Abstract
The Aberrant Behavior Checklist—Community (ABC-C; Aman, Burrow, & Wolford, 1995) has been increasingly adopted as a primary tool for measuring behavioral change in clinical trials for individuals with fragile X syndrome (FXS). To our knowledge, however, no study has documented the longitudinal trajectory of aberrant behaviors in individuals with FXS using the ABC-C. As part of a larger longitudinal study, we examined scores obtained on the ABC-C subscales for 124 children and adolescents (64 males, 60 females) with FXS who had two or more assessments (average interval between assessments was approximately 4 years). Concomitant changes in age-equivalent scores on the Vineland Adaptive Behavior Scales (VABS) were also examined. As expected for an X-linked genetic disorder, males with FXS obtained significantly higher scores on all subscales of the ABC-C and significantly lower age-equivalent scores on the VABS than females with FXS. In both males and females with FXS, scores on the Irritability/Agitation and Hyperactivity/Noncompliance subscales of the ABC-C decreased significantly with age, with little to no change occurring over time on the Lethargy/Social Withdrawal, Stereotypic Behavior, and Inappropriate Speech subscales. The decrease in scores on the Hyperactivity/Noncompliance domain was significantly greater for males than for females. In both males and females, age-equivalent scores on the VABS increased significantly over this developmental period. These results establish a basis upon which to evaluate long-term outcomes from intervention-based research. However, longitudinal direct observational studies are needed to establish whether the severity of problem behavior actually decreases over time in this population.
Keywords: fragile X syndrome, aberrant behavior, longitudinal data, adaptive behavior
1. Introduction
Children with intellectual disabilities (ID) show a greater tendency to engage in problem behavior than individuals without ID (Dekker, Koot, van der Ende, & Verhulst, 2002; de Ruiter, Dekker, Verhulst, & Koot, 2007; Dykens, 2000; Emerson, 2003) and there is evidence in the literature that the prevalence of problem behavior among individuals with certain genetic conditions associated with ID is higher than in individuals with idiopathic ID (Arron, Oliver, Moss, Berg, & Burbidge, 2011; Bodfish & Lewis, 2002; Powis & Oliver, 2014). For example, individuals with fragile X syndrome (FXS) – the most common known form of inherited intellectual disability, affecting approximately 1 in 4000 individuals worldwide (Turner, Webb, Wake, & Robinson, 1996) – have been reported to engage in significantly higher levels of problem behaviors such as hyperactivity, stereotypy, self-injury, and inappropriate speech than age-, and IQ-matched controls (Baumgardner, Reiss, Freund, & Abrams, 1995; Lachiewicz, Spiridigliozzi, Gullion, Ransford & Rao, 1994; Sansone et al., 2012; Sudhalter, Cohen, Silverman, & Wolf-Schein, 1990). In addition to ID, researchers have documented a distinct behavioral phenotype associated with FXS (Reiss & Freund, 1992; Smith, Barker, Seltzer, Abbeduto, & Greenberg, 2012; Wolff et al., 2012), of which the core features include social anxiety, withdrawal and eye-gaze aversion (Hall, DeBernardis, & Reiss, 2006), elevated rates of self-injurious behavior (Hall, Lightbody, & Reiss, 2008; Symons, Clark, Hatton, Skinner, & Bailey, 2003), and hyperactivity and attention deficit (Hatton et al., 2002; Thurman, McDuffie, Hagerman, & Abbeduto, 2014). Due to varied methods of measurement and analysis, and age-ranges sampled, however, the persistence of problem behavior in this population from childhood to adulthood is not well understood (Bailey et al., 2012; Fisch et al., 1999; Hatton et al., 2002; Sansone et al., 2012; Wheeler et al., 2014).
FXS is caused by a mutation to the FMR1 gene on the long arm of the X chromosome; located at the Xq27.3 “fragile” locus and consisting of abnormal multiple CGG replications. Expansions of approximately 55 to 200 repeats are associated with the fragile X “premutation” for which individuals are considered carriers and show few or no symptoms of the disorder, whereas larger expansions (>200 CGG repeats) are associated with the “full mutation” (Oberle et al., 1991). Consequently, the full mutation produces abnormal DNA hypermethylation of cytosines in the FMR1 promoter region, transcriptional silencing of the FMR1 gene and thwarted production of the gene’s product, the fragile X mental retardation protein (FMRP; Verkerk et al., 1991). Although the syndrome affects males and females, males with FXS have only one X chromosome and significantly lower FMRP and are, therefore, more affected by the disorder.
