Abstract
Objective:
Nigrostriatal terminal losses are known to progress most rapidly in early-stage Parkinson disease (PD) and then plateau, whereas cortical pathology continues and may provide a better marker of PD progression in later stages. We investigated cortical gyrification indices in patients with different durations of PD, since cortical folding may capture complex processes involving transverse forces of neuronal sheets or underlying axonal connectivity.
Methods:
Longitudinal cohort structural MRI were obtained at baseline, 18 months, and 36 months from 70 patients with PD without dementia and 70 control participants. Cortical local gyrification index (LGI) was compared between controls and PD subgroups based upon duration of illness (DOI, <1 year [PDE, n = 17], 1–5 years [PDM, n = 19], >5 years [PDL, n = 24]) and adjusted using false discovery rate. Associations between LGI and clinical measurements were assessed using multiple linear regression. Areas having significantly reduced LGI also were analyzed using baseline data from a newly established cohort (PD n = 87, control n = 66) to validate our findings.
Results:
In the longitudinal cohort, PDL had significantly reduced overall gyrification, and bilaterally in the inferior parietal, postcentral, precentral, superior frontal, and supramarginal areas, compared to controls (p < 0.05). Longitudinally, loss of gyrification was accelerated in PDM participants, compared to controls. LGI showed robust correlations with DOI and also was correlated with PD-related clinical measurements. Similar results were obtained in the validation sample.
Conclusions:
Loss of cortical gyrification may be accelerated within the first few years after PD diagnosis, and become particularly prominent in later stages. Thus, it may provide a metric for monitoring progression in vivo.
In Parkinson disease (PD), degeneration of dopamine terminals is thought to progress rapidly within the first few years after diagnosis and then plateau.1 Thus, in more advanced stages of disease, non-nigrostriatal brain changes may serve as better markers of PD progression. Evidence suggests that widespread pathologic changes occur in the cortex, including apoptotic signaling, Lewy pathology, reduction in other neurotransmitters, and interneuron loss.2–4 It is unclear, however, how cell death relates to the pattern of cortical Lewy pathology, and whether cortical changes can be used to gauge PD progression.5
Lewy pathology has been documented in specific cortical layers (i.e., preferentially in deep layers of high-order sensory association areas).5,6 Some previous imaging studies have demonstrated decreased cortical thickness in PD,7,8 but reported results have been inconsistent and have not shown robust associations with disease progression in the absence of dementia. These inconsistencies may be attributable to several factors. First, cortical thickness may be less sensitive in areas where cortex pathology is not transmural. Second, thickness measurements may not reflect more complex changes of cortex surface architecture.9 Thus, the distinction between structural metrics is important because they may reflect different aspects of cortical neurodegeneration. In the current study, we aimed to characterize changes in cortical gyrification during PD progression by studying participants with different durations of illness. We hypothesized that reductions in gyrification would follow the spatiotemporal distribution described in studies of Lewy pathology.5,6
