Abstract
Background
A relationship of blood uric acid (UA) with hypertension and cardiovascular risk is under debate thus salt intake is hypothesized to contribute to such associations.
Methods
In this cross-sectional study, stratified cluster random sampling elicited a sample of 1805 Kazakhs with 92.4% compliance. Hypertension and moderate-or-high total cardiovascular risk (mTCR) were defined according to guidelines. Sodium intake was assessed by urinary sodium excretion. Prevalence ratios (PRs) were used to express associations of UA with hypertension and mTCR.
Results
In the highest tertile of sodium intake in women, the adjusted PRs (95% confidence intervals) of low to high quartiles compared with the lowest quartile of UA, were 1.22(0.78–1.91), 1.18(0.75–1.85), and 1.65(1.09–2.51) for hypertension and 1.19(0.74–1.90), 1.39(0.91–2.11), and 1.65(1.10–2.47) for mTCR (P for trend <0.05). However, these findings were not shown for other sodium intake levels. There were similar results in men. PRs markedly increased with a concomitant increase in UA and sodium intake and there was a significant interaction (P = 0.010) for mTCR with PRs of 1.69(1.10–2.60) for men and 3.70(2.09–6.52) for women in those with the highest compared with the lowest quartile of UA and tertile of sodium intake. Similar findings were shown for hypertension.
Conclusions
This study implied that a high salt intake may enhance the associations of UA with hypertension and cardiovascular risk.
Introduction
A relationship between uric acid (UA) and hypertension, which leads to at least 1.5 million cardiovascular (CV) deaths per year [1], has been under debate for decades. Most observational studies have reported that elevated UA levels is likely linked with hypertension in Caucasian [2,3], African-Americans [3], and Chinese Han [4], and particularly with the incident hypertension in adolescents [2,5]. However, some studies have reported no such association in men or the elderly [6,7], in particular the Framingham Study, which thought the UA level was an independent predictor of incident hypertension and longitudinal BP progression [2], but did not indicate a causal role of UA in CV risk [8]. Therefore, the associations of UA with hypertension and related CV risk remain less well established, and UA is not considered as a risk factor for hypertension and CV diseases in current guidelines [1,9,10].
A high-salt intake is well established as the most important risk factor of hypertension. A rat model that hyperuricemia induced salt-sensitivity, which is linked to hypertension and CV risk, has been established [11]. Recently, an increased salt intake was associated with increased UA levels, which enhanced the association of a high-salt diet with hypertension [12]. However, whether a high-salt diet plays a role on the association between UA and hypertension has not been explored.
We hypothesized a high-salt diet contributes to associations of UA with hypertension and related CV risk. The present study explored influence of diet salt on such association in Kazakhs, a people across Eurasia, who are well known to have a high level of sodium intake and prevalence of hypertension.
Methods
Study population
Hong Dun town is the base of this study locating in the urban-rural fringe of Altay county-level city in the North Xinjiang, China. 12 Kazakh-based administrative villages and, one township office which almost covers all citizens of this town, were included in this study after excluding villages with less than 100 Kazakh individuals who live together with other ethnic groups.
Stratified random-cluster sampling was performed based on a computer-generated allocation algorithm (Fig 1). In sampled units, all Kazakh people aged 30 years or over were recruited for the study with a consideration of sample size based on the prevalence (46.8%) of hypertension among Kazakhs aged 30–70 years [13]. Kazakh was defined as a person whose biological parents were both Kazakh and who had Kazakh paternal/maternal biological grandparents. Pregnant women, persons bedridden aged over 80 years, disabled persons, or persons with mental disorders or other severe disease (as determined by doctors) were excluded. There were 1805 people who met eligibility criteria and 1668 people completed the survey. This represented 92.4% compliance; specifically, 94.4% (637/675) from pastoral villages, 90.2% (838/929) from agricultural villages, and 96.0% (193/201) from the township office.
Fig 1. Flow chart for recruitment of participants.
Between October 2012 and February 2013, participants were invited to take a face-to-face questionnaire survey that included demographic information, hypertensive or CV risk factors, and medical history. Physical examinations, including weight, height, waist, blood pressure (BP) measurements, electrocardiography (ECG), and laboratory tests, were performed. All participants gave their written informed consent to participate in this study whose ethical approval was from the Ethics Committee of Institute of Basic Medical Sciences Chinese Academy of Medical Sciences.
Acquirement of information on familial aggregation
Familial relationship information was collected in April 2013 based on current living backgrounds, including and limited to consanguineous and marital relationships of three generations within subjects recruited. When a husband and his wife were both oriented from the same village, the couples would be classificated into the husband’s or the wife’s family at random. These relationships were recorded from the highest through the third generation. Members of the highest generation within one family could be couples or siblings with their spouses. Therefore, 303 families that had at least two members and included 1370 family-based subjects were confirmed.
