Limb-shaking (LS) TIA is a rare form of TIA manifesting as involuntary movements involving one or more limbs. Cerebral ischemia in the context of hemodynamic failure has been incriminated.1 Indeed, LS-TIAs are associated with severe carotid steno-occlusive disease and often precipitated by a decrease in blood pressure.1 A limited number of studies using transcranial Doppler,2 133xenon SPECT,3 or 15O-H2O-PET4 have shown reduced regional cerebral blood flow (CBF) and diminished vasomotor reactivity. The latter techniques can only provide a single snapshot of regional CBF and do not allow for continuous assessment of cerebral hemodynamic changes during an actual LS-TIA. Functional near-infrared spectroscopy (fNIRS) is a neuroimaging technique that can noninvasively monitor at the bedside cortical changes in oxyhemoglobin (HbO2), deoxyhemoglobin (HbR), and total hemoglobin (HbT, as a proxy to cerebral blood volume).5 We report the hemodynamic changes observed throughout the course of LS-TIAs using a simultaneous multichannel fNIRS-EEG system.
Case report.
A 61-year-old man was admitted for daily episodes of right upper LS and leg weakness for the last 3 weeks, more often while standing. The patient had been hospitalized 12 years ago for a left hemispheric stroke in association with a 60% stenosis of the M1 segment of the left middle cerebral artery (MCA), which subsequently became occluded later that same year. He recovered well until 2 months prior to admission, at which time he experienced right-sided amaurosis fugax. CT angiography at that time showed a 30% stenosis of the supraclinoid right internal carotid artery (ICA) without any significant stenosis of the external carotid artery.
On admission, neurologic examination was normal. MRI disclosed no acute lesions. Magnetic resonance angiogram revealed interval progression of the right supraclinoid ICA narrowing to a severe stenosis of more than 70% (figure e-1A on the Neurology® Web site at Neurology.org). Dynamic susceptibility contrast T2*-weighted perfusion (figure e-1B) and arterial spin labeling perfusion (figure e-1C) both confirmed decreased relative CBF to the left MCA territory while relative CBF within the right carotid artery territory appeared maintained despite the aforementioned stenosis.
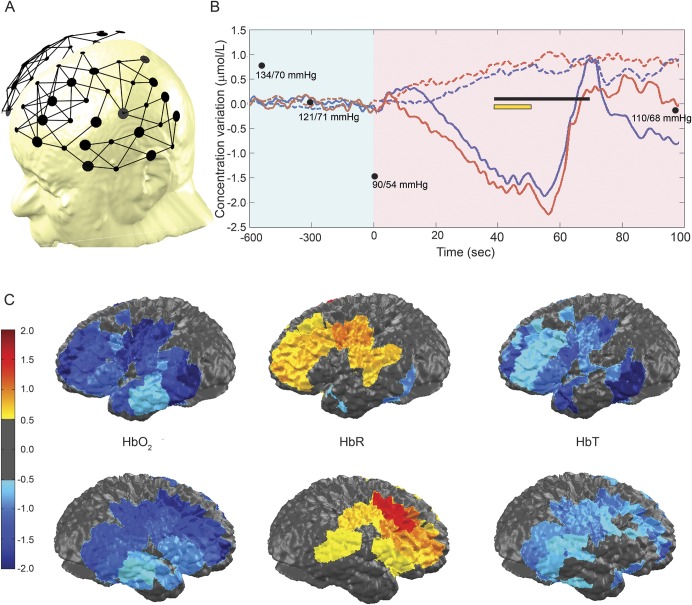
A portable fNIRS-EEG system developed in-house was used to record real-time HbO2, HbR, and HbT over the fronto-temporo-parietal regions bilaterally. This system has been described previously.6 Briefly, our system includes 32 light sources and 32 photodetectors offering a total of 128 NIRS channels with an average interoptode distance of 3 cm. As the patient stood up, blood pressure gradually decreased from 134/70 mm Hg to 90/54 after 10 minutes, at which point he experienced tremor of the right forearm and hand associated with weakness of both legs and mild dysarthria (video). fNIRS showed over bilateral (left more than right) dorsolateral frontal cortices a progressive decrease in HbO2 and HbT as well as an increase in HbR over a 1-minute period prior to the onset of LS (figure 1). These changes normalized within 15 seconds after the patient sat down, and LS subsided. Three additional LS episodes were recorded, which revealed similar hemodynamic changes starting 20–60 seconds prior to LS and weakness. Simultaneous EEG monitoring showed diffuse slow (3–50 μV, 4–6 Hz theta) activity (more predominantly over the left parasagittal regions) at the moment of LS.