METHODS
Longitudinal cohort participants.
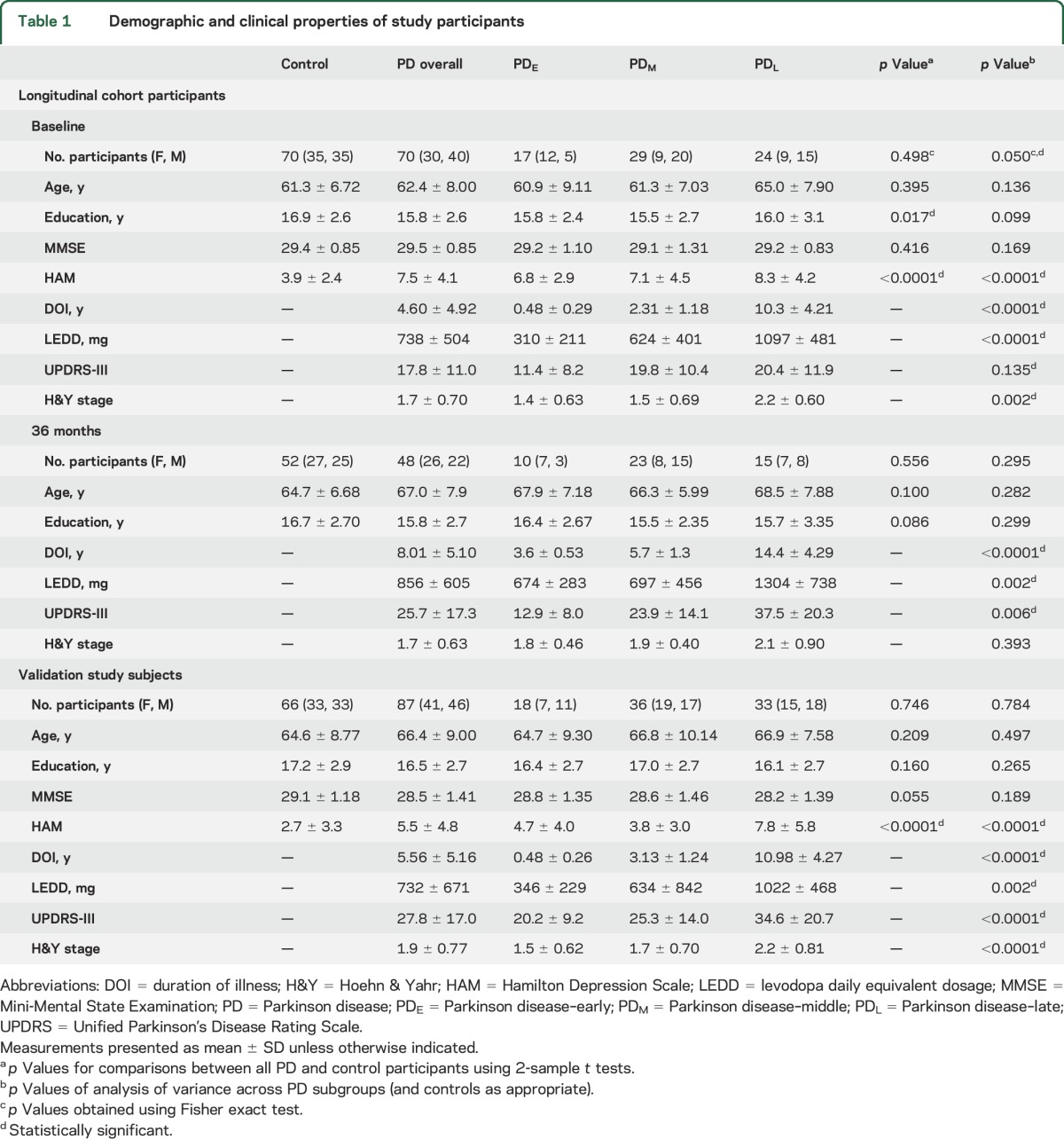
Patients with PD (n = 70) and controls (n = 70) with a Mini-Mental State Examination (MMSE) score ≥26 were selected from a large cohort study based on matching for baseline age distribution, sex ratio, and number of follow-up visits (table 1).10,11 Patients with PD were recruited from a tertiary movement disorders clinic, and controls from spouses and the local community. PD diagnosis was confirmed according to published criteria.12 All participants were free of major and acute medical issues or neurologic disorders other than PD. All brain images were inspected and deemed free of any major structural abnormalities. Hamilton Depression Rating Scale (HAM)13 scores were obtained at each visit. PD subgroups were assigned for comparisons to controls based upon duration of illness (DOI), defined as the number of years since diagnosis in the same fashion that we had done previously (PD–early [PDE] [<1 year], PD–middle [PDM] [1–5 years], PD–late [PDL] [>5 years]).14
Table 1.
Demographic and clinical properties of study participants
Validation study participants.
A validation study was conducted using the baseline data from a newly established cohort under the NIH PD Biomarkers Program (NCT01888185).15 Participants were recruited in a similar manner as the longitudinal cohort participants, except there were more advanced-stage patients. The original population of validation participants included 104 patients with PD and 71 controls. Participants having signs of dementia were excluded using the MMSE score cutoff described above.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained for all participants, in accordance with the Declaration of Helsinki. The research study protocol was approved by the Penn State Hershey Institutional Review Board.
Clinical evaluation.
Unified Parkinson's Disease Rating Scale (UPDRS) motor scores and Hoehn & Yahr (H&Y) stages were obtained for PD in the “on” medication state at each visit. Longitudinal cohort UPDRS motor scores were recorded using the original UPDRS.16 Validation study UPDRS motor scores were recorded using the revised UPDRS.17 Levodopa-equivalent daily dose (LEDD) was calculated according to published criteria.18
MRI data acquisition and analysis.
All participants were scanned using a 3.0T MRI scanner (Trio, Siemens Magnetom, Erlangen, Germany, with an 8-channel phased array head coil) at baseline, 18 months, and 36 months. A magnetization-prepared rapid acquisition gradient echo sequence was used to obtain T1-weighted images with repetition time/echo time = 1,540/2.34, field of view = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm (with no gap), slice number = 176.
