Abstract
Over the past 30 years, research has demonstrated that estrogens are not only important for female reproduction, but play a role in a diverse array of cognitive functions. Originally, estrogens were thought to have only one receptor, localized exclusively to the cytoplasm and nucleus of cells. However, it is now known that there are at least three estrogen receptors (ERs): ERα, ERβ and G-protein coupled ER1 (GPER1). In addition to being localized to nuclei, ERα and ERβ are localized to the cell membrane, and GPER1 is also observed at the cell membrane. The mechanism through which ERs are associated with the membrane remains unclear, but palmitoylation of receptors and associations between ERs and caveolin are implicated in membrane association. ERα and ERβ are mostly observed in the nucleus using light microscopy unless they are particularly abundant. However, electron microscopy has revealed that ERs are also found at the membrane in complimentary distributions in multiple brain regions, many of which are innervated by dopamine inputs and were previously thought to contain few ERs. In particular, membrane-associated ERs are observed in the prefrontal cortex, dorsal striatum, nucleus accumbens, and hippocampus, all of which are involved in learning and memory. These findings provide a mechanism for the rapid effects of estrogens in these regions. The effects of estrogens on dopamine-dependent cognition likely result from binding at both nuclear and membrane-associated ERs, so elucidating the localization of membrane-associated ERs helps provide a more complete understanding of the cognitive effects of these hormones.
Keywords: g protein coupled estrogen receptor 1, estrogen receptor alpha, estrogen receptor beta, dopamine, electron microscopy
The effects of estrogens on cognition, and the mechanisms through which these effects are achieved in the brain, are more varied and complex than was initially believed. Estrogens are a class of steroid hormones that include estrone, estrione, and estradiol (E2), the last of which is the most potent estrogen in female mammals during their reproductive years. There is substantial research examining the role of estrogens in cognition (see Luine, 2014 for review). Interestingly, although estrogens have also been implicated in dopamine-dependent cognition, the brain regions important for this (viz. the striatum (STR), nucleus accumbens (NAc), prefrontal cortex (PFC) and hippocampus) have relatively sparse nuclear labelling for estrogen receptors (ERs; Milner et al 2001, Mitra et al 2003, Shughrue et al 1998, Weiland et al 1997). Some effects of estrogens on dopamine transmission in these regions occur over a relatively long time course (> 10h) and are mediated by nuclear ERs (Luine et al 1998, Korol and Kolo 2002, Quinlan et al 2010) but other estrogen effects are too rapid to occur through binding at the nuclear ERs (Almey at al 2014, Becker and Rudick 1999, Thompson and Moss 1994). This review examines the evidence for membrane-associated estrogen receptors (mERs), the role of both nuclear ERs and mERs in dopamine-dependent cognition, and recent immunoelectron microscopy research localizing mERs to the STR, NAc, PFC, and hippocampus.
Following the discovery of estrogens in 1929 (Butenandt 1929), research on this class of steroid hormones focused on their role in reproduction and the menstrual/estrous cycle in females (Doisy 1972). In 1966, an ER was characterized in breast and uterine tissue (Toft and Gorski 1966), and this ER was also localized to brain regions typically associated with endocrine or reproductive functions, such as the hypothalamus (for review see McEwen and Alves 1999). This receptor, now known as ERα, was observed primarily in cell nuclei, typical for steroid hormone receptors. In the mid-1990s, a second ER, ERß, was discovered, which also was localized to cell nuclei (Kuiper et al 1996). Both ERα and ERß are expressed in the uterus, breast tissue, testicles, prostate, cardiovascular system, and to a lesser extent in the bone and the lungs (Rollerova and Urbancikova 2000). More relevant to this review, these ERs are expressed in the pituitary and many brain regions, including the hypothalamus, the hippocampus, the amygdala, and the PFC, among others (Kuiper et al 1998, Montague et al 2008, Shughrue et al 1998, Shughrue and Merchenthaler 2001, Spencer et al 2008). Estrogen activation of ERs either directly or indirectly contribute to certain diseases and disorders (Brann et al., 2007) and have numerous behavioural effects including: increasing agonistic behaviours, improving spatial learning and memory tasks, and initiating copulatory behaviours (Luine et al 1998, Clipperton-Allen et al 2011, Clipperton Allen et al 2010, Gervais et al 2013, Almey et al 2014, Brann et al 2007, Cornil and Charlier 2010).
The original conceptualization of ERα and ERβ was as typical steroid/nuclear receptor located in the cytoplasm of cells when not activated. When estrogens bind to these receptors, the newly formed receptor-ligand complexes dimerize and translocate to the nucleus where they bind to estrogen response elements (EREs) on DNA (Kumar and Chambon 1988) to regulate the transcription of proteins (Nilsson et al 2001). It is difficult to predict the effects of binding at an ERE, since EREs can have different transcriptional effects, and numerous co-activators and co-repressors alter the transcriptional effects of binding at EREs (Kuiper et al 1996, Rollerova and Urbancikova 2000). Nonetheless, there is evidence that estrogens alter the production of multiple proteins in the central nervous system, including growth factors (Varea et al 2010, Woolley 1999), cytokines (Kovacs et al 2002), and apoptotic factors in the brain (Kiess and Gallaher 1998, Vasconsuelo et al 2011). In addition to acting through these nuclear ERs to elicit genomic effects, ERs can be found at the cell membrane, where estrogen-binding induces rapid non-genomic effects such as altering membrane permeability (Fu and Simoncini 2008, Wong and Moss 1992) and activating second messenger cascades (Edwards and Boonyaratanakornkit 2003, Fu and Simoncini 2008).
The earliest evidence for membrane ERs (mERs) was from Pietras and Szego (1975), who demonstrated that application of E2 to endometrial cells causes rapid depolarization as a result of an increase in intracellular Ca2+ influx. The authors then used subcellular fractionation techniques to isolate the cell membrane from the cytoplasm, and demonstrated that ERα is present in membrane fractions from endothelial tissue suggesting that ERα is associated with cell membranes (Pietras and Szego, 1980). Shortly afterwards, estrogens were shown to exhibit rapid cellular effects in the central nervous system, as the application of E2 to parvocellular neurons in the medial preoptic area, arcuate nucleus, and the ventromedial hypothalamus resulted in fast hyperpolarization of these cells (Kelly et al 1980, Kelly et al 1976). These findings inspired further research on these rapid effects of estrogens, which then revealed that application of estrogens to neurons from the arcuate and ventromedial hypothalamus or the amygdala resulted in hyperpolarization of these cells in the presence of transcription blockers (Kelly et al 1980, Minami et al 1990, Nabekura et al 1986). Additionally, the membrane-impermeable E2-bovine serum albumin (BSA) conjugate binds to receptors in the hypothalamus, cerebellum, and olfactory bulb (Zheng and Ramirez 1997). Since estrogens’ genomic effects cannot occur rapidly in the presence of transcription blockers, or via E2-BSA, these early studies concluded that estrogens must bind at some then-unknown membrane-associated ER to elicit these rapid, non-genomic effects.
1. Membrane associated estrogen receptors
1.1 ERα and ERβ: steroid receptors in a novel location
There is now evidence that ERα and ERß are both found at the cell membrane, as well as in nuclei and in the cytoplasm, where they were originally localized. These two receptors are found as either homo- (ERα-ERα or ERβ-ERβ) or hetero- (ERα-ERβ) dimers at the membrane, and they are membrane-associated, but not actually embedded within the cell membrane (Boonyaratanakornkit and Edwards 2007). mERα and mERβ can induce a number of intracellular events typically induced by activating G-protein coupled receptors. mERs are thought to activate G-protein coupled receptors to regulate L-type Ca2+ channels and activate protein kinase A (PKA), protein kinase C (PKC), and mitogen activated protein kinase (MAPK) signalling cascades (Coleman and Smith 2001, Fu and Simoncini 2008, Yang et al 2008). The mechanisms through which ERα and ERβ become associated with the cell membrane remain unclear, but two are believed to be pivotal: the post translational lipid modification of these ERs, and their interaction with membrane/cytoplasmic scaffolding proteins (Boonyaratanakornkit 2011). Note that research on ER membrane association has predominantly focused on ERα.
The primary form of lipid modification associated with mERs is palmitoylation. Palmitoylation refers to the addition of the fatty acid, palmitic acid, to specific residues of proteins, typically membrane-associated proteins (Basu 2004). If palmitoylation is inhibited in hippocampal cell cultures, rapid estrogen-induced phosphorylation of cyclic AMP response element binding protein (CREB) is eliminated (Meitzen et al 2013). Furthermore, palmitoylation occurs at specific cysteine sites of ERα and ERβ receptors; when these palmitoylation sites are mutated, rapid estrogen-induced CREB phosphorylation and activation of MAPK and PI3 kinase are blocked (Meitzen et al 2013, Pedram et al 2002). Additionally, E2-induced decreases in synaptosomal membrane-associated ERα in the hippocampus occur through depalmitoylation (Tabatadze et al 2013). Together, these findings indicate that palmitoylation of specific cysteine residues of the ERs is critical for the rapid effects of membrane-associated ERs. A truncated version of ERα was discovered in endothelial cells; this truncated ERα is 46-kDa as opposed to the typical 67-kDa ERα. This 46-kDa version of ERα is preferentially palmitoylated, and is more effective at rapidly activating endothelial nitric oxide synthase than the traditional ERα (Li et al 2003), but does not mediate transcriptional responses (Figtree et al 2003). This suggests that there is a smaller isoform of ERα primarily associated with the cell membrane.
