Abstract
Aim
To validate the association between visceral obesity and pathogenesis of chronic kidney disease (CKD) among individuals aged 40 years and above, and the potential of visceral adiposity index (VAI) to predict CKD.
Methods
This study was based on a cross-sectional epidemiologic study in the People’s Republic of China. A total of 1,581 residents aged over 40 years were included and divided into four groups based on VAI quartile intervals, namely, Groups I, II, III, and IV (eg, Group I included patients with their VAIs in the lowest quartile). Logistic regression analysis was performed.
Results
VAI is positively correlated with the albumin-to-creatinine ratio and the prevalence of CKD (P<0.001), and is inversely related to estimated glomerular filtration rate (P<0.001). Using Group I as control, odds ratios (ORs) were calculated to quantify the risk of developing CKD as VAI increased (Group II 1.08 [P>0.05], Group III 1.57 [P<0.05], Group IV 2.31 [P<0.001]). Related factors like age and sex were normalized in the logistic model before calculation. ORs became 1.16 (P>0.05), 1.59 (P<0.05), and 2.14 (P<0.05), respectively, for each group after further normalization considering smoking, drinking, physical activity, education, and the history of hypertension, coronary heart disease, and diabetes. The same results were not observed after fasting blood glucose and blood pressure levels were included in the normalization. There was no significant difference in the ORs for different groups: 0.94 (P>0.05), 1.11 (P<0.05), and 1.68 (P>0.05), respectively.
Conclusion
VAI is highly correlated with the prevalence of CKD in the population aged 40 years and above. It can be used to predict the pathogenesis of CKD, which is dependent on fasting blood glucose and blood pressure levels.
Keywords: visceral adiposity index, visceral obesity, chronic kidney disease
Introduction
Chronic kidney disease (CKD) has emerged as a global public health problem, with the number of new cases increasing exponentially in the last few decades. Public policies targeting primary prevention, early diagnosis, and intervention for CKD are in urgent need.1 The epidemic of obesity, meanwhile, has been shown to be one of the principal causes of the markedly increased CKD prevalence.2 Conversely, the role of body-fat distribution in the pathogenesis of CKD is less clear. It has been shown that general body obesity, quantified by the body mass index (BMI), is associated with the development of CKD.3 It is also noted that abdominal obesity may be more specifically important, which could be assessed by waist circumference (WC).4–7 Despite good correlation with abdominal adiposity, such anthropometric measurements do not differentiate the visceral from subcutaneous adiposities.8 Moreover, the effects of the visceral adipose tissue (VAT) cannot be precisely predicted by BMI and WC in clinical practice.9 Visceral obesity, rather than peripheral/subcutaneous type, reflects the metabolic changes in the body more accurately.5,6,10 Computed tomography (CT) and magnetic resonance imaging (MRI) are two of the most sensitive methods to measure visceral fat. However, both procedures are inadequate to screen large populations, since they require expensive and specialized equipment that may expose the individuals to ionizing radiation.11
Notably, VAT has shown the strongest consistency with visceral obesity measured by MRI.12,13 In order to surrogate the imaging techniques, predictive equations were validated to estimate VAT from simple measures including WC, BMI, and metabolic indexes (triglycerides [TGs], high-density lipoprotein [HDL], etc).12 In this setting, such predictive equations can be regarded as simple, convenient, cost-effective, and reliable measurement tools to quantify VAT.9,14 VAT has been documented to correlate with the occurrence of chronic diseases, such as diabetes, cerebrovascular disease (CVD), and hypertension.15,16 Stępień et al17 reported the method using this index to evaluate the association between obesity and CKD.
The objective of the present study was to investigate whether the estimated VAT by visceral adiposity index (VAI) is associated with reductions in glomerular filtration rate (GFR) and the prevalence of CKD among residents aged 40 years and above at Wanzhai Town in the People’s Republic of China.16,18 Meanwhile, we sought to propose that VAI could be used as a simple economic reference index for the screening of CKD in clinical practice.
Methods
This study was approved by the ethics committee of the Third Affiliated Hospital of Southern Medical University. All the participants provided written informed consent to participate in this study.
Study population
Data were drawn from a population-based, cross-sectional survey conducted in Wanzhai Town, Zhuhai. Zhuhai is located on the southern coast of the People’s Republic of China. There are six communities in Wanzhai Town, and three of them were randomly selected for this survey. This survey was conducted between June and October 2012. An additional inclusion criterion was an age ≥40 years. Exclusion criteria were: taking lipid-lowering medications or angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists for nearly 1 month, incomplete basic information or laboratory data, menstruation, symptomatic urinary tract infection, and urinary tract obstruction. A total of 1,581 residents were recruited. All residents were asked to stop the antidiabetic or antihypertensive treatment for 4 weeks before examination.
