Abstract
Objective
Evidence on the effectiveness of behavioral weight management programs often comes from uncontrolled program evaluations. These frequently make the assumption that, without intervention, people will gain weight. The aim of this study was to use data from minimal intervention control groups in randomized controlled trials to examine the evidence for this assumption and the effect of frequency of weighing on weight change.
Methods
Data were extracted from minimal intervention control arms in a systematic review of multicomponent behavioral weight management programs. Two reviewers classified control arms into three categories based on intensity of minimal intervention and calculated 12‐month mean weight change using baseline observation carried forward. Meta‐regression was conducted in STATA v12.
Results
Thirty studies met the inclusion criteria, twenty‐nine of which had usable data, representing 5,963 participants allocated to control arms. Control arms were categorized according to intensity, as offering leaflets only, a single session of advice, or more than one session of advice from someone without specialist skills in supporting weight loss. Mean weight change at 12 months across all categories was −0.8 kg (95% CI −1.1 to −0.4). In an unadjusted model, increasing intensity by moving up a category was associated with an additional weight loss of −0.53 kg (95% CI −0.96 to −0.09). Also in an unadjusted model, each additional weigh‐in was associated with a weight change of −0.42 kg (95% CI −0.81 to −0.03). However, when both variables were placed in the same model, neither intervention category nor number of weigh‐ins was associated with weight change.
Conclusions
Uncontrolled evaluations of weight loss programs should assume that, in the absence of intervention, their population would weigh up to a kilogram on average less than baseline at the end of the first year of follow‐up.
Introduction
Evidence from randomized controlled trials shows that behavioral weight management programs involving diet, exercise, and behavioral counselling can lead to significant weight loss in adults with overweight or obesity 1. However, many programs have not been evaluated in randomized trials due to the complexity and costs associated with trial design, conduct, and analysis 2, 3. In the absence of a control group, these observational reports 4, 5, 6, 7 leave either readers to make their own judgement on the weight of a population left untreated or the investigators to make explicit assumptions about what would happen to the population had they not received the intervention being evaluated.
For example, a cost‐effectiveness analysis of a primary care‐based behavioral weight management program assumed that, had participants not enrolled in the intervention, they would have gained 1 kg year−1 8. Two other evaluations used data from the CARDIA cohort (n = 5,115; age: 18‐30 years; mean baseline BMI: 24.4 ± 3.9 kg m−2) to suggest that, if untreated, individuals would steadily gain weight over time, though neither paper quantified the rate of weight gain 9, 10. Yet analysis of a large, pooled multi‐cohort database suggests such assumptions are unwarranted. The collaborative analysis of 57 prospective cohort studies found that, although participants who were classified as overweight (BMI ≥ 25 and <30 kg m−2) at baseline gained 0.9 kg after 10 or more years, participants with a baseline BMI of 30‐50 kg m−2 lost an average of −0.4 kg over the same period 11. However, using data from population cohort studies is not appropriate for investigating what may happen to people who are aiming to lose weight because the population was enrolled without this being an inclusion criterion. There is no data on what a group of people with the desire to enroll in a weight management program might achieve without a formal program. As such, it is difficult to evaluate the effectiveness of weight loss programs when tested in uncontrolled evaluations.
In addition, the control arm in an randomized controlled trial may contain elements that constitute an effective intervention since the process of recruitment to a trial and measurement of body weight might motivate weight loss. For example, cross‐sectional studies show an association between doctors’ advice to lose weight and their patients’ attempts to do so 12, and in qualitative research many people report the regular weigh‐in as an important aspect of weight management programs 13. This suggests that it would be helpful for physicians to raise the issue of weight, weigh people, and remind their patients that they will check their weight again in the future. Such interventions may have important public health benefits because of their low cost and high reach but any randomized trial to examine the efficacy of such minimal interventions with very small effect sizes would have to be very large and perhaps impractical as a result. Nonetheless, formal weight management programs need to demonstrate efficacy over and above this “brief intervention.”
Here we aimed to examine the weight loss achieved during the first 12 months of follow‐up by people enrolled in randomized trials who were assigned to minimal intervention control groups in order to inform future uncontrolled program evaluations. Additionally, in order to inform the development and provision of brief interventions, we used meta‐regression to provide observational evidence on the potential value of both advice on weight loss from someone without special knowledge of how to achieve it and of scheduled weigh‐ins for weight management.