Interactions between ERs and certain scaffolding proteins also are believed to play a critical role in the association of ERα and ERβ with the cell membrane. The scaffolding proteins receiving the most attention for their role in facilitating membrane-association of ERs are caveolins (Boonyaratanakornkit 2011). Caveolins are the primary structural components of caveolae, which are 50-100nm invaginations of the cell membrane. The structure of caveolae is thought to promote protein-protein interactions and integrate receptors and signaling molecules to facilitate rapid and specific signal transduction (Okamoto et al 1998). ERα is localized in caveolar subfractions of endothelial plasma membranes (Chambliss et al 2000), and confocal microscopy has observed extensive colocalization of caveolins and ERα (Pedram et al 2002). Caveolins are hypothesized to facilitate transport of ERα from the cytoplasm to caveolae, as endothelial cells expressing caveolin have a significantly higher ratio of membrane to cytoplasmic ERα (Pedram et al 2002), and knocking down caveolin in the arcuate nucleus of the hypothalamus reduces the expression of mERα (Christensen and Micevych 2012). Additionally, estrogens affect the production of caveolins; application of physiological levels of E2 significantly increases levels of caveolin and increases caveolin-ERα associations (Razandi et al 2002). These results suggest that estrogens increase levels of caveolin, which in turn facilitates transport of ERα to the cell membrane (Razandi et al 2002). Interestingly, when the palmitoylation site on ERα is mutated, the physical association between ERα and caveolin is reduced (Pedram et al 2007); this suggests that palmitoylation of ERα facilitates interactions between this receptor and caveolins.
Palmitoylation of mERs and associations with caveolins allow these receptors to associate with cell membranes, but does not explain the effects of binding at these mERs on neuronal transmission. The rapid effects of estrogens have been shown to be sensitive to G protein manipulation, which suggests that mERα and mERβ may be able to alter G protein receptor activity (Kelly and Wagner 1999). Research suggests that binding at mERα and mERβ stimulates metabotropic glutamate receptors (mGluRs; Meitzen and Mermelstein 2011). mGluRs are G-protein-coupled receptors categorized into three families: mGluRI (mGluR1 and 5) that are Gq receptors, and mGluRII (mGluR2 and 3) and III (mGluR4, 6, and 7), which are Gi/o receptors (Niswender and Conn 2010). In cultured hippocampal and striatal cells, application of E2 increases CREB phosphorylation within 30 seconds, an effect that is mediated by ERα (Boulware et al 2005, Grove-Strawser et al 2010). In cultured hippocampal neurons this effect of E2 estradiol (E2) is replicated by applying an mGluR1 agonist, and blocked by applying an mGluR1 antagonist to the cells (Boulware et al 2005). In contrast, in striatal neurons the E2-induced increase in CREB phosphorylation is mimicked by mGluR5 agonists and blocked by mGluR5 antagonists (Grove-Strawser et al 2010). Additionally E2 can have bidirectional effects on CREB phosphorylation in these brain regions. In hippocampal cultures E2 also inhibits CREB phosphorylation via binding at ERα and ERβ, an effect blocked by mGluR2 antagonists and mimicked by mGluR2 agonists (Boulware et al 2005). In striatal cultures the E2-induced decrease in CREB function is mediated by mGluR3 receptors (Grove-Strawser et al 2010). More recently it was shown that binding at ERα in the CA1 of the hippocampus activates mGluR1, mobilizing components of the endocannabinoid system, leading to reduced GABA release (Tabatadze et al 2013). The authors interpreted these findings to suggest that some of the rapid effects of E2 in the hippocampus and STR are mediated by mER interactions with different members of the mGluRI and mGluRII receptor families (Meitzen and Mermelstein 2011).
mGluRs are frequently associated with caveolins, and inhibiting caveolin expression or activity inhibits E2 effects on CREB phosphorylation, indicating that the association between E2 and mGluRI and/or mGluRII is dependent on caveolins (Boulware et al 2007). Taken together, these studies suggest that the classical ERs are palmitoylated, which may promote the interaction between these ERs and caveolins (see Fig 1; Meitzen et al 2013). Caveolins facilitate transport of mERs to caveolae where these receptors form associations with mGluRs; binding at these mERs alters CREB activity via activation of mGluRs (Meitzen et al 2013). There are other mechanisms involved in ER-membrane associations; multiple proteins including the adapter protein Shc, the calmodulin binding protein Striatin, and the modulator of non-genomic activity of estrogen receptor, have also been implicated in the membrane association of estrogen receptors (Boonyaratanakornkit 2011, Boonyaratanakornkit and Edwards 2007). However, the palmitoylation of mER, and mER associations with caveolins and mGluRs, provide the most complete explanation for how mERs become associated with the cell membrane, and occur in dopamine-innervated regions (Boulware et al 2005, Grove-Strawser et al 2010, Meitzen and Mermelstein 2011, Huang and Woolley 2012).
Fig 1.
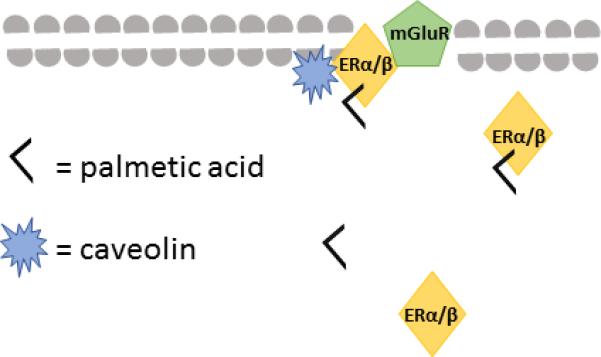
Schematic representation of how ERα or ERβ may be bound to the neuronal membrane via associations with caveolin following palmitoylation of the receptor.
1.2 GPER1: a membrane-bound G protein-coupled estrogen receptor
In addition to mERα and mERβ, a third membrane-associated ER was discovered. An orphan G protein-coupled receptor, G protein coupled receptor 30 (GPR30; a protein migrating at 30 kDa) is now designated as G protein-coupled ER 1 or GPER1. GPER1 first was identified in breast tissue (Carmeci et al 1997), and has a single binding site specific to estrogens (Prossnitz et al 2008). Initially, GPER1 was observed at the endoplasmic reticulum in neurons, so it was hypothesized that binding at GPER1 modulated the effects of estrogens at ERα or ERß (Revankar et al 2005, Sakamoto et al 2007). GPER1 is also found on the plasma membrane of cells in the hippocampus and hypothalamus, indicating that binding at GPER1 could have direct effects on neuronal transmission in these brain regions (Funakoshi et al 2006, Prossnitz et al 2008, Waters 2015). The effects of binding at GPER1 are not fully elucidated, but evidence demonstrates that application of E2 to COS (fibroblast-like cell line) or HeLa cells transfected with GPER1 rapidly increases Ca2+ influx (Bologa et al 2006, Funakoshi et al, 2006). Additionally, binding at GPER1 activates the phosphoinositide 3-kinase (PI3K) second messenger- signalling cascade (Prossnitz et al 2008), the MAPK signalling cascades (Filardo et al 2000) and the PKA signalling cascade (Fu and Simoncini 2008). Recently, we found that GPER1 in the hippocampus interacts with the PSD-95 and the spine scaffolding protein SAP97 that would position GPER1 for non-genomic signalling at the spine synapse (Akama et al 2013, Waters 2015). Additionally, application of E2 and the GPER1 agonist, G1, to cultured cortical neurons attenuated NMDA-induced excitotoxicity via activation of MAPK signalling pathways (Liu et al, 2012). These findings demonstrate that activation of GPER1, like mERα and mERß, increases intracellular Ca2+ and activates multiple second messenger cascades, suggesting that binding at this receptor could have widespread effects on neuronal function.
1.3 Are there more membrane-associated estrogen receptors to be discovered?
Other potential membrane-associated receptors have been identified. The first is referred to as ER-X. This receptor type is distinguished from ERα, ERß and GPER1 by its molecular weight, which is 63 kDa compared to 67kDa, 60kDa, and 44kDa respectively (Filardo et al 2007, Toran-Allerand et al 2002). The putative ER-X can also be distinguished from other ERs because the two stereoisomers of E2, 17α- and 17ß- E2 have equal affinity for this receptor, whereas 17ß-E2 has 100 times greater affinity for ERα and ERß than 17α-E2 (Toran-Allerand et al 2002). Another potential membrane-associated ER is a G-protein coupled receptor called Gq-mER that is found in the arcuate nucleus of the hypothalamus and is activated by has been the selective ER modulator, STX (Qiu et al 2003). STX has a 20-fold greater affinity for this receptor than it does for either ERα or ERβ, and STX has rapid effects in the hypothalamus of GPER1 knockout mice, suggesting that it is binding to an undiscovered ER (Qiu et al 2003). Gq-mER is thought to shown to play an important role in estrogens’ effects on metabolic function (Smith et al 2013), and affect multiple homeostatic processes including reproduction, stress, sleep, as well as motivated behaviours (Qiu et al 2008). These potential ERs (i.e. ER-X and Gq-mER) may provide alternate mechanisms via which estrogens can rapidly affect neuronal function, and ultimately cognition. However, there is not sufficient research to speculate on their role in cognition, so the remainder of this review will focus on ERα, ERβ, and GPER1, and their role in dopamine-dependent cognition in females.
