Abstract
Objective
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) demonstrated a higher periprocedural stroke and death (S+D) rate among patients randomized to carotid artery stenting (CAS) than to carotid endarterectomy (CEA). Herein, we seek factors that affect the CAS-CEA treatment differences, and potentially to identify a subgroup of patients where CAS and CEA have equivalent periprocedural S+D risk.
Methods
Patient and arterial characteristics were assessed as effect modifiers of the CAS-CEA treatment difference in 2,502 patients by the addition of factor-by-treatment interaction terms to a logistic regression model.
Results
Lesion length and lesions that were contiguous or were sequential and non-contiguous extending remote from the bulb were identified as influencing the CAS-to-CEA S+D treatment difference. For those with longer lesion length (≥12.85 mm) the risk of CAS was higher than CEA (odds ratio [OR] = 3.42; 95% confidence interval [CI], 1.19-9.78). Among patients with sequential or remote lesions extending beyond the bulb, the risk for S+D was higher for CAS relative to CEA (OR = 9.01; 95% CI: 1.20 – 67.8). For the 37% of patients with lesions that were both short and contiguous, the odds of S+D in those treated with CAS was non-significantly 28% lower than for CEA (OR = 0.72; 95% CI: 0.21 – 2.46).
Conclusion
The higher S+D risk for those treated with CAS appears to be largely isolated to those with longer lesion length and/or those with sequential and remote lesions. In the absence of those lesion characteristics, CAS appears to be as safe as CEA with regard to periprocedural risk of S+D.
Introduction
Data from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) have previously shown that the composite 4-year primary endpoint event rates (stroke, death, and myocardial infarction (MI)) during the periprocedural period, plus ipsilateral stroke over the 4-year follow-up were not different when comparing carotid endarterectomy (CEA) and carotid artery stenting (CAS) (Hazard ratio or HR = 1.11; 95% CI: 0.81 – 1.51; p = 0.51).1 However, the 4-year stroke plus death (S+D) rate was higher with CAS than CEA (HR = 1.50; 95% CI: 1.05 – 2.15; p = 0.03), a difference driven almost exclusively by treatment differences in the S+D rate in the periprocedural period (HR = 1.90; 1.21 = 2.98; p = 0.005).1 Peri-procedural events comprised 73% (55 of 75) of all 4-year S+D events in the CAS group, and peri-procedural events comprised 58% (29 of 50) of all 4-year S+D events in the CEA group. With the majority of S+D events occurring during the peri-procedural period, the higher periprocedural S+D rate for patients treated with CAS had a substantial impact on the overall study findings, and for this reason this paper focuses on potential effect modifiers during the periprocedural period.
We have reported factors associated with a differential treatment efficacy of 4-year endpoints2 and factors associated with differential outcomes in the periprocedural period.3 Up to now we have not been able to identify subgroups of patients with a differential CAS-to-CEA treatment efficacy for the periprocedural S+D endpoint (i.e., effect modifiers of periprocedural risk). For example, while age has been shown to be associated with differences in the primary composite outcome over the 4-year horizon, it was not a significant effect modifier during the periprocedural period for either the composite outcome (pinteraction = 0.13) or for the stroke outcome (pinteraction = 0.27).2 Likewise for sex, our previous reports described sex as a significant effect modifier for the periprocedural composite outcome (pinteraction = 0.04); however, this difference was comprised of a complex pattern where women had a non- significantly greater periprocedural S+D risk with CAS than CEA, and also had a non-significantly smaller protection from periprocedural myocardial infarction with CAS than CEA. Specifically, the periprocedural S+D risk was non-significantly larger (pinteraction = 0.28) for CAS than CEA in women than for men.3 Finally, the treatment differences among asymptomatic patients (HR = 1.88; 95% CI: 0.79 – 4.42) and symptomatic patients (HR = 1.89; 95% CI: 1.11 – 3.21) were quite similar, underscoring that symptomatic status is not acting as an effect modifier of treatment differences.1
Hence, we have not identified factors affecting the periprocedural S+D outcome that were pivotal to our overall findings. The potential that there could be effect modifiers of the S+D periprocedural risk are profound, as it would open the possibility of identification of subgroups of patients with equivalent CAS and CEA periprocedural S+D risk. Likewise, it could provide the opportunity to identify a subgroup of patients where the higher S+D risk with CAS treatment is concentrated, providing insights to where CAS treatment should be specifically avoided. Herein, we extend this effort to assess if other characteristics of the patient, or characteristics of the target artery, can be used to guide the selection of the procedure to minimize the S+D periprocedural endpoint.
