Abstract
Objective:
We hypothesized that nonalcoholic fatty liver disease (NAFLD) is independently associated with cognitive impairment in a representative sample of the general US population regardless of the presence of cardiovascular disease (CVD) or its risk factors.
Methods:
This was a cross-sectional study of 4,472 adults aged 20–59 years who participated in the Third National Health and Nutritional Examination Survey. The participants underwent assessment of liver enzyme activity and hepatic steatosis by ultrasound, and underwent cognitive evaluation using the following computer-administered tests: the Simple Reaction Time Test (SRTT), the Symbol-Digit Substitution Test (SDST), and the Serial Digit Learning Test (SDLT). We defined NAFLD as moderate/severe steatosis as determined by ultrasound in the absence of hepatitis B or C or excessive alcohol consumption. We used multiple linear regression models to examine the association between NAFLD and cognitive function while controlling for potential confounders.
Results:
Participants with NAFLD showed lower overall performance on the SDLT (β = 0.726, 95% confidence interval [CI] 0.105–1.347), while associations with SRTT and SDST did not reach significance. Increased activity of the liver enzymes alanine aminotransferase (β = 0.018, 95% CI 0.006–0.030) and aspartate aminotransferase (β = 0.021, 95% CI 0.005–0.037) correlated with lower performance on the SDLT, while increased alanine aminotransferase was also correlated with lower performance in the SDST (β = 0.002, 95% CI 0.0001–0.004).
Conclusions:
NAFLD was independently associated with lower cognitive performance independent of CVD and its risk factors. Given the scarcity of risk factors associated with age-related cognitive decline, these findings may have significant implications.
In the United States and globally, nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease.1,2 By definition, NAFLD occurs in the absence of excessive alcohol consumption,3 and is associated with cardiovascular disease (CVD) and its risk factors including type 2 diabetes, obesity, hyperlipidemia, and hypertension.4,5 Such risk factors are known to contribute to cognitive impairment with or without the mediation of CVD.6,7 Furthermore, considering findings from previous studies suggesting that NAFLD could be an independent CVD risk factor,8 it appears reasonable to hypothesize that NAFLD is independently associated with cognitive impairment. To our knowledge, the relationship between NAFLD and cognitive impairment has not been investigated previously.
In the current study, we analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III), covering a representative sample of the general US population. Our aim was to investigate the relationships between NAFLD determined by ultrasonography and cognitive impairment as assessed by 3 computerized tests. As a secondary objective, we investigated the relationships between liver enzyme activity, another common surrogate marker of inflammatory NAFLD, and cognitive impairment.
METHODS
Participants.
NHANES III was conducted in the United States between 1988 and 1994 and represents a complex, multistage, clustered, stratified, probability-sampled survey undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. NHANES III was designed to obtain a representative sample of the US population living in households.9,10
Standard protocol approvals, registrations, and patient consents.
All participants were required to provide signed informed consent. The Institutional Review Board of NCHS approved NHANES III.
Cognitive function testing and definition of outcomes.
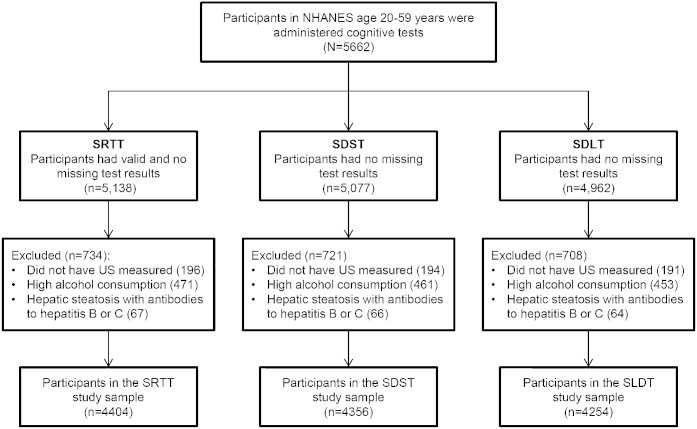
The evaluation of cognitive function was conducted for a random half-sample of NHANES III examinees between 20 and 59 years old who participated in the physical examination conducted at the mobile examination center (n = 5,662; figure). Individuals with survey identification numbers that were odd-numbered received cognitive tests, with the exclusion of those who were unable to speak English or Spanish, as well as those determined to be legally blind.11
Figure. Details of the study sample used for cognitive testing.