Methods
Search and inclusion criteria
We identified the studies included in this review from those in a large systematic review of the effectiveness of multicomponent behavioral weight management programs. The review of multicomponent weight management programs has been published elsewhere, along with a full account of its methods 14.
To be included in the review of multicomponent weight management programs, studies had to recruit adults (≥18 years) with a BMI of ≥25 kg m−2 (or a BMI of ≥23 kg m−2 in Asian populations). Interventions had to involve multiple contacts with the provider and be clearly defined as multicomponent weight management programs. Studies in women who were pregnant, people with eating disorders, and those where the weight loss program was used specifically to treat a medical condition, such as sleep apnea or diabetes, were excluded. Studies were required to include a measure of weight change at 12 months or greater from baseline.
Definitions of control intensity
To be included in this analysis of weight change in control groups, trials had to have a minimal contact control arm, which ranged from no intervention to multiple contacts with someone without specific training in weight management. Often, these were described as “usual care” in the included studies, but given the variation in these definitions, we established criteria to define control group intensity. Control groups were coded as:
No intervention at all; self‐help material only (including leaflets and static websites); or seeing someone more than once for discussion of something other than weight management;
Single weight management session including discussion/advice/counseling ±self‐help material; or
Seeing a professional (e.g., general practitioner [GP], practice nurse) without specific training in delivering weight management advice and without a defined program to follow, more than once for weight management, ± self‐help material.
Statistical analysis
We calculated mean weight change from baseline to 3, 6, and 12 months. Weight was extracted or calculated from complete case data 15 as baseline observation carried forward (BOCF). Where 12‐month data were not available, we used data at up to 18 months in their place. Mean difference in weight change between the intervention and control groups has been evaluated elsewhere 14.
This was an exploratory not confirmatory analysis so all nominally statistically significant P values should be interpreted as indicative and not confirmation of a hypothesis. We used random effects meta‐analysis to calculate mean weight change for all studies combined. We used the methods described by Riley et al. to calculate 95% prediction intervals 16. Random effects analysis assumes that there is not a single underlying mean but rather that means from studies vary, in this case as a function of the population enrolled. The prediction intervals give the range of weights within which 95% of control population means would be expected to lie.
Random effects meta‐regression was conducted using STATA v12 for all studies with usable data. Control group categories A, B, and C were of increasing intensity, with A the lowest and C the highest, and therefore we tested for a linear trend. A second meta‐regression investigated all categories as binary variables with control category A as the reference group. Finally, we examined the association between number of weigh‐ins during 12 months and weight loss, so we added this variable and other potential study level confounders to the model using a stepwise method.
Results
Of the 37 studies included in the review of behavioral weight management programs, 30 met our inclusion criteria for this analysis, 29 of which had sufficient outcome data to be included in the analysis.
Characteristics of included studies
An overview of the 30 included studies can be seen in Table 1. Half of the studies were conducted in the USA, and all but three of the remaining studies were conducted in Europe. One multicenter study was conducted in the UK, Germany, and Australia 42.
Table 1.
Characteristics of included studies: Participantsa
| Study ID | Country | Total N | % Female | Mean age | BMI, mean (SD) | Participant inclusion criteriab | Control N |
|---|---|---|---|---|---|---|---|
| Control group category A (Leaflet/advice only and/or non-weight-related follow‐up) | |||||||
| Bertz 2012 17 | Sweden | 68 | 100% | 32 | 30.2 (3.4) | Women 8‐12 weeks post‐partum | 17 |
| Fitzgibbon 2010 18 | USA | 213 | 100% | 46 | 39.8 (5.8) | African‐American women | 106 |