Methods
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) randomized 2,502 patients with either symptomatic (n = 1,321) or asymptomatic (n = 1,181) significant carotid stenosis to revascularization by either CAS or CEA. The protocol was approved by the institutional review boards/ethics committees at participating sites, and all participants provided signed informed consent. Patients were followed for a composite outcome of any stroke (regardless of arterial distribution), MI or death during a periprocedural period, plus stroke ipsilateral to the study artery over a follow-up extending to 4-years after randomization (median follow-up was 2.5 years). Medical records for suspected stroke or myocardial infarction events were retrieved, and centrally adjudicated by committees of physicians blinded to treatment allocation. Additional details of the study protocol are available elsewhere.4
Demographic factors (age, gender, and race), symptomatic status, patient risk factors (hypertension, diabetes, dyslipidemia, smoking history) and percent arterial stenosis were available in 2,502 patients from the CREST database. A qualifying degree of stenosis for eligibility could be established by either angiography or ultrasound, with angiography used for randomization for 438 of the 1240 (35%) CEA patients; a randomization or procedure-related angiogram was available at the core laboratory for 1140 of the 1262 (90%) patients randomized to CAS. Together, this resulted in angiography being available on a subset of 1,578 patients, where detailed arterial characteristics could be assessed including the presence of ulcerated plaque, stenosis, sequential or remote lesions, and lesion length. Lesion length was measured as the distance (in mm) from the proximal to the distal shoulder of the lesion in the most unforeshortened projection. Lesion ulceration was identified if there was a small crater within the plaque or the presence of a luminal flap. The opening to the ulcer may have a narrow mouth that extends into a widened cavity or it may have a mouth that is as wide as the cavity. The type of aortic arch was not part of the clinical documentation.
Accordingly, an analysis was conducted on all 2,502 patients with the primary goal of assessing factors that may act as an effect modifier of the periprocedural S+D CAS versus CEA treatment difference. Because analysis was restricted to the periprocedural period, study dropouts were not an issue and analysis could be performed with logistic regression (rather than proportional hazards analysis as in the primary CREST paper1). The potential that the factor could be acting as an effect modifier was assessed by the addition of factor-by-treatment interaction terms to the logistic regression model, with an a priori p-value of 0.10 for significance of the interaction term. The individual and joint effects of factors found to be acting as an effect modifier were assessed using the dichotomous outcome of event/no-event during the periprocedural period.
While all patients randomized to CAS received an angiogram, many patients have a qualifying stenosis by ultrasound, are randomized to CEA, and proceed to the surgery without conventional angiography. These CEA patients without preoperative angiography are excluded from the analysis. In recognition that those patients randomized by angiography may, in some way, be different from those randomized by ultrasound, a subset analysis was carried out for those patients randomized by angiography alone. Finally, since all CAS patients will have had angiography at the time of the procedure prior to intervention and since some of those patients will have been randomized based upon ultrasound and others by angiography, this provided the opportunity to further compare the two groups in order to determine whether or not the method used to qualify a patient for randomization resulted in a change in factor characteristics.
Results
During the course of the study there were a total of 122 endpoint strokes of which 81(66.4%) occurred in the perioperative interval.