2. Distribution of estrogen receptor containing cells in the CNS
Light microscopic immunocytochemical and in situ hybridization studies have shown that cells with ERα, ERβ, and GPER1 are found throughout the brain, from the most rostral regions of the forebrain to the cerebellum. It would not be practical to list all regions containing ERs, but regions where high levels of these receptors are consistently observed are described. Interestingly, although these studies observe nuclear labelling for ERα and ERβ throughout the brain, reports of extra-hypothalamic mERα and mERβ using light microscopic methods are limited.
Cells with ERα are most commonly localized to the bed nucleus of the stria terminalis, the medial amygdala, the preoptic area, and various hypothalamic nuclei. High levels of this receptor were also observed in the periaqueductal grey and parabrachial nucleus, and lower levels are observed in the locus coeruleus (Mitra et al 2003, Shughrue et al 1998). The reported distribution of ERβ differs slightly from ERα, but the regions with the most dense labelling are similar, as this receptor has been observed primarily in the lateral septum, the bed nucleus of the stria terminalis, the medial and basolateral amygdala, the preoptic region and other hypothalamic nuclei, and the trigeminal nuclei (Creutz and Kritzer 2002, Milner et al 2010, Mitra et al 2003, Shughrue et al 1999, Shughrue and Merchenthaler 2001). Intermediate levels of ERβ labelled cells are found in the hippocampus and cerebral cortex (Milner et al 2005, Milner et al 2010, Mitterling et al 2010, Shughrue and Merchenthaler 2001). Some studies have observed ERβ labelled cells in the ventral tegmental area, the locus coeruleus and in granulosa cells of the cerebellum (Mitra et al 2003, Shughrue and Merchenthaler 2001).
GPER1 also is observed throughout the brain, with high levels in the olfactory bulbs, and hypothalamus and various cortical regions including the motor, somatosensory piriform cortices, the hippocampus, and the habenular nucleus of the epithalamus (Brailoiu et al 2007, Hazell et al 2009, Xu et al 2009, Waters et al 2015). More caudally, GPER1 is observed in the nucleus of the solitary tract, and the Purkinje and granule cells of the cerebellum (Hazell et al 2009, Spary et al 2013). Light microscopy observes GPER1 is seen at cytoplasmic sites, and associated with the plasma membrane (Funakoshi et al 2006).
3. Estrogens affect dopamine-dependent diseases and cognitive processes
Estrogens affect a wide array of cognitive processes by altering transmission in various neurotransmitter systems. There is growing evidence that estrogens affect dopamine-dependent cognitive processes. Implications of estrogens’ involvement in dopamine dependent diseases comes from clinical observations of sex differences in susceptibility to Parkinson's, schizophrenia, and addiction.
Parkinson's disease is caused by decreased dopamine transmission in the STR, and Parkinson's patients show hippocampal atrophy and decreased markers of neurogenesis in the dentate gyrus (for review see Regensburger et al 2014). A higher incidence of Parkinson's in males (Shulman and Bhat, 2006), however, Parkinson's symptoms in females increase following menopause when endogenous estrogen production decreases (Ragonese et al 2004). Moreover, women respond better to L-3,4-dihydroxyphenylalanine (L-DOPA), the first line treatment for Parkinson's disease, when it is administered with transdermal E2 (Blanchet et al 1999).
Schizophrenia is also hypothesized to result from dysregulated dopamine transmission, with increased dopamine activity in the NAc and STR, and decreased dopamine transmission in the PFC (Howes and Kapur 2009). Individuals with schizophrenia show numerous hippocampal abnormalities including hippocampal atrophy, and symptom-related changes in hippocampal metabolic activity, among others (for review see Harrison 2004). Women exhibit later onset and less severe symptomatology than men (Hafner, 2003). However, these symptoms increase when estrogen levels decrease, both during the postpartum period and following menopause (Kulkarni et al 2012, Matevosyan 2011). Moreover, women respond better to antipsychotic drugs when they are administered in conjunction with E2 (Akhondzadeh et al 2003, Kulkarni et al 2014). Lastly, addiction is also related to dysfunctional dopamine transmission; repeated drug use is associated with a decrease in dopamine release in the STR and NAc (Volkow et al 2009), and significant decreases in adult hippocampal neurogenesis leading to changes in the striatal-cortical-frontal circuitry (Chambers 2013). There are sex differences in the development of addiction, as women escalate use of drugs, including opiates, psychostimulants, and nicotine more rapidly than men (Hernandez-Avila et al 2004, Lynch et al 2002). Women also report a greater response to amphetamines during the luteal phase of the menstrual cycle, when estrogens are high (Justice and de Wit 1999). Collectively, these findings suggest that estrogens play a role in central dopamine function, as these disorders are all associated with dysfunctional dopamine transmission and hippocampal atrophy/decreased hippocampal function.
3.1 Estrogens alter dopamine-dependent cognitive processes in rats
There is evidence that estrogens affect many dopamine-dependent cognitive processes, including selective attention object recognition memory and memory system bias. The majority of research has examined the genomic effects of estrogens, administering E2 ~12 hours prior to testing, but research examining the rapid effects of E2 on cognition will be described when available. Latent inhibition, a measure of selective attention, is dependent on dopamine transmission within the mesocorticolimbic pathway. Lesions and local infusion of a dopamine antagonist in the PFC enhance latent inhibition (Broersen et al 1996, George et al 2010) whereas lesions of the NAc or hippocampus abolish latent inhibition (Gal et al 1997, Kaye and Pearce,1987, Oswald et al, 2002). Moreover, dopaminergic activity in the anterior STR is positively correlated with behaviour in a latent inhibition task (Jeanblanc et al 2003). Interestingly, increases in plasma levels of estrogens, either during the proestrus phase of the estrous cycle or following E2 replacement in ovariectomized rats, disrupt the expression of latent inhibition (Nofrey et al 2008, Quinlan et al 2010, Almey et al 2013).
Object recognition memory is another dopamine-dependent cognitive process affected by estrogens. Systemic and intra-PFC administration of a D1 antagonist can impair object recognition memory, reflected in poor performance on a novel object preference task (Besheer et al 1999, Nagai et al 2007). Correspondingly, systemic administration of a D1 agonist enhances long-term object recognition memory when administered immediately after training (de Lima et al 2011) or 10 minutes before testing (Hotte et al 2005). Administration of E2 to ovariectomized rats leads to improved object recognition memory (Gervais et al 2013, Jacome et al 2010), an effect that is mimicked by diarylproprionitrile (DPN), an ERβ agonist, suggesting the effects of E2 are at least partially due to binding at ERβ (Inagaki et al 2010, Luine et al 1998). Interestingly, this dose of DPN resulted in a 100% increase in dopamine in the PFC, suggesting that estrogen-induced improvements in recognition memory are due, in part, to increased dopamine (Inagaki et al 2010, Luine et al 1998).
Estrogens also affect the use of place or response memory to navigate an environment. Rats use either spatial or egocentric cues to locate a reward. If spatial cues are used to locate a reward, this is referred to as a place memory, which is mediated by the hippocampus; if egocentric cues are used, this is called a response memory, which is mediated by the dorsal STR (Packard et al 1989, Packard and White 1991, White and McDonald 2002, see also review by Korol and Pisani in this issue). An infusion of amphetamine, an indirect-dopamine agonist, into the hippocampus biases rats toward using place memory, but an amphetamine infusion into the STR biases rats toward using response memory (Packard and White 1991). Interestingly, when estrogen levels are high, either during the proestrus phase of the estrous cycle or following E2 replacement in ovariectomized females, rats are biased toward using place memory (Korol and Kolo 2002, Quinlan et al 2008). More recently we showed that an infusion of E2 directly into the PFC biases female rats towards use of place memory, indicating that E2 acts rapidly (<15 minutes) to affect memory system bias, possibly via reciprocal projections with the STR and hippocampus (Almey et al 2014). These preclinical studies provide evidence that estrogens affect dopamine-dependent cognitive processes. Some of these cognitive effects of E2 occur rapidly (<4 hours; Almey et al 2014, Gervais et al 2013, Inagaki et al 2010, Jacome et al 2010), suggesting that the cognitive effects of estrogens result from binding at both nuclear and mERs, leading to genomic and non-genomic effects.
3.2 Estrogens modulate central dopamine transmission
There is accumulating evidence that estrogens alter dopamine function at various stages in transmission by affecting dopamine availability, dopamine receptor density, and the affinity of the dopamine transporter. Again, the majority of research has examined the genomic effects of estrogens, administering E2 ~12 hours prior to testing, but research examining the rapid effects of E2 on dopamine transmission will be described when available. In the STR, tonic dopamine availability is increased when estrogen levels are high (Xiao and Becker 1994); this increase in dopamine is mediated via both long-term and rapid effect of estrogens, as maximal dopamine increases are observed when E2 is administered ~12 hours prior to testing, and then again 30 minutes prior to testing (Becker and Rudick 1999). When E2 is applied to tissue from the STR this rapidly decreases dopamine uptake by decreasing the affinity of the dopamine transporter (Disshon et al 1998), providing a potential mechanism for E2-induced increases in dopamine availability. Additionally, ovariectomized rats receiving estrogen replacement have higher binding at dopamine D1 and D2 receptors, but lower binding at D3 receptors, in the STR (Landry et al 2002, Le Saux et al 2006, Levesque and Di Paolo 1989, Levesque et al 1989). This increase in dopamine D2 receptor binding occurs without any changes in dopamine D2 mRNA levels (Le Saux et al 2006), indicating that this change occurs through non-genomic mechanisms.
