Abstract
Genome-wide insight into insect pest response to the infection of Beauveria bassiana (fungal insect pathogen) is critical for genetic improvement of fungal insecticides but has been poorly explored. We constructed three pairs of transcriptomes of Plutella xylostella larvae at 24, 36 and 48 hours post treatment of infection (hptI) and of control (hptC) for insight into the host-pathogen interaction at genomic level. There were 2143, 3200 and 2967 host genes differentially expressed at 24, 36 and 48 hptI/hptC respectively. These infection-responsive genes (~15% of the host genome) were enriched in various immune processes, such as complement and coagulation cascades, protein digestion and absorption, and drug metabolism-cytochrome P450. Fungal penetration into cuticle and host defense reaction began at 24 hptI, followed by most intensive host immune response at 36 hptI and attenuated immunity at 48 hptI. Contrastingly, 44% of fungal genes were differentially expressed in the infection course and enriched in several biological processes, such as antioxidant activity, peroxidase activity and proteolysis. There were 1636 fungal genes co-expressed during 24–48 hptI, including 116 encoding putative secretion proteins. Our results provide novel insights into the insect-pathogen interaction and help to probe molecular mechanisms involved in the fungal infection to the global pest.
Introduction
The diamond-back moth Plutella xylostella (Lepidoptera: Yponomeutidae) coevolves with crucifer [1] and hence is one of most devastating insect pests threatening economically important crops, such as cabbage, cauliflower and rapeseed [2]. The pest damage results in an annual economic loss of 4–5 billion US dollars worldwide [3,4]. This insect pest is also notorious with its high resistance to almost all classes of chemical insecticides [5,6] and crystal toxins of Bacillus thuringiensis [7]. Thus, it is critical to develop alternative strategies for the pest control [4].
One of alternative strategies against the pest is to make use of filamentous fungal insect pathogens, such as Beauveria bassiana and Metarhizium anisopliae which have been widely used as fungal insecticides [8–10]. Such fungi start infection by conidial adhesion to the insect integument, followed by germination and hyphal penetration through the host cuticle [11,12] by means of the action of various cuticle-degrading enzymes [13–17]. Upon entry into the host hemocoel, penetrating multicellular hyphae turn into unicellular blastospores, which must overcome high osmolarity and defensive immunity-derived oxidation encountered in the host hemolymph for yeast-like budding propagation until the host dies from mycosis [18–20]. Subsequently, the blastospores turn back into hyphae to penetrate the integument again for outgrowth and produce conidia on the cadaver surface for the initiation of a new infection cycle. This infection mode endows the insect pathogens with an ability to kill not only piercing insects but also chewing pests, such as P. xylostella [21]. The fungal lethal action is a process of host-pathogen interaction involved in cuticular penetration, blastospore propagation and host death due to the depletion of hemolymph nutrition. This process is namely a latent period of several days varying with insect species and body size, making the fungal lethal action slower than the action of a chemical insecticide. There has been no recorded insect resistance to fungal insect pathogens, perhaps a consequence of the slower lethal action.
The major disadvantage of fungal insecticides has been alleviated by rapid progress in entomopathogenic fungal biotechnology. Fungal virulence has been enhanced in transgenic fungal strains expressing exogenous chitinase, hybrid chitinase or protease (Pr1A) in B. bassiana [22–24]. The integration of a scorpion neurotoxin into fungal candidate strains has resulted in a great increase of toxicity to several lepidoptera pests [25–27] and malaria parasites within mosquitoes [28]. In spite of the normal route of cuticular penetration, fungal strains can acquire per os virulence for expansion of target pest spectrum by the integration of an insect midgut-specific toxin, such as the vegetative insecticidal protein Vip3Aa1 from B. thuringiensis [29–31]. A fungal insecticide based on a Vip3Aa1-expressing strain can compete with a chemical insecticide to protect a cabbage crop from full-season damages caused by an insect pest complex comprising P. xylostella, caterpillars and aphids [32]. Overexpression of an endogenous Mn2+-cofactored superoxide dismutase (MnSOD) in a B. bassiana strain resulted in not only enhanced virulence but increased tolerance to oxidative stress and UV-B irradiation [33]. Since the transgenic strains improved by exogenous gene expression are subject to strict safety evaluation prior to registration, it is ideal to explore more endogenous genes, such as the MnSOD gene, for use in genetic improvement of fungal insecticides. However, this is impeded by a lack of deep insight into the host-pathogen interaction due to unavailability for genomic information of fungal insect pathogens and many important insect pests a few years ago.
The annotated genomes of B. bassiana [34] and P. xylostella [35] provide an excellent opportunity for a genome-wide insight into the host-pathogen interaction by means of next generation sequencing (NGS) technology [36,37]. This study seeks to study the transcriptomic response of P. xylostella to the B. bassiana infection by analyzing digital gene expression (DGE) libraries (transcriptomes) of total RNAs derived respectively from the P. xylostella larvae at 24, 36 and 48 hours post treatment of infection (hptI) and of control (hptC). We identified a large number of differentially expressed genes (DEGs) in the pest response to the infection of B. bassiana and many fungal DEGs involved in the course of host infection.
Materials and Methods
Insect stock
The P. xylostella strain Fuzhou-S, which was used in the P. xylostella genome sequencing project [35], was maintained on caged cabbage plants at 25 ± 1°C in a light/dark cycle of 14:10 h under a fluctuating relative humidity of 65–85%. The third-instar larvae from the plants were prepared for the following use.
Treatment of larvae with conidial suspension
The wild-type strain B. bassiana ARSEF 2860, which attacks many piercing and chewing insects and hence has been subjected to genome sequencing and annotation [34], was cultivated for full conidiation on Sabouraud dextrose agar (4% glucose, 1% peptone and 1.5% agar) plus 1% yeast extract at 25°C in a light/dark cycle of 12:12 h. Conidia harvested from the culture were suspended in 0.02% Tween 80 and standardized to 1 × 108 conidia/ml. Three cohorts (replicates) of ~35 larvae on cabbage leaf discs (~10 cm in diameter) were separately exposed to an equal-volume (1 ml) spray of conidial suspension (treatment) or 0.02% Tween 80 (control) from the top nozzle of an Automatic Potters Spray Tower (Burkard Scientific Ltd, Uxbridge, UK) at a uniform working pressure of 0.7 kg/cm2. The sprayed larvae were reared in situ in large Petri dishes at 25°C under a photoperiod of 14:10 h and monitored daily for mortality records, which were corrected based on the background mortality in the control. Fresh leaf discs were supplied for their feeding whenever necessary during the period of rearing.