A significant advance in our understanding of FXS was brought forth with the isolation of the Fmr1 gene in a knockout (KO) mouse model of FXS, designed to characterize the structure and function of FMRP and dendritic abnormalities found in humans with FXS. The KO model has led to the discovery that loss of FMRP – which typically functions as a repressor of translation of specific messenger ribonucleic acid (mRNA) – allows for translation of mRNA near synapses, activation of metabotropic glutamate receptors (mGluRs), and subsequently increased long-term depression of transmission at hippocampal synapses (Bear, Huber, & Warren, 2004). An emerging theory therefore implicates mGluR antagonists in the potential alleviation of impairments in the FXS phenotype, and by extension, the aberrant behavior associated with FXS (Choi et al., 2011; Meredith, de Jong, & Mansvelder, 2011). As such, investigators have been charged with identifying suitable measures of behavioral outcome in clinical trials testing the theory. Following extensive research establishing concurrent and construct validity, inter-rater and test-retest reliability (Aman, Singh, Stewart, & Field, 1985), and a gradual, but increasing adoption of the tool in clinical trials, the Aberrant Behavior Checklist—Community (ABC-C; Aman, Singh, Stewart, & Field, 1985) has garnered much attention for its potential sensitivity in measuring treatment response and detecting changes in behavior (cf., Aman, 2012; McCracken et al., 2002; Research Units on Pediatric Psychopharmacology Autism Network, 2005; Schroeder, Rojahn, & Reese, 1997; Shedlack, Hennen, Magee, & Cheron, 2005). Furthermore, recommendations recently published by a working group of FXS experts identified the ABC-C as one of the few measures available that came closest to meeting optimal criteria for use in clinical trials (Berry-Kravis et al., 2013).
However, there is a paucity of longitudinal investigations using the ABC-C. In one study conducted by Anderson, Maye, and Lord (2011), longitudinal analyses of scores obtained on the ABC-C were conducted with three diagnostic groups: autism, broader autism spectrum disorder (ASD), and nonspectrum developmental disabilities (DD). Caregivers were asked to rate their child on the ABC-C at age 9 and then every four months via mailed questionnaires and those with a minimum of two assessment points were included in the sample. Trajectories were presented on three of the five ABC-C subscales, i.e., Hyperactivity/Noncompliance, Irritability/Agitation, and Lethargy/Social Withdrawal. Results showed that individuals with autism displayed higher levels of aberrant behavior than individuals with ASD and DD, with decreasing trends observed over time on the Irritability/Agitation and Hyperactivity/Noncompliance subscales across all groups. However, a different pattern emerged on the Lethargy/Social withdrawal subscale. Individuals in both the autism and ASD groups showed an increasing trend in Lethargy/Social withdrawal with age (especially those in the ASD group), indicating a need for services addressing social withdrawal beyond early intervention and into early adulthood in this population (Anderson et al., 2011).
Although there have been studies that have begun to map the trajectory of development in FXS (Bailey, Hatton, & Skinner, 1998; Dykens et al., 1989; Fisch et al., 1999; Hagerman et al., 1989; Klaiman et al., in press), to our knowledge, none have measured the trajectory of aberrant behavior using longitudinal methods in a large sample of individuals with FXS using the ABC-C. Considering that pharmaceutical trials are already underway, the time is ripe for an investigation of the developmental trajectory of aberrant behavior in this population, both for specification purposes and to establish a basis upon which to evaluate long-term outcomes from intervention-based research. Revealing the developmental course of aberrant behavior in individuals with genetic disorders – particularly by utilizing longitudinal methods – is vital to understanding the specific needs and therapeutic standards that apply to the disorder.
The purpose of the current study was to assess the trajectory of specific categories of aberrant behavior from childhood to early adulthood in individuals with FXS using the ABC-C. The study also aimed to establish normative data on the trajectory of aberrant behavior in FXS using this measure. We also administered the Vineland Adaptive Behavior Scales (VABS; Sparrow, Balla, & Cicchetti, 1984) at each time point to characterize concomitant developmental change in adaptive behavior and to examine whether levels of adaptive behavior predicted the trajectory of aberrant behavior. We therefore aimed to examine the rate of change in aberrant and adaptive behavior in males and females with FXS.