The relationship between estrogens and dopamine transmission in the NAc is similar to that observed in the STR (Thompson and Moss 1994), as E2 replacement administered to ovariectomized rats is associated with increased tonic (Madularu et al 2014) and phasic (Thompson and Moss 1994) levels of dopamine in the NAc. Systemic administration of E2 increases NAc phasic dopamine release within 15 minutes, indicating this is a rapid effect mediated by mERs (Thompson and Moss 1994). E2 replacement administered to ovariectomized rats was shown to attenuate dopamine reuptake in the NAc (Thompson 1999), providing a potential explanation for the E2-induced dopamine availability in the NAc. Additionally E2 treatment increases dopamine D2 receptor binding in the NAc (Le Saux et al 2006), paralleling findings in the STR.
Estrogens also alter dopamine transmission in the PFC. Females exhibit the highest baseline dopamine levels during estrus, when estrogens are declining, and the lowest basal dopamine levels during proestrus, when estrogen levels are high (Dazzi et al 2007). However, rats in proestrus have higher ethanol-induced dopamine release in the PFC, suggesting that estrogens decrease basal dopamine, but increase dopamine release in the PFC (Dazzi et al 2007). Additionally, dopamine D1 receptor density as well as dendritic spine density is increased in the PFC when ovariectomized females are treated with E2 (Levesque et al 1989; Wallace et al 2006).
The hippocampus receives a small dopaminergic afferent, and more substantial GABAergic afferents from the VTA (Rocchetti et al 2015). Additionally, glutamatergic afferents from the hippocampus to the NAc regulate the firing of dopaminergic neurons in the VTA (Floresco et al 2001). Moreover, hippocampal projections to the PFC are thought to play a role in amplifying neuronal activity in the PFC (Ishikawa and Nakamura 2003). These findings suggest that hippocampal afferents can modulate dopamine activity throughout the mesocorticolimbic pathway.
As discussed extensively in previous reviews (McEwen and Alves, 1999; McEwen and Milner, 2007; Spencer et al., 2008; McEwen et al., 2012), the hippocampus is also sensitive to the effects of estrogen. Female rats, either in the proestrus phase of the estrous cycle or following E2 replacement after ovariectomy had significantly elevated spine synapse density in CA1 pyramidal neurons (Gould et al 1990; Woolley et al 1990). The effect of E2 on synaptic spine density in the hippocampus has been shown to occur rapidly, within 30 minutes of subcutaneous E2 administration (MacLusky et al 2005). In vivo studies demonstrate that female rats administered an infusion of E2 in the hippocampus demonstrate better recollection for the platform location in a watermaze task (Packard and Teather 1997), and demonstrate a bias towards use of place memory to navigate a maze (Zurkovsky et al 2007).
Although estrogens have been shown to affect dopamine-dependent cognitive processes in the STR, PFC and hippocampus, these brain are not recognized for having high levels of ERs. Generally, light microscopy and in situ hybridization observe low levels of ERα immunoreactivity (IR) in the STR and NAc, and almost none in the PFC (Mitra et al 2003, Shughrue et al 1998; see also figures 2 & 4), although other light microscopy studies report moderate levels of ERα in the PFC (Montague et al, 2008). Light microscopy studies observe ERβ–IR at low levels in the PFC, and very low levels in the STR and NAc (Mitra et al 2003, Milner et al 2010; see also figures 2 & 4). Similarly, in situ hybridization studies observe low levels of ERβ in the STR and NAc, and extremely low levels of the receptor in the PFC (Shughrue et al 1999). Moderate levels of ERα-IR profiles were observed in interneurons of the hippocampus using light microscopy, with the highest density in the stratum radiatum and dentate hilus (Solum and Hanada 2001, Weiland et al 1997), and ERβ-IR is observed throughout the hippocampus at moderate levels (Zhang et al 2002). The immunolabeling for ERα and ERβ observed in these light microscopy experiments was primarily nuclear labelling (Mitra et al 2003, Zhang et al 2002); these nuclear receptors are likely responsible for the genomic actions of estrogens in these regions.
Fig 2.
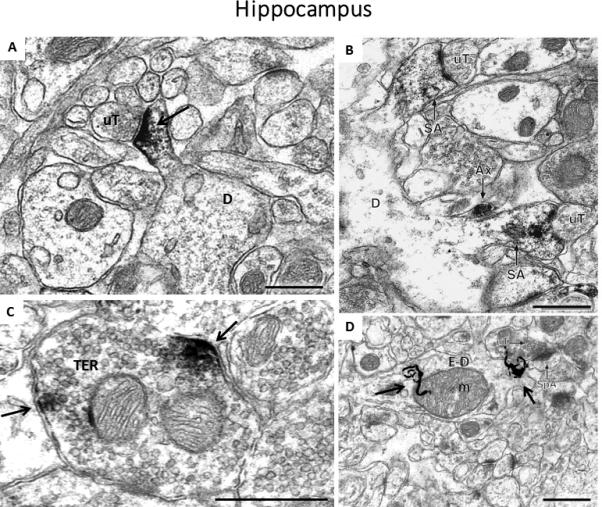
Images of labeling for estrogen receptors and estradiol in the hippocampus of female rats. A) Immunoperoxidase labeling for ERβ in a dendritic spine (D) is contacted by an unlabeled terminal (uT) in CA1 stratum radiatum, B) Immunoperoxidase labeling for ERα is found in two dendritic spines identifiable by the presence of spine apparati (SA), which arise from the same dendrite (D). Both labeled spines are contacted by unlabeled terminals (uT), and an ERα-labeled axon (Ax) is found nearby, C) Clusters of immunoperoxidase labeling for GPER1 are found in small synaptic vesicles near the plasma membrane of a terminal, D) Autoradiographic silver grains (black squiggly lines) denoting 125I-estradiol binding in stratum radiatum of the CA1 region of the hippocampus in a dendrite (E-D) overlying a mitochondrion (m). Black arrows = immunoperoxidase labeling/radioactive marker, Scale Bar = 500nm. The image in A) is taken from: Milner, T.A., Ayoola, K., Drake, C.T., Herrick, S.P., Tabori, N.E., et al. 2005. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 491, 81-95, B) is taken from: Milner, T.A., McEwen, B.S., Hayashi, S., Li, C.J., Reagan, L.P., Alves, S.E. 2001. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 429, 355-71., C) is taken from: Waters, E.M., Thompson,, L.I., Paterl, P., Gonzalez, A.D., Ye, H., Filardo, E.J., Clegg, D.J., Goreka, J., Akama, K.T., McEwen, B.S., Milner, T.A. 2015. G-Protein-Coupled Estrogen Receptor 1 Is Anatomically Positioned to Modulate Synaptic Plasticity in the Mouse Hippocampus. J. Neurosci. 35., D) is taken from: Milner, T.A., Lubbers, L.S., Alves, S.E., McEwen, B.S. 2008. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology 149, 3306-12.
Fig 4.

A) Representation of estrogen receptor localization within pre- and post-synaptic profiles in the dorsal striatum. Although estrogens are known to increase dopamine release in this area, almost no estrogen receptors were observed on dopamine terminals. Thus, estrogens are likely to affect dopamine release via changes in presynaptic transmission of GABA or cholinergic neurons. B) ERα-immunoreactivity (IR) in a dendritic spine (SP) that is contacted by an unlabeled axon terminal (uTER), and an axon terminal (TER) that forms an asymmetric synapses with an unlabeled dendritic spine (uSP); C) ERα-IR in a dendritic shaft (DEN) where it is affiliated with the plasma membrane and a mitochondrion (mit), and in a glial process (GL); D) ERβ-IR in an axon terminal where it is associated with the plasma membrane and with a mitochondrion (mit) (TER), and an unmyelinated axon (AX); E) GPER1-IR is found in Golgi bodies (Golgi) in a soma (SOM). Black arrow, immunoperoxidase labeling for estrogen receptors; Scale Bar, 500nm. Images taken from Almey, A., Filardo, E.J., Milner, T.A., Brake, W.G. 2012. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153, 5373-83.
However, there are rapid behavioural effects of estrogen in the STR, NAc, PFC and hippocampus that could not occur through binding nuclear ERs (Almey et al 2014, Cornil and Charlier 2010, MacLusky et al 2005, Thompson 1999, Thompson and Moss 1994). Immunolabeling for GPER1 is observed at relatively high levels in the hippocampus, moderate levels in the PFC, and lower levels in the STR and NAc (Hazell et al 2009), presumably at the cell membrane or associated with cytoplasmic organelles (Almey et al 2012, Otto et al 2008). Therefore, binding at GPER1 could be responsible for some of the rapid effects of E2 in these brain regions, but these rapid effects of E2 also suggest mERα or mERβ may be present as well. Some light microscopy studies observe non-neuronal mER-IR profiles (Milner et al 2005, Wagner, Silverman, and Morell 1998, Zhang et al 2002), but the majority do not (Almey et al 2014, Almey et al 2013, Cruetz and Kritzer 2002, Mitra et al 2003, Shughrue et al 1998, Weiland et al 1997), which suggests that light microscopy does not have sufficient resolution to detect low levels of mERs. Immunoelectron microscopy methods have higher resolution than light microscopy (See Box 1), and can discriminate discreet labeling for mERs in the brain (Milner 2011). Consequently, we used this technique to clarify the subcellular localization of mERs to specific neuronal profiles in the hippocampus and the terminal regions of the mesocorticolimbic and nigrostriatal pathways (see sections 4.2 and 4.3). The effects of estrogen on cognition likely result from a combination of genomic and non-genomic actions; there is an impetus to clarify the distribution of mERs, to better understand this non-genomic component of estrogen's effects.