T1-weighted images were processed automatically using FreeSurfer (version 5.1.0).19 The longitudinal pipeline was utilized to process longitudinal cohort images by first creating unbiased within-subject templates. The within-subject templates were then used to initialize image processing (skull stripping, Talairach transforms, atlas registration, spherical surface maps) for scans at each visit.20,21
Local gyrification index (LGI) was used as a measurement of cortical folding. Historically, LGI was defined as the ratio of cortical surface over the outer contour (perimeter) of a 2D brain section.22 LGI offers a method to quantify gyrification as it varies across the surface of a 3D cortical mesh (∼150,000 vertices).23 For each vertex vi, a circular region of interest was defined on the mesh surface having radius 25 mm and center vertex vi. Outer and pial surface areas (AO, AP) were computed as the sum of surface areas assigned to vertices that fell within the region of interest. LGI was defined as the ratio of AP/AO. The final computation of LGI at each vertex was calculated by inverse weighting based upon distance. Thus, the LGI computed for each vertex vi contains information from both the center vertex vi and vertices that are nearby. However, this is to be expected, since gyrification is aimed to represent the combined structural properties of neighboring gyri and the sulci between them.23
Statistical analysis.
Sex and age were compared between patients with PD and controls using Fisher exact test and 2-sample Student t test, respectively. Analysis of variance was used to assess differences among PD subgroups or controls. We performed longitudinal analyses of cortical structure at the vertex level using a validated framework (spatiotemporal linear mixed effects model) that leverages covariance among neighboring vertices and can yield increases in statistical power while providing good control of false-positive rate.24 Briefly, each hemisphere was divided into ∼30,000 regions of homogenous covariance from ∼150,000 vertices. Fast expectation maximization iterations were applied to obtain more accurate parameter estimates, which were averaged within each region.25 Hypothesis testing utilized the Satterthwaite-based approximation of a scaled F statistic. p Values were adjusted using an expected false discovery rate of 0.05.26,27
The final mixed effects model used for group comparisons included the following: linear and quadratic terms of age at baseline, sex, years elapsed since baseline, years of education, HAM at each visit, intracranial volume (ICV), the terms for PD stages, the respective interaction terms for PD stages and years elapsed, the term for interaction between age at baseline and years elapsed, and the term for interaction between sex and years elapsed. ICV was included as a covariate because it was associated with overall LGI (p < 0.0001).
General linear hypothesis testing using the F statistic was utilized to conduct group and subgroup comparisons.28 Overall LGI was defined as the average of LGI across all cortical vertices. Regional and overall gyrification indices were analyzed using R version 3.1.1.29
The relationships between clinical measurements and cortical structural measures among PD participants at baseline were assessed (1) descriptively using locally weighted regression and 95% bootstrapped confidence regions and (2) quantitatively using multiple linear regression for each variable of interest independently (covariates included age, sex, education years, ICV, and HAM as appropriate).
The validation study was conducted in cortical areas that were found to be significantly associated with advanced PD stage in the longitudinal cohort. For this analysis, we utilized the bilateral regional means of LGI for the respective cortical areas. The general linear model was used to conduct these validation analyses and included the following variables: linear and quadratic terms of age at baseline, sex, years of education, HAM, ICV, and the terms for PD stages. Outliers having extremely low gyrification values (2 standard deviations < sample mean) were excluded from the study (1 control, 2 PDM, 2 PDL in the validation sample).
RESULTS
Demographic characteristics of study participants.
In the longitudinal cohort, patients with PD and controls were not significantly different in age or sex frequencies at any visit (table 1). Controls had more years of education than patients with PD, but education did not correlate with any cortical metrics in either the patients with PD or controls. From the baseline visit, the 18-month dropout rate was 20.0% and 17.1% for patients with PD and controls, respectively, and 14.3% and 10.3%, respectively, from 18 to 36 months. The total number of visits did not differ between patients with PD and controls (p = 0.210). The demographic characteristics of those who dropped out did not differ between patients with PD and controls. Patients with PD demonstrated the expected progression of symptoms as reflected by increased UPDRS-III scores and LEDD. Patients with PD had increased depression scores compared to controls (p < 0.0001). Among patients with PD, disease stage and DOI were significantly associated with depression scores at all visits (p < 0.0001).
In the validation study sample, PD subgroups and controls were relatively similar in age, sex frequencies, and education, but patients with PD had trend-level lower MMSE scores compared to controls (p = 0.055).
Longitudinal cohort analysis of cortical gyrification.
We first investigated LGI differences between patients with PD and controls using vertex-level analyses. Compared to controls, patients with PD overall had reduced LGI in the left inferior parietal, superior frontal, frontal pole, and rostral anterior cingulate, and the right inferior parietal, precentral, rostral middle frontal, and fusiform areas (p < 0.05). There were no significant differences in LGI between completed PD participants and PD participants lost to follow-up (p ≥ 0.20).
