Abstract
Chromium oxide nanoparticles (Cr2O3NPs) are widely used in polymers and paints. In the present study, we aimed to determine the toxicity of Cr2O3NPs in murine fibrosarcoma (L929) cells. The cytotoxicity of Cr2O3NPs was measured by MTT and neutral red uptake assays; Cr2O3NPs had significant cytotoxic effects on L929 cells. Enhancement of intracellular reactive oxygen species was observed in L929 cells after exposure to Cr2O3NPs. Cr2O3NPs produced caspase-3, indicating that exposure to Cr2O3NPs induced apoptosis. After exposure to Cr2O3NPs, the cellular glutathione level decreased and lipid peroxidation, superoxide dismutase, and catalase increased in a dose- and time-dependent manner. By using single-cell gel tests, we also observed increased DNA damage in a Cr2O3NP exposure-duration- and dose-dependent fashion. Cell toxicity and DNA damage may be useful biomarkers for determining the safety of Cr2O3NPs in human and animal health.
Keywords: Cr2O3NPs, L929 cells, MTT assay, oxidative stress
Introduction
Engineered nanoparticles are extensively used in the industrial sector. Chromium oxide nanoparticles (Cr2O3NPs) are used in pigments, thermal protection coating, and sensing of humidity.1,2 It has been reported that interaction of nanoparticles with biological molecules depends on their shape and size.3,4 To use nanoparticles for therapeutic applications, it is important to assess their toxicity in cancer cells and normal cells. Nanoparticles can induce effects that are harmful to human health, specifically depending on the particle size.5 Warheit et al6 and Oberdorster et al7 reported that oxidative stress and inflammation occur in animals upon inhalation of nanoparticles. The toxic effects of Cr2O3NPs (eg, DNA damage) are of specific concern because alterations in genetic materials have the potential to cause cell death, cancer, and adverse reproductive effects. The comet assay is a widely accepted technique used to determine the genotoxicity of xenobiotic compounds in living organisms and to determine the toxic potential of different doses of carcinogens or genotoxic materials in animals.8 In vitro examinations have validated the physiological response to nanoparticles in animals and furnished results demonstrating increased oxidative stress in nanoparticle-treated cell lines.9,10
The present study was conducted to assess the toxicity of Cr2O3NPs in murine fibrosarcoma (L929) cells. We measured levels of reactive oxygen species (ROS), superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) in L929 cells in response to Cr2O3NPs. DNA damage effects were evaluated using the comet assay.
Materials and methods
Chemicals and reagents
Cr2O3NPs; 2′,7′-dichlorofluorescin diacetate (DCFH-DA); 5,5′-dithiobis(2-nitrobenzoic acid) and GSH were acquired from Sigma-Aldrich (St Louis, MO, USA).
Characterization of Cr2O3NPs
Cr2O3NP was suspended in culture media (1 mg/mL) and dispersed by using a sonicator for 20 minutes with 40 W power, at normal temperature. Size and shape characterization of Cr2O3NPs was studied using high-resolution transmission electron microscope (HRTEM). Size of Cr2O3NP in DMEM was examined by using dynamic light scattering (DLS) (Nano-Zeta Sizer-HT, Malvern Instrument, Malvern,UK).
L929 cell culture and treatment
Murine fibrosarcoma (L929) cell line was obtained from American Type Culture Collection (Rockville, MD, USA). L929 cells with 80% growth were collected and cultured into 96-well plates and petri dishes. We treated L929 cells to various doses (0, 10, 50, and 100 μg/mL) for 12 and 24 hours.
Morphological analysis of cells
Morphology of L929 cell lines were seen under an inverted microscope (Leica DMIL, Leica Microsystem, Wetzlar, Germany) after Cr2O3NPs treatment for 24 hours.