Box 1. What is Electron Microscopy?
Electron microscopy is similar to other types of microscopy, in that it magnifies images of small samples, allowing for visualization of fine details. Electron microscopes focus a beam of accelerated electrons on a sample – electrons pass through certain components of a sample but scatter when they hit other components of the sample. The electron beam that exits the sample carries information that is magnified with optical lenses and projected on a fluorescent screen. Because the wavelength of electrons is 100,000 times shorter than that of visible photons of light, electron microscopes have ~2000 times greater resolution than light microscopes, and consequently can reveal the structure of much smaller objects.
Electron microscopy allows for visualization of neurons, so immunoreactivity for proteins can be localized to specific types of profiles (axons vs dendrites), and even specific regions of neuronal profiles (i.e. associated with the cell membrane). This makes electron microscopy ideal for investigating the distribution of mERs, since this technique allows for visualization of these receptors at the plasma membrane, associated with synaptic vesicles and cellular organelles, and in close proximity to synapses, confirming that ERα and ERβ are localized to extra-nuclear sites.
4. Ultrastructural localization of ERs in the brain
To date, electron microscopic studies have examined the distribution of mERs in regions that directly receive dopaminergic projections, including the STR, NAc, and PFC, or in the hippocampus, which has reciprocal connections with these dopaminergic nuclei (Floresco et al. 2001, Rocchetti et al 2015). These studies will be briefly summarized.
4.1 Early ER localization studies in hypothalamus
The earliest incidence of ultrastructural localization of ERs examined the distribution of ER (later called ERα) in hypothalamic tissue (Blaustein et al 1992, Langub and Watson 1992). Landub and Watson (1992) focussed on nuclear labeling for ERα in the medial preoptic area and the median eminence. While examining the ultrastructural localization of nuclear ERα in the hypothalamus, a second research group noticed that ERα-IR profiles were also localized to extranuclear sites in both dendrites and terminals (Blaustein et al 1992). These findings implied that estrogens could have rapid, non-genomic effects on transmission in the hypothalamus, in agreement with the observation that increases in estrogens during proestrus reduced gonadotropin releasing hormone and lutenizing hormone in 30 minutes, an effect so rapid that it could not be elicited via genomic ERs (Condon et al 1988, Sarkar and Fink 1980). This initial electron microscopic research in the hypothalamus was not pursued, despite that it provided a mechanism for the rapid effects of estrogens in this region. However it did suggest that electron microscopy is a powerful tool for observing mERs in the brain.
4.2 Ultrastructural analysis of ERs in the hippocampus
Ultrastructural analysis of the distribution of ERs recommenced in the hippocampus in the late 90s, based on evidence from McEwen and colleagues demonstrating that estrogens induce rapid structural changes in the hippocampus as discussed above. mERα was localized to the cytoplasmic surface of the membrane in interneurons throughout the hippocampus, with the highest density in the stratum radiatum of the CA1 (see Fig 2B; Milner et al 2001). In the hippocampus, 50% of all mERα-IR profiles in the hippocampus are presynaptic profiles, i.e. axons and terminals. Presynaptic terminals with mERα-IR form both asymmetric and symmetric synapses, suggesting that estrogens affect both excitatory and inhibitory transmission in the hippocampus (Milner et al 2001). Additionally 25% of the total mERα-IR profiles are post-synaptic, i.e. dendrites and dendritic spines (Milner et al 2001), providing a potential mechanism for the E2-induced changes in synaptic spine density observed in the hippocampus (Gould et al 1990). The remaining 25% of mERα-IR profiles in the hippocampus are glial, including astrocytes and microglia (Milner et al 2001), suggesting a mechanism for E2 involvement in glial-mediated neuroprotection (Arevalo et al 2010). After this initial study in rats, subsequent studies have shown that ERα-IR profiles in the mouse hippocampus are similarly distributed (Mitterling et al 2010). Some of the mERα-IR profiles observed in the hippocampus are localized to cholinergic axons and terminals, identified via the vesicular acetylcholine transporter, which suggests that mERα is positioned to have rapid effects on cholinergic transmission in this region (Towart et al 2003). Additional electron microscopic autoradiography studies have shown that 125I-estradiol binds to both pre- and post-synaptic profiles in the hippocampus supporting a functional role for nonnuclear ERs (Milner et al 2008).
Ultrastructural studies have demonstrated that mERα is associated with small synaptic vesicles in a subset of GABAergic axons and terminals in the CA1 of the hippocampus; findings from this experiment suggest that estrogens may bind at these receptors to mobilize vesicles towards synapses (Hart et al 2007). Further research demonstrated that some of the mERα-IR vesicles contain GABA (Tabatadze et al 2013), and that E2 acts via ERα to reduce GABA release through interactions with the cannabinoid system (Huang and Woolley 2012). Together these findings demonstrate that mERα is observed in the hippocampus, where it is positioned to affect presynaptic transmission in GABAergic and cholinergic neurons, and affect E2-induced increases in spine density.
The success of these studies on mERα distribution in the hippocampus led to studies examining the distribution of mERβ and GPER1 in the rodent hippocampus. mERβ-IR was also observed at extranuclear sites in the hippocampus, in the CA1, CA3, and dentate gyrus (see Fig 2A; Milner et al 2005, Mitterling et al 2010). Profiles with mERβ-IR were primarily post-synaptic: ~25% of the total mERβ-IR profiles were dendritic shafts, and ~15% of the mERβ-IR profiles were dendritic spines. There was a greater percentage of mERβ localized to dendritic spines in the CA1 region of the hippocampus (Milner et al 2005), where E2-induced changes in spine synaptic density are observed (Gould et al 1990). Ten percent of all mERβ-IR profiles were axon terminals, and 20% of the mERβ-IR profiles were axons, suggesting that binding at mERβ would also affect presynaptic transmission in the hippocampus. Similar to mERα, mERβ-IR was localized to endomembranes, including the membranes of endoplasmic reticulum and mitochondria, and has been observed in glial cells in the hippocampus (Milner et al 2005). See earlier reviews (McEwen and Alves, 1999; McEwen and Milner, 2007; Spencer et al., 2008; McEwen et al., 2012) for an in-depth discussion of estrogen effects in the hippocampus.
Following the discovery and characterization of the newest ER, GPER1, light microscopic studies observed GPER1-IR in several subregions of the rodent hippocampus (Brailoiu et al 2007, Funakoshi et al 2006). Moreover, electron microscopic studies observed GPER-IR at the plasma membrane of pyramidal cells in the CA2 (Funakoshi et al 2006). Recently, collaborative studies with the Milner lab have furthered these findings, demonstrating that GPER1-IR is localized to pre- and post-synaptic sites in both the rat and mouse hippocampus (see Fig 2C; Akama et al 2013, Waters 2015). Notably, GPER1 in this region is exclusively extranuclear and found in pyramidal cells and interneurons throughout the hippocampus (Waters 2015). Within perikarya, GPER1-IR is affiliated with the plasma membrane and endoplasmic reticulum. Like mERα and mERβ, GPER1-IR is localized to dendritic spines, suggesting that estrogens could alter dendritic spine morphology by binding at GPER1. Moreover, GPER1 is found in axons and clusters of vesicles in axon terminals, especially in CA3, where it could regulate synaptic transmission (Waters et al 2015).
Together, electron microscopic studies have demonstrated that profiles containing mERα, mERβ and GPER1 are abundant in the hippocampus. Moreover, although the distribution of these three receptors is overlapping, it is largely complementary. Light and electron microscopic studies also have revealed that ERs are in hippocampal neurons that undergo adult neurogenesis which are known to be important in cognitive processes (for review see Leuner et al 2006). Systemic administration of estrogens significantly increases cell proliferation in the dentate gyrus in a dose-dependent manner (Gould et al 2000, Tanapat et al 2005). Using in situ hybridization, Isgor and Watson (Isgor and Watson 2005) demonstrated that ERα and ERβ mRNA are expressed in new cells in the hippocampus. Electron microscopic studies have revealed that newly generated cells in the subgranular region of the dentate gyrus express mERβ-IR at the plasmalemmal membrane and the membrane of cellular organelles (Herrick et al 2006). These findings implicate estrogens in the genesis, and/or maturation of cells in the hippocampus.
Additionally, estrogens have been shown to increase production of pre- and post-synaptic proteins in the dorsal region of the CA1 (Brake et al 2001), an effect that is mediated by both ERα and ERβ (Spencer-Segal et al 2012, Waters et al 2009). This provides further evidence that estrogens are involved in synaptogenesis in the hippocampus, suggesting that estrogens play a role in the formation of new synaptic connections. It has also been shown that systemic E2 administration results an increase in phosphorylated Akt 6 hours following administration, and an increase in phosphorylated TrkB 48 hours following administration—an effect that occurs via binding at both ERα and ERβ in the hippocampus (Spencer-Segal et al 2012, Spencer et al 2008). Moreover, electron microscopic studies have shown that estrogens regulate the levels and trafficking of pAkt and pTrkB in hippocampal neurons (Spencer-Segal et al 2011, Yildirim et al 2011, Znamensky et al 2003). Both of these signalling pathways are implicated in synaptic plasticity, so E2-induced activation of these pathways provides another mechanism through which estrogens may cause synaptic strengthening or remodelling. Current theories on the neural mechanisms responsible for memory formation postulate that synaptic plasticity, synaptogenesis, and neurogenesis work in concert in the hippocampus, allowing for memory formation and retention. Therefore, the estrogen-induced changes in these brain plasticity mechanisms could impact hippocampal-dependent memory. Interestingly, post-embedding electron microscopic studies have revealed that aging negatively affects trafficking of mERα (Adams et al 2002) and mERβ (Waters et al 2011) in synapses within the hippocampus; together these findings provide a potential mechanism for the cognitive impairments observed in post-menopausal women.
