Abstract
Objective:
Even though statin pretreatment is associated with better functional outcomes and lower risk of mortality in acute ischemic stroke, there are limited data evaluating this association in acute ischemic stroke due to large artery atherosclerosis (LAA), which carries the highest risk of early stroke recurrence.
Methods:
Consecutive patients with acute LAA were prospectively evaluated from 7 tertiary-care stroke centers during a 3-year period. Statin pretreatment, demographics, vascular risk factors, and admission and discharge stroke severity were recorded. The outcome events of interest were neurologic improvement during hospitalization (quantified as the relative decrease in NIH Stroke Scale score at discharge in comparison to hospital admission), favorable functional outcome (FFO) (defined as modified Rankin Scale score of 0–1), recurrent stroke, and death at 1 month. Statistical analyses were performed using univariable and multivariable Cox regression models adjusting for potential confounders. All analyses were repeated following propensity score matching.
Results:
Statin pretreatment was documented in 192 (37.2%) of 516 consecutive patients with LAA (mean age: 65 ± 13 years; 60.8% men; median NIH Stroke Scale score: 9 points, interquartile range: 5–18). Statin pretreatment was associated with greater neurologic improvement during hospitalization and higher rates of 30-day FFO in unmatched and matched (odds ratio for FFO: 2.44; 95% confidence interval [CI]: 1.07–5.53) analyses. It was also related to lower risk of 1-month mortality and stroke recurrence in unmatched and matched analyses (hazard ratio for recurrent stroke: 0.11, 95% CI: 0.02–0.46; hazard ratio for death: 0.24, 95% CI: 0.08–0.75).
Conclusion:
Statin pretreatment in patients with acute LAA appears to be associated with better early outcomes regarding neurologic improvement, disability, survival, and stroke recurrence.
Taking statins before stroke may improve early outcomes including early neurologic deterioration, mortality, and disability in patients with acute ischemic stroke (AIS).1,2 In a recent meta-analysis, statin pretreatment was found to reduce mortality risk, while increasing good functional outcome at 3 months after stroke onset.3 In another systematic review, the beneficial effect of statin pretreatment in AIS was more profound in patients with high vascular risk and in patients with ideal low-density lipoprotein levels.4
Despite the fact that large artery atherosclerotic (LAA) stroke carries the highest risk of early recurrent stroke in comparison to other AIS subtypes,5,6 the potential beneficial effect of statin pretreatment has not been investigated in this specific stroke subgroup. Of note, a recent multicenter study indicated that statin pretreatment reduced the risk of recurrent cerebral ischemia in patients with TIA due to symptomatic carotid artery stenosis but had no protective effect in TIA patients without carotid artery stenosis.7 In view of the former considerations, we conducted a prospective, international, multicenter study that aimed to evaluate the potential relationship between statin pretreatment and early outcomes in patients with acute LAA.
METHODS
Study population.
We prospectively evaluated consecutive first-ever AIS patients with LAA from 7 tertiary stroke care centers who were enrolled during a 3-year study period (June 2011 to June 2014). Additional information regarding the participating centers is available in the e-Methods on the Neurology® Web site at Neurology.org. LAA was diagnosed according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.8 Additional information regarding the LAA definition is available in the e-Methods.
Vascular neuroimaging (including neurosonology examinations [cervical duplex ultrasound, transcranial sonography] as well as magnetic resonance angiography or CT angiography of cervical and cerebral vessels) was performed during hospitalization as part of standard diagnostic workup in all cases.9–11 Patients with TIA, recurrent stroke, missing information on statin pretreatment, and lack of follow-up information were excluded from further evaluation. Additional information regarding the variables that were recorded in our study is available in the e-Methods.
In-hospital management.
All patients were treated during hospitalization according to the most current (before study onset) American Heart Association (AHA) recommendations12 for AIS in all participating centers. Statin pretreatment was not discontinued during hospitalization in all participating centers. Statins were also initiated at discharge in the indicated patients without history of statin pretreatment according to current AHA recommendations for secondary stroke prevention.13 Carotid endarterectomy was performed in patients with LAA who had symptomatic extracranial carotid artery stenosis (≥50%) according to AHA recommendations14 within 2 weeks from symptom onset in patients with no or minor residual disability (grade 0–2 on the modified Rankin Scale [mRS]) as previously described.11 All remaining patients with LAA who had extra- or intracranial atherosclerotic disease in all participating centers were managed conservatively in accordance with current AHA12 guidelines.
Follow-up.
We prospectively followed all patients at the outpatient stroke clinic of the aforementioned stroke centers and evaluated their clinical status at 30 days after symptom onset, as previously described.9–11 We prospectively captured the following outcome events during the first 30 days of ictus: (1) neurologic improvement during hospitalization, (2) death, (3) recurrent stroke, and (4) favorable functional outcome (FFO) during the first month following the index event. Neurologic improvement was quantified as the relative decrease in NIH Stroke Scale (NIHSS) score at hospital discharge in comparison to hospital admission ([NIHSSadm−NIHSSdis]/NIHSSadm × 100%).15,16 Recurrent strokes were diagnosed as cerebrovascular events occurring suddenly, lasting >24 hours, and resulting in increased preexisting neurologic deficits or causing new neurologic symptoms and signs.10 The presence of a new lesion on follow-up brain imaging that involved an anatomical site or vascular territory that was unaffected on the admission CT scan was a prerequisite, along with the clinical findings, for the diagnosis of recurrent stroke.9,10 The mRS score at 1 month was estimated for all patients. FFO at 30 days was defined as an mRS score of 0 or 1.17 All outcome events were assessed by attending-level stroke neurologists at the individual participating centers who were unaware of information regarding statin pretreatment.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the corresponding ethics committees of the participating institutions, and informed consent was obtained from all patients (or guardians of patients, when consent could not be obtained directly from the patients) participating in the study.18
Statistical analysis.