MTT assay
Viability of L929 cells was assessed after treatment of Cr2O3NP by MTT assay.11 Cell viability was calculated using the following formula:
NRU assay
Viability of L929 cells after treatment of Cr2O3NP for 12 and 24 hours was assessed by neutral red uptake (NRU) assay.12
Measurement of ROS generation
Generation of ROS was measured in L929 cells after exposure of Cr2O3NPs for 12 and 24 hours using DCFH-DA dye.13
Measurement of oxidative biomarkers
L929 cells (~6×106) were grown in a flask (75 cm2) and administered Cr2O3NPs (0, 10, 50, and 100 μg/mL) for 12 and 24 hours. Exposed L929 cells were collected and washed with chilled phosphate-buffered saline. The collected cells were lysed with a lysing solution. The cells and the lysing solution were cryocentrifuged at 104× g for 10 minutes at 4°C, following which they were placed on ice for oxidative stress. Protein content in cell lysate was determined by using Lowry et al’s method.14
Lipid peroxide (LPO) was evaluated by estimating the production of MDA.15 SOD level was measured according to Alarifi et al’s method.16 GSH level was evaluated by using Elman’s methods.17
Condensation of chromosome and caspase-3 activity
We observed condensed chromosome in L929 cells by using Hoechst 33342 (Thermo Fisher Scientific) staining after exposure to Cr2O3NPs for 12 and 24 hours. Images of condensed chromosome were captured by fluorescence microscopy (Nikon, Tokyo, Japan). Caspase-3 enzyme was quantified by using Alarifi and Ali’s method.18
Comet assay
DNA damage was assessed by comet technique according to Ali et al12 method.
Data analysis
The data was presented as average ± standard error (SE). Data were analyzed by one-way analysis of variance (ANOVA), followed by the Dunnett’s test. Statistical significance was denoted as P<0.05.
Results
Size of Cr2O3NPs
The size of the Cr2O3NPs used in this work was determined using HRTEM. Most of the nanoparticles were spherical, but some of them were polygonal. The mean diameter of the Cr2O3NPs was 36.8 nm. Table 1 shows the hydrodynamic diameter and zeta potential of the Cr2O3NPs in DMEM.
Table 1.
DLS measurements of Cr2O3NPs in culture media
| DLS measurements | Value |
|---|---|
| Hydrodynamic size | 253±4.0 nm |
| Zeta potential | −13.6 mV |
Abbreviations: Cr2O3NPs, chromium oxide nanoparticles; DLS, dynamic light scattering.
Morphological alteration of L929 cells
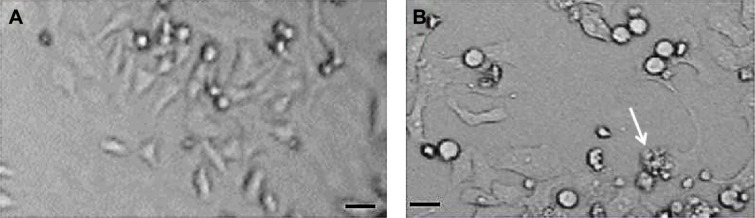
Figure 1 shows the shape and size of untreated L929 cells (Figure 1A) and cells exposed to Cr2O3NPs for 24 hours (Figure 1B). L929 cells became spherical and separated from the surface of the culture plates after exposure to 100 μg/mL of Cr2O3NPs (Figure 1B).
Figure 1.
Morphology of L929 cells.
Notes: (A) Control. (B) At 50 μg/mL of Cr2O3NPs for 24 hours. Scale bar (▬) is 50 μm; magnification 40×. The arrow indicates a damaged cell.
Abbreviation: Cr2O3NPs, chromium oxide nanoparticles.
Cytotoxicity
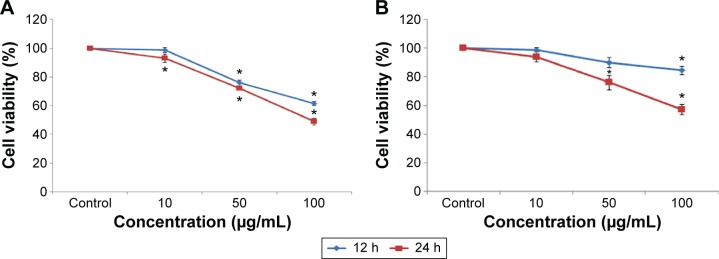
Cr2O3NP-induced cytotoxicity in L929 cells was measured by the MTT and NRU assays. Both MTT (Figure 2A) and NRU (Figure 2B) results indicated a dose- and time-dependent cytotoxicity after exposure of L929 cells to Cr2O3NPs.
Figure 2.
Cytotoxicity of Cr2O3NPs in L929 cells for 12 and 24 hours.
Notes: (A) MTT and (B) NRU tests. *P<0.05 vs control.
Abbreviations: Cr2O3NPs, chromium oxide nanoparticles; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRU, neutral red uptake; h, hours.