Data collection
Data were collected based on residents’ health records, including age, sex, medical history, family history, and personal history. The registration was followed by physical examination and collection of blood and urine samples.
Physical examination
Blood pressure (BP) was measured in the seated position with a calibrated mercury sphygmomanometer after at least 5 minutes’ rest. According to the guidelines of the World Health Organization, WC was measured at the level midway between the lower rib margin and the iliac crest in the midaxillary line, with the participants standing with their feet 25–30 cm apart and breathing out gently. BP and WC were measured three times and their average values were determined. BMI was calculated as weight (kilograms) divided by the square of the height (meters).
Laboratory variables
First morning urine samples were collected; urine protein and creatinine levels were measured and the albumin-to-creatinine ratio (ACR) was calculated. Fasting blood samples were collected for detecting AST/ALT [aspartate aminotransferase/alanine transaminase], C-reactive protein (CRP), low-density lipoprotein (LDL) cholesterol, TGs, uric acid, HDL cholesterol, creatinine, very-low-density lipoprotein cholesterol, glucose, insulin, and total cholesterol. All specimens from collection sites were transported to the central laboratory in the Third Affiliated Hospital of Southern Medical University within 3 hours and stored at 2°C–8°C until analysis.
Calculation
Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting plasma glucose (mmol/L) × fasting insulin (mU/L)/22.5.19 GFR was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) equation [175× (serum creatinine)−1.234 × (age)−0.179 ×0.79 (if female)].20 ACR (mg/g) was calculated as the ratio of urinary albumin to urinary creatinine. VAI was calculated as follows:21
| (1) |
| (2) |
Diagnostic criteria
CKD was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 by the MDRD formula and/or ACR ≥30 mg/g.22 Hypertension was determined by elevated BP (more than 140/90 mmHg) or previous diagnosis of hypertension and receiving antihypertensive treatment. Diabetes mellitus was defined as a fasting serum glucose ≥7.0 mmol/L and/or self-reported diagnosis of type II diabetes. The education level is categorized into three stages: illiterate, primary school or junior high school, and high school or above.
Statistical analysis
Acquired data were analyzed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). Continuous variables were represented as mean ± standard deviation (SD), and categorical variables were represented as proportions of each subgroup. Continuous variables were analyzed via one-way analysis of variance (ANOVA), and categorical variables were analyzed via the chi-square test.
Logistic regression models were used to determine whether VAI is associated with CKD. VAI was divided into four quartiles and considered a categorical variable.23 Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, lifestyle (smoking status, alcohol consumption, physical activity, and education), hypertension, diabetes mellitus, and coronary heart disease (CHD). To determine whether fasting blood glucose (FBG) and BP affect the relationship between VAI and CKD, age, sex, lifestyle, history of hypertension, diabetes mellitus, CHD, FBG, and BP were included in model 3 included in model 3. P-values <0.05 were considered statistically significant.18
Results
A total of 1,581 residents (585 men and 996 women) were included in the present study. Their ages ranged from 40 to 95 years, and the mean age was 57.47±11.08 years. It was noted that the employment rate was higher for men among local residents, and employees were offered free physical examinations by employers. This could explain the observed sex bias in our sample. BMI was obtained for each participant to estimate the prevalence of general obesity (43.1%), with the cutoff value set at 24 kg/m2. Besides, medication history was also recorded: 150 subjects were taking antihypertensive agents, 40 subjects were taking hypoglycemic drugs, one subject was taking sleeping pills, and 28 subjects were taking Chinese medicine.
Baseline characteristics of each group
Table 1 shows the clinical, biological, and anthropometric characteristics for the residents categorized into four groups, as described in the “Methods” section. In general, VAI scoring positively correlated with age, BMI, WC, BP, FBG, cholesterol, TGs, LDL, CRP and blood uric acid levels, and ACR (P<0.05). However, HDL level and GFR were negatively correlated with VAI values (P<0.001). It is noted that the prevalence of hypertension is higher for residents with their VAI values in the upper quartile, which is the same as that for diabetes (P<0.05).
Table 1.