Assessment of hypertension and related CV risk
After an overnight fasting, BP was measured with an appropriate arm cuff and a mercury column sphygmomanometer on the left arm after a resting period of at least 10 min in the supine position. BP was defined as the mean of two readings, or three readings if there was a difference of more than 5 mmHg between the initial readings [1].
2013 guidelines on hypertension from the European Society of Hypertension and the European Society of Cardiology (ESH/ESC Guidelines) were used at the stage of analysis [10]. Hypertension was defined as a systolic BP ≥ 140 mmHg, a diastolic BP ≥ 90 mmHg, or both, or the use of antihypertensive medications within the last 2 weeks. BP was categorized as optimal, normal, high normal, and Grade 1–3 hypertension, by the highest BP level, whether systolic or diastolic. Moderate-or-high total cardiovascular risk (mTCR) based on BP category, CV risk factors, asymptomatic organ damages (ODs) and presence of diabetes, and symptomatic cardiovascular diseases (CVDs) or chronic kidney diseases (CKDs) was stratified (Table 1) and meant a progressively increasing absolute likehood (≥ 15%) of developing a major CV event within the next 10 years [9,10].
Table 1. Definition of moderate or higher total cardiovascular risk.
| Other risk factors, asymptomatic ODs or diseases | BP, mm Hg | |||
|---|---|---|---|---|
| High normal: systolic BP 130–139 or Diastolic BP 85–89 | Grade 1 hypertension: systolic BP 140–159 or Diastolic BP 90–99 | Grade 2 hypertension: systolic BP 160–179 or Diastolic BP 100–109 | Grade 3 hypertension: systolic BP ≥180 or Diastolic BP ≥110 | |
| No other RF | Low risk | Moderate risk | High risk | |
| 1–2 RF | Low risk | Moderate risk | Moderate to high risk | High risk |
| ≥3 RF | Low to moderate risk | Moderate to high risk | High risk | High risk |
| OD, CKD stage 3 or diabetes | Moderate to high risk | High risk | High risk | High to very high risk |
| Symptomatic CVD, CKD stage ≥4 or diabetes | Very high risk | Very high risk | Very high risk | Very high risk |
BP: Blood pressure; CKD: chronic kidney disease; CVD: cardiovascular disease; OD: organ damage; RF: risk factor.
We defined male sex, age (men ≥ 55 years; women ≥ 65 years), smoking (at least one cigarette per day), dyslipidemia (total cholesterol [TC] > 4.9 mmol/L, and/or low-density lipoprotein cholesterol > 3.0 mmol/L, and/or high-density lipoprotein cholesterol < 1.0 mmol/L for men and < 1.2 mmol/L for women, and/or triglyceride > 1.7 mmol/L), impaired fasting glucose (5.6–6.9 mmol/L without hypoglycemic drug treatment), and obesity (body mass index [BMI] ≥ 30 kg/m2) or abdominal obesity (waist circumference ≥ 90 cm for men and ≥ 85 cm for women [1]) as CV risk factors; pulse pressure (in the persons aged ≥ 60 years) ≥ 60 mmHg, electrocardiographic left ventricular hypertrophy, CKD with eGFR 30–60 mL∙min-1∙1.73m-2 as asymptomatic ODs; fasting plasma glucose ≥ 7.0 mmol/L or the use of hypoglycemic medications within the last 2 weeks as diabetes mellitus; and stroke from a questionnaire, myocardial infarction from ECG, and CKD with eGFR < 30 mL∙min-1∙1.73m-2 as established CV or renal disease. Staging of CKD with eGFR was done on the basis of K/DOQI clinical practice guidelines [14].
Sodium intake and laboratory measurements including UA
Venous blood samples were collected after an overnight fast of at least 10 h, and plasma was immediately separated. Sodium intake was assessed by urinary sodium excretion from the second urine sample after waking, urine creatinine (Cr) concentration, and the 24-h urinary Cr excreted as estimated from height, body weight, and age [15]. Each 43 mmol of sodium is approximately equivalent to 1 g of sodium or 2.5 g of salt (sodium chloride). Plasma UA concentration was measured with the uricase/POD method (Roche Diagnostics, Indianapolis, IN, USA) and a coefficient of variation of 4.0% was achieved. Hyperuricemia was defined as > 416 mmol/L in men and >357 mmol/L in women [16].
All chemistry measurements, including plasma UA concentrations were measured with a Beckman Coulter AU2700 Clinical Chemistry Analyzer (CA, USA). All electrolytes were measured with a Caretium XI-921CT Electrolyte Analyzer (Shenzhen, China).