NHANES = Third National Health and Nutrition Examination Survey; SDLT = Serial Digit Learning Test; SDST = Symbol Digit Substitution Test; SRTT = Simple Reaction Time Test; US = ultrasonography.
To evaluate cognitive function, 3 computerized tests were used. The tests used were taken from the Neurobehavioral Evaluation System 2, developed by Baker and Letz,12,13 and have been used frequently in epidemiologic studies. Further details of the protocol have been published previously.14 All cognitive tests were conducted with a standardized protocol by trained technicians in either English or Spanish. A practice phase preceded each test. The Simple Reaction Time Test (SRTT) assesses response time, or visual-motor speed, with an output measured in milliseconds. The test involves an “a” appearing in the center of a blank computer monitor, after which the participants are instructed to press a button as fast as possible. The output for the SRTT was the average reaction time, with 50 trials conducted in total and results from the first 10 trials excluded. Reaction times that were ≥750 or ≤50 milliseconds, or individuals who had mean scores involving <20 trials, were determined by NHANES III to be invalid.11 The Symbol Digit Substitution Test (SDST) involves a set of 9 symbols matched to the digits 1 to 9 and is designed to assess visual attention and coding ability. A series of symbols are shown to the participant, who is required to match the symbol with the correct corresponding digit as fast as possible. Different pairings of digits and symbols were used over 4 trials, with the final output scored as the average total time for overall completion, in seconds.11–13 The Serial Digit Learning Test (SDLT) displays a digital series on a computer screen, and is designed to test learning, recall, and concentration. Participants were requested to enter the full sequence on a keyboard from memory. With the exception of a practice trial, all tests used the same 8-digit sequence. When each participant responded correctly for 2 consecutive trials or after 8 trials in total were completed, testing ceased, and the sequence of digits entered was recorded. The output of the SDLT is the sum of the errors entered by the participants during the test.11–13
Assessment of NAFLD and liver enzymes.
NHANES III participants underwent gallbladder examination by ultrasound with a Toshiba SSA-90A (Tustin, CA) machine with a 3.75 and 5.0 MHz transducer.15 Further details of the protocol have been described previously.16,17 Data concerning the presence of kidney to liver contrast, echogenic walls within the small intrahepatic vessels and deep beam attenuation, as well as the brightness of the liver parenchyma, and definition of the gallbladder walls was obtained. These data were processed using a standardized algorithm in order to categorize the extent of steatosis initially as a 4-level classification (none, mild, moderate, or severe steatosis) and then as a 2-level classification: none to mild or moderate to severe. The intrarater and interrater κ statistics for the reliability of the 2-level variable have been determined to be 0.77 (95% confidence interval [CI] 0.73–0.82) and 0.70 (0.64–0.76), respectively.17
Liver enzymes including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed with a Hitachi 737 automated multichannel chemistry analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Further details of the assays and quality control procedures have been described previously.18
Covariates.
Standardized questionnaires were conducted with all participants and included data for age, sex, race/ethnicity, income, education, alcohol consumption, smoking, prevalent medical conditions, drug use, and physical activity.19 An average daily estimate for alcohol consumption was obtained by multiplying the number of drinks on an average drinking day by the number of drinking days reported over the past 12 months and dividing the result by 365. Never drinkers were those who answered no to the following question: “In your entire life, have you had at least 12 drinks of any kind of alcoholic beverage?” Increased alcohol consumption was categorized as 1 or more drinks per day for women or 2 or more drinks per day for men.20
Standardized measurements of weight, waist circumference, height, and systolic/diastolic blood pressure were obtained, with body mass index (BMI) calculated. A history of CVD was self-reported as a history of heart failure, acute myocardial infarction (MI), or stroke. A second-generation enzyme immunoassay (Abbott Laboratories, Chicago, IL) was used to detect the presence of hepatitis C antibodies, and confirmed by MATRIX assay (Abbott Laboratories), while a solid phase competitive immunoassay (Abbott Laboratories) was used to detect antibodies to the hepatitis B core antigen.