We presented continuous parametric data using their mean values together with their corresponding SDs. We used median values with their corresponding interquartile ranges for the presentation of nonparametric data and percentages for all dichotomous variables. Statistical comparisons between different subgroups were performed using the χ2 test, unpaired t test, or Mann–Whitney U test, where appropriate.
We used the Kaplan–Meier product-limit method to calculate the cumulative probabilities of both mortality and 30-day stroke recurrence after the initial event. The factors that were associated with the risk of stroke recurrence and 30-day mortality were identified using Cox proportional hazards analyses. Associations are presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs).
We used univariable and multivariable logistic regression analyses to search for possible predictors of FFO. In multivariable analyses, we tested the statistical significance hypothesis under the likelihood ratio test with an α value of 0.05. We reported all associations as odds ratios (ORs) with their corresponding 95% CIs.
For the propensity score analysis, we constructed a multivariable logistic regression model to account for statin use before stroke onset among all stroke patients with LAA. The aim of propensity score analyses was to minimize potential imbalances in the distribution of potential confounders between statin users and nonusers as previously described in detail.19 Using the final matched dataset, new fully adjusted cox proportional hazards and logistic regression models were generated to determine the association of prestroke statin use with 30-day mortality, stroke recurrence, and FFO. Additional information regarding the statistical analyses (Cox regression and logistic regression models as well propensity score matching approach) is available in the e-Methods.
The Stata statistical software release 13 for Windows (StataCorp LP, College Station, TX) was used for all statistical analyses.
RESULTS
Unmatched analysis.
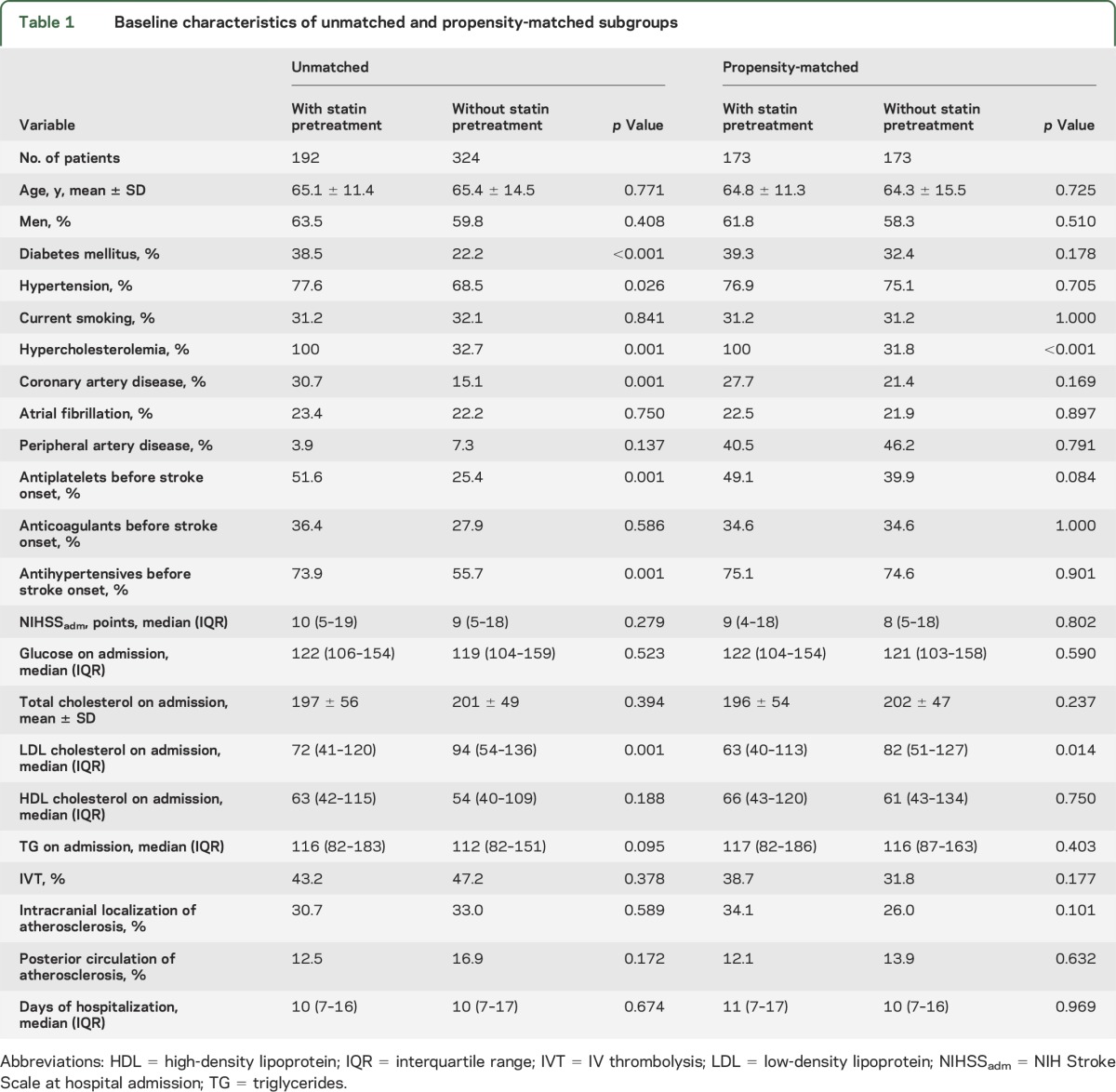
A total of 516 LAA stroke patients (mean age: 65.3 ± 13.4 years; 60.8% men; 42.2% Asians, 57.8% Caucasians; median NIHSS score: 9, interquartile range: 5–18) were included during the study period. Medication data were available for all patients, with no missing values. All patients reported to be taking their prescribed medications the previous days before admission. None of the patients received intra-arterial revascularization procedures. Statin intake before stroke onset was reported in 192 (37.2%) of these patients. Patients with statin pretreatment before stroke onset were found to have higher rates of diabetes mellitus (p < 0.001), hypertension (p = 0.026), hypercholesterolemia (p = 0.001), coronary artery disease (p = 0.001), antiplatelets (p = 0.001), and antihypertensives (p = 0.001) administration before admission, and to have lower low-density lipoprotein levels on admission (p = 0.001), when compared to patients who did not receive statins before stroke onset (table 1). The 2 groups did not differ in age (p = 0.771), sex (p = 0.408), baseline stroke severity (p = 0.279), intracranial location of atherosclerosis (p = 0.589), and duration of hospitalization (p = 0.674). The rates of carotid endarterectomy were similar among patients with and without statin pretreatment (63.0% vs 57.7%; p = 0.235).