Statistical analyses
Prevalence ratios (PRs) with 95% confidence intervals (95% CIs) were used to express associations of plasma UA with hypertension and mTCR. For calculating PRs, robust Poisson method-based models, excluding missing data less than 0.5% of all subjects, and UA quartiles were used. For evaluating effect modification of sodium intake on the associations of UA with hypertension and mTCR, interactions and the main effects of sodium intake and UA were illustrated in robust Poisson models, then tertile of sodium intake and a common reference group, defined as one with the lowest quartile of UA and the lowest tertile of sodium intake, were used in stratified analysis. Families were combined into such Poisson models as repeat measurements [17]. For evaluating familiar intraclass correlation, intraclass correlation coefficients (ICCs), i.e. the covariance parameter estimate for family divided by the sum of the family and residual covariance estimates, were calculated on two-level logistic models where “family” was a random effect or Level 2 unit [18].
SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all analyses. All P-values were two sided except P trend tests based on robust Poisson method, in which one-sided P values were used, and a P-value < 0.05 was considered statistically significant.
Results
This population had male proportions of 46.7% and average ages of 46.5±12.3 years. The prevalence of hypertension and hyperuricemia was 45.3% and 6.2% (50.1% and 7.6% for men, 41.1% and 4.9% for women). The average salt intake daily of this population is 17.6 ± 14.2 g. Other sex-specific population characteristics were shown in Table 2.
Table 2. Study sample characteristics.
| Men (n = 779) | Women (n = 889) | Total (n = 1668) | |
|---|---|---|---|
| Age, years | 46.5 ± 12.1 | 46.6 ± 12.5 | 46.5 ± 12.3 |
| Occupational background, % | |||
| Nomad | 38.4 | 38.0 | 38.2 |
| Farmer | 52.6 | 48.1 | 50.2 |
| Non-manual worker | 9.0 | 13.8 | 11.6 |
| BP variables | |||
| SBP, mm Hg | 137 ± 22 | 135 ± 26 | 136 ± 24 |
| DBP, mm Hg | 87 ± 13 | 84 ± 13 | 85 ± 13 |
| Hypertension, % | 50.1 | 41.1 | 45.3 |
| mTCR, % | 55.3 | 42.9 | 48.7 |
| Antihypertensive agent, % | 8.1 | 17.4 | 13.1 |
| UA variables | |||
| UA level, mmol/L | 306 ± 74 | 236 ± 69 | 269 ± 80 |
| Hyperuricemia, % | 7.8 | 5.1 | 6.4 |
| Salt intake daily, g | 18.9 ± 17.6 | 16.5 ± 10.1 | 17.6 ± 14.2 |
| Smoking, % | 55.5 | 2.6 | 27.3 |
| Drinking, % | 28.8 | 0.6 | 13.7 |
| BMI, kg/m2 | 25.7 ± 4.3 | 26.9 ± 5.1 | 26.3 ± 4.8 |
| TC, mmol/L | 5.28 ± 0.95 | 4.95 ± 0.93 | 5.10 ± 0.96 |
| Fasting blood glucose, mmol/L | 5.33 ± 0.80 | 5.14 ± 0.74 | 5.23 ± 0.77 |
| Blood Cr, mmol/L | 74.0 ± 11.8 | 62.3 ± 34.9 | 67.7 ± 27.4 |
Values are means ± SD and percent.
Overall family-based ICC values for hypertension and mTCR were 0.041 and 0.033 with a dramatic difference on sex (Table 3). For example, 0.055 of ICC was observed in men; however, this value was approximately equal to zero in women.
Table 3. Intraclass correlation of the family based on two-level logistic models.
| Dependent variable | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| Random variance | ICC | Random variance | ICC | Random variance | ICC | |
| Hypertension | 0.192 | 0.055 | < 0.001 | < 0.001 | 0.141 | 0.041 |
| mTCR | 0.152 | 0.044 | < 0.001 | < 0.001 | 0.113 | 0.033 |
ICC: intraclass correlation coefficients; Residual variance: π2 / 3 = 3.289.
We established sex-specific and age-adjusted simple models only including major effects with their interaction item or not as shown in Table 4. Both UA and salt levels as major effects were correlated with hypertension and mTCR with a statistical significance; their interaction item when being included in the models also showed a statistical or marginal significance. The findings were accordant in multivariable models adjusted for influencing factors such as age, occupation, smoking (only for men), alcohol intake (only for men), BMI, plasma Cr, TC, fasting glucose, and antihypertensive treatment for hypertension and age, occupation, and antihypertensive treatment for mTCR.