NAFLD was defined as moderate/severe steatosis as determined by ultrasound in the absence of elevated alcohol consumption (≥1 drink per day for women and ≥2 drinks per day for men) as previously described21,22 and in the absence of a positive test for viral hepatitis B or hepatitis C.
Statistical analysis.
Statistical analysis was conducted using SAS, version 9.2, PROC SURVEY procedures (SAS Institute, Inc., Cary, NC) and took into consideration the complex sampling design used by NHANES. Specifically, these include strata, cluster, and weight statements, which account for the unequal probability of selection from the survey cluster design, adjustments for nonresponse and oversampling of target populations, and adjustments to independent population controls. The baseline characteristics of the participants by NAFLD status were compared using PROC SURVEYFREQ for categorical variables or PROC SURVEYMEANS for continuous variables. In the PROC SURVEYFREQ, we used the Rao-Scott χ2 test to test for differences in categorical variables and the Wald F test in the PROC SURVEYREG to test for differences in continuous variables.
In order to evaluate the relationship between NAFLD and cognitive impairment, we performed multivariate linear regression analysis (SAS PROC SURVEYREG). In model 1, we adjusted for sex, age, race, and education, and in model 2, we further adjusted for BMI, waist circumference, hypertension, diabetes mellitus, hypercholesterolemia, history of acute MI, and stroke.