Samples of infected and uninfected larvae for RNA extraction
The mortality curve of the larvae after spray was used to prepare insect samples for RNA extraction. Several cohorts of larvae were sprayed with the conidial suspension (treatment) or 0.02% Tween 80 (control) and reared as above. Samples of 50 surviving larvae were collected from the fungal treatment and the control at 24, 36 and 48 h respectively, forming three pairs of hptI and hptC samples.
Construction and analysis of DGE libraries
Each sample of 50 larvae was immediately ground in liquid nitrogen. Total RNA was extracted from each ground sample with an RNAisoTM Plus Reagent (TaKaRa, Dalian, China), purified with a Qiagen RNeasy Mini Kit (Qiagen, Germantown, MD, USA) plus on-column treatment with DNase I, and reversely transcribed into cDNA under the action of a PrimeScript RT® Reagent kit (TaKaRa). The resultant cDNA was separated into large and small parts. The large part was sequenced at Beijing Genomics Institute (Shenzhen, China) for constructing a DGE library by means of an Illumina HiSeq™ 2000 platform (San Diego, CA, USA). The small part was used as a template to verify the sequencing by assessing transcript levels of selected genes through quantitative real-time PCR (qRT-PCR) experiments.
All raw DGE reads gained by sequencing the cDNA samples were filtered to generate clean tags, which were mapped with SOAP2 program [38] to the respective genome databases of P. xylostella [35] and B. bassiana [34] at the level of no more than five-base mismatching. All the data were normalized as reads per kilobase transcriptome per million mapped reads (RPKM). The whole DGE library database was registered in Gene Expression Omnibus (GEO) and available under the accession code GSE72383.
All P. xylostella DEGs were identified by horizontal comparison of paired DGE libraries (i.e., 24, 36 and 48 hptI versus 24, 36 and 48 hptC respectively) or longitudinal comparison between each pair of the libraries at two sequential time points (i.e., 36 versus 24 hptI or 48 versus 36 hptI) based on the standards of P ≤ 0.001, FDR ≤ 0.001 and |log2 ratio| ≥ 1. All identified host DEGs were functionally annotated with known or putative gene information in the non-redundant NCBI protein databases and subjected to Gene Ontology (GO) analysis (http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis (http://www.genome.jp/kegg/).
Similarly, all B. bassiana DEGs were identified through the GO term and KEGG enrichment analyses of the fungal DGE libraries at 36/24 hptI and 48/36 hptI respectively.
Validation of DGE libraries
Seven DEGs were arbitrarily taken from each of the host DGE libraries at the three time points. Their transcript levels in the 10-fold dilution samples of the same cDNAs used for sequencing were quantified in a Mastercycler® Ep-Realplex (Eppendorf, Hamburg, Germany) cycler via qRT-PCR with SRBR® Premix Ex TaqTM (TaKaRa) and paired primers (Table A in S1 File). A gene (Px008022.1) encoding 60S ribosomal protein L32 was used as an internal reference. The mean log2 R of each gene in each cDNA from the infected larvae over that in the cDNA from the control was calculated and compared with the value in the corresponding pair of DGE libraries to judge a validity of the constructed libraries. Three samples of each cDNA were used as replicates in the qRT-PCR experiment.
Results and Discussion
Survival trend of P. xylostella larvae heavily infected by B. bassiana
The B. bassiana strain was assayed for its virulence to the third-instar larvae of P. xylostella by spraying the highly concentrated suspension (1×108 conidia/ml) to cohorts of ~35 larvae in the spray tower. This spray was expected to maximize both the fungal infection to the larvae and the fungal mass accumulation in the infected larvae, which were then used for total RNA extraction. As illustrated in Fig 1, the spray resulted in a corrected mortality of ~5%, 33% and 56% at 24, 48 and 72 hptI, respectively, and reached 90% at 132 hptI. The mortality trend of the sprayed larvae suggests a critical period of 24–48 hptI for the host-pathogen interaction.
Fig 1. Corrected mortality trend of third-instar P. xylostella larvae infected by B. bassiana.
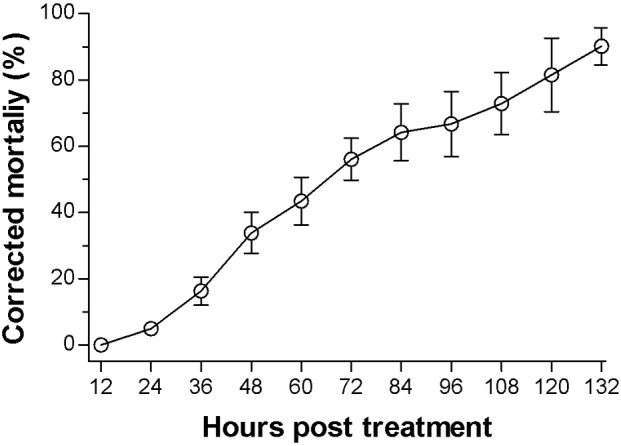
Error bars: SD of the mean from three replicates.
Features of DGE libraries
Total RNAs were extracted from three pairs of 50 P. xylostella larvae surviving at 24, 36 and 48 hptI and hptC respectively and sequenced by means of the NGS technique, resulting in a total number of 65 million raw tags (Table 1). Percent distribution for the copy number of tags in each sequenced sample (Figure A in S1 File) coincides well with the reported feature of mRNA distribution [39–41]. All clean tags from each sequenced sample were mapped to the genomes of P. xylostella [35] and B. bassiana [34]. As a result, paired DGE libraries derived from the infected and uninfected (control) larvae comprised 14166 and 14382 host genes at 24 hptI and hptC, 14773 and 13995 at 36 hptI and hptC, and 14837 and 13903 at 48 hptI and hptC, respectively. Up to 3148, 3613 and 4922 fungal genes were also expressed in the infected larvae at 24, 36 and 48 hptI respectively, constituting three other DGE libraries specific to the fungal infection.