Table 1.
Baseline characteristics of unmatched and propensity-matched subgroups
A total of 29 cases of recurrent stroke were identified in our study populations. There were 28 cases of ischemic stroke (97%) and one case of intracerebral hemorrhage (3%). The most common etiopathogenic mechanism of recurrent ischemic strokes was LAA (n = 20, 71%), small vessel disease (n = 2, 7%), cardioembolism (n = 2, 7%), infarct of other determined etiology (n = 1, 4%), and infarct of undetermined etiology (n = 3, 11%). Stroke recurrence was in the same vascular territory in all patients with recurrent cerebral infarction due to LAA (n = 20, 71%). A total of 6 cases with recurrent strokes were fatal (21%). Data regarding stroke severity among stroke survivors with recurrent stroke was not systematically collected during the 30-day follow-up period.
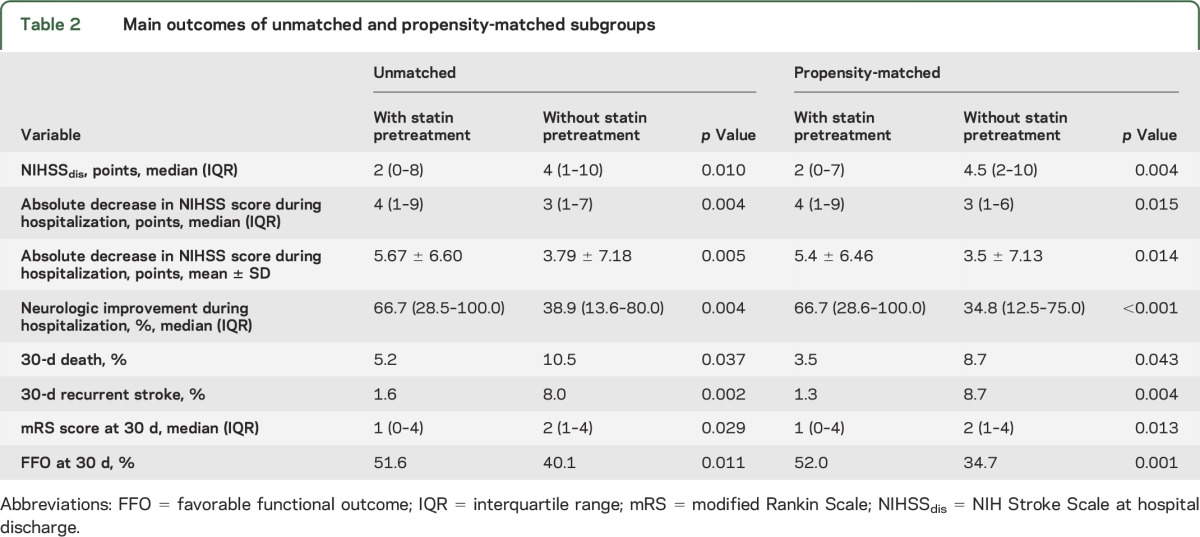
Patients with LAA pretreated with statins had lower 30-day mortality (p = 0.037) and 30-day recurrent stroke (p = 0.002) rates, lower NIHSSdis (p = 0.010), greater absolute NIHSS decrease during hospitalization (p = 0.004), and lower mean mRS score at 30 days (p = 0.029) in comparison to patients who were treated with statins before stroke onset in the initial unadjusted analysis (table 2). Neurologic improvement during hospitalization was greater in patients with than without statin pretreatment (66.7% vs 38.9%; p = 0.004). The rate of FFO was higher in patients with statin pretreatment (51.6% vs 40.1%; p = 0.011).
Table 2.
Main outcomes of unmatched and propensity-matched subgroups
In survival analysis, the cumulative 30-day mortality was found to be higher in patients who were not pretreated with statins before stroke onset (10.5% [7.2%–13.8%] vs 5.2% [2.1%–8.3%]; log rank = 4.24, df = 1, p = 0.039) (figure, A). Patients who were not pretreated with statins also had a higher cumulative 30-day stroke recurrence (8% [5.1%–10.9%] vs 1.6% [0%–3.4%]; log rank = 9.69, df = 1, p = 0.002) (figure, B).
Figure. Kaplan–Meier curves of patients without and with statin pretreatment before stroke onset.