In a secondary analysis to evaluate the relationships between liver enzymes (as additional surrogate markers for NAFLD) and cognitive test scores, we performed multivariate linear regression analysis using liver enzyme data instead of NAFLD.
RESULTS
Demographics.
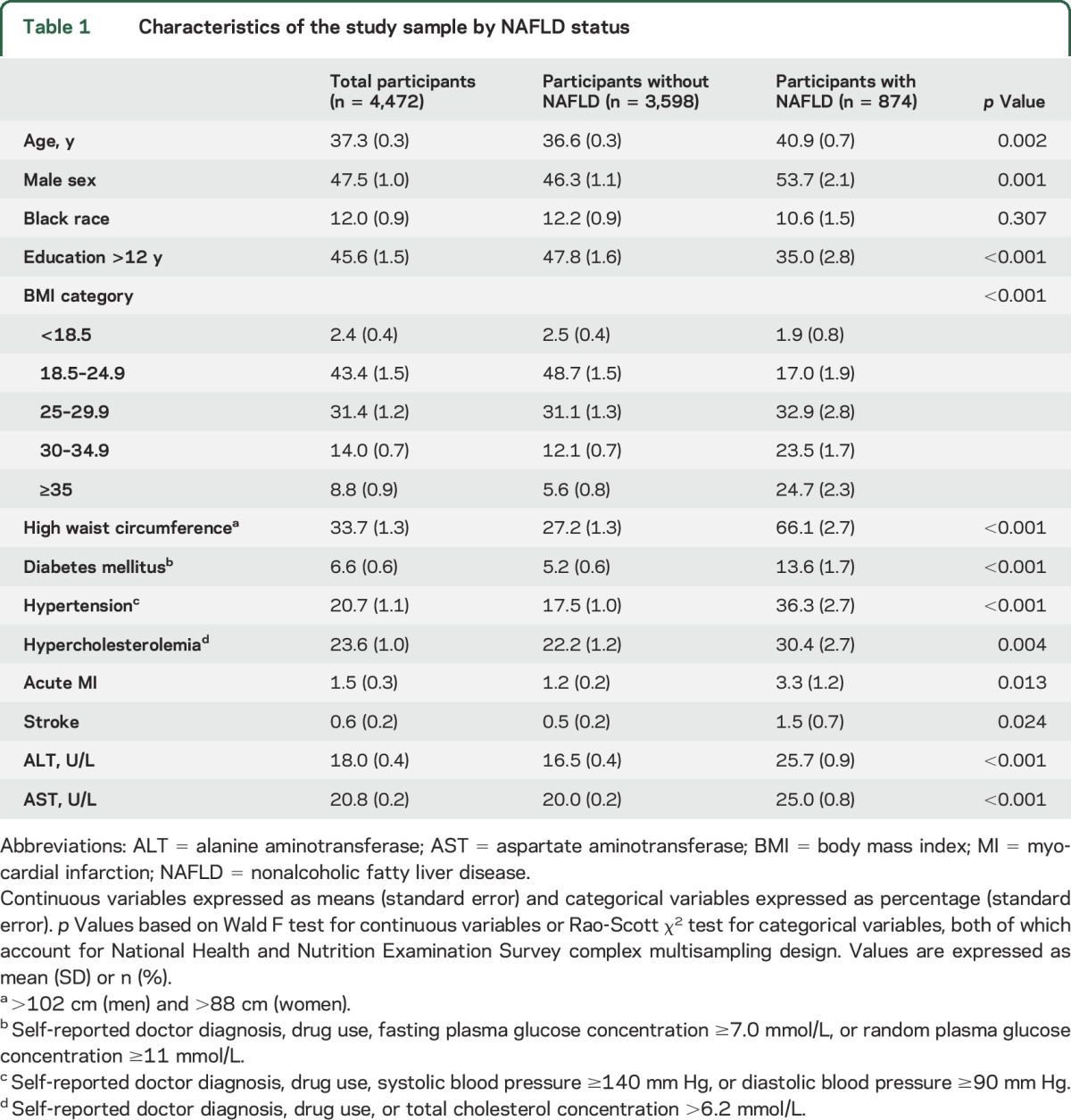
Table 1 summarizes the characteristics of the participants according to NAFLD status. Of the initial study population (4,472 adult participants from NHANES III), 874 individuals fulfilled the ultrasound definition of NAFLD. Participants with NAFLD were older and more likely to have a higher BMI, higher waist circumference, and higher prevalence of diabetes mellitus, hypertension, hypercholesterolemia, acute MI, and stroke than those without NAFLD.
Table 1.
Characteristics of the study sample by NAFLD status
Association between NAFLD and cognitive function.
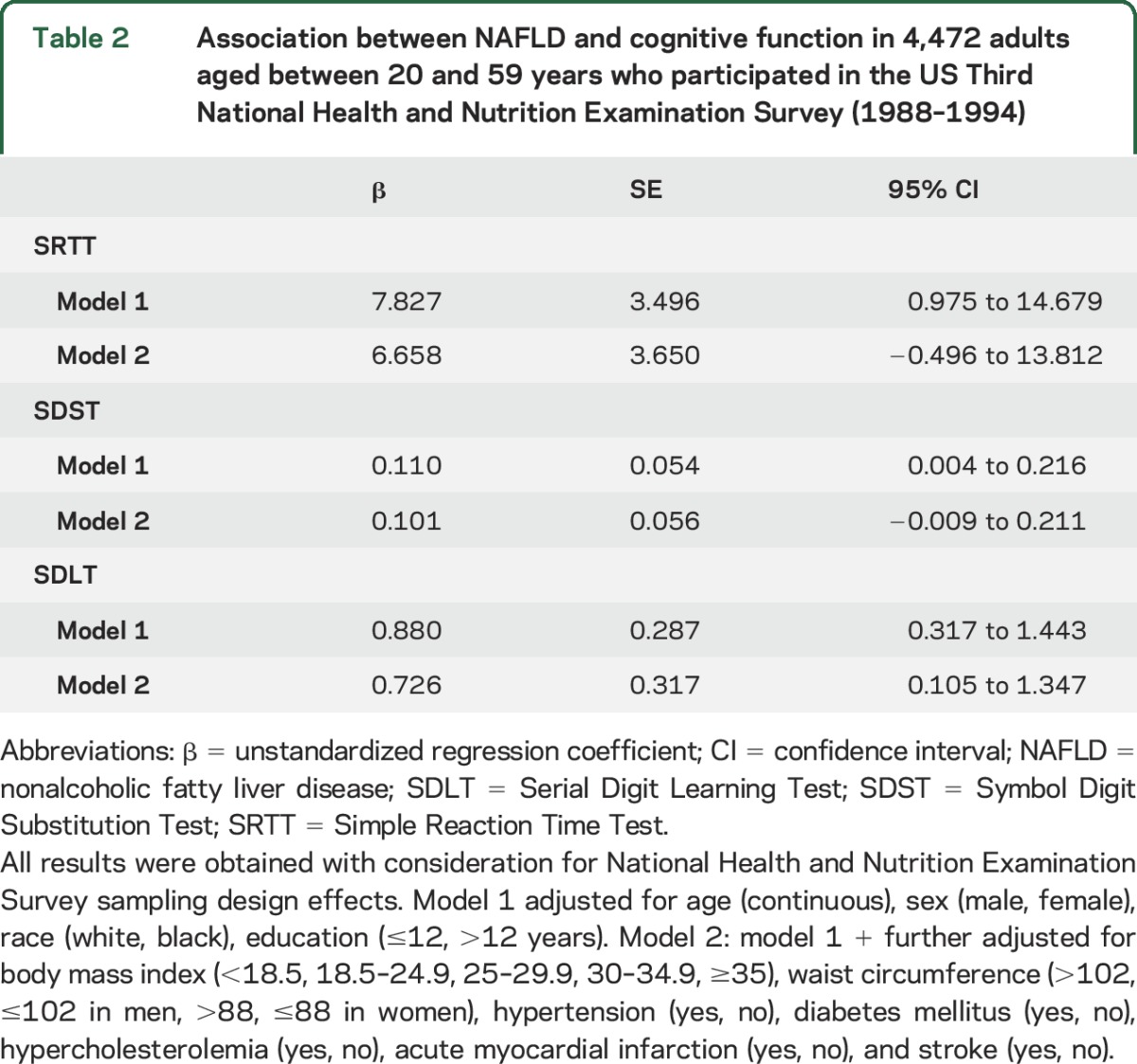
Compared to participants without NAFLD, participants with NAFLD had lower performances on SRTT, SDST, and SDLT after controlling for age, sex, race, and education (table 2). After further controlling for BMI, waist circumference, hypertension, diabetes