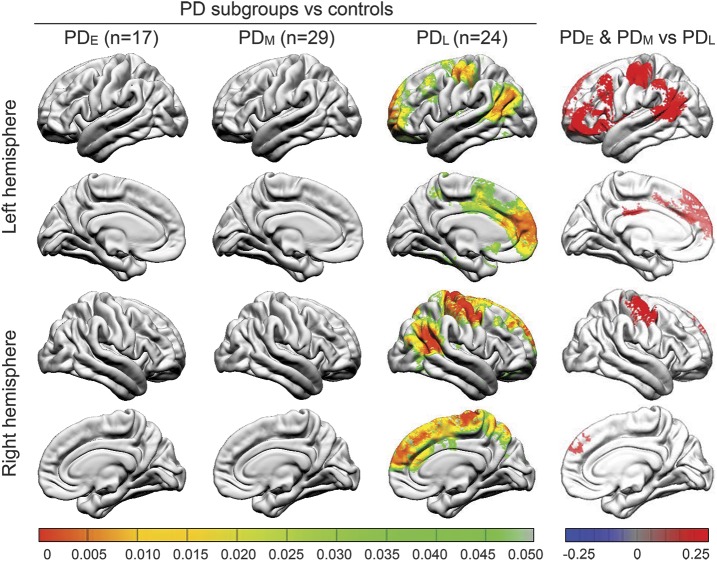
At baseline and 36-month visits, LGI was not significantly reduced in any region in PDE or PDM subgroups vs controls. There were substantial differences in LGI between PDL and controls, however, at baseline bilaterally in overall LGI, inferior parietal, postcentral, precentral, superior frontal, and supramarginal areas (p < 0.05) (figure 1, table 2). At the 36-month visit, these bilateral differences persisted and also extended to include bilaterally the transverse temporal, fusiform, inferior temporal, and pars orbitalis regions (p < 0.05). Comparisons of LGI between PDL and controls at the 18-month visit revealed patterns of reduced LGI that were intermediate between baseline and 36-month visits. Figure e-1 on the Neurology® Web site at Neurology.org depicts the longitudinal trajectories of LGI in cortical regions where LGI was significantly reduced in PDL.
Figure 1. Comparison of local gyrification index between Parkinson disease (PD) subgroups and controls (left) and among PD subgroups (right) at baseline.
PD–late (PDL) participants (duration of illness >5 years) demonstrated significantly reduced gyrification bilaterally in the inferior parietal, precentral and postcentral, and superior frontal areas, compared to controls at baseline visit (left). PD subgroup vs control color maps represent adjusted p values using an expected false discovery rate of 0.05. PDL participants demonstrated significantly reduced gyrification in several neocortical areas, compared to PD–early (PDE) and PD–middle (PDM) participants at baseline (right). Post hoc PD subgroup comparison color maps represent significant β values using a false discovery rate–adjusted p value threshold of 0.05.
Table 2.
Comparisons of regional local gyrification index among PD subgroups and controls
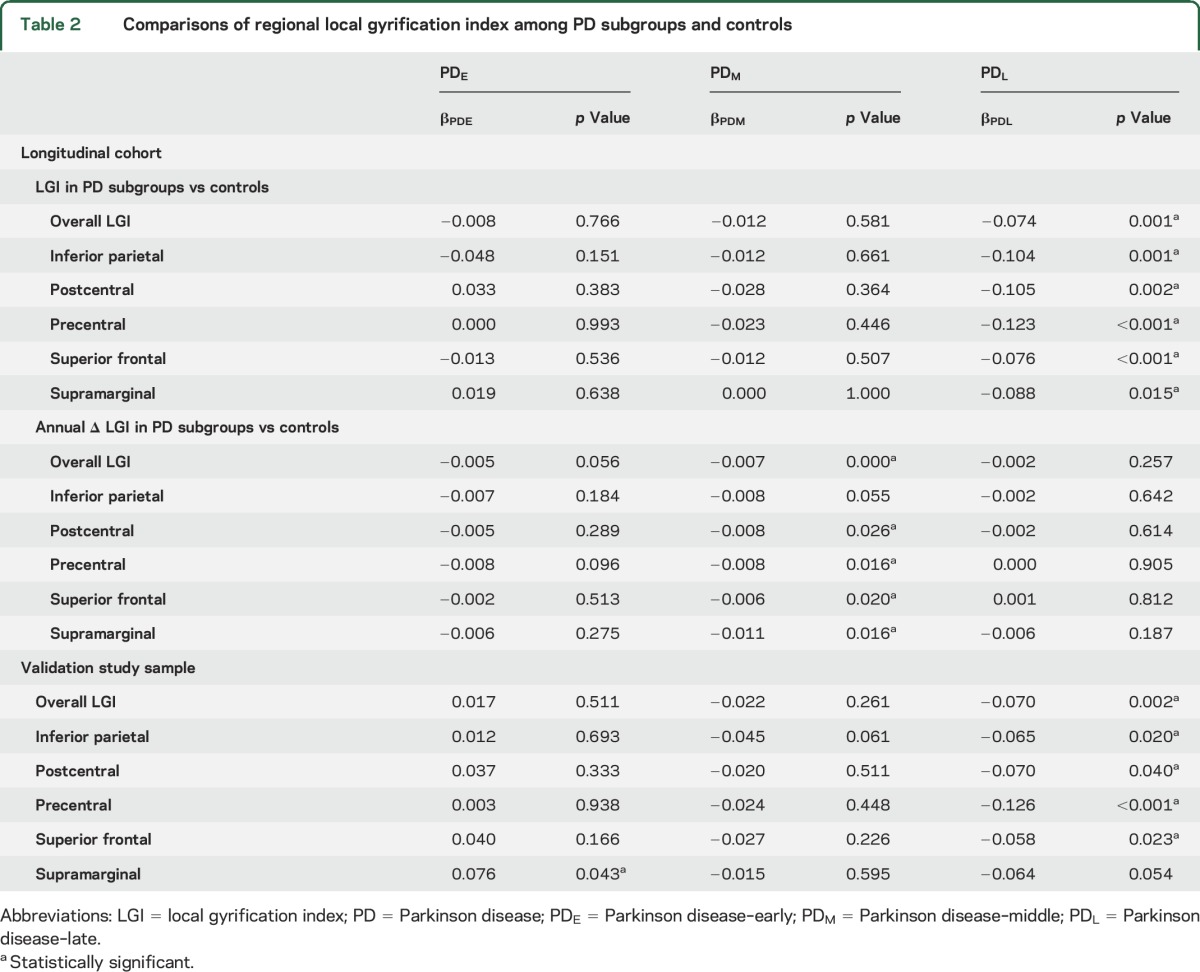