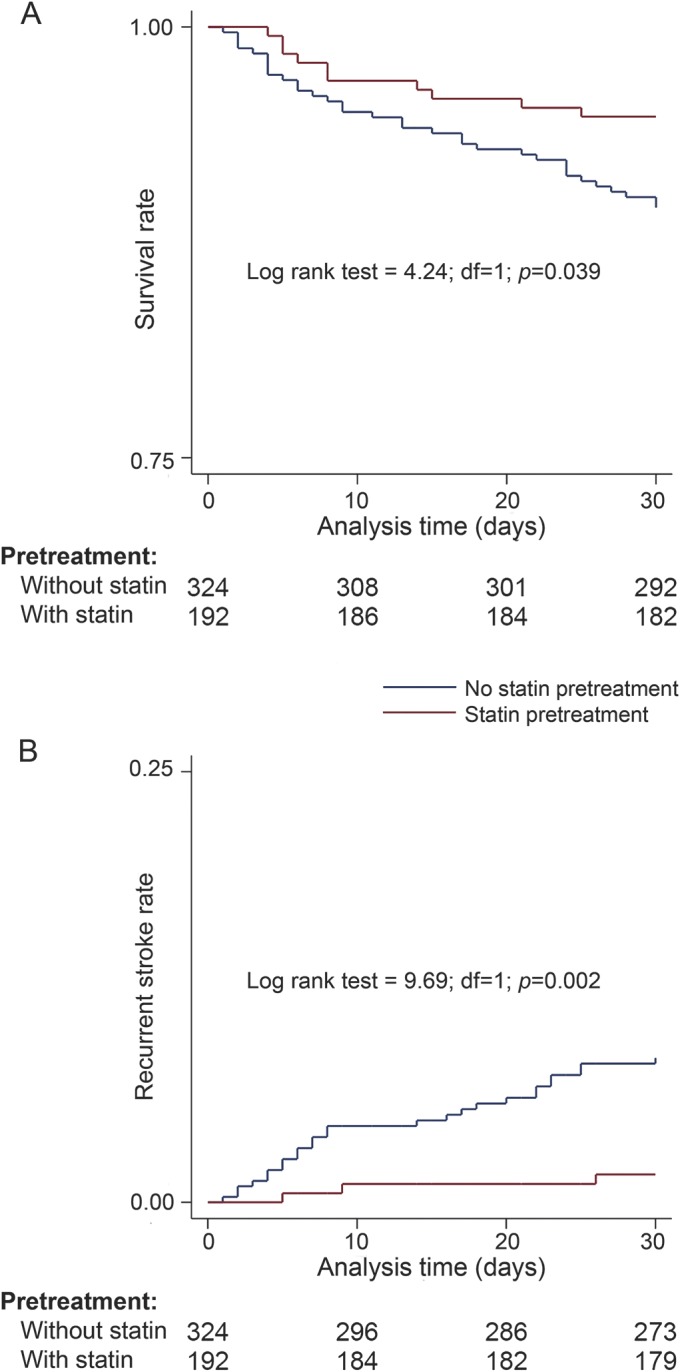
(A) Survival at 30 days and (B) the risk of recurrent stroke at 30 days of patients without and with statin pretreatment before stroke onset.
Table 3 and table e-1 summarize the predictors of 30-day mortality and 30-day stroke recurrence in univariable and multivariable Cox regression analyses. In univariable analysis, the 30-day mortality risk (table e-1) was found to be associated with current smoking (p = 0.023), coronary artery disease (p = 0.003), atrial fibrillation (p = 0.027), statin pretreatment (p = 0.044), antiplatelets pretreatment (p = 0.033), NIHSSadm (p < 0.001), serum glucose on admission (p = 0.015), total cholesterol on admission (p = 0.008), HDL cholesterol on admission (p = 0.002), triglycerides on admission (p = 0.044), and IV thrombolysis (IVT) (p < 0.001). In the multivariable model, only the history of coronary artery disease (HR = 2.10, 95% CI: 1.10–4.36, p = 0.046), statin intake before stroke onset (HR = 0.30, 95% CI: 0.13–0.69, p = 0.005), and NIHSSadm (HR = 1.07, 95% CI: 1.02–1.12, p = 0.009) were independently associated with risk of 30-day mortality (table e-1).
Table 3.
Univariable and multivariable analysis for the 30-day risk of recurrent stroke
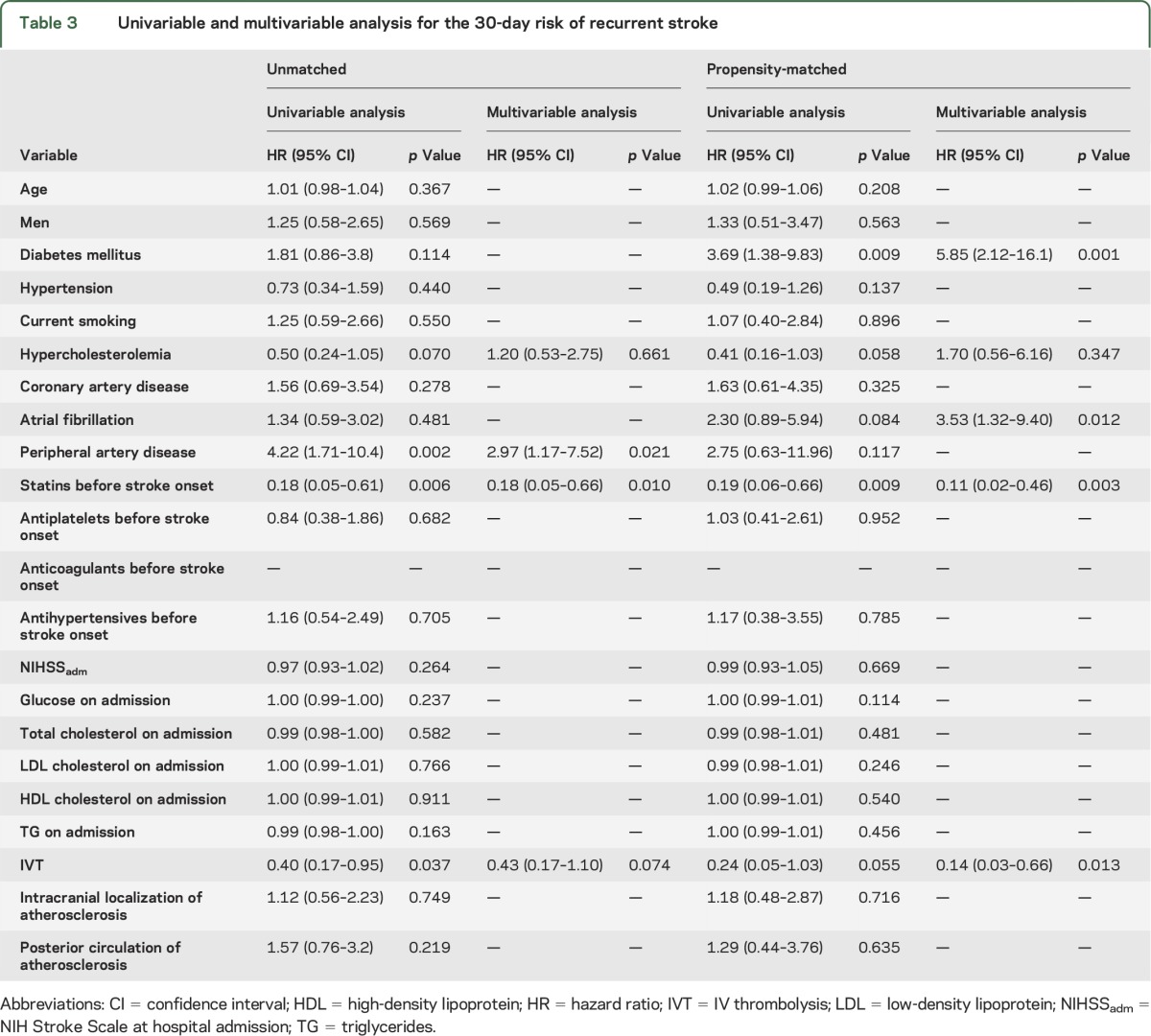