A description of the study population by factors considered as potentially affecting (i.e., effect modifiers) the CAS-to-CEA periprocedural S+D treatment difference is provided in Table I. There were no differences between the patients randomized to CEA and CAS with the exception that more patients assigned to CEA were dyslipidemic (P = 0.048) and had slightly less stenosis (p=0.01). As noted in the methods, not all CEA patients had an angiogram, and as such the arterial characteristics are not available on approximately 65% of these patients. Analysis in previous reports has shown age, sex and symptomatic status to be related to treatment differences or event rates when comparing CEA and CAS.1, 2, 5 There were no significant differences in these factors when comparing patients with and without angiograms (table II). Analysis in a previous paper has failed to show a relationship between other demographic and risk factors with either event rates or treatment differences in the CREST population, and therefore other atherosclerotic risk factors are unlikely to act as confounding factors in these analyses.6
Table I.
Baseline Comparison of Factors Potentially Associated With Increased Procedural Risk. Sample sizes vary slightly for specific characteristics (rows) because of missing data on specific items for a small number of patients.
| Factor | CAS | CEA | P-value | |
|---|---|---|---|---|
| Demographic and risk factors (nCAS = 1,262; nCEA = 1240) | Age (mean ± SD) | 68.9±8.96 | 69.2±8.75 | 0.40 |
| Female sex | 36.1 | 33.6 | 0.20 | |
| Symptomatic | 52.9 | 52.7 | 0.89 | |
| Diabetes | 30.6% | 30.4% | 0.95 | |
| Hypertension | 85.8% | 86.1% | 0.85 | |
| Dyslipidemia | 82.9% | 85.8% | 0.048 | |
| Smoke | 26.5% | 26.1% | 0.83 | |
| History of CVD or CABG | 43.6% | 46.4% | 0.18 | |
| Weight, pounds (mean ± SD) | 179.9 ± 37.4 | 179.5 ± 37.1 | 0.78 | |
| Arrhythmia | 6.1% | 6.5% | 0.62 | |
| LVH | 6.1% | 5.7% | 0.68 | |
| Arterial characteristics available from measurement in the core facility (nCAS = 1,141; nCEA = 438) | Lesion length (mm) | 13.8 ± 5.7 | 13.4 ±5.6 | 0.31 |
| Percent Diameter Stenosis (mean ± SD) | 73.7±11.1 | 72.0±11.1 | 0.01 | |
| Sequential or remote lesion | 33.3% | 34.3% | 0.71 | |
| Narrow ulcer | 4.1% | 3.9% | 0.87 | |
CAS, carotid artery stenting; CEA, carotid endarterectomy; CVD, cardiovascular disease; CABG, coronary artery bypass graft; LVH, left ventricular hypertrophy.
Continuous data are presented as mean ± standard deviation, and categorical data are presented as %.
Table II.
Description of patients assigned to CEA by whether they were randomized by an angiogram or ultrasound at baseline.
| Factor | Periprocedural Stroke and Death | |||
|---|---|---|---|---|
| Randomized by Angiogram (%) (n = 470) |
Randomized by Ultrasound (%) (n = 763) |
P-value | ||
| Participant characteristics | Age (mean ± SD) | 69.1±8.9 | 69.3±8.7 | 0.69 |
| Female sex | 32.8 | 34.2 | 0.60 | |
| Symptomatic | 57.0 | 49.7 | 0.01 | |
| Diabetes | 33.1 | 28.8 | 0.11 | |
| Hypertension | 88.7 | 84.3 | 0.03 | |
| Dyslipidemia | 87.2 | 85.1 | 0.30 | |
| Smoke | 24.7 | 26.7 | 0.43 | |
| History of CVD or CABG | 50.4 | 44.0 | 0.03 | |
| Weight, pounds | 181.8 ± 36.6 | 178.1 ± 37.4 | 0.09 | |
| Arrhythmia | 8.1 | 5.5 | 0.08 | |
| LVH | 4.4 | 6.4 | 0.18 | |
CVD, cardiovascular disease; CABG, coronary artery bypass graft; LVH, left ventricular hypertrophy.
Continuous data are presented as mean ± standard deviation, and categorical data are presented as %.