The success of ultrastructural analysis in the hippocampus led to experiments examining the distribution of mERs in other brain regions where estrogens are known to have effects, despite a paucity of nuclear receptor staining. Prior reviews have discussed the localization of ERs in autonomic circuits (McEwen et al 2012), so it is not discussed here. Additionally, ultrastructural analysis revealed that there was extranuclear mERα observed in serotonergic neurons of the raphe nuclei (Milner 2003), providing a mechanism for rapid effects of estrogens on serotonergic transmission in this region.
4.3 Ultrastructural analysis of mERs within dopamine terminal regions
Since ultrastructural analysis allowed for visualization of mERs in the hippocampus and autonomic brain regions, the ultrastructural distribution of mERs in the terminal regions of the mesocorticolimbic and nigrostriatal pathways was investigated. The initial experiments examined the distribution of mERs in the STR of female rats. These experiments observed little to no ERα- or ERβ-IR using light microscopy (see Fig 3), but abundant mERα-IR and moderate levels or mERβ-IR, at exclusively extranuclear sites, using electron microscopy (Almey et al 2012). Additionally, GPER1-IR was observed in the perikarya via light microscopy, and correspondingly GPER1-IR was observed at extranuclear sites using electron microscopy (Almey et al 2012). All three ERs were seen at all types of neuronal profiles (See Fig 4 B-E), but they were most commonly observed in presynaptic profiles, with axons and terminals being the majority (55-65%) of total profiles labeled with mERα, mERβ, and GPER1 (Almey et al 2012). Additionally, ~25% of all mERα and GPER1-IR profiles were glia, similar to findings in the hippocampus (Waters et al 2015, Milner et al 2001), providing a potential mechanism for E2 effects on glial-mediated neuroprotection (Arevalo et al 2010, Liu et al 2011). A similar number of mERα-IR and GPER1-IR profiles were observed in the STR (~95 profiles in a 2416μm area); profiles with mERα- and GPER1-IR were observed ~5 times more frequently than profiles with mERβ-IR (~20 profiles in a 2416μm area) suggesting the majority of the effects of estrogens in the STR are via mERα or GPER1. Despite previous findings that estrogens affect dopaminergic transmission in the STR, mERα and GPER-1 are not localized to dopaminergic presynaptic profiles in this brain area (Almey et al 2012). However, a proportion of cholinergic interneurons contained mERα- and GPER1-IR in the STR; 13% of all GPER1-IR dendrites contained vesicular acetylcholine transporter (VAChT) and 3% of GPER1-IR terminals contained VAChT, while 5% of the total mERα-IR dendrites contained VAChT and 5% of mERα containing terminals also contained VAChT (Almey et al 2012). Since less than 10% of the ER labeled profiles are in cholinergic neurons, the remaining ER-labeled profiles in the STR must belong to another neuron type. Recent unpublished observations find ~15% of mERα-IR dendrites, and ~20% of GPER1-IR dendrites also contain immunoreactivity for GABA, the most common type of interneuron in the STR (Almey, Milner, and Brake, unpublished observations). Acetylcholine and GABA both have strong modulatory effects on dopamine transmission in the STR, so estrogens could indirectly affect dopamine transmission by altering cholinergic and/or GABAergic transmission (see Fig 4A).
Fig 3.
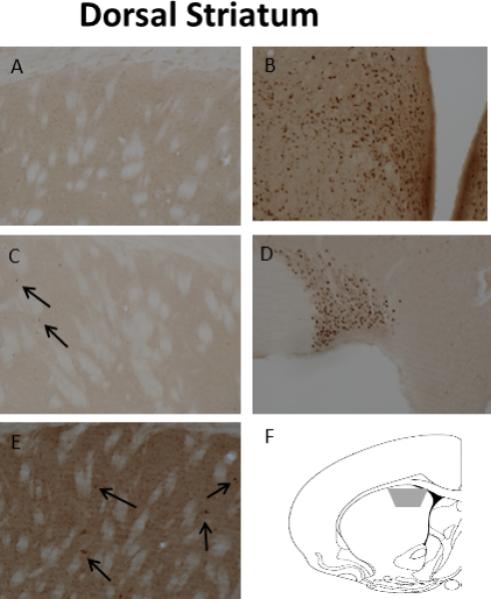
Light microscopic localization of ERs in the dSTR. A) Neither nuclear nor extranuclear ERα-IR is detected. B) Dense nuclear ERα-IR in the ventromedial and arcuate nuclei of the hypothalamus. C) No extranuclear ERβ-IR is detected; however, rarely a nucleus with ERβ-IR (arrow) is detected. D) Dense nuclear ERβ-IR in the supraoptic nucleus. E) Dense extranuclear GPER-1-IR is detected in the neuropil; moreover, several cells with GPER-1-IR (arrows) are seen. F) A coronal schematic of the striatum [atlas level 14; AP +1.00mm from bregma (Paxinos and Watson, 1998)] indicated the region (grey trapezoid) analysed by EM. Images taken from Almey, A., Filardo, E.J., Milner, T.A., Brake, W.G. 2012. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153, 5373-83.
The ultrastructural distribution of mERs was also examined in the medial PFC (mPFC) following behavioural experiments demonstrating rapid behavioural effects of infusions of E2 to this brain area (Almey et al 2014). Although light microscopy observed relatively low levels of ERα and ERβ (see Fig 5), at the ultrastructural level, mERα, mERβ, and GPER1 are localized to extranuclear sites in neuronal and glial profiles in the mPFC of female rats (see Fig 6 B-E; Almey et al 2014). Although mERs are observed at all types of neuronal profiles, the majority of profiles are at axons and terminals; 60-70% of the total profiles with immunoreactivity for mERα, mERβ, and GPER1 are presynaptic, suggesting that estrogens alter transmission in the mPFC via presynaptic mechanisms (Almey et al 2014). Immunoreactivity for all three ERs was also observed in postsynaptic profiles, but at much lower levels. The presynaptic profiles that are immunoreactive for ERα, ERβ, and GPER1 could be dopaminergic axons and terminals. This would provide a mechanism via which estrogens could alter dopamine availability in the mPFC to affect dopamine-dependent cognitive processes; dual labelling studies are required to determine whether these ERs are in fact localized to dopaminergic neurons in the mPFC. ~25% of all profiles with mERs in the mPFC are in glial cells, paralleling findings in the STR and hippocampus (Almey et al 2012, Milner et al 2005, Milner et al 2001). Profiles labeled with GPER1 are the most abundant ER in the mPFC of female rats: GPER1 immunoreactivity is twice as abundant as mERα-labeled profiles, and 4 times more abundant than mERβ. Future research using agonist/antagonists for mERs is required to determine how binding at each receptor type contributes to the effects of E2 in the PFC (see fig 6A).
Fig 5.
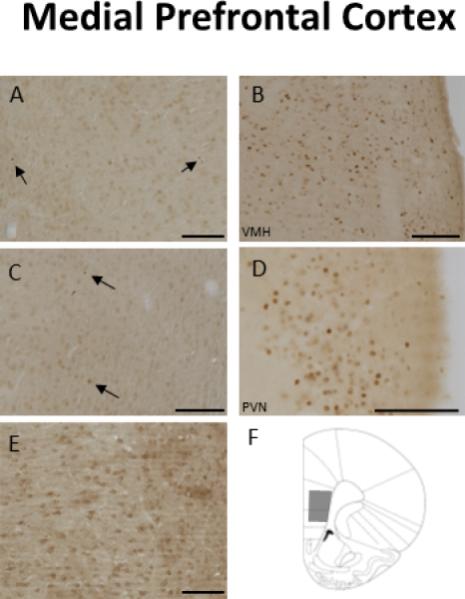
Light microscopic examination of ER localization in the mPFC. A) No extranuclear immunoreactivity (IR) for ERα, but there were very low levels of nuclear ERα-IR (arrows), B) Dense ERα-IR in the ventromedial hypothalamus, C) No extranuclar ERβ-IR was observed in the mPFC, but a couple of labeled nuclei were observed (arrows), D) Dense nuclear ERβ-IR in the hypothalamus, E) Dense extranuclear GPER1-IR is detected in the neuropil, F) A coronal schematic depicting the area of the mPFC (grey trapezoid) analyzed by electron microscopy. Scale Bar, 100μm. Taken from Almey, A., Cannell, E., Bertram, K., Filardo, E., Milner, T.A., Brake, W.G.. 2014. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155, 4422-32.
Fig 6.