2. Method
2.1. Participants
Data for this study were obtained from a longitudinal investigation of brain and behavior development in children and adolescents with FXS conducted at Stanford’s Center for Interdisciplinary Brain Sciences Research (CIBSR) from 1998 to 2011. Participants were included in the analysis if at least two assessment points on the ABC-C were available and were spaced more than a month apart (the questionnaire specifies that responses reflect behavior “in the previous 4 weeks”). A total of 124 individuals with FXS (64 males, 60 females) who had at least two assessments were included in the study. The mean time interval between the first and second assessments was 4.17 years (SD = 2.39 years, range = 1.20 to 11.96 years) for males with FXS and 3.78 years (SD = 2.07 years, range = .70 to 11.32 years) for females with FXS. Sixty-six individuals (35 males, 31 females) had at least three assessments and the mean time interval between the second and third assessments was 4.24 years (SD = 1.53 years, range = 1.66 to 9.22 years) for males with FXS and 3.96 years (SD = 1.76 years, range = .09 to 7.96 years) for females with FXS. Finally, 12 participants (5 males, 7 females) had a fourth ABC-C assessment1 and the mean time interval between the third and fourth assessments was 3.34 years (SD = 1.20 years, range = 2.23 to 5.23 years) for males with FXS and 4.99 years (SD = 2.60 years, range = 1.41 to 9.44 years) for females with FXS. Individuals who had three or more assessments were no different from those who had only two assessments in terms of age, medication use, age-equivalent score on the VABS, or ABC-C subscale scores. All had a diagnosis of FXS confirmed by Southern Blot DNA analysis (Kimball Genetics, Inc.). FMRP expression (i.e., percentage of sampled lymphocytes showing FMRP expression) were measured using the method devised by Willemsen and colleagues (i.e., blood smears were made and FMRP was analyzed in the cytoplam of lymphocytes; Willemsen et al., 1997).
Table 1 shows the demographic characteristics of the cohort at baseline.
Table 1.
Mean, (SD), and range of chronological age, developmental age, FMRP expression, and medication usage at baseline for males and females with FXS.
| Males | Females | t/X2 | p-value | |
|---|---|---|---|---|
| Age (years) | 11.76 (4.19) range = 2 to 26 |
11.46 (4.44) range = 3 to 25 |
.39 | .70 |
| VABSa | 5.05 (2.50) range = 1 to 11 |
8.24 (3.96) range = 2 to 17 |
5.08 | <.001 |
| FMRP (%) | 14.10 (14.84) range = 1 to 75 |
55.35 (18.84) range = 15 to 96 |
12.99 | <.001 |
| Medication use (%) | 62.5 | 37.5 | 4.21 | .04 |
VABS Adaptive Behavior Composite Age-Equivalent Score
As expected for an X-linked genetic disorder, males with FXS obtained significantly lower scores on the VABS (p = < .001) and had significantly lower percentage of FMRP expression than females (p = < .001). A significantly greater proportion of males with FXS were taking psychoactive medications than females with FXS (p = < .05) with 62.5% of males and 37.5% of females taking at least one psychoactive medication. These medications included stimulants (77.3%; 40 males, 18 females), antidepressants (65.3%; 31 males, 18 females), antipsychotics (34.7%; 16 males, 10 females), alpha agonists (26.7%; 15 males, 5 females), anxiolytics (17.3%; 9 males, 4 females), and mood stabilizers (12%; 6 males, 3 females).
2.2. Measures
2.2.1. Aberrant Behavior Checklist
The Aberrant Behavior Checklist—Community version (ABC-C; Aman, Burrow, & Wolford, 1995) was used to measure the severity of problem behavior at each time point. The 58-item rating scale is a multidimensional and empirically-derived tool (Aman, Singh, Stewart, & Field, 1985) commonly employed to assess problem behavior in individuals with ID. Items resolve into five subscales or categories of aberrant behavior: Irritability/Agitation, which includes 15 items relating to disruptive, aggressive and self-injurious behavior (e.g., Injures self on purpose, Aggressive to other children or adults, Screams inappropriately, Temper tantrums/outburst, etc.); Lethargy/Social Withdrawal, which includes 16 items relating to social withdrawal behavior (e.g., Listless, sluggish, inactive, Seeks isolation from others, Preoccupied; stares into space); Stereotypic Behavior, which includes 7 items (e.g., Meaningless, recurring body movements, Stereotyped behavior; abnormal, repetitive movements, Odd, bizarre in behavior); Hyperactivity/Noncompliance, which includes 16 items relating to hyperactive, impulsive, and non-compliant behavior and attention deficit (e.g., Excessively active at home, school, work, or elsewhere, Boisterous, Impulsive, Restless, unable to sit still); and Inappropriate Speech, which includes 4 items (e.g., Talks excessively, Repetitive speech, Talks to self loudly, Repeats a word or phrase over and over). Given that items are rated on four-point scale of severity (0 = not at all a problem, 1 = the behavior is a problem, but slight in degree, 2 = the problem is moderately serious, 3 = the problem is severe in degree), the maximum possible score is 45 for Irritability/Agitation, 48 for Lethargy/Social Withdrawal, 21 for Stereotypic Behavior, 48 for Hyperactivity/Noncompliance, and 12 for Inappropriate Speech, with higher subscale scores indicating more severe aberrant behavior. The child’s primary caregiver completed the ABC-C at each assessment point.