We next compared LGI among PD subgroups (figure 1). PDL had reduced LGI at baseline in the precentral, postcentral, and superior frontal areas bilaterally; the left inferior parietal, pars orbitalis, superior temporal banks, lateral orbitofrontal, and rostral middle frontal; and right caudal middle frontal areas compared to combined PDE and PDM subgroups (p < 0.05). At the 36-month visit, there were significant differences in LGI between PDE/PDM and PDL in the right precentral and postcentral areas (p < 0.05).
Longitudinal analysis revealed accelerated overall LGI loss in the PDM subgroup (p < 0.001) and nonsignificant accelerated loss in PDE (p = 0.056) compared to controls. Loss of LGI also was accelerated in the postcentral, precentral, superior frontal, and supramarginal areas among PDM (p < 0.05) compared to controls, and nonsignificant accelerated loss was present in the inferior parietal area (p = 0.055) (table 2).
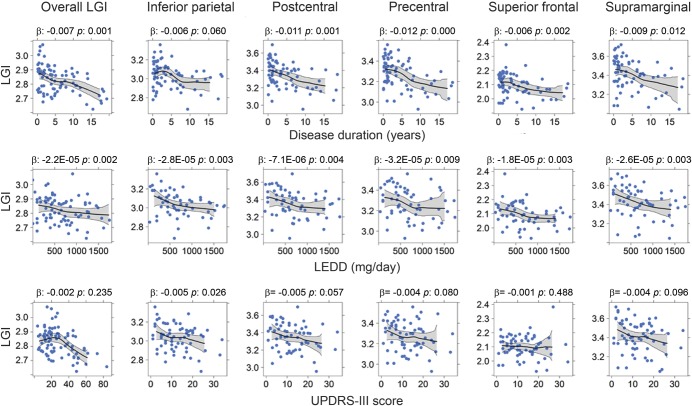
Several clinical measurements were correlated with LGI. DOI was correlated negatively with overall LGI and regional LGI of the postcentral, precentral, superior frontal, and supramarginal areas (p < 0.05). LEDD was correlated negatively with LGI in all of the aforementioned areas and UPDRS showed correlations with LGI of the inferior parietal area (p = 0.026) (figure 2).
Figure 2. Relationships of baseline regional local gyrification index and clinical measurements among Parkinson disease (PD) participants in the longitudinal cohort.
Descriptive plots illustrate the relationship between regional local gyrification and clinical measurements among PD participants in the longitudinal cohort. Center lines (black) and gray areas represent the moving average and 95% bootstrapped confidence region for local gyrification index as a function of clinical measurements. The β and p values were obtained via multiple linear regression for each clinical variable. Two data points were not shown in the levodopa-equivalent daily dose (LEDD) plots because they had LEDD >1,800 mg/d. LGI = local gyrification index; UPDRS = Unified Parkinson's Disease Rating Scale.