Table 4. Major effects of UA and salt intake and their interaction.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UA, mmol/L | Salt intake, gram | Interaction | UA, mmol/L | Salt intake, gram | Interaction | |||||
| PR | P value | PR | P value | P value | PR | P value | PR | P value | P value | |
| Age-adjusted simple model | ||||||||||
| Major effects without interaction item | ||||||||||
| Hypertension as dependent variable | 1.031 | 0.004 | 1.093 | 0.002 | – | 1.031 | 0.004 | 1.115 | < 0.001 | – |
| mTCR as dependent variable | 1.024 | 0.012 | 1.091 | 0.001 | – | 1.028 | 0.008 | 1.111 | < 0.001 | – |
| Major effects with interaction item | ||||||||||
| Hypertension as dependent variable | 1.045 | 0.001 | 1.004 | < 0.001 | 0.024 | 1.055 | 0.005 | 1.013 | 0.120 | 0.132 |
| mTCR as dependent variable | 1.039 | 0.002 | 1.004 | < 0.001 | 0.019 | 1.075 | < 0.001 | 1.022 | 0.002 | 0.010 |
| Multivariable model | ||||||||||
| Major effects without interaction item | ||||||||||
| Hypertension as dependent variable* | 1.007 | 0.333 | 1.067 | 0.075 | – | 0.999 | 0.912 | 1.107 | < 0.001 | – |
| mTCR as dependent variable** | 1.024 | 0.015 | 1.096 | 0.014 | – | 1.021 | 0.032 | 1.114 | < 0.001 | – |
| Major effects with interaction item | ||||||||||
| Hypertension as dependent variable* | 1.016 | 0.242 | 1.002 | 0.025 | 0.129 | 1.021 | 0.064 | 1.013 | 0.103 | 0.146 |
| mTCR as dependent variable** | 1.034 | 0.009 | 1.003 | 0.004 | 0.057 | 1.068 | < 0.001 | 1.023 | < 0.001 | 0.010 |
UA and salt intake as major effects were included in age-adjusted simple model with interaction item or not. UA level was categorized from its P25 with a step of 20 mmol/L. Salt intake daily was categorized from 5 g with a step of 5 g in major effect-based model, however, defined as a continuous variable with a unit of gram in interaction item-based model.
*Adjusted for age, occupation, smoking (only for men), alcohol intake (only for men), BMI, plasma Cr, TC, fasting glucose, and antihypertensive treatment.
**Adjusted for age, occupation, and antihypertensive treatment.–Not applicable. mTCR: moderate-or-high total cardiovascular risk.
Salt-based stratified results with multivariable models, including major effects and other influencing factors, were shown in Table 5. Although no overall association was observed in men, in the highest tertile of sodium intake, the PRs (95% CIs) from low to high quartiles of UA, comparing with lowest quartile of UA, were 1.05 (0.77–1.42), 1.14 (0.82–1.58), and 1.24 (0.94–1.64) for hypertension (P for trend = 0.140) and 1.05 (0.82–1.36), 0.98 (0.73–1.32), and 1.28 (0.99–1.65) for mTCR (P for trend = 0.035). In women with an overall association, such PRs were 1.22 (0.78–1.91), 1.18 (0.75–1.85), and 1.65 (1.09–2.51) for hypertension (P for trend = 0.008) and 1.19 (0.74–1.90), 1.39 (0.91–2.11), and 1.65 (1.10–2.47) for mTCR (P for trend = 0.019). However, these significant or marginally significant linear trends, regardless of sex, were not shown in other sodium intake levels.
Table 5. Association of UA, modified by sodium intake, with hypertension and mTCR.
