to the Jeffrey Modell Foundation for promoting awareness of Primary Immune Deficiencies.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Electronic supplementary material The online version of this article (doi:10.1007/s10875-014-0102-8) contains supplementary material, which is available to authorized users.
The authors declare that they have no conflict of interest.
Contributor Information
Lisa Kobrynski, Department of Pediatrics, Emory University School of Medicine, 2015 Uppergate Dr., Atlanta, GA 30322, USA.
Rachel Waltenburg Powell, Office of Public Health Genomics, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Scott Bowen, Office of Public Health Genomics, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.Stadtmauer G, Cunningham-Rundles C. Outcome analysis and cost assessment in immunologic disorders. JAMA. 1997;278(22):2018–23. [PubMed] [Google Scholar]
- 2.Baumgart KW, Britton WJ, Kemp A, et al. The spectrum of primary immunodeficiency disorders in Australia. J Allergy Clin Immunol. 1997;100:415–23. doi: 10.1016/s0091-6749(97)70257-4. [DOI] [PubMed] [Google Scholar]
- 3.Eades-Perner AM, Gathmann B, Knerr V, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2004–06. Clin Exp Immunol. 2007;147(2):306–12. doi: 10.1111/j.1365-2249.2006.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasth A. Primary immunodeficiency disorders in Sweden: cases among children, 1974–1979. J Clin Immunol. 1982;2:86–92. doi: 10.1007/BF00916891. [DOI] [PubMed] [Google Scholar]
- 5.Gathmann B, Grimbacher B, Beauté J, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157:3–11. doi: 10.1111/j.1365-2249.2009.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geller-Bernstein C, Etzioni A. Pediatric allergy and immunology in Israel. Ped All Immunol. 2013;24:187–94. doi: 10.1111/pai.12044. [DOI] [PubMed] [Google Scholar]
- 7.Ishimura M, Takada H, Doi T, et al. Nationwide survey of patients with primary immunodeficiency diseases in Japan. J Clin Immunol. 2011;31(6):968–76. doi: 10.1007/s10875-011-9594-7. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick P, Riminton S. Primary immunodeficiency diseases in Australia and New Zealand. J Clin Immunol. 2007;27(5):517–24. doi: 10.1007/s10875-007-9105-z. [DOI] [PubMed] [Google Scholar]
- 9.Lee WI, Huang JL, Jaing TH, et al. Distribution, clinical features and treatment in Taiwanese patients with symptomatic primary immuno-deficiencies (PIDs) in a nationwide population based study during 1985–2010. Immunobiology. 2011;216(12):1286–94. doi: 10.1016/j.imbio.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Stray-Pedersen A, Abrahamsen TG, Frøland SS. Primary immuno-deficiency diseases in Norway. J Clin Immunol. 2006;20(6):477–85. doi: 10.1023/a:1026416017763. [DOI] [PubMed] [Google Scholar]
- 11.Joshi AY, Iyer VN, Hagan JB, et al. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84(1):16–22. doi: 10.4065/84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27(5):497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- 13.CEREDIH The French PID study group. The French national registry of primary immunodeficiency diseases. Clin Immunol. 2010;135(2):264–72. doi: 10.1016/j.clim.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Leiva LZ, Zelazco M, Oleastro M, et al. Primary immunodeficiency diseases in Latin america: the second report of the LAGID registry. J Clin Immunol. 1997;27:101–8. doi: 10.1007/s10875-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 15.Winkelstein JA, Marino MC, Johnston RB, et al. Chronic granulomatous disease: a report on a national registry of 368 patients. Medicine. 2000;79(3):155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Winkelstein JA, Marino MC, Ochs H, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine. 2003;82:373–84. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 17.Winkelstein JA, Marino M, Lederman H, et al. X-linked agammaglobulinemia: report on a united states registry of 201 patients. Medicine. 2006;85:193–207. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 18.Yarmohammadi H, Estrella L, Doucette J, Cunningham-Rundles C. Recognizing primary immunodeficiencies in clinical practice. Clin Vacc Immunol. 2006;13:329–32. doi: 10.1128/CVI.13.3.329-332.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar JDM, Buckland M, Guzman D, et al. The United Kingdom primary immune deficiency (IKPID) registry: report of the first 4 years’ activity 2008–2012. Clin Exp Immunol. 2013;175:68–78. doi: 10.1111/cei.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bousfiha AA, Jeddane L, Ailal F, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immun. 2013;33:1–7. doi: 10.1007/s10875-012-9751-7. [DOI] [PubMed] [Google Scholar]