Basic characteristics of participants in the four groups
| VAI value | Group I (N=398), VAI value <0.92 | Group II (N=392), VAI value =0.92–1.47 | Group III (N=399), VAI value =1.47–2.37 | Group IV (N=392), VAI value ≥2.37 | P-value |
|---|---|---|---|---|---|
| Age (years)b | 56.49±11.21 | 57.57±11.23 | 57.07±10.57 | 58.76±11.21 | 0.029 |
| Male, n (%) | 175 (44.0) | 129 (32.9) | 150 (37.6) | 131 (33.4) | <0.005 |
| Hypertension, n (%) | 67 (17.0) | 88 (22.5) | 103 (25.9) | 137 (35.1) | <0.001 |
| Diabetes, n (%) | 19 (4.8) | 24 (6.1) | 37 (9.3) | 42 (10.7) | 0.006 |
| CAD, n (%) | 8 (2.0) | 15 (3.8) | 12 (3.0) | 12 (3.1) | 0.519 |
| Education, n (%) | 119 (30.1) | 121 (31.1) | 131 (33.2) | 110 (28.3) | 0.514 |
| Smoking, n (%) | 50 (12.8) | 48 (12.5) | 41 (10.3) | 52 (13.5) | 0.569 |
| Sports, n (%) | 236 (62.8) | 244 (66.3) | 247 (64.2) | 229 (61.9) | 0.620 |
| Drinking, n (%) | 88 (22.3) | 89 (23.2) | 80 (20.2) | 88 (22.7) | 0.749 |
| BMI (kg/m2)b | 21.97±3.21 | 23.03±3.14 | 24.53±3.36 | 24.84±3.06 | <0.001 |
| WC (cm)b | 78.08±8.72 | 82.65±9.38 | 87.17±9.20 | 89.29±9.88 | <0.001 |
| S-BP (mmHg)b | 125.02±19.17 | 129.72±19.39 | 132.69±19.12 | 135.81±18.82 | <0.001 |
| D-BP (mmHg)b | 75.25±9.63 | 77.54±9.45 | 80.31±10.50 | 82.08±10.49 | <0.001 |
| FBG (mmol/L)b | 4.82±0.83 | 4.99±0.99 | 5.22±1.31 | 5.48±1.59 | <0.001 |
| Cholesterol (mmol/L)b | 5.30±0.95 | 5.51±0.90 | 5.61±1.04 | 5.71±1.08 | <0.001 |
| LDL (mmol/L)b | 3.14±0.82 | 3.42±0.81 | 3.48±0.92 | 3.10±0.89 | <0.001 |
| HDL (mmol/L)b | 1.82±0.36 | 1.59±0.26 | 1.41±0.20 | 1.35±0.25 | <0.001 |
| TG (mmol/L)a | 0.76 (0.62–0.88) | 1.09 (0.97–1.24) | 1.50 (1.32–1.79) | 2.52 (2.09–3.27) | <0.001 |
| CRP (mg/L)a | 0.62 (0.31–1.31) | 0.93 (0.472–32) | 1.45 (0.68–2.84) | 1.64 (0.83–3.37) | <0.001 |
| GFR (mL/min/1.73 m2)b | 99.29±22.59 | 98.49±21.18 | 94.80±19.13 | 92.93±21.20 | <0.001 |
| BUA (μmol/L)b | 322.65±88.19 | 331.32±87.45 | 372.98±95.37 | 388.89±99.44 | <0.001 |
| ACR (mg/g)a | 7.00 (5.00–12.00) | 8.00 (5.00–15.00) | 9.00 (6.00–17.00) | 10.00 (6.00–23.00) | <0.001 |
| HOMA-IRa | 1.27 (0.87–1.77) | 1.58 (1.13–2.41) | 2.15 (1.42–3.08) | 2.81 (1.86–4.08) | <0.001 |
Notes:
Median and interquartile range (25th to 75th percentiles) were used to show continuous variables having a skewed distribution, such as HOMA-IR, serum CRP, ACR, and serum TG. Kruskal-Wallis H(K) test was used for skewed distribution.
The continuous variables were presented as mean ± standard deviation if they had a normal distribution.
Abbreviations: VAI, visceral adiposity index; CAD, coronary artery disease; education, high school and above; BMI, body mass index; WC, waist circumference; S-BP, systolic blood pressure; D-BP, diastolic blood pressure; FBG, fasting blood glucose; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TGs, triglycerides; CRP, C-reactive protein; GFR, glomerular filtration rate; BUA, blood uric acid; ACR, albumin-to-creatinine ratio; HOMA-IR, homeostasis model assessment-insulin resistance.
The relationship between VAI and the prevalence of CKD
The prevalence rates of CKD in the four groups are presented in Figure 1. They were 11.8%, 12.8%, 16.5%, and 23.7%, respectively, for Groups I–IV, which implies the significant correlation of VAI value with occurrence of CKD (P<0.001).
Figure 1.