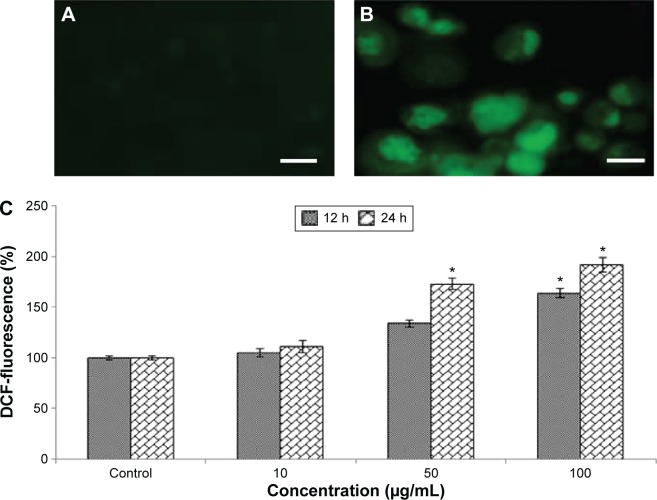
Oxidative stress and production of ROS
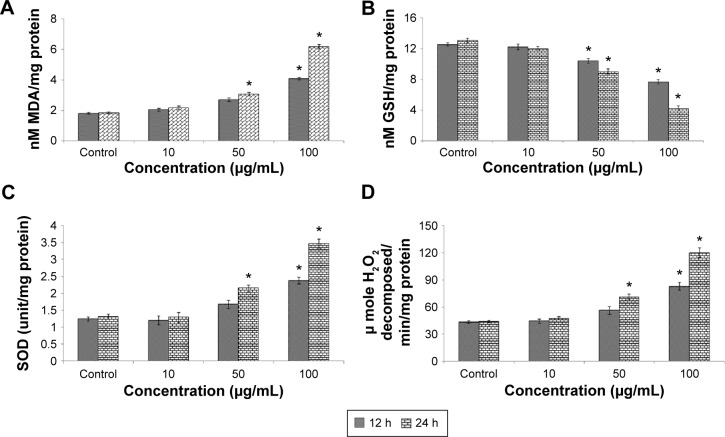
Induction of oxidative stress by Cr2O3NPs was evaluated. Cr2O3NPs generated ROS in duration and concentration basis (Figure 3A–C). Due to exposure of Cr2O3NP, GSH was decreased (Figure 4B) and LPO, SOD and catalase were increased in L929 cells (Figure 4A, C, and D).
Figure 3.
Cr2O3NPs induced ROS in L929 cells.
Notes: (A) Control (B) at 100 μg/mL of Cr2O3NPs for 24 hours (C) % ROS generation due to Cr2O3NPs in L929 cells. Scale bar (▬) 50 μm; magnification 40×.
*P<0.05 vs control.
Abbreviations: Cr2O3NPs, chromium oxide nanoparticles; DCF, dichlorofluorescein; ROS, reactive oxygen species.
Figure 4.
(A) LPO, (B) GSH, (C) SOD, and (D) catalase in L929 cells after exposure to Cr2O3NPs for 12 and 24 hours.
Note: *P<0.05 vs control.
Abbreviations: Cr2O3NPs, chromium oxide nanoparticles; GSH, glutathione; LPO, lipid peroxidation; SOD, superoxide dismutase; h, hours.
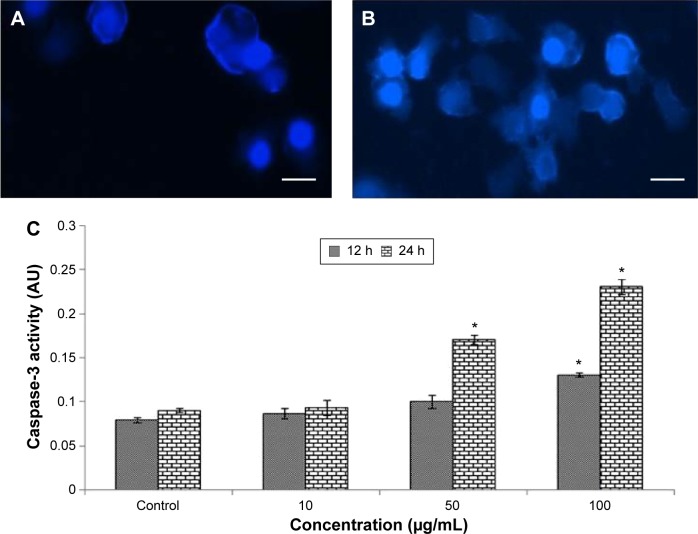
Caspase-3 activity and condensation of chromosome
Chromosome lesions were observed in L929 cells after treatment with Cr2O3NPs (Figure 5A and B). On treatment with Cr2O3NPs, the level of caspase-3 raised (Figure 5C).