In the initial univariable Cox regression analyses, the following factors emerged as potential predictors of 30-day stroke recurrence risk (table 3): hypercholesterolemia (p = 0.070), peripheral artery disease (p = 0.002), pretreatment with statins (p = 0.006), and IVT (p = 0.037). In multivariable analyses, only peripheral artery disease (HR = 2.97, 95% CI: 1.17–7.52, p = 0.021) and statin pretreatment (HR = 0.18, 95% CI: 0.05–0.66, p = 0.010) were independently associated with the risk of 30-day stroke recurrence (table 3).
Univariable and multivariable logistics models were used to identify independent predictors of FFO at 1 month (table 4). In the initial univariable analyses, age (p < 0.001), diabetes mellitus (p = 0.004), current smoking (p = 0.007), coronary artery disease (p = 0.053), atrial fibrillation (p = 0.060), statin intake before stroke onset (p = 0.012), antiplatelet intake before stroke onset (p = 0.021), antihypertensives before stroke onset (p = 0.041), NIHSS score on admission (p < 0.001), glucose levels on admission (p = 0.036), IVT (p < 0.001), and the intracranial localization of atherosclerosis (p = 0.094) were associated with FFO. In the multivariable analysis, only diabetes mellitus (OR = 0.41, 95% CI: 0.24–0.70, p = 0.001), statin pretreatment (OR = 3.32, 95% CI: 2.02–5.46, p < 0.001), NIHSSadm (OR = 0.82, 95% CI: 0.78–0.85, p < 0.001), and IVT (OR = 3.04, 95% CI: 1.70–5.41, p < 0.001) were associated with FFO at 30 days (table 4).
Table 4.
Univariable and multivariable analysis for the probability of favorable functional outcome at 30 days
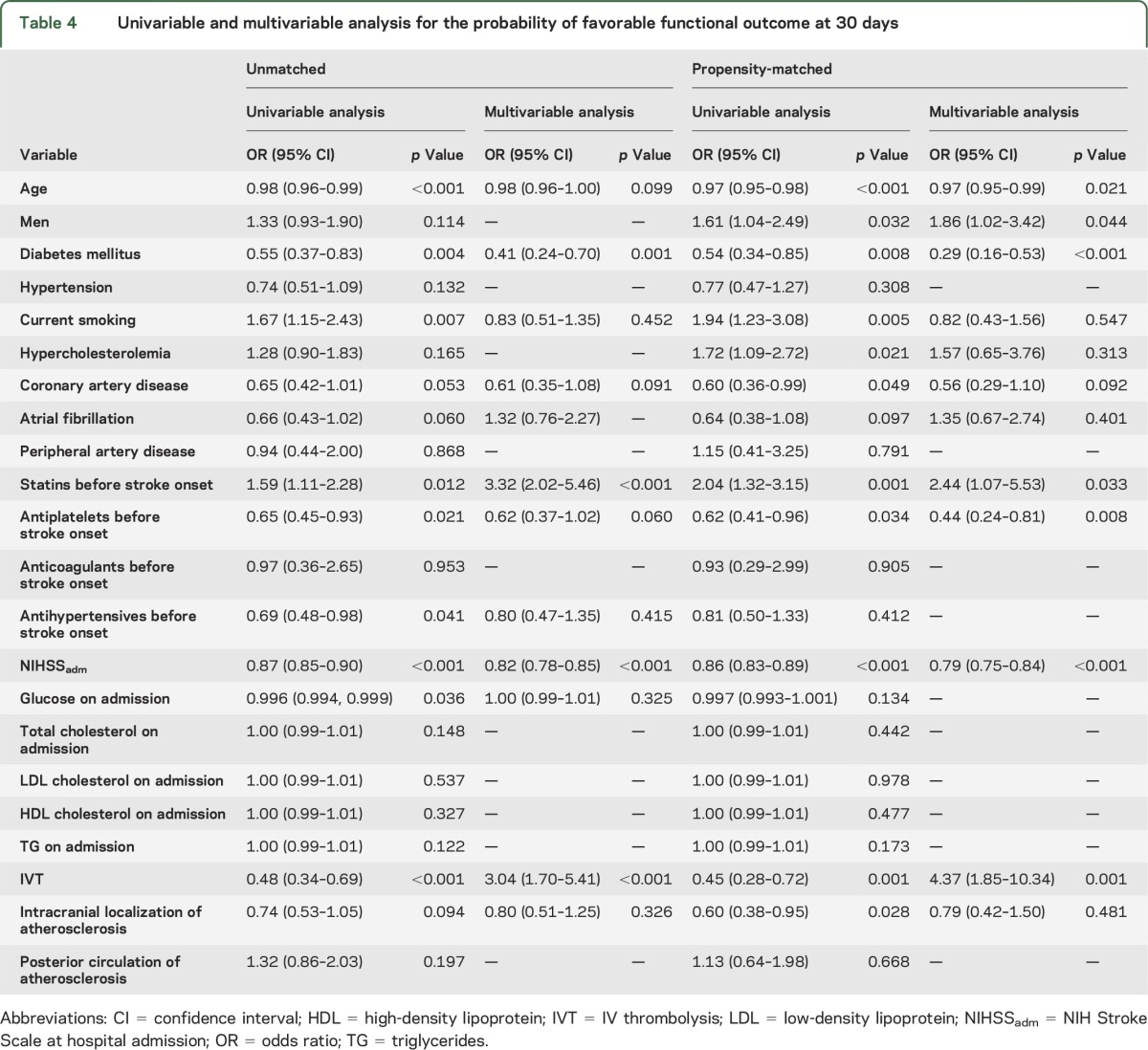
Propensity-matched analysis.