mellitus, hypercholesterolemia, acute MI, and stroke (table 2), these results were unchanged for SDLT (β = 0.726, 95% CI 0.105–1.347) but the associations between NAFLD and SRTT and SDST lost statistical significance (SRTT, β = 6.658, 95% CI −0.496 to 13.812; SDST, β = 0.101, 95% CI −0.009 to 0.211).
Table 2.
Association between NAFLD and cognitive function in 4,472 adults aged between 20 and 59 years who participated in the US Third National Health and Nutrition Examination Survey (1988–1994)
Association between liver enzyme activity and cognitive function.
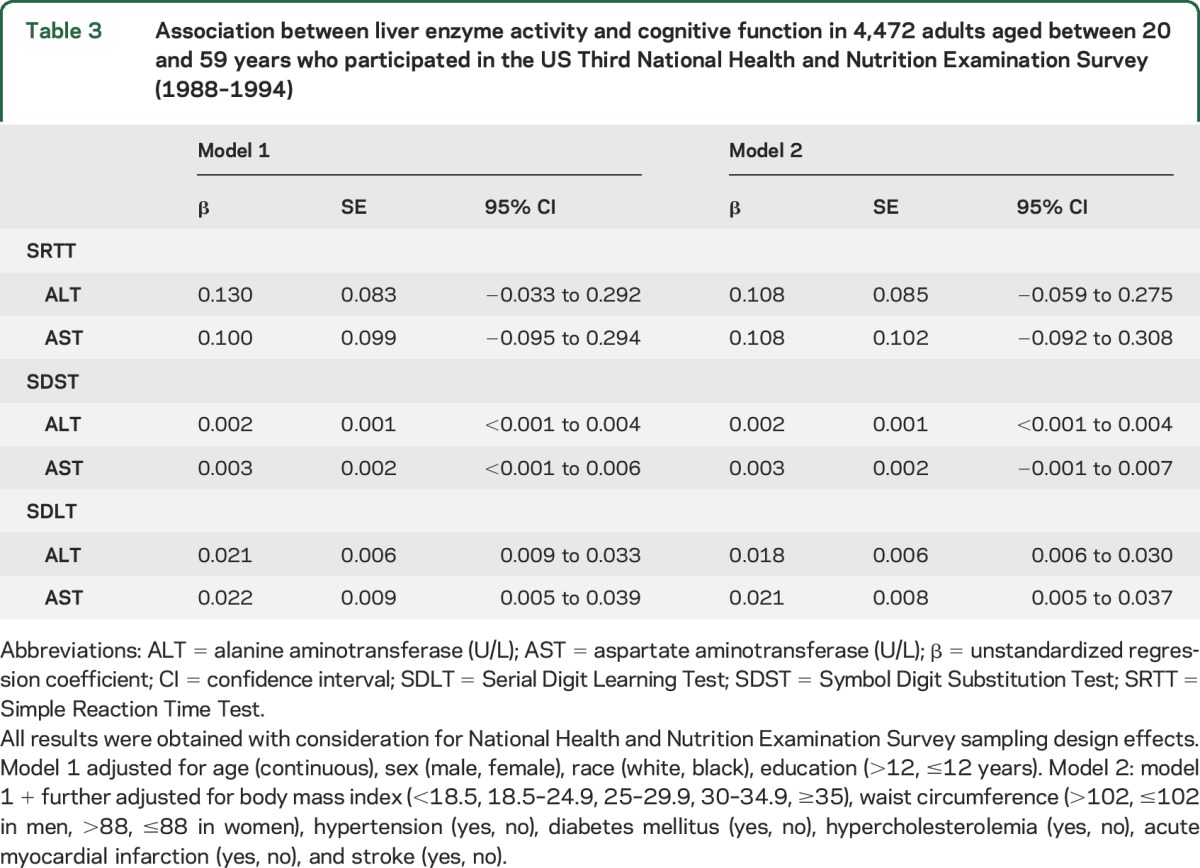
In model 1, increased liver enzyme activity was correlated with lower performance on the SDST and SDLT (table 3). In model 2, increased liver enzyme activity was correlated with lower performance on the SDLT (ALT, β = 0.018, 95% CI 0.006–0.030; and AST, β = 0.021, 95% CI 0.005–0.037). Increased ALT activity was also correlated with lower performance on the SDST (β = 0.002, 95% CI 0.0001–0.004) (table 3).
Table 3.
Association between liver enzyme activity and cognitive function in 4,472 adults aged between 20 and 59 years who participated in the US Third National Health and Nutrition Examination Survey (1988–1994)
Because participants with minimal hepatic encephalopathy might be included in these analyses, we investigated the relationships between NAFLD and cognitive impairments only in participants with no advanced NAFLD fibrosis. We calculated liver fibrosis score because this score can accurately predict the absence of advanced fibrosis in NAFLD.23 The regression formula (risk score) is as follows:
NAFLD fibrosis score = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (×109/l) − 0.66 × albumin (g/dL).
We performed further analyses in participants with no advanced NAFLD fibrosis (NAFLD fibrosis score <−1.445).23 The new results were generally similar with previous results (tables e-1 and e-2).
DISCUSSION
This cross-sectional study of a large representative sample of the US population identified an independent association between NAFLD and cognitive function. Our major finding was that NAFLD is independently associated with inferior learning, recall, and concentration function. The magnitude of the association with cognition was modest, but persisted even when CVD and its risk factors (known to affect cognition) were included in models. Furthermore, liver enzyme activity, a surrogate marker for NAFLD, was associated predominantly with lower performance on the SDLT and SDST. Considering the lack of modifiable risk factors for age-related cognitive decline, and the very high prevalence of NAFLD, these findings have important implications for public health.