The association of factors with periprocedural S+D and the potential that these factors are acting as effect modifiers of the CAS-to-CEA treatment efficacy differences in S+D are shown in Table III. For each factor, the first column identifies the number of events and number of patients available in the analysis. The second column presents the hazard ratio (HR) for periprocedural events in the pooled CAS and CEA population, showing a 1.60 (95% CI: 1.24 – 2.07) times increase in the periprocedural S+D risk of per 10-year increase in age, a 2.49 (95% CI: 1.54 – 4.02) times increase in risk for symptomatic patients relative to asymptomatic patients, and 1.04 (95% CI: 1.00 – 1.08) times increase risk per millimeter of lesion length; with other factors not significantly associated with risk of periprocedural S+D events (P > 0.05). The third column assesses the potential for the factor to be acting as an effect modifier of the CAS-to-CEA treatment difference, where there was evidence that the CAS-to-CEA treatment difference was affected by only lesion length (P = 0.075) and whether the lesion was composed of sequential lesions or lesions extending remote to the carotid bulb, versus being a contiguous lesion (P = 0.095). The last two columns give the HR (95% CI) for each factor by treatment.
Table III.
The frequency of events, available sample size, hazard ratio for association with a S+D periprocedural endpoint in the pooled CAS and CEA population, and assessment of potential effect modification of the CAS versus CEA treatment difference (factor × treatment interaction).
| Factor | Periprocedural Stroke and Death | |||||
|---|---|---|---|---|---|---|
| Overall (n=2502; 84 events) |
CAS (n=1262; 55 events) |
CEA (n=1240; 29 events |
||||
| # Events / N patients |
HR (95% CI) of Factora |
Factor × Treatment Interaction P-valueb |
HR (95% CI) of Factorc |
HR (95% CI) of Factor c |
||
| Participant characteristics (from baseline case report forms) | Age per 10 years | 84/2502 | 1.60 (1.24,2.07) | 0.18 | 1.81 (1.32, 2.48) | 1.26 (0.82, 1.95) |
| Female sex | 84/2502 | 1.27 (0.82,1.96) | 0.27 | 1.51 (0.89, 2.57) | 0.89 (0.40,1.95) | |
| Symptomatic (relative to Asymptomatic) | 84/2502 | 2.49 (1.54,4.02) | 0.98 | 2.49 (1.38, 4.52) | 2.47 (1.09,5.58) | |
| Diabetes | 84/2489 | 1.13 (0.72,1.78) | 0.20 | 1.40 (0.81, 2.41) | 0.72 (0.31, 1.69) | |
| Hypertension | 84/2492 | 1.82 (0.84,3.94) | 0.19 | 2.89 (0.90, 9.26) | 1.01 (0.35, 2.90) | |
| Dyslipidemia | 84/2481 | 0.67 (0.40,1.13) | 0.36 | 0.57 (0.31, 1.05) | 1.01 (0.35, 2.89) | |
| Smoke | 83/2460 | 0.66 (0.38,1.14) | 0.15 | 0.48 (0.23, 1.01) | 1.08 (0.48, 2.44) | |
| History of CVD or CABG | 82/2411 | 0.88 (0.56,1.36) | 0.59 | 0.96 (0.56, 1.64) | 0.74 (0.35, 1.58) | |
| Weight per 10 lbs | 82/2455 | 0.97 (0.92,1.03) | 0.76 | 0.97 (0.90, 1.04) | 0.99 (0.89, 1.09) | |
| Arrhythmia | 84/2450 | 1.79 (0.90,3.58) | 0.56 | 1.53 (0.61, 3.83) | 2.32 (0.81, 6.67) | |
| LVH | 77/2231 | 1.09 (0.44,2.69) | 0.65 | 0.93 (0.29, 3.00) | 1.43 (0.34, 6.07) | |
| Arterial Characteristics (from core angiography laboratory assessment) | Lesion Length (per mm) | 60/1564 | 1.04 (1.00,1.08) | 0.07 | 1.05 (1.01, 10) | 0.92 (0.80, 1.06) |
| Percent Stenosis tertile 1 tertile 2 tertile 3 |
60/1564 |
reference 1.28 (0.68, 2.41) 1.20 (0.63, 2.28) |
0.30 |
reference 1.60 (0.78, 3.28) 1.46 (0.71, 3.01) |
reference 0.46 (0.09, 2.35) 0.52 (0.10, 2.68) |
|
| Sequential or remote lesion versus contiguous lesion | 60/1572 | 1.24 (0.74,2.10) | 0.09 | 1.53 (0.88, 2.66) | 0.25 (0.03, 1.96) | |
| Narrow ulcer | 60/1568 | 2.21 (0.88,5.51) | 0.71 | 2.05 (0.74, 5.68) | 3.23 (0.40, 25.9) | |
Hazard ratio indicates the risk of stroke or death associated with factor of interest after adjustment for treatment.