- 21.Baker MW, Laessig RH, Katcher ML, et al. Implementing routine testing for severe combined immunodeficiency within Wisconsin's newborn screening program. Public Health Rep. 2010;125(S2):88–95. doi: 10.1177/00333549101250S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerstel-Thompson JL, Wilkey JF, Baptiste JC, et al. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of Severe Combined Immunodeficiency in population based newborn screening. Clin Chem. 2010;56(9):1466–74. doi: 10.1373/clinchem.2010.144915. [DOI] [PubMed] [Google Scholar]
- 23.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the united states. JAMA. 2014;312(7):729–38. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Census Bureau [August 10, 2014];Profile of general demographic characteristics. 2005 http://www.census.gov/popest/datasets/demographic_profile/0_United_States/2kh00.pdf.
- 25.Gathmann B, Goldacker S, Klima M, et al. German national registry for primary immunodeficiencies (PID). Clin Exp Immunol. 2013;173(2):372–80. doi: 10.1111/cei.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champi C. Primary immunodeficiency disorders in children: prompt diagnosis can lead to lifesaving treatment. J Pediatr Health Care. 2003;16(1):6–21. [PubMed] [Google Scholar]
- 27.Cunningham-Rundles C, Bodian L. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92(1):34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 28.Lindegren ML, Kobrynski L, Rasmussen S, et al. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Recomm Rep. 2004;53(RR-1):1–29. [PubMed] [Google Scholar]
- 29.Waltenburg R, Kobrynski L, Reyes M, Bowen S, Khoury MJ. Primary immunodeficiency diseases: practice and awareness among the general public, United States, 2008. Genet Med. 2010;12:792–800. doi: 10.1097/GIM.0b013e3181f3e2c9. [DOI] [PubMed] [Google Scholar]
- 30.AAP position statement The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatr. 2005;116(5):1245–55. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- 31.de Vries E, the European Society for Immunodeficiencies (ESID) members Patient-centered screening for primary immunodeficiency: a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2011;167:108–19. doi: 10.1111/j.1365-2249.2011.04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989;9(1):22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- 33.Buckley RH. Variable phenotypic expression of mutations in genes of the immune system. J Clin Invest. 2005;115:2974. doi: 10.1172/JCI26956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoine C, Muller S, Cant A, et al. Long-term survival and transplantation of haematopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361(9357):553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 35.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 36.Pai S, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resnick ES, Bhatt P, Sidi P, Cunningham-Rundles C. Examining the use of ICD-9 diagnosis codes for primary immune deficiency diseases in New York state. J Clin Immunol. 2013;33:40–8. doi: 10.1007/s10875-012-9773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham-Rundles C, Sidi P, Estrella L, Doucette J. Identifying undiagnosed primary immunodeficiency diseases in minority subjects using computer sorting of diagnosis codes. J Allergy Clin Immunol. 2004;113:747–56. doi: 10.1016/j.jaci.2004.01.761. [DOI] [PubMed] [Google Scholar]
- 39.Kwan A, Church J, Cowan M, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–50. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel B, Bonagura V, Weinberg GA. Newborn screening for SCID in New York state: experience from the first 2 years. J Clin Immunol. 2014;34:289–303. doi: 10.1007/s10875-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M, Li J, Hagan J, Maddox D, Abraham R. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.