Table 1. Distribution of reads and genes in the DGE libraries.
| Reads mapped to reference genome | 24 h | 36 h | 48 h | |||
|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | |
| Fungal treatment | ||||||
| Total reads | 65228988 | 100.0 | 64624232 | 100.0 | 66880206 | 100.0 |
| Mapped reads | 32824512 | 50.3 | 34905517 | 54.0 | 35485924 | 53.1 |
| Unique match | 29665023 | 45.5 | 31800308 | 49.2 | 31712757 | 47.4 |
| Detected host genes | 14166 | 78.4 | 14773 | 81.8 | 14837 | 82.1 |
| Control | ||||||
| Total reads | 65035304 | 100.0 | 65464942 | 100.0 | 66644942 | 100.0 |
| Mapped reads | 35048892 | 53.9 | 35183307 | 53.7 | 31841983 | 47.8 |
| Unique match | 31639270 | 48.7 | 31695748 | 48.4 | 28419774 | 42.6 |
| Detected host genes | 14382 | 79.6 | 13995 | 77.4 | 13903 | 76.9 |
* The percentage (%) for the mapped reads or the unique match is based on the count of total reads while the percentage for the detected host genes is based on the number of P. xylostella genes (18071).
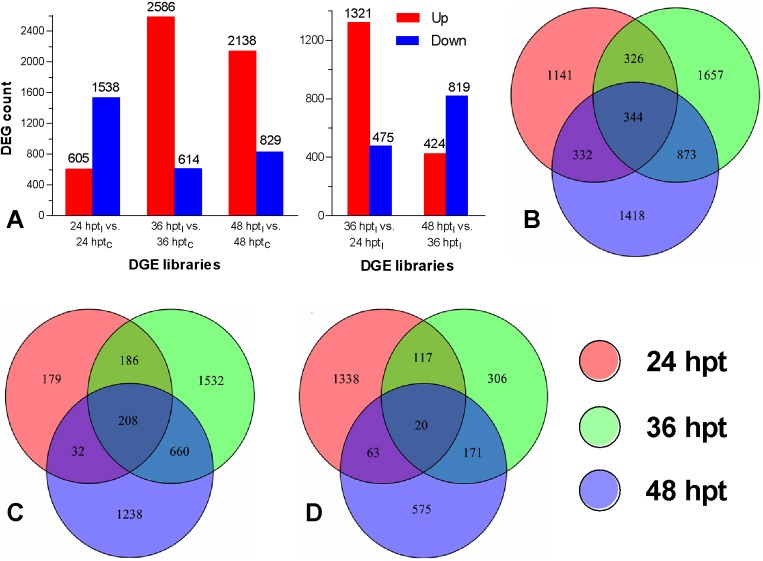
The counts of host genes consistently detected in the DGE libraries at 24, 36 and 48 h were up to 12953 and 13620 in the respective control (Fig 2A) and fungal treatment (Fig 2B). At the three time points, 996 host genes were expressed only in the infected larvae while other 328 genes were expressed only in the uninfected larvae. There were 1950 fungal genes co-expressed in the infected larvae at 24 and 36 hptI, 2735 at 36 and 48 hptI, and 1636 at the three time points (Fig 2C), indicating potential roles of these genes in the fungal response to the host immune defense during the critical period of infection.
Fig 2. Venn diagrams for gene expression in P. xylostella and B. bassiana.

(A, B) Counts of P. xylostella genes expressed respectively in the control (uninfected) and infected larvae at 24, 36 and 48 h post treatment (hpt). (C) Counts of B. bassiana genes expressed in the infected P. xylostella larvae at the three time points.
Host genes identified from the DGE libraries
To identify host genes responsive to the fungal infection, the DGE libraries from the infected larvae were horizontally compared with the control counterparts based on the log2 ratios of host genes (|log2 R| ≥ 1) in each pair of DGE libraries and also longitudinally compared at 36/24 hptI and 48/36 hptI. As illustrated in Fig 3A (left), 605, 2586 and 2138 host genes were upregulated at 24, 36, and 48 hptI/hptC, respectively, while 1538, 614 and 829 host genes were downregulated at the three time points. In the longitudinal comparison, 1321 host genes were specifically upregulated at 36/24 hptI, contrasting to 424 host genes upregulated at 48/36 hptI (Fig 3A, right). The horizontal comparisons revealed that 344 host DEGs were consistently present at the three time points (Fig 3B), including 208 co-upregulated (Fig 3C) and 20 co-downregulated genes (Fig 3D). The host genes co-up- and co-downregulated at 24 and 36 hptI reached 394 and 137, and the counts increased to 868 and 191 at 36 and 48 hptI, respectively. These data implicate that each stage of infection has its specific requirements and interferes with specific biological processes in the host. Previously, The first cuticle penetration of B. bassiana was observed to occur in intersegmental membrane of Solenopsis invicta at 18 h after inoculation, and the penetration expanded on to the insect cuticle around setae at 24 h after inoculation and on to thorax, abdomen and legs soon after that [42]. Unicellular blastospores (hyphal bodies) were present in the hemolymph of Spodoptera exigua at 36 h after B. bassiana infection [43]. Therefore, we speculate that at 24 hptI in this study, the fungal conidia adhered to the host integument could have germinated to initiate cuticular penetration, which could induce the host reaction with the early upregulated genes, followed by many more host genes activated in the defense response to the fungal penetration through the cuticle for entry into the hemocoel at 36 and 48 hptI.
Fig 3. Identification of P. xylostella DEGs at 24, 36 and 48 h post treatment of infection (hptI) and control (hptC).