| Quartiles of UA with median (interquartile range), mmol/L | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | Men | Women | |||||||||
| 227 (203–246) | 288 (276–298) | 326 (315–341) | 391 (375–425) | P for trend† | 163 (149–175) | 212 (200–222) | 249 (238–264) | 321 (293–353) | P for trend† | ||
| Adjusted for age only | |||||||||||
| Hypertension | |||||||||||
| 1st tertile of sodium intake | 1.00 | 0.66 (0.43, 1.02) | 0.88 (0.61, 1.27) | 1.11 (0.81, 1.53) | 0.128 | 1.00 | 2.26 (1.15, 4.42) | 2.39 (1.26, 4.53) | 2.27 (1.18, 4.37) | 0.033 | |
| 2nd tertile of sodium intake | 1.00 | 0.95 (0.64, 1.43) | 1.01 (0.72, 1.42) | 1.10 (0.77, 1.59) | 0.125 | 1.00 | 1.29 (0.85, 1.96) | 1.33 (0.91, 1.94) | 1.40 (0.93, 2.11) | 0.493 | |
| 3rd tertile of sodium intake | 1.00 | 1.02 (0.76, 1.37) | 1.09 (0.81, 1.47) | 1.42 (1.08, 1.86) | 0.019 | 1.00 | 1.03 (0.65, 1.65) | 1.24 (0.80, 1.93) | 1.66 (1.10, 1.51) | 0.002 | |
| Total‡ | 1.00 | 0.88 (0.72, 1.09) | 0.98 (0.81, 1.20) | 1.18 (0.98, 1.40) | 0.019 | 1.00 | 1.34 (1.01, 1.77) | 1.46 (1.12, 1.88) | 1.67 (1.31, 2.14) | 0.008 | |
| mTCR | |||||||||||
| 1st tertile of sodium intake | 1.00 | 0.63 (0.43, 0.92) | 0.83 (0.60, 1.15) | 0.98 (0.75, 1.29) | 0.414 | 1.00 | 2.21 (1.19, 4.12) | 2.48 (1.37, 4.48) | 2.09 (1.10, 3.95) | 0.080 | |
| 2nd tertile of sodium intake | 1.00 | 0.89 (0.62, 1.29) | 1.15 (0.87, 1.51) | 1.16 (0.86, 1.57) | 0.021 | 1.00 | 1.47 (0.97, 2.22) | 1.40 (0.97, 2.01) | 1.43 (0.96, 2.13) | 0.157 | |
| 3rd tertile of sodium intake | 1.00 | 1.04 (0.81, 1.34) | 0.94 (0.70, 1.26) | 1.25 (0.96, 1.63) | 0.081 | 1.00 | 1.08 (0.67, 1.76) | 1.36 (0.88, 2.08) | 1.62 (1.07, 1.45) | 0.009 | |
| Total‡ | 1.00 | 0.86 (0.72, 1.04) | 0.97 (0.81, 1.15) | 1.10 (0.95, 1.29) | 0.049 | 1.00 | 1.44 (1.09, 1.89) | 1.57 (1.22, 2.01) | 1.64 (1.28, 2.11) | 0.001 | |
| Adjusted for multivariables | |||||||||||
| Hypertension* | |||||||||||
| 1st tertile of sodium intake | 1.00 | 0.64 (0.42, 0.98) | 0.74 (0.51, 1.06) | 0.86 (0.64, 1.16) | 0.264 | 1.00 | 1.85 (1.05, 3.26) | 1.62 (0.83, 3.14) | 1.65 (0.75, 3.60) | 0.120 | |
| 2nd tertile of sodium intake | 1.00 | 0.89 (0.59, 1.34) | 0.94 (0.65, 1.38) | 0.84 (0.53, 1.33) | 0.200 | 1.00 | 1.42 (0.91, 2.21) | 1.46 (1.01, 2.11) | 1.60 (0.98, 2.63) | 0.330 | |
| 3rd tertile of sodium intake | 1.00 | 1.05 (0.77, 1.42) | 1.14 (0.82, 1.58) | 1.24 (0.94, 1.64) | 0.140 | 1.00 | 1.22 (0.78, 1.91) | 1.18 (0.75, 1.85) | 1.65 (1.09, 2.51) | 0.008 | |
| Total‡ | 1.00 | 0.88 (0.71, 1.09) | 0.92 (0.75, 1.13) | 0.93 (0.77, 1.13) | 0.500 | 1.00 | 1.46 (1.11, 1.92) | 1.44 (1.11, 1.85) | 1.47 (1.13, 1.92) | 0.307 | |
| mTCR** | |||||||||||
| 1st tertile of sodium intake | 1.00 | 0.65 (0.44, 0.96) | 0.77 (0.57, 1.05) | 0.90 (0.70, 1.16) | 0.262 | 1.00 | 2.12 (1.14, 2.94) | 2.30 (1.25, 4.24) | 1.98 (0.94, 4.18) | 0.330 | |
| 2nd tertile of sodium intake | 1.00 | 0.90 (0.63, 1.29) | 1.25 (0.95, 1.65) | 1.19 (0.89, 1.60) | 0.006 | 1.00 | 1.58 (1.08, 2.32) | 1.53 (1.06, 2.19) | 1.53 (1.06, 2.22) | 0.738 | |
| 3rd tertile of sodium intake | 1.00 | 1.05 (0.82, 1.36) | 0.98 (0.73, 1.32) | 1.28 (0.99, 1.65) | 0.035 | 1.00 | 1.19 (0.74, 1.90) | 1.39 (0.91, 2.11) | 1.65 (1.10, 2.47) | 0.019 | |
| Total‡ | 1.00 | 0.89 (0.74, 1.07) | 0.98 (0.83, 1.17) | 1.08 (0.92, 1.26) | 0.048 | 1.00 | 1.52 (1.18, 1.98) | 1.62 (1.26, 2.08) | 1.63 (1.24, 2.14) | 0.015 | |
*Adjusted for age, residence, smoking, alcohol intake, BMI, urine sodium, plasma Cr, TC, fasting glucose, and antihypertensive treatment.