P-value for the treatment by factor interaction after adjustment for treatment. This indicates whether the factor of interest affects risk for CAS and CEA differentially after adjustments.
Hazard ratio indicates the risk of stroke or death associated with factor of interest
CABG, Coronary artery bypass graft; CI, confidence interval; CVD, cardiovascular disease; LVH, left ventricular hypertrophy.
The details of the effect modification for lesion length and complex lesion characteristics are shown in Table IV. The CAS-to-CEA treatment differences were relatively small for those with shorter lesions (i.e., less than the median lesion length of 12.85 mm), where 2.2% (95% CI: 0.7% – 5.2%) of those treated with CEA had events, compared to 2.8% (95% CI: 1.6% – 4.5%) for those treated with CAS, for an odds ratio of 1.24 (95% CI: 0.45 – 3.45). In contrast, larger CAS-to-CEA treatment differences were observed for those with longer lesions (Figure 1), where 1.9% (95% CI: 0.5% – 4.7%) of those treated with CEA had periprocedural S+D events, compared to 6.1% (4.3% – 8.4%) of those treated with CAS; for an odds ratio of 3.42 (95% CI: 1.19 – 9.78). Among those with short contiguous lesions, there was only a small treatment difference between CAS and CEA, where 2.5% (95% CI: 0.07–6.4) of CEA patients had periprocedural S+D events, compared to 2.0% (95% CI: 0.9–3.9) for those treated with CAS; for an odds ratio of 0.72 (95% CI: 0.21–2.46). This is in contrast to patients with lesions that were contiguous and long (>12.85mm), where there was a major difference between CEA and CAS. Patients treated with CEA had a periprocedural S&D event rate of 3.1% (95% CI: 0.9–6.1) compared to 6.1% (95% CI:3.8–9.1) for those patients treated with CAS for an odds ratio of 2.06 (95% CI: 0.69-6.17). There is also a much larger CAS-to-CEA treatment difference among those with sequential lesions (Figure 2) or lesions extending beyond the carotid bulb, where 0.7% (95% CI: 0.02% – 3.7%) of those treated with CEA had events compared to 5.8% (95% CI: 3.7% – 8.7%) of those treated with CAS; for an odds ratio of 9.01 (95% CI: 1.20 – 67.8).
Table IV.
Details of effect modification for the nature of the lesion and lesion length, where for each treatment number of patients, number of periprocedural stroke and death event are shown, and then the treatment odds ratio adjusted for age, sex and symptomatic status.