Figure 5.
Chromosomal condensation and caspase-3 activity in L929 cells after treatment with Cr2O3NPs.
Notes: (A) Control, (B) at 50 μg/mL of Cr2O3NPs for 24 hours. (C) Caspase-3 activity. *P, 0.05 vs control. Scale bar (▬) 50 μm; magnification 40×.
Abbreviations: AU, arbitrary units; Cr2O3NPs, chromium oxide nanoparticles; h, hours.
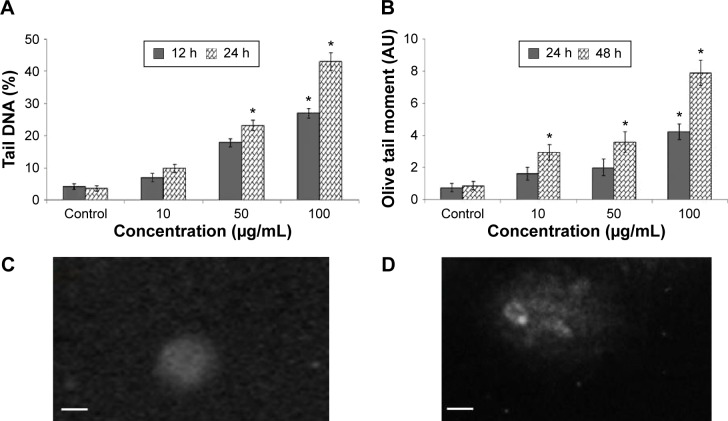
DNA damage
Damage of DNA was determined in tail DNA (%) (Figure 6A) and Olive tail moment (Figure 6B) in untreated and Cr2O3NP-treated L929 cells. L929 cells exhibited more DNA damage after exposure to Cr2O3NPs (Figure 6D) than control group (Figure 6C). Maximum level of DNA damage was recorded in L929 cells exposed to 100 μg/mL of Cr2O3NPs for 24 hours (Figure 6).
Figure 6.
DNA lesions in L929 cells because of Cr2O3NPs.
Notes: (A) Tail DNA (%). (B) Olive tail moment. (C) Control cell. (D) At 100 μg/mL of Cr2O3NPs for 24 hours. Scale bar (▬) is 50 μm; magnification 40×. *P<0.05 vs control.
Abbreviations: Cr2O3NPs, chromium oxide nanoparticles; h, hours.
Discussion
Metal nanoparticles are widely used in industry and medicine, but their applications have been limited because of significant toxicity and chronic secondary effects. It is the physiochemical properties of nanoparticles such as size, surface area, and structure that make them useful in nanomedicine.19 Shi and Dalal20 reported that chromium shows toxicity due to conversion of chromium(IV) to chromium(III) as a consequence of OH⋅ generation. Chromium(IV) crosses plasma membranes through ion channels.
Here, we measured the size and zeta potential of Cr2O3NPs by TEM and DLS, respectively, although the size determined by DLS was larger than that quantified by TEM. Our results demonstrate that toxicity of Cr2O3NPs in L929 cells was time- and dose-dependent, as measured by the cellular reduction of MTT and uptake of neutral red dye. The MTT test is a biomarker for activity of succinate dehydrogenase, a main constituent of the mitochondrial electron transport chain, and is used to measure cell survival; only live cells with undamaged mitochondria can reduce MTT dye, and the total quantity of MTT reduced is decreased in a ratio of the number of live cells. After 12 hours of exposure of cells to Cr2O3NPs, there was relatively little effect seen in the MTT test. However, after 24 hours of treatment, significant dose-dependent reduction in MTT levels were observed. The toxic effects of Cr2O3NPs were validated by the observation of separation of dead cells from the surface of culture plates.