Propensity score matching resulted in 2 subgroups of 173 patients that were balanced for all potential confounding variables, except for the history of hypercholesterolemia (p < 0.001) and the values of total cholesterol on admission (p = 0.014; table 1), which are both directly related to the matching variable of statin pretreatment.
Patients with LAA pretreated with statins had lower 30-day mortality (p = 0.043) and recurrent stroke rates (p = 0.004), lower NIHSSdis (p = 0.004), greater absolute NIHSS decrease during hospitalization (p = 0.015), and lower mean mRS score at 30 days (p = 0.013) with higher rates of 30-day FFO (p = 0.001) in the initial unadjusted propensity score–matched analysis (table 2). Neurologic improvement during hospitalization was greater in patients with than without statin pretreatment (66.7% vs 34.8%; p < 0.001). The rate of FFO was higher in patients with statin pretreatment (52.0% vs 34.7%; p = 0.001).
In the univariable Cox regression analysis, the following factors emerged as predictors of the 30-day mortality risk: age (p = 0.083), coronary artery disease (p = 0.014), atrial fibrillation (p = 0.007), statin pretreatment (p = 0.052), antiplatelets pretreatment (p = 0.006), NIHSSadm (p < 0.001), total cholesterol on admission (p = 0.076), IVT (p = 0.001), and intracranial (p = 0.037) or posterior (p = 0.057) localization of atherosclerosis. In the multivariable model, only statin pretreatment (HR = 0.24, 95% CI: 0.08–0.75, p = 0.014), antiplatelet intake before stroke onset (HR = 8.21, 95% CI: 2.18–30.9, p = 0.002), and NIHSSadm (HR = 1.19, 95% CI: 1.09–1.30, p < 0.001) were independently associated with 30-day mortality risk (table e-1).
In the univariable Cox regression analysis, the following factors emerged as predictors of the 30-day stroke recurrence risk (table 3): diabetes mellitus (p = 0.009), hypercholesterolemia (p = 0.058), atrial fibrillation (p = 0.084), pretreatment with statins (p = 0.009), and IVT (p = 0.055). In multivariable analyses, diabetes mellitus (HR = 5.85, 95% CI: 2.12–16.1, p = 0.001), atrial fibrillation (HR = 3.53, 95% CI: 1.32–9.40, p = 0.012), statin pretreatment (HR = 0.11, 95% CI: 0.02–0.46, p = 0.003), and IVT (HR = 0.14, 95% CI: 0.03–0.66, p = 0.013) were independently associated with the risk of 30-day stroke recurrence (table 3).
In the univariable logistic regression, the likelihood of FFO at 30 days was associated with age (p < 0.001), male sex (p = 0.032), diabetes mellitus (p = 0.008), current smoking (p = 0.005), hypercholesterolemia (p = 0.021), coronary artery disease (p = 0.049), statin intake prior to stroke onset (p = 0.001), antiplatelet intake prior to stroke onset (p = 0.034), NIHSSadm (p < 0.001), IVT administration (p = 0.001), and intracranial localization of atherosclerosis (p = 0.028). In the multivariable analysis age (OR = 0.97, 95% CI: 0.95–0.99, p = 0.021), male sex (OR = 1.86, 95% CI: 1.02–3.42, p = 0.044), diabetes mellitus (OR = 0.29, 95% CI: 0.16–0.53, p < 0.001), statin pretreatment (OR = 2.44, 95% CI: 1.07–5.53, p = 0.033), pretreatment with antiplatelets (OR = 0.44, 95% CI: 0.24–0.81, p = 0.008), NIHSSadm (OR = 0.79, 95% CI: 0.75–0.84, p < 0.001), and IVT administration (OR = 4.37, 95% CI: 1.85–10.34, p = 0.001) were independently associated with FFO at 30 days (table 4).
DISCUSSION
Our study showed that both in unmatched and propensity score–matched analyses, statin pretreatment was associated with better early outcomes in terms of neurologic improvement during hospitalization, 30-day stroke survival, 30-day stroke recurrence, and FFO at 30 days in patients with AIS due to LAA. These findings lend support to current AHA recommendations for AIS management that advocate continuation of statin treatment during hospitalization among AIS patients pretreated with statins.20 This statement is based solely on observational findings,1–3,21,22 while there is paucity of phase III, randomized controlled trial (RCT) data on the safety and efficacy of statins during the first 30 days of ictus.23–26 Thus, our observations provide additional insight regarding the beneficial effects of statin pretreatment in patients with LAA, which carries the highest risk of early neurologic deterioration and early stroke recurrence. Moreover, statin withdrawal during the first 3 days of hospitalization in AIS patients was also associated with increased risk of 3-month death or dependency according to the findings of the RCT.27 Of note, LAA represented by far the most common subgroup (56%, 50/89) of AIS patients who were randomized in this trial.