(A) Counts of DEGs in the DGE libraries of 24 hptI/24 hptC, 36 hptI/36 hptC and 48 hptI/48 hptC (left) or of 36/24 hptI and 48/36 hptI (right). (B–D) Venn diagrams for the total counts of host genes differentially expressed and those upregulated and downregulated at 24, 36 and 48 hptI respectively. Note the counts of those co-upregulated (C) or co-downregulated (D) at the two time points of 36/24 and 48/36 hptI and at the three time points.
To gain insight into the quality of the constructed DGE libraries, we assessed transcript levels of three sets of seven host DEGs taken at each time point via qRT-PCR and compared these transcripts with the corresponding transcripts in the DGE libraries following previous studies [41,44,45]. As a result, the up- or downregulated trend of each set of gene transcripts assessed by qRT-PCR was well in agreement with the corresponding expression trend of the selected genes in the libraries. This comparison confirmed the validity of the DGE libraries constructed in the present study.
Host DEGs enriched in KEGG pathways
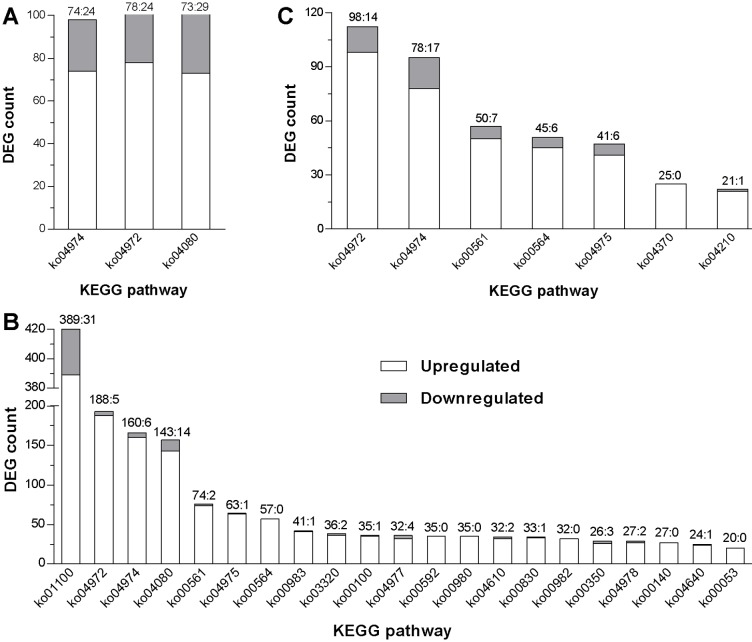
All the DEGs horizontally compared at the three time points were mapped to KEGG pathways at the significant levels of P < 0.01 and Q < 0.01 [41,46]. The host DEGs at 24 hptI were significantly enriched only in three KEGG pathways (Fig 4A; detailed in Table B in S1 File), including protein digestion and absorption, pancreatic secretion and neuroactive ligand-receptor interaction. Interestingly, 74% of those host genes in the pathways were upregulated by the fungal infection, which apparently triggered the early response of P. xylostella at 24 hptI.
Fig 4. KEGG pathways enriched with P. xylostella DEGs.
(A–C) Distribution of host DEGs in enriched KEGG pathways at 24, 36 and 48 h post treatment (hpt) of B. bassiana infection versus control respectively. All identified DEGS and enriched KEGG pathways are detailed in Tables B–D in S1 File.
The host DEGs at 36 hptI were enriched in the aforementioned and 18 other KEGG pathways, including immunity- and detoxification-related pathways (such as complement and coagulation cascades, drug metabolism-other enzymes, metabolism of xenobiotics by cytochrome P450) and metabolism-related pathways (such as glycerolipid metabolism and alpha-linolenic acid metabolism) (Fig 4B; detailed in Table C in S1 File). Previously, such pathways have been shown to play important roles in the response of Ostrinia furnacalis and Bombyx mori to microbial challenge [47,48]. Intriguingly, up to 91% of the enriched host genes were upregulated at 36 hptI, indicating their importance for P. xylostella to cope with the fungal infection at 36 hptI.
Only were seven KEGG pathways were enriched by the host DEGs at 48 hptI (Fig 4C; detailed in Table D in S1 File). Several immunity- or detoxification-related pathways activated at 36 hptI disappeared at 48 hptI, including drug metabolism-other enzymes, complement and coagulation, metabolism of xenobiotics by cytochrome P450, and drug metabolism-cytochrome P450. The number and proportion of upregulated genes also decreased drastically at 48 hptI versus 36 hptI. For instance, those upregulated genes in the pathways of protein digestion/absorption and pancreatic secretion decreased to 78 and 98 at 48 hptI from 160 and 188 at 48 hptI, respectively. The host apoptosis pathway was significantly activated only at 48 hptI.