Xia et al21 reported that the production of ROS is a possible mechanism of Cr2O3NP toxicity, which might induce DNA damage. Cr2O3NPs were found to induce production of intracellular ROS when assessed by H2DCFDA, thus it could be initial action for its toxic effect in L929 cells. Cr2O3NPs contain a transition metal capable of initiating Fenton reactions. The ROS-producing capability of Cr2O3NPs may lead to various impairments of DNA such as mutations and oxidative damage. Elevation of LPO was observed in L929 cells due to Cr2O3NPs. GSH levels in cells declined, while catalase and SOD increased in a time- and dose-dependent manner on exposure to Cr2O3NPs. Single-cell gel tests demonstrated significant enhancement in percentage tail DNA and Olive tail moment, both markers of DNA fragmentation, at higher doses of Cr2O3NPs after exposure for 24 hours.
We observed a correlation between ROS generation and fall in GSH, suggesting that greater generation of ROS induced LPO with a simultaneous decline in glutathione to hunt those free radicals. Kang et al22 reported that oxidative stress and LPO lead to apoptosis and DNA damage. During apoptosis, a chain of biochemical reactions occurs, inducing alteration of L929 cell shape and cell death.
Kroemer et al23 reported some biomarkers to characterize apoptosis, including DNA fragmentation, cell membrane blebbing, condensation of chromosome, and shrinking and disintegration of organelles. In the present study, Hoechst 33342 (Thermo Fisher Scientific) staining of Cr2O3NPs-treated L929 cells indicated nuclear fragmentation and condensation. Chen et al24 reported that, because of their small size and shape, nanoparticles interact with DNA. DNA damage can either induce cell death or carcinogenesis, consequently disrupting normal cell functions. We found DNA-damaging effects of Cr2O3NPs in L929 cells by using the comet test, which identifies double- and single-strand breaks at minute damage of DNA.25
This study indicates that use of manufactured Cr2O3NPs should be cautiously evaluated because of its hazardous effects on animals.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No RGP-180. Review board approval was not sought as our institute does not require ethical committee approval for in vitro research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gibot P, Vidal L. Original synthesis of chromium (III) oxide nanoparticles. J Eur Ceram Soc. 2010;30(4):911–915. [Google Scholar]
- 2.Makhlouf SA, Bakra ZH, Al-attara H, Moustafaa MS. Structural, morphological and electrical properties of Cr2O3 nanoparticles. Mater Sci Eng. 2013;178:337–343. [Google Scholar]
- 3.Pan Y, Neuss S, Leifer A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Xu D, Li W, Yu J, Chen Yu. Effect of size, shape, and surface modification on cytotoxicity of gold nanoparticles to human HEp-2 and canine MDCK cells. J Nanomater. 2012:375496. [Google Scholar]
- 5.Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warheit DB, Laurence BR, Reed KL, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 7.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krewski D, Westphal M, Al-Zoughool M, Croteau MC, Andersen ME. New directions in toxicity testing. Annu Rev Public Health. 2011;32:161–178. doi: 10.1146/annurev-publhealth-031210-101153. [DOI] [PubMed] [Google Scholar]
- 9.Lin W, Huang YW, Zhou XD, et al. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217:252–259. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Hu X, Gao Y, Ji Y. ZnO nanoparticles treatment induces apoptosis by increasing intracellular ROS levels in LTEP-a-2 Cells. Biomed Res Int. 2015;2015:423287. doi: 10.1155/2015/423287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Ali D, Ray RS, Hans RK. UVA-induced cyototoxicity and DNA damaging potential of benz (e) acephenanthrylene in human skin cell line. Toxicol Lett. 2010;199(2):193–200. doi: 10.1016/j.toxlet.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using micro-plate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol. 2013;32(4):296–307. doi: 10.1177/1091581813487563. [DOI] [PubMed] [Google Scholar]
- 17.Elman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Alarifi S, Ali D. Mechanisms of multi-walled carbon nanotubes-induced oxidative stress and genotoxicity in mouse fibroblast cells. Int J Toxicol. 2015;34(3):258–265. doi: 10.1177/1091581815584799. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson K, Stone V, Tran CL, et al. Nanotoxicology. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi XL, Dalal NS. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI) Biochem Biophys Res Commun. 1989;163:627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- 21.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2007;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 22.Kang SJ, Kim BM, Lee YJ, Chung H. Barium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp Cell Res. 2005;305(1):51–62. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]