| Treatment | Number of Pts |
Periproc S+D |
Rate (% / 95% CI) |
Odds Ratio (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| Individual Effects | Nature of the lesion | Contiguous | CEA | 288 | 8 | 2.8 (1.2 – 5.4) | 1.35 (0.61, 3.02) |
| CAS | 757 | 29 | 3.8 (2.6 – 5.5) | ||||
| Sequential or Remote | CEA | 150 | 1 | 0.7 (0.02 – 3.7) | 9.01 (1.20,67.8) | ||
| CAS | 377 | 22 | 5.8 (3.7 – 8.7) | ||||
| Lesion Length | Shorter Lesion (<12.85 mm) | CEA | 222 | 5 | 2.2 (0.7 – 5.2) | 1.24 (0.45, 3.45) | |
| CAS | 557 | 16 | 2.8 (1.6 – 4.5) | ||||
| Longer Lesion (≥12.85 mm) | CEA | 215 | 4 | 1.9 (0.5 –4.7) | 3.42 (1.19, 9.78) | ||
| CAS | 570 | 35 | 6.1 (4.3 – 8.4) | ||||
| Joint Effects of the Nature of the Lesion and the Length of the Lesion | Contiguous + Short | CEA | 158 | 4 | 2.5 (0.07 – 6.4) | 0.72 (0.21 – 2.46) | |
| CAS | 404 | 8 | 2.0 (0.9 – 3.9) | ||||
| Contiguous + Long | CEA | 129 | 4 | 3.1 (0.9–6.1) | 2.06 (0.69 –6.17) | ||
| CAS | 346 | 21 | 6.1 (3.8–9.1) | ||||
| Remote/Sequential + Short | CEA | 64 | 1 | 1.6 (0.04 – 8.4) | 3.59 (0.44–29.56) | ||
| CAS | 153 | 8 | 5.2 (2.3 – 10.0) | ||||
| Remote/Sequential + Long | CEA | 86 | 0 | 0.0 (0.0 – 4.2) | Not defined due to zero count | ||
| CAS | 224 | 14 | 6.3 (3.5 – 10.3) | ||||
S+D, stroke and death; CI, confidence interval; CAS, carotid artery stenting; CEA, carotid endarterectomy.
Figure 1.
Two examples of long lesions, beginning at the bulb of the internal carotid artery and extending 27 and 30 mm distally.
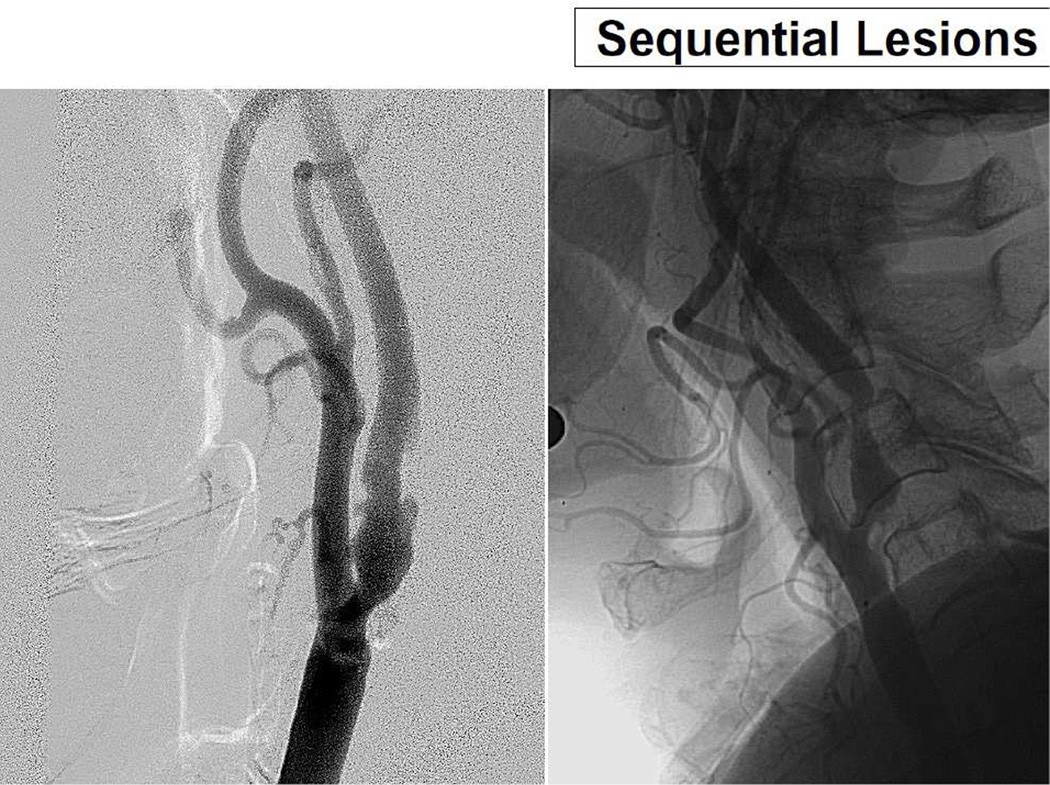
Figure 2.