It remains possible that the association between NAFLD and cognitive impairment is an epiphenomenon, because cardiovascular risk factors and diseases are related to both NAFLD and cognition. However, in our study, the association with cognition still existed after controlling for these possible confounders. The pathobiology of the relationship between NAFLD and cognitive impairments remains unknown. Insulin resistance might explain the association between NAFLD and cognitive impairment, because insulin resistance plays critical roles in the pathogenesis of NAFLD and Alzheimer disease (AD).4,24 A preclinical study has suggested that increased insulin resistance induced by exposure to nitrosamine leads to nonalcoholic steatohepatitis (NASH) and AD in rats.25 Alternatively, NAFLD might affect cognitive impairment via inflammatory processes. Previous studies have shown that expanded and inflamed liver fat, especially in patients with NASH, releases inflammatory cytokines and adipokines, possibly accompanied by abnormal levels of lipoproteins, endothelial dysfunction, and oxidative stress, suggesting that NAFLD is a marker of inflammation.26–28 Other previous studies have suggested that inflammation might be one of the most important causes of degenerative dementia.29,30 In addition, carotid intimal thickness might provide a link, given that carotid intimal thickness is associated with both NAFLD and cognitive impairment.31,32
Interestingly, the association between NAFLD and cognitive impairment varied across the cognitive tests. That is, NAFLD was associated only with SDLT scores, independent of cardiovascular risk factors and diseases, while the association with SRTT and SDST scores disappeared after adjusting for metabolic components and cardiovascular diseases. SRTT, SDST, and SDLT are designed to assess psychomotor speed, visual attention, and learning, recall, and concentration function, respectively. Previous studies suggest that SDLT scores are generally influenced by the presence of medial temporal lesions in the hippocampus, although SDLT performance also requires some frontal related processes. Therefore, our findings suggest that NAFLD might affect brain function through region-specific processes rather than diffuse cortical dysfunction. Further studies are required to examine this hypothesis in detail.
The results from the analyses examining the association between liver enzyme activity and cognition were largely consistent with the determination of NAFLD by ultrasound; namely, that there was an independent association with SDLT. The only difference, however, was that liver enzyme activity, but not the presence of NAFLD, was associated with SDST. Liver enzymes have been used as noninvasive surrogates for NAFLD in the context of no elevated alcohol consumption,2 although their use involves some degree of limited sensitivity and specificity.33,34 These markers are thought to reflect inflammatory status, and thus support the hypothesis that inflammation in the liver, more than simply the presence of fat, is the major driver of the observed association. Future studies with detailed assessments of liver fat and inflammation are needed to further distinguish differences within these associations by the level of damage.
Our study assessed a large and nationally representative sample of healthy middle-aged adults, with standardized administration of 3 computerized tests to evaluate cognitive functioning and a thorough medical workup for liver disease, including ultrasound, serologic testing, and standardized questionnaires to define NAFLD; other evaluation of medical conditions also allowed further adjustment of potential demographic, metabolic, and cardiovascular diseases. However, we acknowledge some limitations. First, the cross-sectional nature of the study does not permit us to ascertain causality in the relationship between NAFLD and cognition. It also leaves open the possibility for unmeasured confounding, particularly with regards to dietary and lifestyle risk factors. We also could not determine the relationship between NAFLD and deficits in specific cognitive domains, as participants did not undergo detailed neuropsychological tests across a range of cognitive functions. Liver ultrasound is the most widely used method to assess hepatic steatosis in large population-based studies, with a sensitivity of 84.8% and a specificity of 93.6%.35 However, some limitations have been reported, including lower sensitivity among people with obesity.36 Another common limitation of ultrasound is its operator dependency. In the NHANES, data were collected by trained staff following rigorous, standardized protocols to ensure that measurement errors are minimized, and to also minimize operator-dependent variations. Furthermore, liver ultrasound cannot distinguish between progressive NAFLD with advanced hepatic fibrosis/cirrhosis and benign steatosis. As a consequence, our study may have included cirrhotic patients with minimal hepatic encephalopathy. However, given the nature of NHANES, is it highly unlikely that very sick patients such as those with cirrhosis and complications would be participating in the survey. NHANES participants included in the present study were required to undergo a 5-hour examination at a mobile examination unit. Moreover, in further analyses, the results based on participants with no advanced NAFLD fibrosis were similar to those based on total participants. Future studies with liver biopsy data are needed to further demonstrate an association between advanced NAFLD and cognition. Despite these limitations, we are left with the suggestion that NAFLD might be a potential independent risk factor for cognitive impairment. Such an association could provide insight regarding the role of NAFLD in cognitive impairment and assist with more effective management of individuals with NAFLD. This is particularly important given the rising incidence of obesity and metabolic syndrome, which could portend rising rates of NAFLD. Furthermore, the assessment of NAFLD might be a novel and helpful approach for dementia risk stratification.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- MI
myocardial infarction
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NCHS
National Center for Health Statistics
- NHANES III
Third National Health and Nutrition Examination Survey
- SDLT
Serial Digit Learning Test
- SDST
Symbol Digit Substitution Test
- SRTT
Simple Reaction Time Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Seo: study concept and design; acquisition, analysis, and interpretation of data. Dr. Gottesman: study concept and design, critical revision of the manuscript. Dr. Clark: critical revision of the manuscript. Dr. Hernaez: critical revision of the manuscript. Dr. Chang: critical revision of the manuscript. Dr. Kim: analysis and interpretation. Dr. Ha: analysis and interpretation. Dr. Guallar: study concept and design. Dr. Lazo: study concept and design, critical revision of the manuscript, study supervision.