2.2.2. Vineland Adaptive Behavior Scales
The Vineland Adaptive Behavior Scales (VABS) – Parent Interview (VABS; Sparrow, Balla, & Cicchetti, 1984) was administered at each time point in order to establish a measure of adaptive functioning. The child’s primary caregiver was the informant at each assessment point. The VABS assesses level of adaptive skills in three primary domains: Communication Skills, Daily Living Skills, and Socialization Skills corresponding to the three broad domains of adaptive functioning outlined by the American Association of Intellectual and Developmental Disabilities. For the present study, we used the Adaptive Behavior Composite age-equivalent scores to track developmental change over time. (For information concerning the trajectories of the domains of the VABS in males and females with FXS, see Klaiman et al., in press). The test taking of the VABS conformed to the interview format.
2.3. Statistical Analyses
We employed standard linear mixed effects modeling (Raudenbush & Bryk, 2002; Singer & Willett, 2003) to model longitudinal trajectories of ABC-C outcomes and VABS age-equivalent score. To improve estimations, the analyses included data from all participants, aged 2 to 26 years. However, the data were sparse outside the 10-to-20 year age range, therefore, we report statistical inferences focusing on this developmental period. We first conducted unconditional analyses without including any predictors of intercept or slope. Each domain was analyzed separately using growth modeling utilizing repeated measures and individually varying time scores (actual age at each assessment). (For a brief introduction to the principles of this approach, see Curran, Obeidat, & Losardo, 2010.) Specifically, we used a random intercept and slope model assuming a linear trend over time. We examined the raw data and conducted likelihood ratio tests to examine whether a quadratic term was necessary for each domain. Likelihood ratio tests indicated no improvement in fit by applying a quadratic model. Missing data points were treated as missing at random conditional on observed information using maximum likelihood estimation (Little & Rubin, 2002). We used the Mplus program version 7.11 (Muthén & Muthén, 1998–2010) to conduct maximum likelihood estimation for all of our longitudinal mixed effects analyses. Finally, in the conditional analyses, we included three time-invariant baseline covariates: VABS age-equivalent score, percentage of FMRP expression, and medication use, to examine whether intercepts and slopes of aberrant behavior varied as a function of these variables. For these analyses, we applied a Bonferroni correction, with alpha level set at 0.01.
3. Results
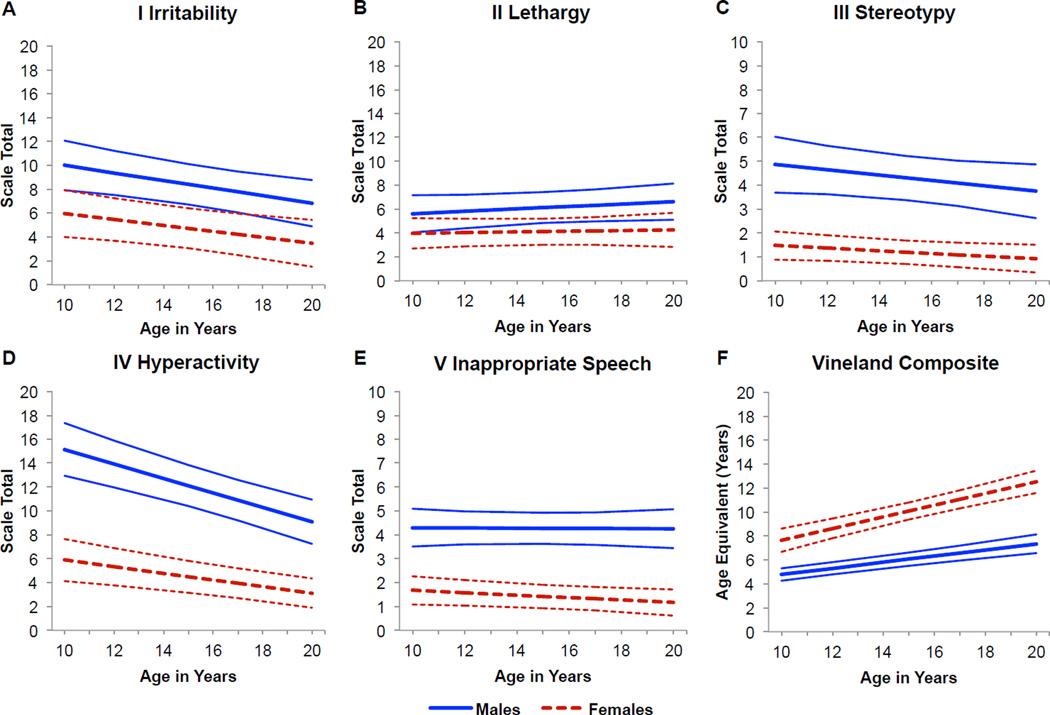
The results of the mixed effects longitudinal analyses and the estimated trajectories for the five ABC-C subscales and VABS age-equivalent scores are presented in Figure 1 and Table 2. (Data concerning the number of observations that were available in each two-year age band, together with the raw means and SDs calculated on each subscale of the ABC-C and the VABS age-equivalent score are included in the Appendix.)