Statin pretreatment was also found to improve postoperative outcome of both symptomatic28 and asymptomatic patients29 undergoing carotid endarterectomy. The favorable effect of statin pretreatment was also evident in the periprocedural and postprocedural outcomes of patients undergoing carotid artery stenting procedures.30,31 The aforementioned positive results could be partially explained by the effect of statins on both intraplaque angiogenesis32 and macrophage cell content within atherosclerotic carotid artery lesions.33
Prior statin intake may preferentially benefit patients with atherothrombotic strokes by favoring the development of collateral pial circulation.34 In addition, statins can also lead to plaque stabilization and potential regression of atherosclerosis as they not only affect the plaque lipid composition but also reduce inflammation, due to their pleiotropic effects.35 Furthermore, statins enhance the upregulation of endothelium nitric oxide synthase, improving endothelia function, and this action may constitute a potential mediator to their favorable effect on cerebral hemodynamics and cerebral autoregulation.36,37 Finally, statins may prevent stroke progression or recurrence during the first days of ictus due to their antithrombotic effects38 and by enhancing endogenous fibrinolysis.37,39
Certain limitations of this report also need to be acknowledged. First, the observational design does not allow us to infer any causal associations between statin pretreatment and improved outcomes in patients with acute LAA. Moreover, the follow-up period (1 month) was a relatively short period for observation of the primary endpoints of recurrent stroke/death. Second, we did not collect information on the duration, dosage, and type of prestroke statin therapy since our aim was to evaluate the overall effect of stroke pretreatment on early stroke outcomes in patients with LAA. Third, there was no central adjudication of outcome events during the 1-month follow-up period. Data on recurrent stroke severity were not available for patients with stroke recurrence at the 30-day follow-up period. Data on leukoaraiosis were not prospectively collected from the patients in our cohort, and thus we cannot assess whether the beneficial effect of statin pretreatment is accentuated in stroke patients with high small vessel disease burden. Fourth, other potential factors that could be related to stroke recurrence (e.g., socioeconomic, inflammatory) were not systematically collected in our dataset. Fifth, we did not collect data for the following potential confounders: prestroke mRS score (although we included only patients with first-ever ischemic stroke, and baseline NIHSS scores were available in all cases), time of last seen well, the exact time window of intervention for carotid endarterectomy (although the vast majority of patients were operated within the first 2 weeks from the index event), and statin initiation at discharge (due to the selected short duration of the follow-up period). Sixth, we included in our cohort of patients with LAA certain cases with coexisting atrial fibrillation if the treating physician considered that the clinical diagnosis of LAA was supported by additional information including history of TIA(s) in the same vascular territory or presence of subclinical infarction in the same vascular territory.8 However, it should be noted that this may have resulted in the misclassification of certain patients with infarction of undetermined etiology to the LAA subgroup.8 Nevertheless, we opted to include these patients in our LAA cohort to avoid potential selection bias and in order to evaluate the association of statin pretreatment with outcomes in a large, unselective cohort of patients with LAA representing everyday clinical practice experience. Moreover, all of our multivariable analyses in both the unmatched and matched cohort have adjusted for atrial fibrillation as a potential confounder. Finally, the reported associations might have been confounded by the noninclusion of specific plaque characteristics (such as plaque echogenicity, stability, or ulceration) in our analyses.11
However, the main strengths of our report must also be considered. The first and most significant is that our study population is a prospective cohort of consecutive patients with acute LAA reflecting a diverse clinical experience from the participating institutions.11 Although the present study design did not include randomization, propensity score matching resulted in subgroups balanced on a large number of baseline characteristics. Moreover, the reported associations between statin pretreatment and outcome events was adjusted for several potential confounders both in unmatched and propensity-matched analyses.
Our findings provide preliminary observational evidence underscoring a potentially beneficial effect of statins in improving early stroke outcomes in AIS patients with an underlying atherothrombotic mechanism. This hypothesis deserves to be further tested in the setting of an RCT.