| Foster‐Schubert 2012 19 | USA | 439 | 100% | 58 | 30.7 (3.9) | Post‐menopausal women | 87 |
| Jeffrey and Wing 1995 20, c | USA | 202 | 50% | 37 | 31.1d | 40 | |
| Jolly 2011 21 | UK | 640 | 71% | 49 | 33.9 (4.4) | 100 | |
| Kuller 2012 22 | USA | 508 | 100% | 57 | 30.9 (3.8) | Post‐menopausal women | 255 |
| Nanchahal 2011 23 | UK | 381 | 73% | 49 | 33.9 (5.6) | 190 | |
| Patrick 2011 24 | USA | 441 | 0% | 44 | 34.3 (4.0) | Men | 217 |
| Rejeski 2011 25 | USA | 288 | 67% | 67 | 32.6 (3.5) | Older adults, evidence of CVD or metabolic syndrome, self‐reported mobility limitation | 93 |
| Silva 2010 26 | Portugal | 239 | 100% | 38 | 31.3 (4.0) | Pre‐menopausal women | 116 |
| Stevens 1993 27 | USA | 564 | 79% | 43 | 29.5 (2.8) | Baseline blood pressure in high normal range | 256 |
| Stevens 2001 28 | USA | 1191 | 34% | 43 | 30.9 (3.2) | As above | 596 |
| Vissers 2010 29 | Belgium | 79 | NR | 45 | 30.8 (3.4) | 21 | |
| Control group category B (Single weight management session) | |||||||
| Appel 2011 30 | USA | 415 | 64% | 54 | 36.8 (5.1) | One or more CVD risk factors | 138 |
| Eriksson 2009 31 | Sweden | 151 | 57% | 54 | 29.4 (5.1) | Additional risk factor for type 2 diabetes | 76 |
| Hersey 2012 32 | USA | 1755 | 74% | NR | 33.6e | 598 | |
| Lindstrom 2003 33 | Finland | 522 | 67% | 55 | 31.1 (4.5) | High risk for type 2 diabetes | 257 |
| Mensink 2003 34 | Netherlands | 114 | 43% | 57 | 29.3 (3.1) | Elevated fasting glucose | 59 |
| Morgan 2011 35 | Australia | 65 | 0% | 36 | 30.5 (3.0) | Men | 31 |
| Penn 2009 36 | UK | 102 | 60% | 57 | 33.5 (4.6) | Impaired glucose tolerance | 51 |
| Ross 2012 37 | Canada | 490 | 71% | 52 | 32.0 (4.2) | 208 | |
| Vermunt 2011 38 | Netherlands | 925 | 60% | 58 | 28.5 (4.1) | Elevated risk of developing type 2 diabetes | 444 |
| Control group category C (Seeing a professional without specific training) | |||||||
| Dale 2008 39 | New Zealand | 79 | 67% | 46 | 36.5 (4.3) | Impaired insulin sensitivity | 23 |
| DPP 40 | USA | 2161 | 69% | 50 | 34.2 (6.7) | Impaired glucose tolerance | 1082 |
| Heshka 2006 41 | USA | 433 | 82% | 45 | 33.6 (3.7) | 212 | |
| Jebb 2011 42 | UK, Germany, and Australia | 772 | 87% | 47 | 31.3 (2.6) | 395 | |
| Munsch 2003 43 | Switzerland | 122 | 75% | 46 | 32.6 (1.8) | 17 | |
| Rock 2010 44 | USA | 442 | 100% | 44 | 34.0 (3.2) | Women | 111 |
| Villareal 2011 45 | USA | 107 | 63% | 70 | 37.3 (4.7) | Aged 65 years or older, mild to moderate frailty | 27 |
| Wadden 2011 46 | USA | 261 | 80% | 52 | 39.0 (4.8) | Have ≥ 2 criteria for metabolic syndrome | 130 |
NR: not reported.
Beyond being adults of both genders with overweight/obesity.
Did not contribute to weight change analyses due to insufficient data.
SD not available.
Across all arms, SD not available.
The studies included 14,169 participants in total. Of these, 5,953 were allocated to control arms. As is common in weight loss studies, the majority of participants were female (69%). Two studies recruited men only and six studies recruited only women. All studies required that participants be at least 18 years or older. The mean age across studies was 49, ranging from 32 to 70 years old. Five studies were aimed at diabetes prevention and required some measure of elevated risk for developing type 2 diabetes beyond overweight/obesity. In two, there was no lower BMI limit, but reported data indicated that >80% of participants in each study arm had overweight or obesity at baseline 31, 40. All other studies had overweight or obesity as an inclusion criterion. The mean BMI across all studies at baseline was 33 (the median was also 33), ranging from 29 to 40. Thirteen of the 30 included studies had a maximum BMI cutoff at baseline; this ranged from 35 to 50 (average 40). The other 17 included studies had no maximum cutoff for baseline BMI.
Thirteen studies had control arms consisting of no additional weight‐related contact (Category A); of these, three received no intervention at all, six received written information only, and four received sessions discussing health issues other than weight loss. One of these studies, Jeffrey and Wing 1995, could not be included in the meta‐regression due to insufficient data with which to calculate BOCF. In nine studies, control arms received one‐off weight management advice (Category B), and in the remaining eight studies, control arms received multiple contacts regarding weight management, delivered by someone without specific training in delivering a weight management program (Category C).
