Abstract
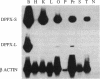
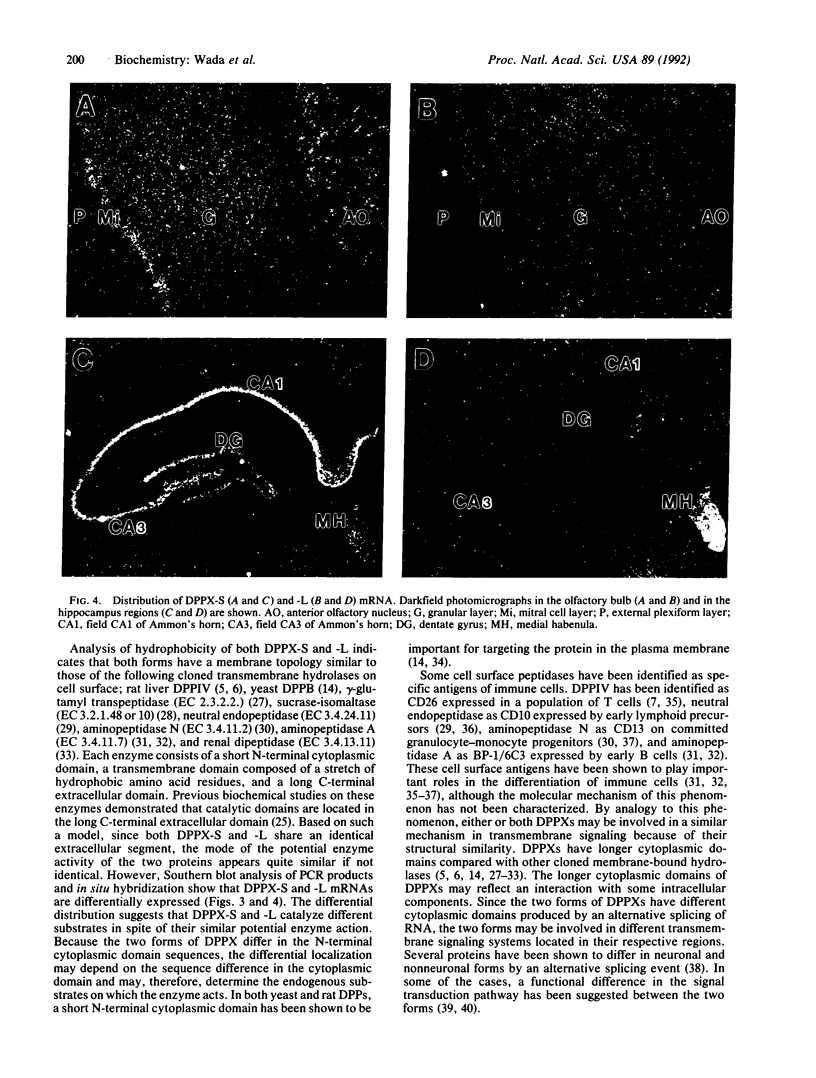
We have identified two cDNAs encoding dipeptidyl aminopeptidase-like proteins (DPPXs) in both bovine and rat brains that have different N-terminal cytoplasmic domains but share an identical transmembrane domain and a long C-terminal extracellular domain. In both species, one of the cDNAs encodes a protein (designated DPPX-S) of 803 amino acid residues with a short cytoplasmic domain of 32 amino acids, and the other cDNA encodes a protein (designated DPPX-L) with a longer cytoplasmic domain--the bovine cDNA encodes 92 amino acids and the rat cDNA encodes 88 amino acids. The membrane topology of DPPX-S and -L is similar to that of other transmembrane peptidases, and DPPX-S share approximately 30% identity and 50% similarity with reported yeast and rat liver dipeptidyl aminopeptidase amino acid sequences, suggesting that DPPX is a member of the dipeptidyl aminopeptidase family. DPPX-S mRNA is expressed in brain and some peripheral tissues including kidney, ovary, and testis; in contrast, DPPX-L mRNA is expressed almost exclusively in brain. No transcripts for either form are found in heart, liver, or spleen. In situ hybridization studies show that the two transcripts have different distributions in the brain. DPPX-L mRNA is expressed in limited regions of brain with the highest level of expression in the medial habenula. More widespread expression is seen for DPPX-S mRNA. The differential distribution of mRNAs for the DPPX-S and -L suggests that these proteins are involved in the metabolism of certain localized peptides and that the cytoplasmic domain may play a key role in determining the physiological specificity of DPPX.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Tawaragi Y., Inuzuka C., Kubota I., Tsujimoto M., Nishihara T., Nakazato H. Primary structure of human microsomal dipeptidase deduced from molecular cloning. J Biol Chem. 1990 Mar 5;265(7):3992–3995. [PubMed] [Google Scholar]
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Chio C. L., Hess G. F., Graham R. S., Huff R. M. A second molecular form of D2 dopamine receptor in rat and bovine caudate nucleus. Nature. 1990 Jan 18;343(6255):266–269. doi: 10.1038/343266a0. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke G. R., Munoz E., Huber B. T., Plaut A. G., Kettner C. A., Bachovchin W. W. Inhibition of dipeptidyl aminopeptidase IV (DP-IV) by Xaa-boroPro dipeptides and use of these inhibitors to examine the role of DP-IV in T-cell function. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1556–1559. doi: 10.1073/pnas.88.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegen M., Niedobitek G., Klein C. E., Stein H., Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990 Apr 15;144(8):2908–2914. [PubMed] [Google Scholar]