**Adjusted for age, residence, and antihypertensive treatment. Tertile 1–3 of sodium intake were defined as < 237, 237–346, and >346 mmol/d in men and < 207, 207–309, and > 309 mmol/d in women.
†UA was defined as a continuous variable (mmol/L).
‡Sodium intake (mmol/d) was adjusted.
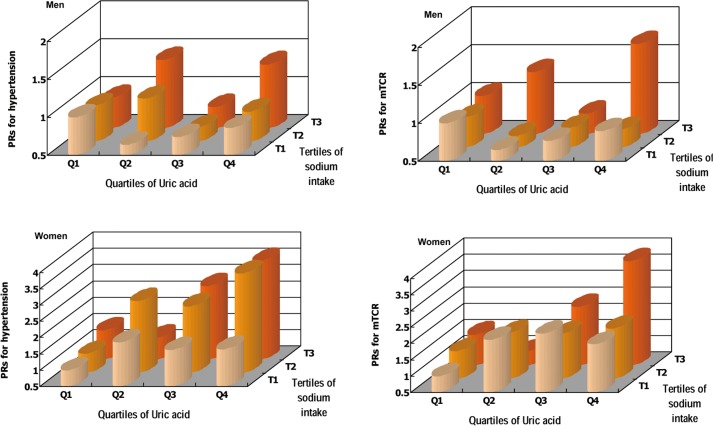
The PRs for mTCR markedly increased with a concomitant increase in UA and sodium intake when comparing with the common reference group, thus the strongest associations were observed with PRs of 1.69 (1.10–2.60) for men and 3.70 (2.09–6.52) for women in the highest quartile of UA and the highest tertile of sodium intake group (Fig 2). Similar findings were observed for hypertension, although the strongest associations were observed in the first or second highest tertile of sodium intake group, with a PR of 1.40 (1.07–1.84) for men and 3.56 (1.37–9.27) for women.
Fig 2. Adjusted PRs for hypertension and mTCR according to multivariables and familial aggregation.
A common reference group was used. Multivariables for hypertension were included age, residence, smoking, alcohol intake, BMI, sodium intake, Cr, TC, fasting glucose, and antihypertensive treatment. Multivariables for mTCR were included age, residence, sodium intake, and antihypertensive treatment. Tertile 1–3 of sodium intake were defined as < 237, 237–346, and >346 mmol/d in men and < 207, 207–309, and > 309 mmol/d in women. Quartile 1–4 of plasma UA were defined as <258, 258–301, 302–349, and >349 mmol/L for men; and <188, 188–226, 227–272, and >272 mmol/L for women.
Discussion
To our knowledge, this study indicated for the first time that a high-salt intake enhanced the associations of blood UA with hypertension and in particular related CV risk. Therefore, if a high-salt diet is not reduced, lowering UA levels may be not enough to prevent hypertension and CV outcomes.
Our analysis showed all of a high UA level, a high-salt intake, and their synergistic action could positively contribute to hypertension and mTCR (Table 4). Up to date, two studies have explored the relationship between UA and sodium metabolism. The first study cross-sectionally indicated that UA levels were inversely and independently associated with proximal tubular sodium excretion, and a greater degree of sodium reabsorbed at nephron sites proximal to the distal tubule was associated with higher circulating levels of UA [19]. The other study, which was conducted in a prospective population, found that a higher sodium intake was associated with greater longitudinal increases in UA [12]. Studies suggested that hyperuricemia is associated with endothelial dysfunction, activation of the renin-angiotensin system (RAS), and pre-glomerular vascular diseases [20]; these likely involve pathogenic mechanisms of salt-sensitive hypertension [11,21]. Also, abnormal renal hemodynamic responses to high-salt intake and alterations of renal sodium handling have been suggested as potential causal mechanisms for the variation in BP responses to sodium [22]. Eventually, salt-sensitive hypertension results in a substantially increased risk of CV events, such as myocardial infarction and stroke of more than twice compared with non-salt-sensitive hypertension [23].