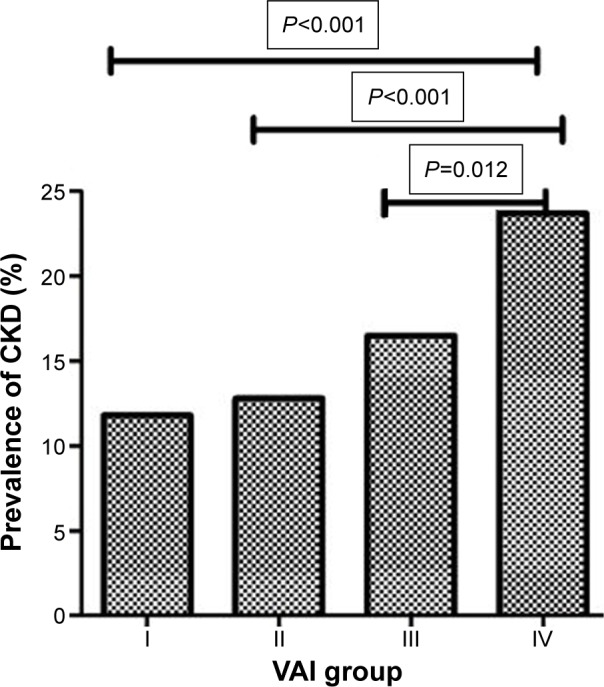
Association between VAI score and the prevalence of CKD (%).
Abbreviations: VAI, visceral adiposity index; CKD, chronic kidney disease.
Logistic regression analysis of the correlations of VAI values with the prevalence of CKD
As shown in Table 2, with Group I (VAI <0.92) as control, the other three groups (II, III, and IV) were defined as 0.92≤ VAI <1.47, 1.47≤ VAI <2.37, VAI ≥2.37, respectively. After normalizing for age and sex, odds ratios (ORs) in Groups II, III, and IV were 1.08 (P=0.727), 1.57 (P=0.034), and 2.31 (P<0.001), respectively. After further normalization with smoking, drinking, physical activity, the degree of education, and the history of hypertension, CHD, and diabetes was considered, ORs for each group became 1.16 (P=0.551), 1.59 (P=0.047), and 2.14 (P=0.001), respectively. Similar results were not observed after FBG and BP were included in the normalization, and there was no significant difference in the ORs for different groups, which were 0.94 (P=0.81), 1.11 (P=0.71), and 1.68 (P=0.053), respectively.
Table 2.
Logistic regression analysis for the association between VAI and CKD
| VAI | Model 1a
|
Model 2b
|
Model 3c
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Group I (<0.92) | 1 (Control) | 1 (Control) | 1 (Control) | |||
| Group II (0.92–1.47) | 1.08 (0.70–1.68) | 0.727 | 1.16 (0.72–1.86) | 0.551 | 0.94 (0.54–1.63) | 0.81 |
| Group III (1.47–2.37) | 1.57 (1.03–2.39) | 0.034 | 1.59 (1.01–2.51) | 0.047 | 1.11 (0.65–1.90) | 0.70 |
| Group IV (≥2.37) | 2.31 (1.54–3.46) | <0.001 | 2.14 (1.37–3.31) | 0.001 | 1.68 (0.99–2.83) | 0.053 |
Notes:
Factors considered for normalization: age and sex;
factors considered for normalization: age, sex, high BP, diabetes, CAD, smoking, drinking, sports, and education;
factors considered for normalization: age, sex, history of high BP, diabetes, CAD, smoking, drinking, sports, education, FBG, S-BP, and D-BP.
Abbreviations: VAI, visceral adiposity index; OR, odds ratio; CI, confidence interval; CKD, chronic kidney disease; CAD, coronary artery disease; S-BP, systolic blood pressure; D-BP, diastolic blood pressure; FBG, fasting blood glucose.
Discussion
Visceral obesity is closely associated with metabolism as well as the incidence of CKD.24 VAT was generally assessed by WC, which cannot differentiate the visceral from peripheral adiposities.6 Moreover, the gold standard MRI is hardly used given its limitations of equipment and cost. Thus, a faster, easier, cheaper, and relatively accurate method for the diagnosis of visceral obesity is desperately required. VAT, combined with anthropometric measurements and blood biochemical indexes, exhibits high consistency with MRI in terms of evaluation of visceral obesity.12 As an important clinical index, VAI has been extensively investigated in the fields of many disorders, but is rarely related to CKD. Therefore, in the current study, the association between VAI and CKD was explored and elucidated in a quantitative way, which could validate the potential of VAI to be used as a convenient and reliable reference index for predicting CKD.