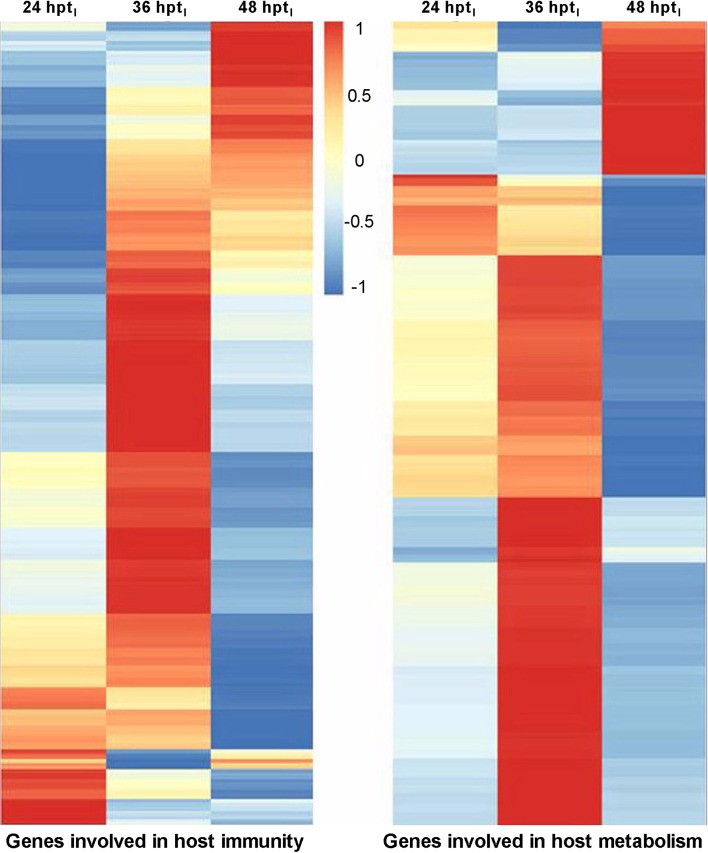
In the paired DGE libraries at 36/24 hptI, many DEGs were enriched in eight immunity- and metabolism-related KEGG pathways (P < 0.05), such as complement and coagulation cascade, Jak-STAT signaling, alpha-linolenic acid metabolism and glycerophospholipid metabolism (Table E in S1 File). Almost all of these DEGs were upregulated, and many more were present at 36 hptI than at 24 hptI. In contrast, an analysis of the paired libraries at 48/36 hptI resulted in 22 immunity- and metabolism-related pathways (Table F in S1 File). During this 12 h period, most of the detected host genes were downregulated in all the immunity-related pathways, such as protein digestion and absorption, drug metabolism-other enzymes, complement and coagulation cascades, and lysosome. This implicates that the host immune system could have been largely impaired at 48 hptI, coinciding well with the subsequent increase of host death (Fig 1). Other enriched pathways were all associated with metabolism, and most genes in the pathways were also downregulated. Up to 505 genes involved in the immune pathways and 211 in the metabolism pathways are illustrated as a heatmap (Fig 5) to show expressional changes at 24, 36 and 48 hptI respectively. Additionally, up to 996 host genes were co-expressed only in the infected larvae at 24, 36 and 48 hptI and enriched in the immunity-related complement and coagulation cascades (P < 0.05) whereas only 328 host genes were co-expressed in the uninfected larvae at the three time points but not enriched in any immunity-related pathway (Table G in S1 File).
Fig 5. Heatmap for the distribution of P. xylostella immunity- and metabolism-related genes expressed at 24, 36 and 48 h after the B. bassiana infection.
Scale bar: Gene expression level in the fungal treatment versus control.
Taken together, the immune defense response of P. xylostella to the infection of B. bassiana increased drastically during a short period of cuticular penetration from 24 to 36 hptI and was largely overcome by the fungal pathogen that could have entered the host hemocoel at 48 hptI. In other words, the short period from 24 to 36 hptI was critical for a success of the fungal infection to the insect pest.
Host genes crucial for immune defense response to fungal infection
In the pathway of protein digestion and absorption, many more host genes were upregulated than downregulated at all the concerned time points (Fig 4). The number of the genes upregulated at log2 R ≥ 2 in this pathway (see Tables B–D in S1 File) reached 20 at 24 hptI (log2 Rmax = 10.9), increased to 117 at 36 hptI (log2 Rmax = 10.4) and then decreased to only 22 at 48 hptI (log2 Rmax = 3.1). Among those upregulated, trypsin genes took a large proportion, followed by the genes encoding serine proteinases and carboxypeptidases. Apparently, these genes play critical roles in the host defense reaction to the fungal infection. Previously, serine proteinases in P. xylostella were considered to circumvent insecticidal plant protease inhibitors through differential expression in response to different plant hosts [35]. The pest defense system could make use of the serine proteinases against either the inhibitors or the fungal infection, which may trigger many more genes for the host defense response.
The complement and coagulation systems are proteolytic cascades composed of the serine proteases in the chymotrypsin family and play important roles in host-pathogen interactions. Complement and coagulation are activated at the respective sites of infection and bleeding in vertebrates [49]. We found 32 upregulated genes and two downregulated genes significantly enriched in the pathway of complement and coagulation cascades at 36 hptI (Fig 4B; detailed in Table C in S1 File). Intriguingly, most of the upregulated host genes encode serine proteinases, coagulation factors and hemocytin. In particular, three genes encoding transmembrane serine proteases were upregulated by >1000-fold at the time point (log2 R: 10.3–13.5). Previously, Drosophila complement and clotting systems were shown to be similar in structure and function, and serine proteases could be the ancestor of both [50,51]. The coagulation factors and hemocytin are known to play vital roles in vertebrate and insect immunity [49,52]. Several upregulated genes encoding coagulation factors and hemocytin at 36 hptI implicate that they may involve in the host immune defense against the fungal entry into the host hemocoel, warranting future studies.
Moreover, up to 42 detoxification-related genes have been found in the P. xylostella genome, including glutathione S-transferases (GST), cytochrome P450 monooxygenases (P450) and carboxylesterases (COE) [35]. Of those, two were upregulated and six were downregulated at 24 hptI. The upregulated genes increased to 30 at 36 hptI but dropped to 10 at 48 hptI (Table H in S1 File). These highlight a significance of the GST, P450 and COE genes for the pest defense response during the fungal infection. Several other genes encoding UDP glucosyltransferases (UGT), sulfatase-modifying factors (SUMF) and glucosinolate sulphatases (GSS) are also on the list of the detoxification-related genes [35], and some of them were also upregulated at 36 or 48 hptI, suggesting them to be involved in the pest defense response to the fungal infection
The GO annotation at P < 0.05 resulted in a classification of 45 DEGs to four GO terms (intracellular ligand-gated calcium channel activity, ryanodine-sensitive calcium-release channel activity, calcium ion transmembrane transporter activity and calcium channel activity) at 24 hptI and of 1346 DEGs to six GO terms (peptidase activity, hydrolase activity, catalytic activity, serine-type peptidase activity, serine hydrolase activity and peptidase activity) at 36 hptI (Table 2; detailed in Tables I and J in S1 File). Most of involved genes in the GO terms at 24 hptI were downregulated, hinting that the host calcium channels could have been impaired by the early fungal attack for cuticular penetration. All the GO terms at 36 hptI were linked to the activities of various enzymes, of which most genes were upregulated. These imply that the upregulated genes could play vital roles for the insect immunity defense against B. bassiana at 36 hptI, which was a critical time point for the fungus-insect interaction. However, no meaningful GO terms were enriched for the DEGs at 48 hptI perhaps due to the immune defense system collapsed by the fungal attack at that time.