Supplementary Material
GLOSSARY
- AHA
American Heart Association
- AIS
acute ischemic stroke
- CI
confidence interval
- FFO
favorable functional outcome
- HR
hazard ratio
- IVT
IV thrombolysis
- LAA
large artery atherosclerosis
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- RCT
randomized controlled trial
Footnotes
Editorial, page 1082
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Tsivgoulis: drafting the manuscript, acquisition of data, analysis and interpretation, study concept and design. Dr. Katsanos: drafting the manuscript, analysis and interpretation. Dr. Sharma: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Krogias: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Mikulik: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Vadikolias: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Mijajlovic: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Safouris: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Zompola: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Faissner: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Weiss: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Giannopoulos: critical revision of the manuscript for important intellectual content. Dr. Vasdekis: critical revision of the manuscript for important intellectual content. Dr. Boviatsis: critical revision of the manuscript for important intellectual content. Dr. A.W. Alexandrov: critical revision of the manuscript for important intellectual content. Dr. Voumvourakis: critical revision of the manuscript for important intellectual content. Dr. A.V. Alexandrov: critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
Dr. Georgios Tsivgoulis, Dr. Robert Mikulik, and Dr. Viktor Weiss have been supported by European Regional Development Fund—Project St. Anne's University Hospital, Brno–International Clinical Research Center (FNUSA-ICRC) (CZ.1.05/1.1.00/02.0123).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Flint AC, Kamel H, Navi BB, et al. Inpatient statin use predicts improved ischemic stroke discharge disposition. Neurology 2012;78:1678–1683. [DOI] [PubMed] [Google Scholar]
- 2.Flint AC, Kamel H, Navi BB, et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke 2012;43:147–154. [DOI] [PubMed] [Google Scholar]
- 3.Ni Chroinin D, Asplund K, Asberg S, et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke 2013;44:448–456. [DOI] [PubMed] [Google Scholar]
- 4.Lakhan SE, Bagchi S, Hofer M. Statins and clinical outcome of acute ischemic stroke: a systematic review. Int Arch Med 2010;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569–573. [DOI] [PubMed] [Google Scholar]
- 6.Shin DH, Lee PH, Bang OY. Mechanisms of recurrence in subtypes of ischemic stroke: a hospital-based follow-up study. Arch Neurol 2005;62:1232–1237. [DOI] [PubMed] [Google Scholar]
- 7.Merwick Á, Albers GW, Arsava EM, et al. Reduction in early stroke risk in carotid stenosis with transient ischemic attack associated with statin treatment. Stroke 2013;44:2814–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 9.Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology 2010;74:1351–1357. [DOI] [PubMed] [Google Scholar]
- 10.Tsivgoulis G, Bogiatzi C, Heliopoulos I, et al. Low ankle-brachial index predicts early risk of recurrent stroke in patients with acute cerebral ischemia. Atherosclerosis 2012;220:407–412. [DOI] [PubMed] [Google Scholar]
- 11.Tsivgoulis G, Krogias C, Georgiadis GS, et al. Safety of early endarterectomy in patients with symptomatic carotid artery stenosis: an international multicenter study. Eur J Neurol 2014;21:1251–1257. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 13.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276. [DOI] [PubMed] [Google Scholar]
- 14.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Stroke 2011;42:e420–e463. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrov AV, Nguyen HT, Rubiera M, et al. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke 2009;40:2738–2742. [DOI] [PubMed] [Google Scholar]
- 16.Nam HS, Lee KY, Han SW, et al. Prediction of long-term outcome by percent improvement after the first day of thrombolytic treatment in stroke patients. J Neurol Sci 2009;281:69–73. [DOI] [PubMed] [Google Scholar]
- 17.Tsivgoulis G, Zand R, Katsanos AH, et al. Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive metaanalysis. Stroke 2015;46:1281–1287. [DOI] [PubMed] [Google Scholar]
- 18.Tsivgoulis G, Sharma VK, Mikulik R, et al. Intravenous thrombolysis for acute ischemic stroke occurring during hospitalization for transient ischemic attack. Int J Stroke 2014;9:413–418. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies of causal effects. Biometrika 1983;70:41–45. [Google Scholar]
- 20.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Sanchez P, Fuentes B, Martinez-Martinez M, et al. Treatment with statins and ischemic stroke severity: does the dose matter? Neurology 2013;80:1800–1805. [DOI] [PubMed] [Google Scholar]
- 22.Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke 2005;36:1298–1300. [DOI] [PubMed] [Google Scholar]
- 23.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–969. [DOI] [PubMed] [Google Scholar]
- 25.Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB. A randomized placebo controlled trial of early treatment of acute ischemic stroke with atorvastatin and irbesartan. Int J Stroke 2012;7:104–111. [DOI] [PubMed] [Google Scholar]
- 26.Montaner J, Chacón P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol 2008;15:82–90. [DOI] [PubMed] [Google Scholar]
- 27.Blanco M, Nombela F, Castellanos M, et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology 2007;69:904–910. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005;36:2072–2076. [DOI] [PubMed] [Google Scholar]
- 29.Heyer EJ, Mergeche JL, Bruce SS, et al. Statins reduce neurologic injury in asymptomatic carotid endarterectomy patients. Stroke 2013;44:1150–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takayama K, Taki W, Toma N, et al. Effect of pitavastatin on preventing ischemic complications with carotid artery stenting: a multicenter prospective study—EPOCH-CAS Study. Cardiovasc Intervent Radiol 2014;37:1436–1443. [DOI] [PubMed] [Google Scholar]
- 31.Reiff T, Amiri H, Rohde S, Hacke W, Ringleb PA. Statins reduce periprocedural complications in carotid stenting. Eur J Vasc Endovasc Surg 2014;48:626–632. [DOI] [PubMed] [Google Scholar]
- 32.Koutouzis M, Nomikos A, Nikolidakis S, et al. Statin treated patients have reduced intraplaque angiogenesis in carotid endarterectomy specimens. Atherosclerosis 2007;192:457–463. [DOI] [PubMed] [Google Scholar]
- 33.Puato M, Faggin E, Rattazzi M, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke 2010;41:1163–1168. [DOI] [PubMed] [Google Scholar]
- 34.Sargento-Freitas J, Pagola J, Rubiera M, et al. Preferential effect of premorbid statins on atherothrombotic strokes through collateral circulation enhancement. Eur Neurol 2012;68:171–176. [DOI] [PubMed] [Google Scholar]
- 35.Shanmugam N, Roman-Rego A, Ong P, Kaski JC. Atherosclerotic plaque regression: fact or fiction? Cardiovasc Drugs Ther 2010;24:311–317. [DOI] [PubMed] [Google Scholar]
- 36.Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab 2012;32:1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher M, Moonis M. Neuroprotective effects of statins: evidence from preclinical and clinical studies. Curr Treat Options Cardiovasc Med 2012;14:252–259. [DOI] [PubMed] [Google Scholar]
- 38.Schafer A, Fraccarollo D, Eigenthaler M, et al. Rosuvastatin reduces platelet activation in heart failure: role of no bioavailability. Arterioscler Thromb Vasc Biol 2005;25:1071–1077. [DOI] [PubMed] [Google Scholar]
- 39.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol 2000;20:556–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