STUDY FUNDING
Supported by R01 DK083393-01 from NIH/NIDDK. M.L. and R.H. were supported by the American Diabetes Association. S.W.S. was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2768) and Korean Neurological Association (KNA-15-MI-08).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis 2009;13:511–531. [DOI] [PubMed] [Google Scholar]
- 2.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008;28:339–350. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372–384. [DOI] [PubMed] [Google Scholar]
- 4.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005;54:3541–3546. [DOI] [PubMed] [Google Scholar]
- 5.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646–650. [DOI] [PubMed] [Google Scholar]
- 6.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 7.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS: Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57–62. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 9.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination survey. Vital Health Stat 2 1992;113:1–35. [PubMed] [Google Scholar]
- 10.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94: series 1: programs and collection procedures. Vital Health Stat 1 1994;32:1–407. [PubMed] [Google Scholar]
- 11.Krieg EF, Jr, Chrislip DW, Letz RE, et al. Neurobehavioral test performance in the Third National Health and Nutrition Examination Survey. Neurotoxicol Teratol 2001;23:569–589. [DOI] [PubMed] [Google Scholar]
- 12.Baker EL, Letz RE, Fidler AT, Shalat S, Plantamura D, Lyndon M. A computer-based neurobehavioral evaluation system for occupational and environmental epidemiology: methodology and validation studies. Neurobehav Toxicol Teratol 1985;7:369–377. [PubMed] [Google Scholar]
- 13.Letz R. The Neurobehavioral Evaluation System 2 User's Manual. Winchester, MA: Neurobehavioral Systems; 1990. [Google Scholar]
- 14.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007;18:2205–2213. [DOI] [PubMed] [Google Scholar]
- 15.Third National Health and Nutrition Examination Survey. Gallbladder ultrasonography procedure manual. Philadelphia: Westat; 1988. [Google Scholar]
- 16.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Third National Health and Nutrition Examination Survey: hepatic steatosis assessment procedure manual. Atlanta: National Center for Health Statistics; 2010. [Google Scholar]
- 18.Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Atlanta: Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health, National Center for Health Statistics; 1996. [Google Scholar]
- 19.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-1994: Department of Health and Human Services Publication No. (PHS) 94-1308: Vital and health statistics 1994: Series 1, No. 32. Atlanta: Department of Health and Human Services; 1994.
- 20.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960–967. [DOI] [PubMed] [Google Scholar]
- 21.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–1029. [DOI] [PubMed] [Google Scholar]
- 22.Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage: The Dionysos Study Group. Gut 1997;41:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 24.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–152. [DOI] [PubMed] [Google Scholar]
- 25.Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- 26.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473–480. [DOI] [PubMed] [Google Scholar]
- 27.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880. [DOI] [PubMed] [Google Scholar]
- 28.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 2008;51:1947–1953. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 2014;45:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 2014;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Sayed SA, El-Folly RF, Ahmed AM. Assessment of the co-incidence between non alcoholic fatty liver disease and carotid atherosclerosis. J Egypt Soc Parasitol 2014;44:187–195. [DOI] [PubMed] [Google Scholar]
- 32.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke 2009;40:3180–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286–1292. [DOI] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 2004;14:635–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.