Figure 1.
Trajectory of scores on the five aberrant behavior domains assessed by the Aberrant Behavior Checklist – Community: (A) I. Irritability/Agitation, (B) II. Lethargy/Social Withdrawal, (C) III. Stereotypic Behavior, (D) IV. Hyperactivity/Noncompliance, (E) V. Inappropriate Speech are shown from age 10 to age 20 years. VABS Adaptive Behavior Composite age-equivalent score in years (F) are represented for the same age range. Trajectories and confidence intervals are represented by solid lines for males with FXS and dashed lines for females with FXS.
Table 2.
Unconditional Analyses
Estimated mean scores at age 10 and change per year for each ABC-C subscale and VABS age equivalent for males and females with FXS.
| Males | Mean Score at age 10 |
Change in Score Per Year (Slope) |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | p-value | Effect Size | |||
| I. Irritability/ Agitation |
10.00 | 7.92, | 12.08 | −0.32 | −0.54, | −0.10 | 0.00 | −0.55 |
| II. Lethargy/Social Withdrawal |
5.60 | 4.04 | 7.16 | 0.10 | −0.07, | 0.27 | 0.24 | 0.25 |
| III. Stereotypic Behavior |
4.86 | 3.69, | 6.03 | −0.11 | −0.25, | 0.03 | 0.11 | −0.50 |
| IV. Hyperactivity Noncompliance |
15.16 | 12.95, | 17.36 | −0.61 | −0.82, | −0.39 | 0.00 | −2.74 |
| V. Inappropriate Speech |
4.30 | 3.51, | 5.09 | −0.01 | −0.10, | 0.09 | 0.92 | −0.03 |
| VABS Age Equivalent |
4.78 | 4.26, | 5.30 | 0.26 | 0.19, | 0.33 | < 0.001 | 3.04 |
| Females | Mean Score at age 10 |
Change in Score Per Year (Slope) |
||||||
| Estimate | 95% CI | Estimate | 95% CI | p-value | Effect Size | |||
| I. Irritability/ Agitation |
5.97 | 4.02, | 7.92 | −0.25 | −0.45, | −0.05 | 0.02 | −0.44 |
| II. Lethargy/Social Withdrawal |
3.97 | 2.69, | 5.24 | 0.03 | −0.13, | 0.19 | 0.71 | 0.07 |
| III. Stereotypic Behavior |
1.47 | 0.88, | 2.07 | −0.06 | −0.12, | 0.01 | 0.10 | −0.25 |
| IV. Hyperactivity Noncompliance |
5.90 | 4.14, | 7.66 | −0.28 | −0.42, | −0.14 | 0.00 | −1.26 |
| V. Inappropriate Speech |
1.67 | 1.08, | 2.27 | −0.05 | −0.11, | 0.01 | 0.10 | −0.26 |
| VABS Age Equivalent |
7.65 | 6.68, | 8.61 | 0.49 | 0.36, | 0.62 | < 0.001 | 5.82 |
3.1. Aberrant Behavior Trajectories
3.1.1. Irritability/Agitation Subscale
At age 10 years, the estimated Irritability/Agitation subscale score was 10.00 for males with FXS and 5.97 for females with FXS, representing a significant difference between the groups (p = 0.01). The average rate of change per year was significant for both males and females, with scores decreasing by 0.32 points per year on average for males (p = 0.004; d = −0.55) and by 0.25 points per year on average for females (p = 0.02; d = −0.44; Figure 1a). However, the rate of change over time was not significantly different between males and females over time (p = 0.65). These data indicated that males with FXS showed significantly higher levels of problem behavior on this subscale than females and that these problems decreased at the same rate over time.
3.1.2. Lethargy/Social Withdrawal Subscale
At age 10 years, the estimated score on the Lethargy/Social Withdrawal subscale was 5.60 for males with FXS and 3.97 for females with FXS, representing a non-significant difference between the groups (p = 0.11). Males and females showed a non-significant change in scores over time, with an average increase of 0.10 points per year for males (p = 0.24, d = 0.25) and an average increase of 0.03 points per year for females (p = 0.71; d = 0.03; Figure 1b). There was no difference in the rate of change over time between males and females (p = 0.53). These data indicated that both males and females with FXS obtained relatively low scores on the Lethargy/Social Withdrawal subscale and that these scores remained low and stable over time.
3.1.3. Stereotypic Behavior Subscale
At age 10 years, the estimated score on the Stereotypic Behavior subscale was 4.86 for males with FXS and 1.47 for females with FXS, representing a significant difference between the groups (p = < 0.001). On average, the rate of change was −0.11 points per year for males (p = 0.11; d = −0.50) and −0.06 points per year for females (p = 0.10; d = −0.25; Figure 1c). The rate of change was not significantly different between males and females over time (p = 0.41). These data indicated that males showed significantly higher levels of problem behavior on this subscale than females, but that scores in both groups remained low and stable over time.