The median number of weight measures in the first year across all studies was three, ranging from two to six weigh‐ins. Table 2 provides further details of the nature of the contact and information provided to the control group in each included study, as well as on the number of weight measures over 12 months.
Table 2.
Characteristics of included studies: Contact and information offered to control participants
| Study ID | Control condition | Study weight measures over 12 months |
|---|---|---|
| Control group category A (Initial contact with leaflet/advice only and/or non-weight-related follow‐up) | ||
| Bertz 2012 17 | No additional contact or information | 3 |
| Fitzgibbon 2010 18 | Regular newsletters covering general health information; phone call from staff member every month relating to newsletter information | 3 |
| Foster‐Schubert 2012 19 | No additional contact or information | 2 |
| Jeffrey and Wing 1995 20 a | No additional contact or information | 3 |
| Jolly 2011 21 | Offered voucher for 12 free entries to local sports center; no additional contact | 3 |
| Kuller 2012 22 | Six general health education sessions in year one and several times over following years to discuss women's health | 3 |
| Nanchahal 2011 23 | Weight management booklet at baseline; no additional contact | 3 |
| Patrick 2011 24 | Offered access to website with general health information; no additional contact. | 3 |
| Rejeski 2011 25 | 18 sessions over 18 months covering general topics related to aging and health | 3 |
| Silva 2010 26 | 29 face‐to‐face health education sessions in thematic courses; weight loss not focus | 3 |
| Stevens 1993 27 | No additional contact or information | 3 |
| Stevens 2001 28 | No additional contact or information | 3 |
| Vissers 2010 29 | No additional contact or information | 4 |
| Control group category B (Single weight management session) | ||
| Appel 2011 30 | Session with weight loss coach; received brochures and list of recommended websites promoting weight loss | 3 |
| Eriksson 2009 31 | Education session by doctor, physiotherapist, and dietician | 2 |
| Hersey 2012 32 | Advice session; provided with a booklet about encouraging exercise and weight loss and access to a basic (non‐interactive) website | 3 |
| Lindstrom 2003 33 | General lifestyle weight management information provided at baseline in an individual or group session lasting 30‐60 minutes | 2 |
| Mensink 2003 34 | One‐off, oral and written information on diet, weight loss, and physical activity | 2 |
| Morgan 2011 35 | Group information session regarding weight loss at baseline, plus program booklet | 4 |
| Penn 2009 36 | Advice session from dietician and physiotherapist; leaflets | 2 |
| Ross 2012 37 | One‐off general advice from physicians on merits of physical activity as strategy for obesity reduction | 3 |
| Vermunt 2011 38 | Session of advice from GP about benefits of healthy diet and exercise | 3 |
| Control group category C (Seeing a professional without specific training) | ||
| Dale 2008 39 | At 8 and 12 months, some advice regarding lifestyle changes; provider not specified | 4 |
| DPP 40 | Placebo controlled with written lifestyle advice provided at baseline and alongside an annual individual session | 3 |
| Heshka 2006 41 | Two consultations with a dietician (baseline and 12 weeks); included as control as authors state dietician provided basic, publicly available information and did not use training to personalize or help set individual goal | 4 |
| Jebb 2011 42 | Weight loss advice from primary care professional at local GP practice (minimum six visits over 12 months) | 5 |
| Munsch 2003 43 | Non‐specific comments about general measures to lose weight from GP on multiple occasions; no specific technique, tools, or written material were used | 3 |
| Rock 2010 44 | Consultation at baseline with research staff where given written information; monthly check‐ins by email or phone | 3 |
| Villareal 2011 45 | General information about a healthy diet provided during monthly visits with the staff | 3 |
| Wadden 2011 46 | Quarterly primary care visits over 24 months to address coexisting illnesses; at each visit, primary care practitioner spent 5‐7 minutes reviewing weight change and discussing info in handouts; given pedometer and calorie counting book. | 6 |
aDid not contribute to weight change analyses due to insufficient data.