Validation study sample analysis of cortical gyrification.
For the validation study, we utilized bilateral averages of LGI in regions that were shown to be reduced bilaterally in the longitudinal cohort study. Compared to controls, PDL demonstrated significantly lower overall LGI and in the inferior parietal, postcentral, precentral, and superior frontal areas (p < 0.05), although the LGI differences in the supramarginal area did not reach statistical significance (p = 0.054) (table 2).
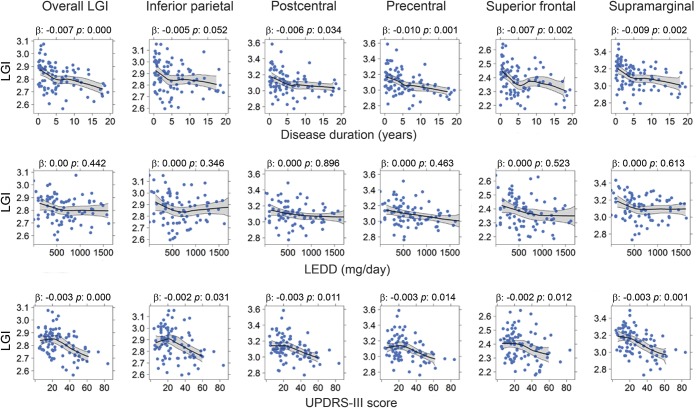
DOI was correlated with overall LGI and regional LGI of the postcentral, precentral, superior frontal, and supramarginal areas (p < 0.05), and the correlation for the inferior parietal area did not reach statistical significance (p = 0.052) (figure 3). UPDRS-III scores were correlated negatively with overall LGI, and with regional LGI of the inferior parietal, postcentral, precentral, superior frontal, and supramarginal areas (p < 0.05).
Figure 3. Relationships of regional local gyrification index and clinical measurements among Parkinson disease (PD) participants in the validation study sample.
Descriptive plots illustrate the relationship between regional local gyrification and clinical measurements among PD participants in the validation study sample. Center lines (black) and gray areas represent the moving average and 95% bootstrapped confidence region for local gyrification index as a function of clinical measurements. The β and p values were obtained via multiple linear regression for each clinical variable. Two data points were not shown in the levodopa-equivalent daily dose (LEDD) plots because they had LEDD >1,800 mg/d. LGI = local gyrification index; UPDRS = Unified Parkinson's Disease Rating Scale.
DISCUSSION
This study demonstrated that reduced cortical gyrification is related to disease progression in participants with PD without dementia. Losses of gyrification were accelerated early after diagnosis, and became prominent in later stages of disease, suggesting that measurements of cortical folding may be useful for monitoring disease progression. Interestingly, we found that the loss of gyrification was particularly prominent in the neocortical regions that are thought to be relatively spared from Lewy pathology in PD.5,30 In contrast, we did not observe altered gyrification in areas known to be more heavily affected by Lewy pathology (i.e., brain base and temporal areas). Taken together, these findings raise the possibility that cortical folding abnormalities may reflect pathologic processes not attributable solely to Lewy pathology.
Although recent data provided initial evidence that gyrification of the cortex is reduced in PD (average DOI ∼ 3.9 years),31 the association between cortical folding and disease progression remained unclear. Indeed, another recent study reported no differences between patients with PD and controls, although there were some areas of correlation between a composite measure of disease progression that included dementia in PD and cortical gyrification of the left middle frontal, superior parietal, superior frontal, supramarginal, lateral occipital, inferior parietal, and right superior frontal and superior parietal areas.8 Whereas these findings may have been attributable to the inclusion of dementia (mean MMSE = 18.3 in the PD dementia group), our study excluded dementia at baseline. Participants with PDE or PDM did not have reduced gyrification at baseline, but demonstrated accelerated loss of gyrification longitudinally. PDL had prominently reduced gyrification bilaterally in several cortical areas despite the lack of dementia (MMSE ∼ 28–29). Disease duration, LEDD, and motor scores also were associated with reduced overall LGI in PD. Together, these findings suggest that accelerated loss of cortical folding occurs shortly after PD diagnosis and may be associated with disease progression prior to the occurrence of dementia. Accordingly, metrics of cortical folding may provide sensitive measurements to gauge ongoing cortical neurodegeneration as PD progresses.