Two examples of sequential lesions. One area of stenosis at the bulb of the internal carotid artery and another area of stenosis distally separated by a relatively normal intervening segment.
The bottom portion of Table IV shows the joint effects of the nature and length of the lesion. While considering these factors simultaneously results in relatively small sample sizes, 562 of 1,565 (36%) patients reported in the table had lesions that were both contiguous and short lesions, and the periprocedural odds of S+D in this group was (non-significantly) 28% lower among those treated with CAS relative to CEA (OR = 0.72; 95% CI: 0.21 – 2.46). The odds for S+D was approximately 2–3 times higher for patients with one of the characteristics who were treated by CAS, specifically 2.06 times (95% CI: 0.69 – 6.17) times for those with lesions that were contiguous but long, and 3.59 times (95% CI: 0.44 – 29.56) for those that were short but remote or sequential. CAS-to-CEA treatment differences were most extreme among those with lesions that were both remote or sequential and were long, where there were no S+D periprocedural events among the 86 (0.0%) patients treated with CEA (95% CI: 0.0% – 4.2%), but 14 events among the 224 (6.3%) treated with CAS (95% CI: 3.5% – 10.3%), with an undefined odds ratio because of the zero events in the CEA group.
The supplemental analysis where analysis is restricted to those randomized by angiography is consistent with the overall analysis (see Supplemental Table I). Periprocedural S+D continued to not differ in those with short lesions (OR = 1.02; 95% CI: 0.30 – 3.46), but S+D risk was higher with CAS among those with longer lesions (OR = 3.39; 95% CI: 1.10 – 10.47). Likewise, CAS versus CEA risk did not differ among those with contiguous lesions (OR = 1.25; 95% CI: 0.49 – 3.18), but was significantly higher with CAS in patients with sequential or remote lesions (OR = 8.28; 95% CI: 1.04 – 65.99). There also seems to be little concern that those randomized by angiography differ from those randomized by ultrasound. Among the 15 patient and plaque characteristics considered, only percent stenosis by angiogram (mean 72.4 vs 74.6; p=0.001) and left ventricular hypertrophy differed (3.8% vs 7.7%; p = 0.01) between CAS patients with qualifying lesions established by angiography compared to CAS patients with qualifying lesions established by ultrasound respectively. (See supplemental table II).
Discussion
Our primary finding was documenting a clear differential treatment efficacy as depending on lesion length and the nature of the lesion. Because of this differential treatment efficacy we stratified the analysis by these factors and assessed CAS-to-CEA risk within each strata, finding larger (significant) differences for longer lesions or sequential/remote lesions, and smaller (non-significant) differences for shorter or contiguous lesions. These data suggest that the higher S+D periprocedural event rate (that is the primary contributor to the higher 4-year S+D event rate) among CAS-treated patients appears to be strongly related to the lesion length and the nature of the lesion. For those who have short contiguous lesions(<12.85mm), there was only a marginal (non-significant) increase risk for treating patients with CEA relative to CAS (Table IV). In contrast, for those with lesions with sequential components or extending remote to the carotid bulb (regardless of the length), or for those with longer lesions (regardless of their nature), the risk of S+D events was significantly higher among those treated with CAS relative to those with CEA. These findings were independent of how the patients were randomized (angiography or ultrasound). Hence, it appears that most of the higher S+D periprocedural risk associated with CAS is relatively isolated among those with the longer or more complex lesion characteristics. Since these lesions were identified by contrast angiography, the information obtained in those patients who have angiography prior to the decision to select either CEA or CAS can be applied. For those patients whose first angiogram is obtained at the time of planned CAS, the finding of a long lesion or complex lesions as defined in this report should guide the interventionalist to stop after the diagnostic angiogram and refer the patient for CEA.