Our results demonstrated that ACR elevated remarkably as VAI increased, and GFR decreased at the same time (Table 1 and Figure 1). Logistic regression analysis showed that the prevalence of CKD among residents in Groups III and IV (VAI ≥1.47) correlated with VAI independently (P<0.05), whereas similar correlation was not observed (P>0.05) after normalization with FBG and BP included in the model. This suggests that VAI would be considered a predictive index for the occurrence of CKD, which is highly dependent on FBG and BP, and the cutoff value for VAI could be 1.47. Furthermore, in Group IV (VAI ≥2.37), OR was 1.68 (P=0.053) after normalization with FBG and BP. However, the P-value was close to 0.05, and the association of VAI and CKD was not certain, which could be caused by the small sample size in this group.
As a predictive index for visceral obesity, VAI is considered to be correlated with the occurrence of metabolic syndrome and other metabolic diseases.13 Obesity is a risk factor for the pathogenesis of multiple disorders. For example, obesity leads to renal dysfunction via high infiltration of fat in the kidney, excessive accumulation of free fatty acids in tissue, and the abnormal circulatory level of adipokines secreted by adipose tissue.25 Furthermore, obesity is involved in inflammation26,27 and vascular diseases28 via the sympathetic nervous system and angiotensin system. Additionally, metabolic disorders could also result from obesity, including IR,29 hyperlipidemia, hypertension,30 hyperuricemia,10 and inflammation, which are also the important risk factors affecting the occurrence and progression of CKD. Consistent with previous reports, we found the possible association between VAI and BP, FBG, HOMA-IR, CRP, and serum uric acid levels. Thus, the correlation between obesity and CKD could be mediated by numerous factors.
Conclusion
In conclusion, among the residents aged 40 years and above, VAI is closely correlated with the occurrence of CKD. VAI could act as a predictive index for the screening of CKD, which is dependent on levels of FBG and BP. Convenient and reliable, the measurement of VAI is expected to be a promising indicator for the prediction of CKD clinically at all levels of medical and health services. However, it is noted that the establishment of VAI calculation was based on the white population. Adjustment of the VAI calculation method for the Chinese population needs further investigation.
Acknowledgments
This work was supported by grants from the EU FP7 Program, UroSense 286386 (2011); ISN GO R&P Grant (06-019) (2014); South Wisdom Valley Innovative Research Team Program (CXTD-001) (2014); and Science and Technique Program of Tianhe District, Guangzhou (number 114KW041).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: common, harmful, and treatable – World Kidney Day 2007. Am J Kidney Dis. 2007;49:175–179. doi: 10.1053/j.ajkd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Hsu C, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Despres J-P. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40:821–836. doi: 10.1016/j.biocel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Pouliot MC, Després JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 7.Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52:29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 9.Petta S, Amato M, Cabibi D, et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543–1552. doi: 10.1002/hep.23859. [DOI] [PubMed] [Google Scholar]
- 10.van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Chen YE, Eitzman DT. Imaging body fat: techniques and cardiometabolic implications. Arterioscler Thromb Vasc Biol. 2014;34:2217–2223. doi: 10.1161/ATVBAHA.114.303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 14.Evans PD, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS One. 2012;7:e34699. doi: 10.1371/journal.pone.0034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14:16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 16.Pascot A, Lemieux S, Lemieux I, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 17.Stępień M, Stępień A, Wlazeł RN, et al. Obesity indices and adipokines in non-diabetic obese patients with early stages of chronic kidney disease. Med Sci Monit. 2013;19:1063–1072. doi: 10.12659/MSM.889390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zhou C, Shao X, et al. Hypertriglyceridemic waist phenotype and chronic kidney disease in a Chinese population aged 40 years and older. PLoS One. 2014;9:e92322. doi: 10.1371/journal.pone.0092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley AJ, Williams K, Gonzalez C, et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 20.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Liu H, Liu X, et al. Central obesity, C-reactive protein and chronic kidney disease: a community-based cross-sectional study in southern China. Kidney Blood Press Res. 2013;37:392–401. doi: 10.1159/000355718. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 23.Du T, Sun X, Huo R, Yu X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int J Obes (Lond) 2014;38:840–847. doi: 10.1038/ijo.2013.181. [DOI] [PubMed] [Google Scholar]
- 24.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 26.Razay G, Vreugdenhil A, Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dement Geriatr Cogn Disord. 2006;22:173–176. doi: 10.1159/000094586. [DOI] [PubMed] [Google Scholar]
- 27.Villaret A, Galitzky J, Decaunes P, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres J-P. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 29.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29:151–153. doi: 10.2337/diacare.29.1.151. [DOI] [PubMed] [Google Scholar]
- 30.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]