Previous studies have reported findings of widespread cortical pathology in PD, including reduced levels of neurotransmitters and tyrosine hydroxylase immunoreactive interneurons,3,4 increased apoptotic signaling,2 and Lewy pathology.5 Neocortical areas, however, have been shown to be relatively spared from Lewy pathology.5 The current study demonstrated that the loss of gyrification is especially prominent in the precentral and postcentral areas in PD, suggesting that cortical gyrification might not reflect Lewy pathology directly as we hypothesized. These differences may be explained by several possibilities. First, Lewy pathology may not be correlated with cell death equally throughout all cortical regions. Second, Lewy pathology is known to occur in distinct cortical layers in PD, preferentially in deeper layers in the neocortical areas and in superficial layers of the mesocortex.5 This differential pattern of pathology may contribute to the gyrification losses observed in this study. Few details, however, are available regarding the exact layer-specific pattern of Lewy pathology, warranting further investigation.5 Third, changes in underlying white matter could result in altered cortical folding.32
To understand the stage-dependent changes of gyrification in PD, we subdivided PD patients into 3 subgroups as we have done previously.33 The PDE upper limit of DOI (1 year) was chosen to define PD participants who had not received extended treatment. PDL was defined as PD participants having at least 5 years of disease duration for several reasons. For example, nigrostriatal terminal labeling has been suggested to reach a floor after approximately 5 years,1 although some data suggest that nigral cell death continues,34 and dopamine levels certainly decline throughout disease progression.35 Clinically, dyskinesias, cognitive decline, and dopamine-nonresponsive symptoms tend to be more prominent after the first 5 years (honeymoon phase).36,37 These subgroup categorizations provided balanced subgroup sample sizes that are powerful for equivalence testing.1,38,39 Interestingly, the LGI continued to decline after 5 years (figure e-2). We also repeated our analyses using H&Y stage, with similar results (table e-1). There were also inverse correlations between gyrification indices and clinical measurements (LEDD and UPDRS-III, figures 2 and 3). Together, our results support the notion that gyrification is stage-dependent and associated with PD progression.
The current study had several limitations. Although the overall sample size is large, the sample size for PD subgroup analysis was relatively small. Larger sample sizes and longer follow-up of various disease staging categories will be needed to generalize these findings. In addition, as is common in longitudinal studies, there was significant dropout in both the PD and control groups. The total number of visits, however, did not differ between these groups. There also may be considerable variability in clinical severity and disease duration.40 However, we repeated all of our analyses using H&Y staging and found similar results (figure e-1).
The PDL group also had a relatively high male-to-female ratio. Most relationships between LGI and disease duration persisted when male and female participants with PD were analyzed separately (table e-1). Although the p values in table e-1 may give the impression of discordant results among female participants, the directions of the correlations were the same for all regions regardless of sex or cohort. Furthermore, we found no significant differences in overall, inferior parietal, postcentral, precentral, superior frontal, or supramarginal LGI between male and female controls, and sex had no effect on the rate of LGI change in controls. Nevertheless, to minimize any potential confounding effect, we included sex in our statistical model. We also utilized “on” medication motor scores because some participants could not tolerate “off” medication assessment. Of note, the scores obtained in the practically defined “off” medication state may not represent true “off” medication symptoms, since some drugs may not completely wash out. “On” medication scores may be more representative of the levodopa-unresponsive components of patient symptoms, which may be more closely related to cortical findings. Finally, the study was validated using the baseline data from another newly established cohort, and the longitudinal data are not yet available. Although the Parkinson's Progression Markers Initiative has longitudinal data, this cohort only includes PD participants in very early stages. Independent validation of longitudinal trajectories in advanced stage PD is needed.
This study demonstrated that cortical gyrification is reduced among participants with PD without dementia, and is associated with measurements of PD progression. Loss of cortical gyrification may be accelerated shortly after PD diagnosis and becomes prominent in later stages. These findings suggest that folding metrics may be informative for quantifying cortical changes throughout PD progression. Moreover, the finding of reduced gyrification in areas known to be spared from Lewy pathology is unexpected, raising the possibility that cortical folding abnormalities reflect processes not attributable solely to Lewy pathology in PD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants; Jeffery Vesek for MRI technical support; and study coordinators Eleanore Hernandez, Brittany Jones, Melissa Santos, and Raghda Clayiff for assistance.
GLOSSARY
- DOI
duration of illness
- H&Y
Hoehn & Yahr
- HAM
Hamilton Depression Rating Scale
- ICV
intracranial volume
- LEDD
levodopa-equivalent daily dose
- LGI
local gyrification index
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- PDE
Parkinson disease–early
- PDM
Parkinson disease–middle
- PDL
Parkinson disease–late
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Nicholas W. Sterling: research project conception, research project organization, research project execution, statistical analysis design, statistical analysis execution, statistical analysis review and critique, writing of first draft, manuscript review and critique. Ming Wang: research project conception, research project execution, statistical analysis design, statistical analysis execution, statistical analysis review and critique, manuscript review and critique. Lijun Zhang: research project conception, research project organization, research project execution, statistical analysis review and critique, manuscript review and critique. Eun-Young Lee: research project conception, manuscript review and critique. Guangwei Du: research project conception, research project organization, research project execution, statistical analysis design, statistical analysis review and critique, manuscript review and critique. Mechelle M. Lewis: research project conception, research project organization, research project execution, statistical analysis review and critique, writing of first draft, manuscript review and critique. Martin Styner: statistical analysis review and critique, manuscript review and critique. Xuemei Huang: research project conception, research project organization, research project execution, statistical analysis review and critique, writing of first draft, manuscript review and critique.