3.1.4. Hyperactivity/Noncompliance Subscale
At age 10 years, the estimated score on the Hyperactivity/Noncompliance subscale was 15.16 for males with FXS and 5.90 for females with FXS, representing a significant difference between the groups (p = < 0.001). The rate of change was significantly greater in males than in females (p = .02) with scores decreasing by 0.61 points per year for males (p = < 0.001; d = −2.74) and by 0.28 points per year for females (p = < 0.001; d = −1.26; Figure 1d). These data indicated that males showed significantly higher levels of problem behavior on this subscale than females and that these problems decreased at a greater rate over time for males than for females.
3.1.5. Inappropriate Speech Subscale
At age 10 years, the estimated score on the Inappropriate Speech subscale was 4.30 for males with FXS and 1.67 for females with FXS, representing a significant difference between the groups (p = < 0.001). The average rate of change on this subscale per year was −0.01 points per year for males (p = 0.92; d = −0.03) and −0.05 points per year for females (p = 0.10; d = −0.26; Figure 1e). The rate of change was not significantly different between males and females (p = 0.42). These data indicated that males showed significantly higher levels of problem behavior on this subscale than females, but that scores in both groups remained low and stable over time.
3.2. Adaptive Behavior Trajectory
To determine whether or not changes in aberrant behavior were commensurate with changes in adaptive behavior, we also evaluated the developmental course of adaptive behavior in the cohort. At age 10 years, the estimated developmental age was 4.78 years for males and 7.65 years for females with FXS, representing a significant difference between the groups (p = < 0.001). The rate of change was significantly slower for males than females (p = < 0.001). On average, developmental age increased by 0.26 per year for males with FXS (p = < 0.001; d = 3.04) and by 0.49 per year for females with FXS (p = < 0.001; d = 5.82; Figure 1f). These data indicate that males and females with FXS did not keep pace with the VABS normative sample with respect to age-equivalent scores, given that the slope of the age-equivalent scores in each group were significantly less than 1.0. However, both groups continued to gain adaptive skills throughout development, resulting in significant increases in age-equivalent scores over time.
3.3. Conditional Analyses
To examine the effect of baseline developmental age, FMRP expression, and medication usage on the intercept and trajectories of aberrant behavior, we conducted conditional analyses with VABS age-equivalent score, FMRP expression, and (dichotomous) psychoactive medication usage. Seventeen females and 8 males were excluded from these analyses due to missing covariate information. Table 3 shows the results.
Table 3.
Conditional Analyses
Effect of baseline covariates on intercept (age 10 years) and slope for each subscale of the ABC-C for males and females with FXS.
| Males | I. Irritability/ Agitation |
II. Lethargy/Social Withdrawal |
III. Stereotypic Behavior |
IV. Hyperactivity/ Noncompliance |
V. Inappropriate Speech |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | p-value | B | p-value | B | p-value | B | p-value | B | p-value | |
| Intercept (Age 10) |
||||||||||
| VABS | −1.54 | 0.00 | −0.80 | 0.04 | −0.60 | 0.03 | −1.52 | 0.00 | 0.08 | 0.70 |
| FMRP | 0.13 | 0.02 | 0.00 | 0.99 | 0.01 | 0.64 | 0.04 | 0.37 | −0.02 | 0.31 |
| Medication | −0.03 | 0.99 | −2.02 | 0.21 | −0.88 | 0.45 | 2.34 | 0.26 | −1.99 | 0.02 |
| Slope | ||||||||||
| VABS | 0.07 | 0.09 | −0.02 | 0.62 | 0.02 | 0.60 | 0.07 | 0.13 | −0.03 | 0.20 |
| FMRP | −0.01 | 0.26 | −0.01 | 0.14 | −0.01 | 0.05 | 0.00 | 0.79 | −0.00 | 0.49 |
| Medication | −0.03 | 0.90 | 0.04 | 0.84 | 0.07 | 0.58 | −0.11 | 0.60 | 0.19 | 0.05 |
| Females | I. Irritability/ Agitation |
II. Lethargy/Social Withdrawal |
III. Stereotypic Behavior |
IV. Hyperactivity/ Noncompliance |
V. Inappropriate Speech |
|||||
| B | p-value | B | p-value | B | p-value | B | p-value | B | p-value | |
| Intercept (Age 10) |
||||||||||
| VABS | −0.75 | 0.00 | −0.29 | 0.11 | −0.26 | 0.00 | −0.52 | 0.02 | −0.20 | 0.04 |
| FMRP | −0.03 | 0.65 | −0.03 | 0.47 | −0.03 | 0.16 | −0.10 | 0.04 | 0.00 | 0.78 |
| Medication | 4.60 | 0.12 | 2.58 | 0.09 | 1.00 | 0.16 | 3.83 | 0.16 | 0.82 | 0.30 |
| Slope | ||||||||||
| VABS | −0.00 | 0.93 | −0.03 | 0.14 | −0.00 | 0.95 | 0.01 | 0.70 | −0.01 | 0.27 |
| FMRP | 0.01 | 0.16 | 0.00 | 0.40 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 1.00 |
| Medication | 0.42 | 0.35 | 0.18 | 0.35 | 0.03 | 0.75 | 0.23 | 0.54 | 0.20 | 0.11 |
B = unstandardized regression coefficient. Bold values indicate significance at the .01 level
In males with FXS, baseline VABS age-equivalent score was significantly associated with the Irritability/Agitation and Hyperactivity/Noncompliance subscales of the ABC-C at age 10. That is, individuals with higher developmental ages at baseline obtained significantly lower scores on these subscales at age 10. However, there was no effect of developmental age on the trajectory of aberrant behavior in males with FXS and there was no effect of FMRP expression or medication use on the intercept or trajectory of aberrant behavior.