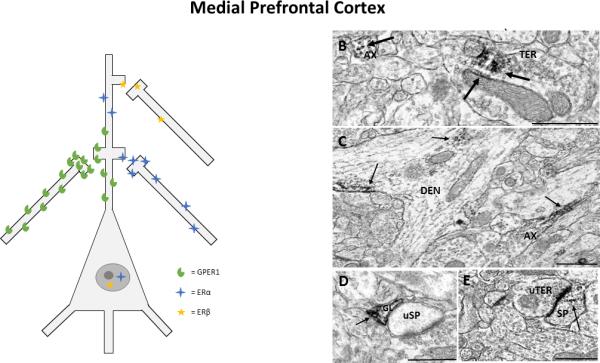
A) Representation of estrogen receptor localization to pre- and post-synaptic profiles in the prefrontal cortex. Estrogen receptors were most commonly localized to axons and terminals in the prefronal cortex, depicted alongside the pyramidal neuron, and were also observed in dendrites and dendritic spines at lower levels, depicted on the apical dendrite of the pyramidal neuron. Low levels of nuclear labelling for ERα and ERβ were observed via light microscopy. B) ERα in an axon (AX) and in a terminal (TER), where immunoreactivity (IR) is observed at small synaptic vesicles and on the membrane of a mitochondrion (mit), C) GPER1-IR in an axon (AX) and in a dendrite (DEN),where it is associated with microtubules and the cell membrane, D) ERβ-IR in a glial cell (GL) that is in apposition to an unlabeled dendritic spine (uSP), E) ERβ-IR in a dendritic spine (SP) that forms an asymmetrical synapse with an unlabeled terminal (uTER). Black arrow, immunoperoxidase labeling for estrogen receptors; Scale Bar, 500nm. Taken from Almey, A., Cannell, E., Bertram, K., Filardo, E., Milner, T.A., Brake, W.G.. 2014. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155, 4422-32.
In addition to these findings, other unpublished electron microscopic studies (Almey, Milner and Brake, unpublished findings) observe mERα and mERβ and GPER1 in the NAc shell and core. A low proportion of these mERs are localized to dopaminergic neurons, and a moderate proportion of mERs are in GABAergic neurons (see Fig 7). The localization of mERs to the STR, mPFC, and NAc shell and core provide a mechanism for estrogens’ rapid effects on dopamine transmission.
Fig 7.

A) Representation of estrogen receptor localization in pre- and post-synaptic regions in the nucleus accumbens. There are low levels of estrogen receptors associated with dopaminergic terminals, so estrogens could affect dopamine transmission directly by binding at these receptors. Additionally, a moderate proportion of ERα and GPER1 are observed in GABAergic terminals and dendrites; estrogens could indirectly affect dopamine release through pre- or post-synaptic changes in GABAergic transmission. B) ERα-immunoreactivity (IR) associated with small synaptic vesicles and a mitochondrion (mit) in an axon terminal (TER) and in an axon (AX), C) GPER1-IR in a large dendrite, where it is associated with microtubules, D) ERβ-IR in two adjacent axons. Black arrow, immunoperoxidase labeling for estrogen receptors; Scale Bar, 500nm.
These experiments demonstrate that electron microscopy is well suited to visualizing mERs in neuronal tissue, which is helpful in light of the fact that the mERs in the hippocampus, STR, NAc, and PFC are not frequently observed using light microscopy. These ultrastructural analyses complement previous light microscopy and in situ hybridization experiments; together they map the distribution of nuclear ERs and mERs, providing a more complete picture of how estrogens affect neurotransmission through genomic and non-genomic mechanisms.
5. Conclusion
In the past 30 years, research has demonstrated that estrogens’ effects extend far beyond reproduction; these hormones affect transmission in various dopaminergic pathways, and are implicated in disease function and multiple cognitive processes. It is clear that estrogen receptors are localized to cell membranes, in addition to being localized to nuclei, which has changed the way that estrogens’ actions in the brain are conceptualized. These membrane-associated receptors are positioned to have rapid neuronal effects, and to mediate some of estrogens’ effects on dopamine-dependent diseases and cognitive processes. In particular, electron microscopic studies have expanded initial observations of mERs in the hippocampus and autonomic nuclei to the STR, PFC and NAc. These studies have yielded critical information for understanding the rapid effects of estrogens on cognitive function, including those mediated by dopamine.
Acknowledgments
GRANT SUPPORT: Supported by a discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (WGB) and NIH grants DA08259, HL096571, HL098351, AG016765 (TAM). The CSBN is a “groupe de recherche” funded by the Fonds de Recherche du Québec – Santé.
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J. Neurosci. 2002;22:3608–14. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J. Bio. Chem. 2013;288:6438–50. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Nejatisafa AA, Amini H, Mohammadi MR, Larijani B, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1007–12. doi: 10.1016/S0278-5846(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology. 2014;155:4422–32. doi: 10.1210/en.2014-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–83. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Hafez N, Hantson A, Brake WG. Deficits in latent inhibition induced by estradiol replacement are ameliorated by haloperidol treatment. Front. Behav. Neurosci. 2013;7:1–8. doi: 10.3389/fnbeh.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–12. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Basu J. Protein palmitoylation and dynamic modulation of protein function. Current Science. 2004;87:212–217. [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol. Biochem. Behav. 1999;64:53–7. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Besheer J, Jensen HC, Bevins RA. Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav. Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Fang J, Hyland K, Arnold LA, Mouradian MM, Chase TN. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: a crossover study. Neurology. 1999;53:91–5. doi: 10.1212/wnl.53.1.91. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–90. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006;2:207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011;76:877–84. doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin. Reprod. Med. 2007;25:139–53. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005;25:5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, et al. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–9. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Heinsbroek RP, de Bruin JP, Olivier B. Effects of local application of dopaminergic drugs into the medial prefrontal cortex of rats on latent inhibition. Biol. Psychiatry. 1996;40:1083–90. doi: 10.1016/S0006-3223(95)00595-1. [DOI] [PubMed] [Google Scholar]
- Butenandt A. Über “Progynon“ ein krystallisiertes weibliches Sexualhormon. Naturwissenschaften. 1929;17 [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–17. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- Chambers RA. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend. 2013;130:1–12. doi: 10.1016/j.drugalcdep.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circulation. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153:3872–7. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Almey A, Melichercik A, Allen CP, Choleris E. Effects of an estrogen receptor alpha agonist on agonistic behaviour in intact and gonadectomized male and female mice. Psychoneuroendocrinology. 2011;36:981–995. doi: 10.1016/j.psyneuen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Clipperton Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. Agonistic behavior in males and females: effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front. Biosci. 2001;6:D1379–91. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- Condon TP, Dykshoorn-Bosch MA, Kelly MJ. Episodic luteinizing-hormone release in the ovariectomized female guinea pig: rapid inhibition by estrogen. Biol. Reprod. 1988;38:121–6. doi: 10.1095/biolreprod38.1.121. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J. Neuroendo. 2010;22:664–73. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J. Comp. Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901. doi: 10.1038/sj.npp.1301150. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Presti-Torres J, Dornelles A, Scalco FS, Roesler R, et al. Modulatory influence of dopamine receptors on consolidation of object recognition memory. Neurobiol. Learn. Mem. 2011;95:305–10. doi: 10.1016/j.nlm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur. J. Pharmacol. 1998;345:207–11. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- Doisy EA. Isolation of a crystalline estrogen from urine and the follicular hormone from ovaries. Am. J. Obstet. Gynecol. 1972;114:701–2. doi: 10.1016/0002-9378(72)90852-6. [DOI] [PubMed] [Google Scholar]
- Edwards DP, Boonyaratanakornkit V. Rapid extranuclear signaling by the estrogen receptor (ER): MNAR couples ER and Src to the MAP kinase signaling pathway. Mol. Interv. 2003;3:12–5. doi: 10.1124/mi.3.1.12. [DOI] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor alpha 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation. 2003;107:120–6. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XD, Simoncini T. Extra-nuclear signaling of estrogen receptors. IUBMB Life. 2008;60:502–10. doi: 10.1002/iub.80. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006;346:904–10. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Gal G, Joel D, Gusak O, Feldon J, Weiner I. The effects of electrolytic lesion to the shell subterritory of the nucleus accumbens on delayed non-matching-to-sample and four-arm baited eight-arm radial-maze tasks. Behav. Neurosci. 1997;111:92–103. doi: 10.1037//0735-7044.111.1.92. [DOI] [PubMed] [Google Scholar]
- George DN, Duffaud AM, Pothuizen HH, Haddon JE, Killcross S. Lesions to the ventral, but not the dorsal, medial prefrontal cortex enhance latent inhibition. Eur. J. Neurosci. 2010;31:1474–82. doi: 10.1111/j.1460-9568.2010.07178.x. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Jacob S, Brake WG, Mumby DG. Systemic and intra-rhinal-cortical 17-beta estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm. Behav. 2013;64:642–52. doi: 10.1016/j.yhbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48:715–20. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10:1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–55. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–62. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J. Neurosci. 2007;27:2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol. 2009;202:223–36. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Research. 2006;1121:46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Hotte M, Naudon L, Jay TM. Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol. Learn. Mem. 2005;84:85–92. doi: 10.1016/j.nlm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr. Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav. 2010;58:415–26. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Watson SJ. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134:847–56. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J. Neurosci. 2003;23:9987–95. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, et al. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol. Learn. Mem. 2010;94:488–98. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Hoeltzel A, Louilot A. Differential involvement of dopamine in the anterior and posterior parts of the dorsal striatum in latent inhibition. Neuroscience. 2003;118:233–41. doi: 10.1016/s0306-4522(02)00823-0. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. Hippocampal lesions attenuate latent inhibition of a CS and of a neutral stimulus. Psychobiology. 1987;15:293–299. [Google Scholar]