Table 2. GO terms for P. xylostella DEGs significantly enriched to 'Molecular Function' categories.
| GO ID | GO Term | DEGs | No. genes | P value |
|---|---|---|---|---|
| 24 hptI/hptC | ||||
| GO:0005218 | intracellular ligand-gated calcium channel activity | 6 | 7 | 0.0186 |
| GO:0005219 | ryanodine-sensitive calcium-release channel activity | 6 | 7 | 0.0186 |
| GO:0015085 | calcium ion transmembrane transporter activity | 18 | 50 | 0.0298 |
| GO:0005262 | calcium channel activity | 15 | 38 | 0.0354 |
| 36 hptI/hptC | ||||
| GO:0008233 | peptidase activity | 101 | 305 | <0.0001 |
| GO:0016787 | hydrolase activity | 356 | 1541 | <0.0001 |
| GO:0003824 | catalytic activity | 755 | 3774 | <0.0001 |
| GO:0008236 | serine-type peptidase activity | 33 | 66 | <0.0001 |
| GO:0017171 | serine hydrolase activity | 33 | 66 | <0.0001 |
| GO:0070011 | peptidase activity, acting on L-amino acid peptides | 68 | 225 | 0.0010 |
Crucial genes involved in the B. bassiana infection to P. xylostella
Up- and downregulated B. bassiana genes (|log2 R| ≥ 1) reached 1934 and 1871 in the DGE libraries at 36/24 hptI, and 3497 and 1071 at 48/36 hptI, respectively. Up to 556 fungal genes were co-upregulated at the three time points, contrasting to only 149 co-suppressed genes. The fungal genes were enriched in 9, 11 and 10 KEGG pathways at 24, 36 and 48 hptI (P < 0.01; Table 3) respectively. Of those, five were co-enriched at the three time points, including energy metabolism and substance biosynthesis (oxidative phosphorylation and TCA cycle), while two or three were specifically enriched at each time point, such as pyruvate metabolism and glyoxylate and dicarboxylate metabolism only at 24 hptI, aminoacyl-tRNA biosynthesis, protein export and RNA polymerase only at 36 hptI, and ribosome biogenesis and 2-oxocarboxylic acid metabolism only at 48 hptI.
Table 3. KEGG enrichment analysis of B. bassiana genes.
| Pathway ID | Description | P value | ||
|---|---|---|---|---|
| 24 hptI | 36 hptI | 48 hptI | ||
| ko03010 | Ribosome | 1.29E-18 | 7.50E-20 | 1.98E-15 |
| ko00190 | Oxidative phosphorylation | 6.33E-08 | 1.71E-06 | 3.99E-07 |
| ko03050 | Proteasome | 3.33E-05 | 2.78E-04 | 1.45E-03 |
| ko01200 | Carbon metabolism | 8.23E-05 | 6.13E-05 | |
| ko00020 | Citrate cycle (TCA cycle) | 9.58E-04 | 3.76E-03 | 1.10E-04 |
| ko04141 | Protein processing in endoplasmic reticulum | 1.56E-03 | 2.81E-05 | 5.06E-03 |
| ko00620 | Pyruvate metabolism | 2.74E-03 | ||
| ko00010 | Glycolysis / Gluconeogenesis | 3.07E-03 | 8.02E-04 | |
| ko00630 | Glyoxylate and dicarboxylate metabolism | 5.74E-03 | ||
| ko00970 | Aminoacyl-tRNA biosynthesis | 5.12E-03 | ||
| ko03008 | Ribosome biogenesis in eukaryotes | 7.44E-05 | ||
| ko01230 | Biosynthesis of amino acids | 4.15E-03 | 1.13E-06 | |
| ko03013 | RNA transport | 5.35E-04 | 8.00E-04 | |
| ko03060 | Protein export | 6.59E-03 | ||
| ko03020 | RNA polymerase | 8.48E-03 | ||
| ko01210 | 2-Oxocarboxylic acid metabolism | 3.07E-03 | ||
The detected fungal genes were enriched in 91, 116 and 101 GO terms at 24, 36 and 48 hptI (P < 0.01; Tables K–M in S1 File), respectively. Two terms specifically enriched at 24 hptI were antioxidant activity and peroxidase activity, which are relevant to 10 antioxidant genes and seven peroxidase genes and indicate an importance of antioxidation for the fungal infection to the pest at the early stage of cuticular penetration. The host insect is assumed to generate superoxide anions harmful to the fungus via its immune defense response to the fungal infection. The expression of antioxidant and peroxidase genes could help the fungus to overcome the harmful effect [33,53–55]. Additionally, 24 fungal genes were enriched in proteolysis at 36 hptI, a time point approaching to the completion of cuticular penetration. Apparently, these genes could be likely involved in the degradation of host cuticle and defense proteins.
Secreted proteins are considered to be crucial effectors likely involved in fungal invasion and virulence [56–58]. In this study, our DGE libraries contained up to 246 fungal genes encoding putative secretion proteins that were co-expressed at two or three time points. Among those, 116 were co-expressed at the three time points (Table N in S1 File) and hence could be likely involved in the cuticular penetration, intrahemocoel proliferation and lethal action of B. bassiana.
Conclusive remarks
Thousands (44%) of B. bassiana genes were differentially expressed at 24–48 hptI for successful infection to P. xylostella. In contrast, the host genes were differentially expressed at a much lower proportion (~15% only) in response to the fungal infection despite a variation over the infection time. The unbalanced low proportion of the host genes expressed in the immune defense against the fungal attack obviously determines the fate of the host-pathogen interaction, i.e., collapse of the host defense systems by B. bassiana and subsequent increase of the host death. This fact highlights a genome-wide background for B. bassiana to attack not only P. xylostella but many more insects and mites [8] and hence for its great potential against global arthropod pests. Our transcriptomic analyses provide the first insight into a genome-wide interaction between B. bassiana and P. xylostella and many clues to further explore molecular mechanisms involved in the host-pathogen interactions.