Weight change
Figures 1, 2, 3 display weight curves for control groups in studies where weight was reported at more than one follow‐up point. The weighted average of weight change for all control groups combined was −1.0 kg (95% CI −1.77 to −0.23, P = 0.011) at 3 months; −0.72 (95% CI −1.17 to −0.27, P = 0.002) at 6 months; and −0.76 kg (95% CI −1.14 to −0.39, P < 0.001) at 12 months. The 95% prediction intervals were wide, encompassing modest weight gain and substantial weight loss (Figure 4).
Figure 1.
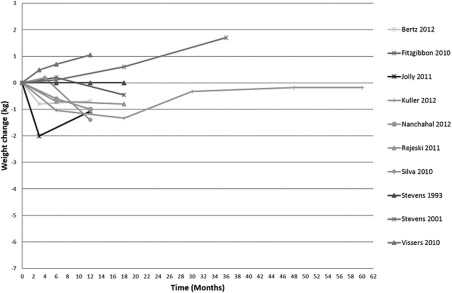
Weight change over time, control group category A.
Figure 2.
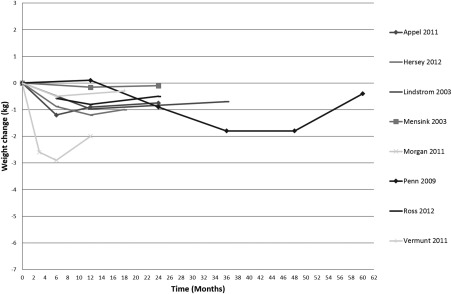
Weight change over time, control group category B.
Figure 3.
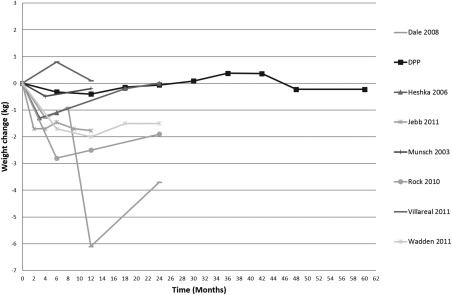
Weight change over time, control group category C.
Figure 4.
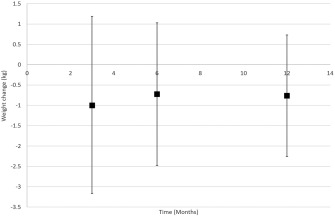
95% prediction intervals for weight loss at 3, 6, and 12 months.
Using meta‐regression we looked for evidence of a linear trend in weight loss with increasing intensity of intervention. At 12 months, increasing intensity was associated with an additional weight loss of −0.53 kg (95% CI −0.96 to −0.09, P = 0.017) per category in an unadjusted model.
At 12 months, we found that each additional weigh‐in was associated with a weight change of −0.42 kg (95% CI −0.81 to −0.03, P = 0.035) in an unadjusted model. No other study characteristics were associated with significant weight change in these control groups.
When both variables were placed in the same model neither intervention category (−0.39 kg; 95% CI −0.86 to 0.08, P = 0.104) nor number of weigh‐ins (−0.28 kg; 95% CI −0.69 to 0.13, P = 0.181) was associated with weight change.
We investigated whether a single session of advice (B) was associated with a greater weight loss than a leaflet or non‐weight‐related contacts (A) at 12 months. There was no evidence of a significant difference in an unadjusted model (−0.25 kg; 95% CI −0.83 to 0.32, P = 0.394) or in one adjusted for the number of weigh‐ins (−0.29 kg; 95% CI −0.91 to 0.33, P = 0.359).
There was a significant difference in 12‐month weight loss between groups having a regular contact with an untrained professional (C) and those having one‐off advice or regular non‐weight‐related contacts (A) in unadjusted (−1.19 kg; 95% CI −2.32 to −0.06, P = 0.039) but not adjusted models (−1.03 kg; 95% CI −2.49 to 0.41, P = 0.164).
None of the findings was qualitatively affected by excluding one study in control category C in which the description of the control intervention was unclear 40.
Discussion
People who volunteered for randomized trials to test the effectiveness of weight loss programs and who were allocated to the control group were about 1 kg lighter on average at the first year of follow‐up. Weight change during that year varied greatly between studies but most studies would be predicted to see weight loss in the control group. There was a suggestion that greater intensity of brief advice on weight loss was associated with greater weight loss but the evidence for this was not strong. Each additional weigh‐in was associated with greater weight loss, but the association was attenuated and not significant when adjusted for intensity of advice given.