- Kelly MJ, Kuhnt U, Wuttke W. Hyperpolarization of hypothalamic parvocellular neurons by 17 beta-estradiol and their identification through intracellular staining with procion yellow. Exp. Brain Res. 1980;40:440–7. doi: 10.1007/BF00236152. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–7. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. Estrogen Modulation of G-protein-coupled Receptors. Trends Endocrinol. Metab. 1999;10:369–74. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Kiess W, Gallaher B. Hormonal control of programmed cell death/apoptosis. Eur. J. Endocrinol. 1998;138:482–91. doi: 10.1530/eje.0.1380482. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav. Neurosci. 2002;116:411–20. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol. Cell Endocrinol. 2002;193:129–35. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrinol. 1998;19(4):253–86. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.33. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Hayes E, Gavrilidis E. Hormones and schizophrenia. Curr. Opin. Psychiatry. 2012;25:89–95. doi: 10.1097/YCO.0b013e328350360e. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988;55:145–56. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Landry M, Levesque D, Di Paolo T. Estrogenic properties of raloxifene, but not tamoxifen, on D2 and D3 dopamine receptors in the rat forebrain. Neuroendocrinology. 2002;76:214–22. doi: 10.1159/000065951. [DOI] [PubMed] [Google Scholar]
- Langub MC, Jr., Watson RE., Jr. Estrogen receptive neurons in the preoptic area of the rat are postsynaptic targets of a sexually dimorphic enkephalinergic fiber plexus. Brain Res. 1992;573:61–9. doi: 10.1016/0006-8993(92)90113-n. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–7. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Levesque D, Di Paolo T. Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Sci. 1989;45:1813–20. doi: 10.1016/0024-3205(89)90522-5. [DOI] [PubMed] [Google Scholar]
- Levesque D, Gagnon S, Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci. Lett. 1989;98:345–50. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc. Natl. Acad. Sci. U S A. 2003;100:4807–12. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SB, Han J, Zhang N, Tian Z, Li XB, Zhao MG. Neuroprotective effects of oestrogen against oxidative toxicity through activation of G-protein-coupled receptor 30 receptor. Clin. Exp. Pharmacol. Physiol. 2011;38:577–85. doi: 10.1111/j.1440-1681.2011.05549.x. [DOI] [PubMed] [Google Scholar]
- Liu SB, Zhang N, Guo YY, Zhao R, Shi TY, Feng SF, Wang SQ, Yang Q, Li XQ, Wu YM, Ma L, Hou Y, Xiong LZ, Zhang W, Zhao MG. G-Protein-Coupled Receptor 30 Mediates Rapid Neuroprotective Effects of Estrogen via Depression of NR2B-Containing NMDA Receptors. J Neurosci. 32:4887–900. doi: 10.1523/JNEUROSCI.5828-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function:Past, present, and future. Horm. Behav. 2014;66:602–18. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Madularu D, Shams WM, Brake WG. Estrogen potentiates the behavioral and nucleus accumbens dopamine response to continuous haloperidol treatment in female rats. Eur. J. Neurosci. 2014;39:257–65. doi: 10.1111/ejn.12401. [DOI] [PubMed] [Google Scholar]
- Matevosyan NR. Pregnancy and postpartum specifics in women with schizophrenia: a meta-study. Arch. Gynecol. Obstet. 2011;283:141–7. doi: 10.1007/s00404-010-1706-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Sheeding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–55. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, et al. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J. Chem. Neuroanat. 2011;42:236–41. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Alves S, Hayashi S, McEwen B. An expanded view of estrogen receptor localization in the brain: Ultrastructural evidence for extranuclear functions in the brain. In: Watson C, editor. The Identities of Membrane Steriod Receptors and Other Proteins Mediating Nongenomic Steriod Action. Springer; US: 2003. [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008;149:3306–12. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, et al. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters B, Robinson D, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol. Biol. 2011;793:23–59. doi: 10.1007/978-1-61779-328-8_3. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17 beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–7. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 2010;518:2729–43. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J. Neuroendo. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–8. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn. Mem. 2007;14:117–25. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, et al. Mechanisms of estrogen action. Physiol. Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofrey BS, Ben-Shahar OM, Brake WG. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn. 2008;66:156–60. doi: 10.1016/j.bandc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Oswald CJP, Yee BK, Rawlins JNP, Bannerman DB, Good M, Honey RC. The influence of selective lestions to components of the hippocampus on orienting response, habituation, and latent inhibition. Eur. J. Neurosci. 2002;15:1983–1990. doi: 10.1046/j.1460-9568.2002.02028.x. [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–56. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J. Neurosci. 1989;9:1465–72. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–13. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav. Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 2002;277:50768–75. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007;282:22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–9. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 2008;109:350–3. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–91. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MG, Duncan A, Loiselle C, Graffe N, Brake WG. Latent inhibition is affected by phase of estrous cycle in female rats. Brain Cogn. 2010;74:244–8. doi: 10.1016/j.bandc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm. Behav. 2008;53:185–91. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Ragonese P, D'Amelio M, Salemi G, Aridon P, Gammino M, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62:2010–4. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol. 2002;16:100–15. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Regensburger M, Prots I, Winner B. Adult hippocampal neurogenesis in Parkinson's disease: impact on neuronal survival and plasticity. Neural plasticity. 2014;2014 doi: 10.1155/2014/454696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, et al. Presynaptic d2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol. Psychiatry. 2015;77:513–25. doi: 10.1016/j.biopsych.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Rollerova E, Urbancikova M. Intracellular estrogen receptors, their characterization and function (Review). Endocr. Regul. 2000;34:203–18. [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, et al. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–50. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J. Endocrinol. 1980;86:511–24. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Biologically active estrogen receptor-beta: evidence from in vivo autoradiographic studies with estrogen receptor alpha-knockout mice. Endocrinology. 1999;140:2613–20. doi: 10.1210/endo.140.6.6876. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–70. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Bhat LMV. Gender disparities in Parkinson's disease. Expert Rev. Neurother. 2006;6:407–416. doi: 10.1586/14737175.6.3.407. [DOI] [PubMed] [Google Scholar]
- Smith AW, Bosch MA, Wagner EJ, Ronnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17beta-estradiol. Am. J. Physiol. Endocrinol. Metab. 2013;305:E632–40. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ERα) in pyramidal neurons of the developing raat hippocampus. Brain Res. Dev. Brain Res. 2001;128:165–75. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Spary EJ, Chapman SE, Sinfield JK, Maqbool A, Kaye J, Batten TF. Novel G protein-coupled oestrogen receptor GPR30 shows changes in mRNA expression in the rat brain over the oestrous cycle. Neurosignals. 2013;21:14–27. doi: 10.1159/000333296. [DOI] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, et al. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–46. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J. Neurosci. 2011;31:6780–90. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol. 2008;29:219–37. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Smejkalova T, Woolley CS. Distribution and posttranslational modification of synaptic ERalpha in the adult female rat hippocampus. Endocrinology. 2013;154:819–30. doi: 10.1210/en.2012-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 2005;481:252–65. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Thompson TL. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res. 1999;834:164–7. doi: 10.1016/s0006-8993(99)01508-5. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J. Neurochem. 1994;62:1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc. Natl. Acad. Sci. U S A. 1966;55:1574–81. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J. Comp. Neurosci. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Varea O, Arevalo MA, Garrido JJ, Garcia-Segura LM, Wandosell F, Mendez P. Interaction of estrogen receptors with insulin-like growth factor-I and Wnt signaling in the nervous system. Steroids. 2010;75:565–9. doi: 10.1016/j.steroids.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Vasconsuelo A, Pronsato L, Ronda AC, Boland R, Milanesi L. Role of 17beta-estradiol and testosterone in apoptosis. Steroids. 2011;76:1223–31. doi: 10.1016/j.steroids.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl. 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Silverman A, Morrell JI. Evidence for estrogen receptor in cell nuclei and axon terminals within the lateral habnula of the rat: Regulation during pregnancy. J. Comp. Neurosci. 1998;392:330–346. [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Paterl P, Gonzalez AD, Ye H, Filardo EJ, Clegg DJ, Goreka J, Akama KT, McEwen BS, Milner TA. G-Protein-Coupled Estrogen Receptor 1 Is Anatomically Positioned to Modulate Synaptic Plasticity in the Mouse Hippocampus. J. Neurosci. 2015;35 doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, et al. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J. Neurosci. Comp. Neurol. 1997;388:603–12. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 2002;77:125–84. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J. Neurosci. 1992;12:3217–25. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr. Opin. Neurobiol. 1999;9:349–54. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci. Lett. 1994;180:155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, et al. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JZ, O'Flatharta C, Harvey BJ, Thomas W. Membrane ERalpha-dependent activation of PKCalpha in endometrial cancer cells by estradiol. Steroids. 2008;73:1110–22. doi: 10.1016/j.steroids.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Lou WY, Akama KT, McEwen BS, et al. Effects of estrogen and aging on the synaptic distribution of phosphorylated Akt-immunoreactivity in the CA1 region of the female rat hippocampus. Brain Res. 2011;1379:98–108. doi: 10.1016/j.brainres.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17beta-estradiol-[125I]bovine serum albumin conjugate. J. Steroid. Biochem. Mol. Biol. 1997;62:327–36. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J. Neurosci. 2003;23:2340–7. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