In females with FXS, baseline developmental age was significantly associated with the Irritability/Agitation and Stereotypic Behavior subscales of the ABC-C at age 10 (i.e., higher age equivalent scores predicted significantly lower scores on these subscales). However, there was no effect of developmental age on the trajectory of aberrant behavior in females. FMRP expression was significantly associated with the trajectory of Hyperactivity/Noncompliance (i.e., greater FMRP expression was associated with a faster decline in hyperactive and noncompliant behavior). There was no effect of medication use on the intercept or trajectory of aberrant behavior.
4. Discussion
We examined trajectories of aberrant behavior in children and adolescents with FXS across multiple assessment points on the ABC-C over the 10 to 20-year age range. Overall, scores on the Irritability/Agitation and Hyperactivity/Noncompliance subscales of the ABC-C decreased significantly over time in both males and females with FXS. Not surprisingly, males obtained significantly higher scores than females with FXS on all subscales except for the Lethargy/Social Withdrawal subscale. The rate of change was not significantly different between males and females with FXS on any of the subscales, except for the Hyperactivity/Noncompliance subscale, for which males evidenced a steeper decrease in scores than females with FXS. Importantly, this reveals that while males with FXS display higher levels of aberrant behavior than females, the rate of change is similar across sexes (with the exception of the Hyperactivity/Noncompliance subscale) and significant decreases in two of the most troubling behavior clusters are evident in both males and females with FXS from childhood to early adulthood. These results support previous cross-sectional research reporting decreases in aberrant behavior over time in this population using the ABC-C (Fisch et al., 1999; Sansone et al., 2012; Wheeler et al., 2014).
Given that the 10 to 20-year age range is a critical developmental period, we also administered the VABS to examine whether changes in adaptive behavior were commensurate with changes in aberrant behavior. As expected, we found that adaptive behavior increased significantly over time, as evidenced by significant gains in age-equivalent scores. Although there was no effect of baseline developmental age on trajectories of aberrant behavior, increased developmental age was a significant predictor of lower Irritability/Agitation in both males and females at age 10, and was a significant predictor of lower Hyperactivity/Noncompliance in males and Stereotypic Behavior in females at age 10. These data indicate that increases in adaptive behavior may be responsible (at least in part) for decreases (or at least the perception of decreases) in aberrant behavior over time. In contrast, there was no effect of baseline medication use on aberrant behavior at age 10 or the trajectory of aberrant behavior and the only significant relationship found between FMRP expression and aberrant behavior was with the trajectory of Hyperactivity/Noncompliance for females with FXS.
The scores obtained on the Lethargy/Social Withdrawal, Stereotypic Behavior, and Inappropriate Speech subscales of the ABC-C did not change for males or females over time, which might imply that these behaviors tend to persist into early adulthood in this population. It is important, however, to consider that the Stereotypic Behavior and Inappropriate Speech subscales contain fewer items than the other three subscales. Because the Stereotypic Behavior and Inappropriate Speech subscales of the ABC-C contain so few items (seven and four, respectively compared to 15, 16 and 16 in other domains), some researchers have chosen to omit those subscales in longitudinal analyses altogether (Anderson et al., 2011). Whether or not the results of the current study call into question the sensitivity of these ABC-C subscales, they have important implications for utilizing them as primary outcomes in treatment evaluations.