STUDY FUNDING
Supported by NINDS (NS060722 and NS082151 to X.H.), the Hershey Medical Center GCRC (NIH M01RR10732), GCRC Construction Grant (C06RR016499), and Pennsylvania Department of Health Tobacco CURE Funds.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol 2009;8:1158–1171. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, He P, Adler CH, et al. Bid signal pathway components are identified in the temporal cortex with Parkinson disease. Neurology 2012;79:1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda T, Takahashi J, Tanaka J. Tyrosine hydroxylase-immunoreactive neurons are decreased in number in the cerebral cortex of Parkinson's disease. Neuropathology 1999;19:10–13. [DOI] [PubMed] [Google Scholar]
- 4.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Res 1983;275:321–328. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 6.Orimo S, Uchihara T, Kanazawa T, et al. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson's disease. Neuropathol Appl Neurobiol 2011;37:791–802. [DOI] [PubMed] [Google Scholar]
- 7.Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS One 2013;8:e54980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2013;84:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385:313–318. [DOI] [PubMed] [Google Scholar]
- 10.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord 2007;22:2314–2324. [DOI] [PubMed] [Google Scholar]
- 11.Goetz CG, Emre M, Dubois B. Parkinson's disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 2008;64(suppl 2):S81–S92. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 15.Ofori E, Du G, Babcock D, Huang X, Vaillancourt DE. Parkinson's disease biomarkers program brain imaging repository. Neuroimage 2015;124:1120–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahn S, Elton R. Unified Parkinson's disease rating scale. In: Fahn S, Jenner P, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1986. [Google Scholar]
- 17.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 19.Bernal-Rusiel JL, Greve DN, Reuter M, Fischl B, Sabuncu MR; for the Alzheimer's Disease Neuroimaging Initiative. Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage 2012;66C:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 2011;57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol 1988;179:173–179. [DOI] [PubMed] [Google Scholar]
- 23.Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging 2008;27:161–170. [DOI] [PubMed] [Google Scholar]
- 24.Bernal-Rusiel JL, Reuter M, Greve DN, Fischl B, Sabuncu MR; Alzheimer's Disease Neuroimaging Initiative. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage 2013;81:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird N, Lange N, Stram D. Maximum likelihood computations with repeated measures: application of the EM algorithm. J Am Stat Assoc 1987;82:97–105. [Google Scholar]
- 26.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983–997. [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300. [Google Scholar]
- 28.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available at: http://www.R-project.org/. Accessed August 2014. [Google Scholar]
- 30.Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer's and Parkinson's diseases. J Alzheimers Dis 2006;9:35–44. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang J, Xu J, et al. Cortical gyrification reductions and subcortical atrophy in Parkinson's disease. Mov Disord 2014;29:122–126. [DOI] [PubMed] [Google Scholar]
- 32.Tallinen T, Chung JY, Biggins JS, Mahadevan L. Gyrification from constrained cortical expansion. Proc Natl Acad Sci USA 2014;111:12667–12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov Disord 2012;27:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlmutter JS, Norris SA. Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Ann Neurol 2014;76:769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington: clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415–455. [DOI] [PubMed] [Google Scholar]
- 36.Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson's disease therapy. Ann Neurol 2003;53(suppl 3):S3–S12; discussion S12–S15. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer RF, Wszolek ZK, Ebadi M. Parkinson's Disease, 2nd ed Philadelphia: Taylor & Francis; 2012. [Google Scholar]
- 38.Lee CS, Schulzer M, de la Fuente-Fernandez R, et al. Lack of regional selectivity during the progression of Parkinson disease: implications for pathogenesis. Arch Neurol 2004;61:1920–1925. [DOI] [PubMed] [Google Scholar]
- 39.Lee CS, Schulzer M, Mak EK, et al. Clinical observations on the rate of progression of idiopathic parkinsonism. Brain 1994;117:501–507. [DOI] [PubMed] [Google Scholar]
- 40.Palmer JL, Coats MA, Roe CM, Hanko SM, Xiong C, Morris JC. Unified Parkinson's Disease Rating Scale-Motor Exam: inter-rater reliability of advanced practice nurse and neurologist assessments. J Adv Nurs 2010;66:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.