High dietary salt intake may play an important role in the activation of the intrarenal RAS thus facilitating an effect of UA on the intrarenal RAS. This is different from activated circulating RAS mainly during relatively low sodium balance. Increased angiotensin II (Ang II), especially following activation of angiotensinogen locally produced by the proximal tubule cells which was likely not affected by sex and age [22,24], has been suggested to result in the subsequent development of salt-sensitive hypertension in the rat [25]. An updated study by Perlstein et al demonstrated that UA level was associated with low basal renal plasma flow and blunted renal vasoconstriction that typically is seen with Ang II infusion during high-salt intake (i.e. ≥ 11.7 g dietary salt per day), lending support to the hypothesis that UA on the condition of a high-salt balance may directly activate the renal RAS thus increase BP [26], but another study conducted in healthy volunteers, who were given 10 g of sodium salt per day on the basis of established salt-sensitive human model, showed a non-significant correlation (r = 0.31) between hyperuricemia and salt-sensitivity [27]. Our population-based study likely provides a new line of evidence of high-salt intake that involves into biomedical mechanism of UA associated with hypertension and related CV risk. When 24-h salt intake was up to a higher level (e.g. ≥ 20.2 g for men or ≥ 18.1 g for women), the prevalence of hypertension and long-term likelihood of CV diseases increased with an increased UA level (Table 5); this trend was highly consistent in either men or women, though they showed a dramatically different overall association between UA and hypertension.
Our findings provide some valuable information for clinical practice.
First of all, salt restriction may be considered in preference to lowering UA levels in both male and female populations with a high-salt diet, even if the association of UA with hypertension and mTCR is presently not confirmed. This is especially important for controlling hypertension and CV diseases for China where most people have an average daily salt-intake of over 12 g [11]. In particular, people of ethnic minorities, such as Kazakhs, Tibetan, and Mongolian, with a high prevalence of hypertension have a fairly high-salt intake due to traditional lifestyle. For instance, 1991 National Hypertension Survey displayed that Kazakhs had the highest female prevalence and the forth highest male prevalence of hypertension. Previous studies showed that diet salt intake of Kazakhs varied from 12.4 g to 40 g per day due to different survey methods and population variation [28,29]. Traditionally, their main diet, with limited fruit and vegetable daily, is milk tea, dried meat, and cooked wheaten food, all of which are added much salt. This is similar to the diet in industrialized countries that possesses a characteristic of high-sodium and low-potassium, which is closely associated with hypertension [30]. Furthermore, studies showed diet salt intake daily of Altay Kazakhs seemly increased 3.5 g within 12 years from 24-h urine-based 12.4 g of the WHO Cardiovascular Diseases and Alimentary Comparison Study (also conducted in Altay) to 15.9 g of ours [30], which was much higher than salt intake level in industrialized countries such as 9–12 g per day in European countries [10]. As a result, salt-restriction could be the most pressing problem to control hypertension among Kazakhs.
For another, the treatment of asymptomatic hyperuricemia is presently under debate. Obviously, this study does not contribute any information to the treatment of asymptomatic hyperuricemia in men; even for women, salt-restriction should be considered prior to the treatment of asymptomatic hyperuricemia.
Multiple strengths lie in this study. First, this study was based on a high-quality design and performing, included a selection of the Kazakh people with a daily high-salt intake as our study population, a 92.4% compliance of a random sample, and a population setting covering pastoral, agricultural, and urban areas that meant good generalization. Second, we adjusted for familial aggregation, besides multiple confounding factors, to improve precision for estimating effects. Finally, the long-term risk of UA on CV outcomes related to hypertension was evaluated by using risk stratifications from ESH/ESC Guidelines, although the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), different from the JNC 6, abandoned this stratification, it is still believed to be helpful for clinical practice and etiological studies by the WHO and Chinese Hypertension League (CHL) [1,9].
This study has some potential limitations. In the context of cross-sectional design, temporality is difficult to establish in this study, so prospective studies are required to examine our results. 24 h urine collection was not used, but the second urine sample after waking here was thought as a satisfactory alternative to 24-h pooled urine in adults for extensive epidemiological surveys [15,31] and used in a large scale epidemiological field [32].
Overall, this study presented and probed into a hypothesis of enhancement from a high salt intake on associations of UA with hypertension and a progressively increasing long-term absolute likelihood of developing a major CV event. In any case, salt-restriction, in preference to the treatment of asymptomatic hyperuricemia, may be recommended among high-salt diet populations such as Kazakhs, though this requires a specific randomized controlled trial.
Acknowledgments
We thank the government of Altay City and Hong Dun Town and the Hong Dun Township Health Center who supported this project. Local village doctors and nurses, Kazakh college students from Xinjiang Medical University who participated in this survey, and all interviewees are acknowledged. P He, W Kang, L Jia, H Qin, H Yu, N Jumabieke, S Jiangtai provided administrative, technical, and material support.