- Hong W. J., Doyle D. Molecular dissection of the NH2-terminal signal/anchor sequence of rat dipeptidyl peptidase IV. J Cell Biol. 1990 Aug;111(2):323–328. doi: 10.1083/jcb.111.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Doyle D. cDNA cloning for a bile canaliculus domain-specific membrane glycoprotein of rat hepatocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7962–7966. doi: 10.1073/pnas.84.22.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. S. A novel dipeptidyl aminopeptidase in rat brain membranes. Its isolation, purification, and characterization. J Biol Chem. 1988 May 15;263(14):6613–6618. [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Joh K., Arai Y., Mukai T., Hori K. Expression of three mRNA species from a single rat aldolase A gene, differing in their 5' non-coding regions. J Mol Biol. 1986 Aug 5;190(3):401–410. doi: 10.1016/0022-2836(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Kato T., Hama T., Nagatsu T. Separation of two dipeptidyl aminopeptidases in the human brain. J Neurochem. 1980 Mar;34(3):602–608. doi: 10.1111/j.1471-4159.1980.tb11186.x. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look A. T., Ashmun R. A., Shapiro L. H., Peiper S. C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989 Apr;83(4):1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvy J. F., Blumberg S. Inactivation and metabolism of neuropeptides. Annu Rev Neurosci. 1986;9:415–434. doi: 10.1146/annurev.ne.09.030186.002215. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Struckhoff G. Purification of two dipeptidyl aminopeptidases II from rat brain and their action on proline-containing neuropeptides. J Neurochem. 1989 Apr;52(4):1284–1293. doi: 10.1111/j.1471-4159.1989.tb01877.x. [DOI] [PubMed] [Google Scholar]
- Mizobuchi M., Frohman M. A., Downs T. R., Frohman L. A. Tissue-specific transcription initiation and effects of growth hormone (GH) deficiency on the regulation of mouse and rat GH-releasing hormone gene in hypothalamus and placenta. Mol Endocrinol. 1991 Apr;5(4):476–484. doi: 10.1210/mend-5-4-476. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Ogata S., Misumi Y., Ikehara Y. Primary structure of rat liver dipeptidyl peptidase IV deduced from its cDNA and identification of the NH2-terminal signal sequence as the membrane-anchoring domain. J Biol Chem. 1989 Feb 25;264(6):3596–3601. [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Bolen J. B. Identification of a novel neuronal C-SRC exon expressed in human brain. Mol Cell Biol. 1990 May;10(5):2035–2040. doi: 10.1128/mcb.10.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Pohlig G., Rothman J. H., Stevens T. H. Structure, biosynthesis, and localization of dipeptidyl aminopeptidase B, an integral membrane glycoprotein of the yeast vacuole. J Cell Biol. 1989 Apr;108(4):1363–1373. doi: 10.1083/jcb.108.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Dechesne C. J., Shimasaki S., King R. G., Kusano K., Buonanno A., Hampson D. R., Banner C., Wenthold R. J., Nakatani Y. Sequence and expression of a frog brain complementary DNA encoding a kainate-binding protein. Nature. 1989 Dec 7;342(6250):684–689. doi: 10.1038/342684a0. [DOI] [PubMed] [Google Scholar]
- Wu Q., Lahti J. M., Air G. M., Burrows P. D., Cooper M. D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc Natl Acad Sci U S A. 1990 Feb;87(3):993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]