The validity of these analyses rests on the comprehensive search for trials of interventions that delivered combined dietary and physical activity interventions. It is possible that interventions we excluded because they involved dietary advice only as the “active” treatment, for example, may have observed different weight loss in control groups, but it is difficult to see a reason why this might be so. We also excluded studies enrolling people who were being treated for a particular medical condition and studies of more intensive interventions and it is perhaps more plausible that people randomized to control groups in these studies may have had greater weight loss than the people in the studies included here. However, participants in the control group of trials of these more intensive interventions are usually randomized to a behavioral program of the kind that represent the “active” intervention of these trials here. In summary, we believe the data show that most populations, and by extension, most people, who would have joined a weight loss program but were randomized to a minimal intervention lose weight and are also at a lower weight at 1 year of follow‐up than at baseline.
Our analysis has several strengths and limitations. The studies we reviewed all suffered loss to follow‐up and presented their results using a variety of methods of imputation or using complete cases only. By standardizing the way statistics were presented, we removed spurious variation due to this. There are two main assumptions that underlie methods to deal with missing data, which are that data are missing at random or they are missing non‐randomly, most probably because people with a “bad” outcome are less willing to attend for follow‐up. Analyzing only those with follow‐up data or multiple imputation assumes data are missing at random. It would have been possible to use either approach here although, for multiple imputation, this would have had to be done at the study level. Multiple imputation at the study level is unlikely to have changed the results because the characteristics of participants in the studies were largely similar 14. Normally, multiple imputation is done at the individual level but this requires access to the full data from the trial. It is conceivable that multiple imputation at the individual level could be more conservative than BOCF, but a study that used several methods of imputing for missing weight data in trials of interventions for weight loss found no great differences between complete case data only and multiple imputation for missing data 47. In this review, we imputed missing data assuming they were not missing at random using BOCF. In the context of a review, this can only move the weight change towards the null, whether it showed mean weight increase or mean weight decrease. Mean weight loss at 12 months was observed in the control group in 90% of studies.
As might be expected, studies in which participants were offered multiple contacts with a health professional who gave untrained and non‐programmatic advice on how to manage weight had a greater number of weigh‐ins. Consequently, when terms reflecting both the intensity of advice and the number of weigh‐ins were added to the equation, none was significant. It is therefore unclear whether the unstructured advice or the simple act of weighing without advice might be contributing to the apparently greater weight loss observed in programs of this type. Previous literature has observed greater weight loss in people trying to lose weight who weigh themselves more frequently 48. In our analysis, the process of being weighed by an independent investigator also includes contact with an external party which may provide tacit accountability and increase motivation. There remains an opportunity to develop regular weighing as a routine intervention in primary care. Perhaps surprisingly, beyond the number of weigh‐ins, there were no differences in outcomes between our different categories of intensity, but this may reflect the difficulties in capturing the relevant aspects of care, for example specific elements of advice provided or the type of professional delivering the advice, which were often not reported in detail. However, we are not aware of other systematic reviews that specifically report on the weight loss in control groups or of evidence testing the differences between these relatively minimal interventions.
By summarizing and comparing weight loss achieved from different trials, our analysis is effectively based on observational data. A summary of weight lost in control groups could only ever be obtained from observational data, and data on the effect of such minimal and tacit interventions as reweighing people might never be the subject of randomized trials because of the very large sample size required. In our study we observed that control groups who received more advice or counseling lost more weight but meta‐regression was unable to exclude chance as the cause of this apparent association.
Our results have important implications for the interpretation of data from uncontrolled evaluations of weight loss interventions, in particular, evaluations that assume weight gain in a comparable but untreated population. Indeed, prospective cohort studies indicate a trend towards weight loss over time in those with obesity 11. However, the reliance on data from cohort studies aiming to establish the natural weight history of the general population may be problematic in uncontrolled program evaluations. Program evaluations include only individuals who want to lose weight and thus have a greater motivation than the general population. This is also the case for participants who volunteer to take part in research to test weight loss interventions but are assigned to a control group. The estimate of weight loss observed here may enable researchers presenting results of uncontrolled evaluations of treatment programs to put the weight loss achieved in context.
In summary, uncontrolled evaluations of weight loss programs should assume that, in the absence of intervention, their population would weigh up to a kilogram less than baseline at 1‐year follow‐up. The variation between studies was great meaning that even 2‐3 kg of weight loss might be observed without a behavioral weight loss program. There is a suggestion, but insufficient evidence to be sure, that regular reweighing and brief advice on how to lose weight may create additional weight loss.