Data Availability
The minimal dataset is ethically or legally restricted according to the authors' ethical approval. Data are available upon request. Requests for the data may be sent to Jingmei Jiang (jingmeijiang@ibms.pumc.edu.cn).
Funding Statement
The study design, data collection and analysis, decision to publish, or preparation of the manuscript were supported by the National Natural Science Foundation of China (grant number 81273181).
References
- 1.Liu LS. Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579–615. Chinese. [PubMed] [Google Scholar]
- 2.Sundström J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005;45:28–33. [DOI] [PubMed] [Google Scholar]
- 3.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension 2006;48:1037–1042. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Sun K, Yang Y, Zhang H, Hu FB, Hui R. Plasma uric acid and hypertension in a Chinese community: prospective study and meta analysis. Clin Chem 2009;55:2026–2034. 10.1373/clinchem.2009.124891 [DOI] [PubMed] [Google Scholar]
- 5.Loeffler LF, Navas-Acien A, Brady TM, Miller ER 3rd, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999–2006. Hypertension 2012; 59:811–817. 10.1161/HYPERTENSIONAHA.111.183244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol 2007;18:287–292. [DOI] [PubMed] [Google Scholar]
- 7.Lu Z, Dong B, Wu H, Chen T, Zhang Y, Wu J, et al. Serum uric acid level in primary hypertension among Chinese nonagenarians/centenarians. J Hum Hypertens 2009;23:113–121. 10.1038/jhh.2008.104 [DOI] [PubMed] [Google Scholar]
- 8.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- 9.Whitworth JA; World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002;40:355–360. [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Scheven L, de Jong PE, Bakker SJ, Curhan GC, Gansevoort RT. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation 2012;125:3108–3116. 10.1161/CIRCULATIONAHA.112.096115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Li N, Yao X, Zhou L, Hong J, Hu J, et al. Epidemiology study on hypertension of Kazakh in pasture area of Fukang Xinjiang. Clin J Hypertens 2010;18:960–964. Chinese [Google Scholar]
- 14.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–822. 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 15.Kawano Y, Tsuchihashi T, Matsuura H, Ando K, Fujita T, Ueshima H; Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (2) Assessment of salt intake in the management of hypertension. Hypertens Res 2007;30:887–893. [DOI] [PubMed] [Google Scholar]
- 16.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353: 2450–2461. [DOI] [PubMed] [Google Scholar]
- 17.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med 2008;65:481, 501–506. 10.1136/oem.2007.034777 [DOI] [PubMed] [Google Scholar]
- 18.Houchens R, Chu B, Steiner C. Hierarchical Modeling using HCUP Data HCUP Methods Series Report # 2007–01 Online. January 10, 2007. U.S. Agency for Healthcare Research and Quality.
- 19.Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M. Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA 1993;270:354–359. [PubMed] [Google Scholar]
- 20.Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med 2009;169:155–162. 10.1001/archinternmed.2008.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 2002;346:913–923. [DOI] [PubMed] [Google Scholar]
- 22.Chamarthi B, Williams JS, Williams GH. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens 2010;28:1020–1026. 10.1097/HJH.0b013e3283375974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997;350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 24.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 2010;28:1422–1428. 10.1097/HJH.0b013e3283392673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 2001;59:2222–2232. [DOI] [PubMed] [Google Scholar]
- 26.Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, et al. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int 2004;66:1465–1470. [DOI] [PubMed] [Google Scholar]
- 27.ter Maaten JC, Voordouw JJ, Bakker SJ, Donker AJ, Gans RO. Salt sensitivity correlates positively with insulin sensitivity in healthy volunteers. Eur J Clin Invest 1999;29:189–195. [DOI] [PubMed] [Google Scholar]
- 28.Batis C, Gordon-Larsen P, Cole SR, Du S, Zhang B, Popkin B. Sodium intake from various time frames and incident hypertension among Chinese adults. Epidemiology 2013;24:410–418. 10.1097/EDE.0b013e318289e047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X, Zhang M, Guo S, Ma R, Ding Y, Guo H, et al. Prevalence of hypertension in Uygur, Kazakhs, and Han people in rural areas of Xinjiang. Chin J Hypertens 2013;21:1164–1168. Chinese. [Google Scholar]
- 30.Liu L, Liu L, Ding Y, Huang Z, He B, Sun S, et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens Res 2001;24:315–322. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993;20:7–14. [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med.2014;371:612–23. 10.1056/NEJMoa1311889 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The minimal dataset is ethically or legally restricted according to the authors' ethical approval. Data are available upon request. Requests for the data may be sent to Jingmei Jiang (jingmeijiang@ibms.pumc.edu.cn).