Supporting Information
Figure A and Tables A–N.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funds from the National Natural Science Foundation of China (Grants 31270537, 31572054 and 31321063) at http://www.nsfc.gov.cn/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ratzka A, Vogel H, Kliebenstein DJ, Mitchell-Olds T, Kroymann J (2002) Disarming the mustard oil bomb. Proc Natl Acad Sci U S A 99: 11223–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talekar NS, Shelton AM (1993) Biology, ecology and management of the diamond back moth. Annu Rev Entomol 38: 275–301. [Google Scholar]
- 3.Zalucki MP, Shabbir A, Silva R, Adamson D, Liu SS, Furlong MJ (2012) Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol 105: 1115–1129. [DOI] [PubMed] [Google Scholar]
- 4.Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol 58: 517–541. 10.1146/annurev-ento-120811-153605 [DOI] [PubMed] [Google Scholar]
- 5.Baxter SW, Badenes-Perez FR, Morrison A, Vogel H, Crickmore N, Kain W, et al. (2011) Parallel evolution of Bacillus thuringiensis toxin resistance in lepidoptera. Genetics 189: 675–679. 10.1534/genetics.111.130971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabashnik BE, Huang FN, Ghimire MN, Leonard BR, Siegfried BD, Rangasamy M, et al. (2011) Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat Biotechnol 29: 1128–1131. 10.1038/nbt.1988 [DOI] [PubMed] [Google Scholar]
- 7.Heckel DG, Gahan LJ, Liu YB, Tabashnik BE (1999) Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc Natl Acad Sci U S A 96: 8373–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol 4: 3–34. [Google Scholar]
- 9.Roberts DW, St Leger RJ (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54: 1–70. [DOI] [PubMed] [Google Scholar]
- 10.Faria M, Wraight SP (2001) Biological control of Bemisia tabaci with fungi. Crop Prot 20: 767–778. [Google Scholar]
- 11.Kirkland BH, Westwood GS, Keyhani NO (2004) Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J Med Entomol 41: 705–711.. [DOI] [PubMed] [Google Scholar]
- 12.Holder DJ, Kirkland BH, Lewis MW, Keyhani NO (2007) Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiol-SGM 153: 3448–3457.. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SC, Leathers TD, El-Sayed GN, Ignoffo CM (1994) Relationships among enzyme activities and virulence parameters in Beauveria bassiana infections of Galleria mellonella and Trichoplusia ni. J Invertebr Pathol 64: 3–17.. [Google Scholar]
- 14.Donatti AC, Furlaneto-Maia L, Fungaro MH P, Furlaneto MC (2008) Production and regulation of cuticle-degrading proteases from Beauveria bassiana in the presence of Rhammatocerus schistocercoides cuticle. Curr Microbiol 56: 256–260.. [DOI] [PubMed] [Google Scholar]
- 15.Da Silva WOB, Santi L, Correa APF, Silva LAD, Bresciani FR, Schrank A, et al. (2010) The entomopathogen Metarhizium anisopliae can modulate the secretion of lipolytic activity in response to different substrate including components of arthropod cuticle. Fungal Biol 114: 911–916.. 10.1016/j.funbio.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Roh JY, Choi JY, Wang Y, Shim HJ, Je YH. (2010) Correlation of the aphicidal activity of Beauveria bassiana SFB-205 supernatant with enzymes. Fungal Biol 114: 120–128.. 10.1016/j.mycres.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 17.Montesinos-Matías R, Viniegra-González G, Alatorre-Rosas R, Loera O (2011) Relationship between virulence and enzymatic profiles in the cuticle of Tenebrio molitor by 2-deoxy-D-glucose-resistant mutants of Beauveria bassiana (Bals.) Vuill. World J Microbiol Biotechnol 27: 2095–2102.. [Google Scholar]
- 18.Pendland JC, Hung SY, Boucias DG (1993) Evasion of host defense by in vivo-produced protoplast-like cells of the insect mycopathogen Beauveria bassiana. J Bacteriol 175: 5962–5969.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis MW, Robalino IV, Keyhani NO (2009) Uptake of the fluorescent probe FM4-64 by hyphae and haemolymph-derived in vivo hyphal bodies of the entomopathogenic fungus Beauveria bassiana. Microbiol-SGM 155: 3110–3120.. [DOI] [PubMed] [Google Scholar]
- 20.Wanchoo A, Lewis MW, Keyhani NO (2009) Lectin mapping reveals stage-specific display of surface carbohydrates in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiol-SGM 155: 3121–3133.. [DOI] [PubMed] [Google Scholar]
- 21.Wang CS, Feng MG (2014) Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol Control 68: 129–135. [Google Scholar]
- 22.Fang WG, Leng B, Xiao YH, Jin K, Ma JC, Fan YH, et al. (2005) Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol 71: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang WG, Feng J, Fan YH, Zhang YJ, Bidochka MJ, St Leger RJ, et al. (2009) Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J Invertebr Pathol 102: 155–159. 10.1016/j.jip.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 24.Fan YH, Fang WG, Guo SJ, Pei XQ, Zhang YJ, Xiao YH, et al. (2007) Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl Environ Microbiol 73: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CS, St Leger RJ (2007) A scorpion neurotoxin increases the potency of a fungal insecticide. Nat Biotechnol 25: 1455–1456. [DOI] [PubMed] [Google Scholar]
- 26.Lu DD, Pava-Ripoll M, Li ZZ, Wang CS (2008) Insecticidal evaluation of Beauveria bassiana engineered to express a scorpion neurotoxin and a cuticle degrading protease. Appl Microbiol Biotechnol 81: 515–522. 10.1007/s00253-008-1695-8 [DOI] [PubMed] [Google Scholar]
- 27.Pava-Ripoll M, Posada FJ, Momen B, Wang CS, St Leger RJ (2008) Increased pathogenicity against coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae) by Metarhizium anisopliae expressing the scorpion toxin (AaIT) gene. J Invertebr Pathol 99: 220–226. 10.1016/j.jip.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Fang WG, Vega-Rodriguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St Leger RJ (2011) Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331: 1074–1077. 10.1126/science.1199115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin Y, Ying SH, Chen Y, Shen ZC, Feng MG (2010) Integration of insecticidal protein Vip3Aa1 into Beauveria bassiana enhances fungal virulence to Spodoptera litura larvae by cuticle and per os infection. Appl Environ Microbiol 76: 4611–4618. 10.1128/AEM.00302-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ZL, Ying SH, Feng MG (2013) Recognition of a core fragment of Beauveria bassiana hydrophobin gene promoter (Phyd1) and its special use in improving fungal biocontrol potential. Microb Biotechnol 6: 27–35. 10.1111/j.1751-7915.2012.00351.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Ying SH, Feng MG (2014) Assessment of oral virulence against Spodoptera litura, acquired by a previously non-pathogenic Metarhizium anisopliae isolate, following integration of a midgut-specific insecticidal toxin. Biol Control 79: 8–15. [Google Scholar]