Acknowledgments
The review protocol for the original review was designed and agreed upon by the National Institute for Health and Care Excellence (NICE) to support the development of NICE Guidance on managing overweight and obese adults—lifestyle weight management services and by members of the Behavioural Weight Management Review Group, which, in addition to the authors, consists of Jane Ogden, Igho Onakpoya, Carolyn Summerbell, and Dawn Phillips. IO also contributed to data extraction. Rafael Perera advised on statistical methods. The opinions expressed in this article are those of the authors and do not represent either NICE's position on these matters or constitute NICE guidance.
See Commentary, pg. 767.
Funding agencies: The work on which this article is based was funded by the National Institute for Health and Care Excellence (NICE) to support the development of NICE Guidance on managing overweight and obese adults—lifestyle weight management services, and the protocol was agreed with them. The UK Medical Research Council (MRC) (U105960389 Nutrition and Health) and the University of Oxford also provided funding. Paul Aveyard is funded by The UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council (MRC), and the Department of Health, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Disclosure: NICE provided support for the original review upon which the submitted work was based. The MRC received grants from Weight Watchers International for work where Susan Jebb was principal investigator. She received no personal remuneration in regard to this work. Susan Jebb has received personal fees from Rosemary Conley and hospitality from Weight Watchers International outside of the submitted work. Paul Aveyard has received hospitality from Weight Watchers and Slimming World outside of the submitted work. Paul Aveyard and Susan Jebb were each authors on one study included in the review.
References
- 1. Hartmann‐Boyce J, Johns D, Aveyard P, et al. Managing Overweight and Obese Adults: The Clinical Effectiveness of Long‐term Weight Management Schemes for Adults. Oxford: University of Oxford; 2013. [Google Scholar]
- 2. Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Inter Med 2005;4142:56‐66. [DOI] [PubMed] [Google Scholar]
- 3. Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci 2013;1281:191‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. JH Lavin, A Avery, SM Whitehead, et al. Feasibility and benefits of implementing a slimming on referral service in primary care using a commercial weight management partner. Public Health 2006;120:872‐881. [DOI] [PubMed] [Google Scholar]
- 5. Hajek P, Humphrey K, McRobbie H. Using group support to complement a task‐based weight management programme in multi‐ethnic localities of high deprivation. Patient Educ Counsel 2010;80:135‐137. [DOI] [PubMed] [Google Scholar]
- 6. Stubbs R, Brogelli D, Barber J, et al. Service evaluation of weight outcomes as a function of initial BMI in 34,271 adults referred to a primary care/commercial weight management partnership scheme. BMC Res Notes 2013;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health 2011;11:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trueman P, Haynes SM, Lyons GF, et al. Long‐term cost‐effectiveness of weight management in primary care. Int J Clin Practice 2010;64:775‐783. [DOI] [PubMed] [Google Scholar]
- 9. Counterweight Program Team . Evaluation of the counterweight programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stubbs RJ, Pallister C, Whybrow S, Avery A, Lavin J. Weight outcomes audit for 34,271 adults referred to a primary care/commercial weight management partnership scheme. Obes Facts 2011;4:113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prospective Studies Collaboration . Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rose SA, Poynter PS, Anderson JW, Noar SM, Conigliaro J. Physician weight loss advice and patient weight loss behavior change: a literature review and meta‐analysis of survey data. Int J Obes (Lond) 2013;37:118‐128. [DOI] [PubMed] [Google Scholar]
- 13. Ahern AL, Boyland EJ, Jebb SA, Cohn SR. Participants' explanatory model of being overweight and their experiences of 2 weight loss interventions. Ann Fam Med 2013;11:251‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartmann‐Boyce J, Johns DJ, Jebb SA, Aveyard P, Behavioural Weight Management Review Group. Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta‐analysis and meta‐regression. Obes Rev 2014;15:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaiser KA, Affuso O, Beasley TM, Allison DB. Getting carried away: a note showing baseline observation carried forward (BOCF) results can be calculated from published complete‐cases results. Int J Obes (Lond) 2012;36:886‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta‐analyses. Br Med J 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 17. Bertz F, Brekke HK, Ellegard L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight‐loss trial in lactating overweight and obese women. Am J Clin Nutr 2012;96:698‐705. [DOI] [PubMed] [Google Scholar]