Figure 1. Functional near-infrared spectroscopy findings during the second episode of limb-shaking (LS) TIA.
(A) Channel configuration. (B) Hemodynamic variations over the dorsolateral frontal cortex and motor areas: no significant variations in total hemoglobin (HbT), oxyhemoglobin (HbO2), or deoxyhemoglobin (HbR) are found during a period of 10 minutes after standing up (condensed in light-shaded blue); progressive decrease in HbT and HbO2 and rise in HbR at t = 0 seconds (light-shaded purple). The black line indicates the period during which the patient experienced LS and lower limb weakness. The yellow line indicates the period during which we averaged hemodynamic changes over the frontal, temporal, and parietal cortices for topographic representation. Solid color line: HbO2. Red: right. Blue: left. Black dots: blood pressure. (C) Topographic views of HbO2, HbR, and HbT averaged from all ipsilateral channels between time 40 to 50 seconds.
Discussion.
In this patient with baseline decreased relative CBF in the left MCA territory from carotid stenosis, fNIRS recording showed gradual brain hypoperfusion (as reflected by the HbT decrease) and hypoxia (as suggested by the increase in HbR) predominantly over the dorsolateral frontal cortex and motor areas prior to LS during postural hypotension. While the relative decrease in HbT and HbO2 were quasi-similar bilaterally, these changes may have had more impact over the left hemisphere as the baseline CBF was much lower there. Overall, our findings are in line with the prevalent hypoperfusion theory.1 The presence of late diffuse EEG slow waves is not unexpected since the EEG becomes abnormal only when CBF declines to 20–30 mL/100 g/min.7
Although our observations stem from a single patient, the fact that we observed similar hemodynamic changes in 4 different spells is compelling. One potential point of criticism is the unknown influence of the skin and extracerebral tissue on cerebral fNIRS signal. Because our patient had no significant stenosis of the external carotid artery, the contribution from superficial layers in the observed hemodynamic changes was most likely minimal. Another important limitation is that fNIRS is unable to assess hemodynamic changes in deeper brain structures such as the basal ganglia, which might also be implicated in the genesis of LS.
This fNIRS case study provides further evidence that LS-TIAs are associated with dynamic cerebral cortical hypoperfusion with resulting hypoxia in dorsolateral frontal and motor cortices.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
Author contributions: A. Kassab: acquisition and analysis of data, drafting of manuscript. J. Tremblay: analysis of data, revision of manuscript for intellectual content. A.Y. Poppe: acquisition and interpretation of data, revision of manuscript for intellectual content. L. Létourneau-Guillon: interpretation of data, drafting of manuscript. A. Gallagher: interpretation of data, revision of manuscript for intellectual content. D.K. Nguyen: conceptualization of study, interpretation of data, revision of manuscript for intellectual content.
Study funding: Supported by the Canadian Institutes of Health Research (CIHR) (MOP 133643).
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Ali S, Khan MA, Khealani B. Limb-shaking transient ischemic attacks: case report and review of literature. BMC Neurol 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persoon S, Kappelle LJ, Klijn CJ. Limb-shaking transient ischaemic attacks in patients with internal carotid artery occlusion: a case-control study. Brain 2010;133:915–922. [DOI] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Young WL, Prohovnik I, Gitelman DR, Correll JW, Mohr JP. Perfusion insufficiency in limb-shaking transient ischemic attacks. Stroke 1990;21:341–347. [DOI] [PubMed] [Google Scholar]
- 4.Zaidat OO, Werz MA, Landis DM, Selman W. Orthostatic limb shaking from carotid hypoperfusion. Neurology 1999;53:650–651. [DOI] [PubMed] [Google Scholar]
- 5.Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 1999;58:541–560. [DOI] [PubMed] [Google Scholar]
- 6.Lareau E, Lesage F, Pouliot P, Nguyen D, Le Lan J, Sawan M. Multichannel wearable system dedicated for simultaneous electroencephalography near-infrared spectroscopy real-time data acquisitions. J Biomed Opt 2011;16:096014. [DOI] [PubMed] [Google Scholar]
- 7.Sharbrough FW, Messick JM, Jr, Sundt TM., Jr Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke 1973;4:674–683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.