The joint effects of the length of the lesion and the nature of the lesion make an even stronger case for using these factors to guide the treatment choice for revascularization. Among the 36% of the patients with lesions that were both short and contiguous, CAS had a (non-significant) lower odds of a periprocedural S+D than CEA (OR 0.72, 95% CI: 0.21–2.46). This suggests that consideration of CAS is competitive with CEA in 36% of patients. In contrast, the risk the CAS-CEA periprocedural S+D risk difference was most extreme among the 310 patients (20% of the total) where the lesion was both long and remote or sequential, where all 14 periprocedural S+D events were among those treated with CAS. As such, it would seem wise to avoid CAS in this smaller group of patients.
Stroke events complicating CAS are the result of embolic debris released from the lesion at the time of intervention. Transcranial Doppler-detected microembolic signals during stenting are detected with increasing frequency during predilatation, post dilation and stent deployment.5 Embolic protection devices significantly reduce but do not eliminate the volume of debris. Excessive manipulation of the plaque, for example, with repeated balloon post dilation has been shown to increase embolic events. It follows, therefore, that depending on technique and technology utilized, the volume of vulnerable plaque, as manifested by long, and discontinuous lesions might result in more events from CAS. On the other hand, the lesions that place CAS at a disadvantage represent no particular technical challenge for CEA since the surgical exposure encompasses the need to manage those lesions and does not represent a greater challenge to open operation.
This study has strengths and limitations. Its greatest strength is the assessment of potential effect modification of periprocedural S+D risk in a large and well-characterized randomized study of CEA and CAS, with core lab analysis of 1578 catheter angiograms. There are several weaknesses. The number of S+D events was low (reflecting clinical success), and this small number of events limits the statistical power to identify factors acting as effect modifiers. Nonetheless, we were able to identify two effect modifiers, providing de facto evidence that the power was sufficient to identify subgroups at differential risk. Another limitation was that a sizable proportion of patients randomized to CEA did not have an angiogram, and so we were unable to examine the arterial characteristics in those patients. However, when the analysis was limited to patients who were randomized on the basis of angiography for both CEA and CAS, the same lesion characteristics, adverse for CAS but not CEA, were identified. Finally, these results represent outcomes from the “fixed wire” RX ACCUNET Embolic Protection System™ and “open cell” RX ACCULINK Carotid Stent System™ utilized in CREST. Whether similar results would be observed with other embolic protection systems and stents in use today cannot be determined.
In conclusion, characteristics of the carotid artery substantially affected the magnitude of the differential risk for periprocedural stroke and death in CREST. Longer lesions and/or sequential lesions extending beyond the carotid bulb predicted differentially higher risk for CAS versus CEA. This higher risk associated with CAS seems to be relatively isolated to patients with these arterial characteristics. We were successful in identifying a sizable proportion of the patients with short and contiguous lesions not extending beyond the carotid bulb (estimated to be over one-third of the patients in the study) where CAS appears as safe as CEA with regard to stroke and death periprocedural events. In addition, we identified a smaller subset of patients with long and complex lesions (approximately 20% of the population) where CAS should be particularly avoided.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in the Plenary Session at the 2015 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, IL, June 17-20, 2015.
References
- 1.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. The New England Journal of Medicine. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC, 3rd, et al. Age and outcomes after carotid stenting and endarterectomy: The Carotid Revascularization Endarterectomy versus Stenting Trial. Stroke. 2011;42:3484–3490. doi: 10.1161/STROKEAHA.111.624155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard VJ, Lutsep HL, Mackey A, Demaerschalk BM, Sam AD, 2nd, Gonzales NR, et al. Influence of sex on outcomes of stenting versus endarterectomy: A subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) Lancet Neurology. 2011;10:530–537. doi: 10.1016/S1474-4422(11)70080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) International Journal of Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard VJ, Voeks JH, Lutsep HL, Mackey A, Milot G, Sam AD, 2nd, et al. Does sex matter? Thirty-day stroke and death rates after carotid artery stenting in women versus men: Results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) lead-in phase. Stroke. 2009;40:1140–1147. doi: 10.1161/STROKEAHA.108.541847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007. p. 121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.