- 18. Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18‐month results. Obesity (Silver Spring) 2010;18:2317‐23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster‐Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight‐to‐obese postmenopausal women. Obesity (Sliver Spring) 2012;20:1628‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeffery RW, Wing RW. Long‐term effects of interventions for weight loss using food provision and monetary incentives. J Consult Clin Psychol 1995;63:793‐796. [DOI] [PubMed] [Google Scholar]
- 21. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomized controlled trial. Br Med J 2011;343:d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuller LH, Pettee Gabriel KK, Kinzel LS, et al. The women on the move through activity and nutrition (WOMAN) study: final 48‐month results. Obesity (Silver Spring) 2012;20:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomized controlled trial in primary care of the Camden weight loss (CAMWEL) programme. Br Med J Open 2012;2:000315044800027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patrick K, Calfas KJ, Norman GJ, et al. Outcomes of a 12‐month web‐based intervention for overweight and obese men. Ann Behav Med 2011;42:391‐401. [DOI] [PubMed] [Google Scholar]
- 25. Rejeski WJ, Brubaker PH, Goff DC Jr., et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Inter Med 2011;171:880‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva MN, Vieira PN, Coutinho SR, et al. Using self‐determination theory to promote physical activity and weight control: a randomized controlled trial in women. J Behav Med 2010;33:110‐122. [DOI] [PubMed] [Google Scholar]
- 27. Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in Phase 1 of the trials of hypertension prevention. Arch Inter Med 1993;153:849‐858. [PubMed] [Google Scholar]
- 28. Stevens VJ, Obarzanek E, Cook NR, et al. Long‐term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Inter Med 2001;134:1‐11. [DOI] [PubMed] [Google Scholar]
- 29. Vissers D, Verrijken A, Mertens I, et al. Effect of long‐term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts 2010;3:93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med 2011;365:1959‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eriksson MK, Franks PW Eliasson M. A 3‐year randomized trial of lifestyle intervention for cardiovascular risk reduction in the primary care setting: the Swedish Bjorknas study. PLoS One 2009;4:e5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hersey JC, Khavjou O, Strange LB, et al. The efficacy and cost‐effectiveness of a community weight management intervention: a randomized controlled trial of the health weight management demonstration. Prevent Med 2012;54:42‐49. [DOI] [PubMed] [Google Scholar]
- 33. Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish diabetes prevention study (DPS): lifestyle intervention and 3‐year results on diet and physical activity. Diabet Care 2003;26:3230‐3236. [DOI] [PubMed] [Google Scholar]
- 34. Mensink M, Blaak EE, Corpeleijn E, Saris WH, de Bruin TW, Feskens EJ. Lifestyle interventions according to general recommendations improves glucose tolerance. Obes Res 2003;11:1588‐1596. [DOI] [PubMed] [Google Scholar]
- 35. Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12‐month outcomes and process evaluation of the SHED‐IT RCT: an internet‐based weight loss program targeting men. Obesity (Silver Spring) 2011;19:142‐151. [DOI] [PubMed] [Google Scholar]
- 36. Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Inter Med 2012;172:414‐424. [DOI] [PubMed] [Google Scholar]
- 38. Vermunt PW, Milder IE, Wielaard F, de Vries JH, van Oers HA, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabet Care 2011;34:1919‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dale KS, Mann JI, McAuley KA, Williams SM, Farmer VL. Sustainability of lifestyle changes following an intensive lifestyle intervention in insulin resistant adults: follow‐up at 2‐years. Asia Pac J Clin Nutr 2009;18:114‐120. [PubMed] [Google Scholar]
- 41. Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self‐help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792‐1798. [DOI] [PubMed] [Google Scholar]
- 42. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomized controlled trial. Lancet 2011;378:1485‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munsch SBE. Evaluation of a lifestyle change programme for the treatment of obesity in general practice. Swiss Med Wkly 2003;133:148‐154. [DOI] [PubMed] [Google Scholar]
- 44. Rock CL. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA 2010;304:1803‐1810. [DOI] [PubMed] [Google Scholar]
- 45. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wadden TA, Volger S, Sarwer DB, et al. A two‐year randomized trial of obesity treatment in primary care practice. New Engl J Med 2011;365:1969‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elobeid MA, Padilla MA, McVie T, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS One 2009;4:e6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self‐monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring) 2007;15:3091‐3096. [DOI] [PubMed] [Google Scholar]