- 32.Liu YJ, Liu J, Ying SH, Liu SS, Feng MG (2013) A fungal insecticide engineered for fast per os killing of caterpillars has high field efficacy and safety in full-season control of cabbage insect pests. Appl Environ Microbiol 79: 6452–6458. 10.1128/AEM.01594-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie XQ, Wang J, Ying SH, Feng MG (2010) A new manganese superoxide dismutase identified from Beauveria bassiana enhances virulence and stress tolerance when over expressed in the fungal pathogen. Appl Microbiol Biotechnol 86: 1543–1553. 10.1007/s00253-010-2437-2 [DOI] [PubMed] [Google Scholar]
- 34.Xiao GH, Ying SH, Zheng P, Wang ZL, Xie XQ, Shang YF, et al. (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep 2: 483 10.1038/srep00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You MS, Yue Z, He WY, Yang XH, Yang G, Xie M, et al. (2013) A heterozygous moth genome provides insights into herbivory and detoxification. Nat Genet 45: 220–225. 10.1038/ng.2524 [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10: 57–63. 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilhelm BT, Landry JR (2009) RNA-seq-quantitative measurement of expression through massively parallel RNA-sequencing. Methods 48: 249–257. 10.1016/j.ymeth.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 38.Li RQ, Yu C, Li YR, Lam TW, Yiu SM, Kristiansen K, et al. (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25: 1966–1967. 10.1093/bioinformatics/btp336 [DOI] [PubMed] [Google Scholar]
- 39.Hegedus Z, Zakrzewska A, Agoston VC, Ordas A, Racz P, Mink M, et al. (2009) Deep sequencing of the Zebrafish transcriptome response to Mycobacterium infection. Mol Immunol 46: 2918–2930. 10.1016/j.molimm.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 40.Luan JB, Li JM, Varela N, Wang YL, Li FF, Bao YY, et al. (2011) Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J Virol 85: 3330–3340. 10.1128/JVI.02507-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XW, Luan JB, Li JM, Bao YY, Zhang CX, Liu SS (2010) De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 11: 400 10.1186/1471-2164-11-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LJ, Lu LH, He YR, Xie MQ (2010) Observation on infection process of Beauveria bassiana on cuticle of the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae), using scanning electron microscope. Acta Entomol Sin 53: 118–124. [Google Scholar]
- 43.Pendland JC, Hung SY, Boucias DG (1993) Evasion of host defense by in vivo-produced protoplast-like cells of the insect mycopathogen Beauveria bassiana. J Bacteriol 175: 5962–5969.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.′t Hoen PAC, Ariyurek Y, Thygesen HH, Vreugdenhil E, Vossen RHAM, de Menezes R, et al. (2008) Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res 36: e141 10.1093/nar/gkn705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Guilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 44: 619–626. 10.2144/000112776 [DOI] [PubMed] [Google Scholar]
- 46.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36 (SI): D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou C, Qin GX, Liu T, Geng T, Gao K, Pan ZH, et al. (2014) Transcriptome analysis of silkworm, Bombyx mori, during early response to Beauveria bassiana challenges. PLoS ONE 9: e91189 10.1371/journal.pone.0091189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen D, Liu Y, Zhou F, Wang G, An C (2014) Identification of immunity-related genes in Ostrinia furnacalis against entomopathogenic fungi by RNA-Seq analysis. PLoS ONE 9: e86436 10.1371/journal.pone.0086436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD (2007) Complement and coagulation: strangers or partners in crime. Trends Immunol 28: 184–192. [DOI] [PubMed] [Google Scholar]
- 50.Dissing M, Giordano H, DeLotto R (2001) Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J 20: 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeMosy EK, Tan YQ, Hashimoto C (2001) Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci U S A 98: 5055–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arai I, Ohta M, Suzuki A, Tanaka S, Yoshizawa Y, Sato R (2013) Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori. J Insect Sci 13: ARTN 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie XQ, Li F, Ying S H, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS ONE 7: e30298 10.1371/journal.pone.0030298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li F, Shi HQ, Ying SH, Feng MG (2015) Distinct contributions of one Fe- and two Cu/Zn-cofactored superoxide dismutases to antioxidation, UV tolerance and virulence of Beauveria bassiana. Fungal Genet Biol 81: 160–171. 10.1016/j.fgb.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 55.Wang ZL, Zhang LB, Ying SH, Feng MG (2013) Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ Microbiol 15: 409–418.. 10.1111/j.1462-2920.2012.02848.x [DOI] [PubMed] [Google Scholar]
- 56.Stergiopoulos I, de Wit PJGM (2009) Fungal effector proteins. Annu Rev Phytopathol 47: 233–263. 10.1146/annurev.phyto.112408.132637 [DOI] [PubMed] [Google Scholar]
- 57.Collette JR, Lorenz MC (2011) Mechanisms of immune evasion in fungal pathogens. Curr Opin Microbiol 14: 668–675. 10.1016/j.mib.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 58.Xia J, Zhang CR, Zhang S, Li FF, Feng MG, Wang XW, et al. (2013) Analysis of whitefly transcriptional responses to Beauveria bassiana infection reveals new insights into insect-fungus interactions. PLoS ONE 8: e68185 10.1371/journal.pone.0068185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A and Tables A–N.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.