Several authors have examined whether the factor structure of the ABC-C, derived for populations of individuals with ID in general, is equivalent for individuals with FXS. Sansone et al. (2012), for example, tested the psychometric properties of the ABC-C in a large sample of individuals with FXS using exploratory and confirmatory factor analyses. Results showed that the factor structure was slightly different, with some items included in different domains and an additional factor (i.e., Social Avoidance; 4 items previously included in the Lethargy/Social Withdrawal subscale) to enhance the specificity and sensitivity of its use in FXS clinical trials. Although this study yielded a somewhat different factor structure of the ABC-C for the FXS population, a subsequent study found that the 6-factor solution resulted in only a slightly improved statistical fit for with this population (Wheeler et al., 2014). We therefore chose to use the original factor structure of the ABC-C in our analyses to allow for comparisons with other populations of individuals with and without ID (see Aman, 2012 for a discussion) and to provide a standard benchmark against which FXS-targeted treatment outcomes might be more easily compared. Interestingly, mean scores obtained at baseline on the five subscale scores in the present study were significantly lower than mean scores obtained in the Sansone et al. study. There were also statistically significant differences for a few of the age group comparisons presented by Wheeler et al., with males scoring lower than Sansone et al.’s sample on most subscales of the ABC-C. As pointed out by Wheeler and colleagues, the data in the Sansone et al. study included data from established clinics for individuals with FXS who presented with potentially greater behavioral challenges. However neither sample is likely to be representative of the FXS population as a whole (Wheeler et al., 2014, p.147).
A comparison of the current results with the cross-sectional trajectories presented by Sansone et al. and Wheeler et al. show strikingly similar patterns in aberrant behavior over time. Of particular interest are the similarities in trajectories between scores on the Lethargy/Social Withdrawal domain (presented here) and the new Social Avoidance domain (presented by Wheeler et al., 2014) showing a slight increase in the severity of these behaviors. Given these similarities, the original factor structure – which combines items related to lethargy and social avoidance – appears to produce the same clinical information. Considering this, and that the new factor structure does not dramatically improve the statistical fit with the fragile X population, use of the original structure might be preferable, as it allows for quantitative comparisons with other clinical populations.
In addition to the aforementioned cross-sectional research on aberrant behavior in FXS, the results of the current study also were similar to the longitudinal results presented by Anderson et al. (2011) on aberrant behavior in individuals with autism, ASD, and DD. Decreasing trends in scores on the Irritability/Agitation and Hyperactivity/Noncompliance subscales of the ABC-C were also evident in the diagnostic groups studied over approximately the same age range as the current study. Interestingly, the autism and ASD group – who often show a similar behavioral phenotype to individuals with FXS – showed the same pattern on the Lethargy/Social Withdrawal subscale of the ABC-C: a slight increase in the severity of social withdrawal behaviors into early adulthood. Taken together, these data support research in FXS indicating that individuals with FXS, particularly males, may require continued social support well into adulthood to decrease, or even stabilize social withdrawal behaviors (Sansone et al., 2012; Wheeler et al., 2014).
It is important to point out that the ABC-C is an informant-based questionnaire that may be subject to bias and recall effects. It requires caregivers to consider the last 4-week period and rate the items on a severity scale relative to the behavior of other children they know or most other children in their child’s classroom. Therefore, a plausible explanation for the decrease in scores on the ABC-C could be that parents are habituating to the severity of their child’s behavior over time. It seems prudent that other, more objective measures of aberrant behavior will need to be developed in the future to determine whether problem behaviors are in fact decreasing in severity over time, and whether the use of observational methods may be a more objective way to detect the efficacy of corresponding treatments. An equally important consideration concerns the fact that, as currently calibrated, scores on the ABC-C are not normed by age and, thus, the results of this and other research using this measure should be considered in this context.
A limitation of the present study was that we did not collect reliability measures (e.g., inter-rater/informant reliability) specific to the current sample. However, both the VABS and ABC-C have demonstrated acceptable reliability (Sparrow, Balla, & Cicchetti, 1984; Aman, Singh, Stewart, & Field, 1985). Another potentially important limitation concerns the fact that we were unable to quantify the extent to which individuals received interventions over the study time period. Although we found no significant effect of baseline medication use on the intercept or trajectory of aberrant behavior, a large proportion of our sample were taking psychoactive medications at baseline (i.e., 62.5% of males and 37.5% of females). It is possible that scores on the ABC-C may have been affected by varying amounts of therapeutic interventions participants received over the study period. Despite these limitations and given the rarity of prospective longitudinal studies using standardized measures, the current analyses provide important new information about patterns of aberrant behavior in young people with FXS. Future research should use these findings to compare results from large-scale, long-term, disease-specific behavioral, pharmacologic, and combined approaches to treatment in fragile X syndrome.
Highlights.
The longitudinal trajectory of aberrant behavior was examined using the ABC-C in individuals with FXS.
Concomitant changes in age-equivalent scores on the Vineland were also examined.
Scores on the Irritability and Hyperactivity subscales of the ABC-C decreased significantly with age.
The decrease in scores on the Hyperactivity domain was greater for males than females with FXS.
Longitudinal studies involving direct observation are needed to confirm these results.
Acknowledgments
This study was supported by grants MH50047, MH064708 (PI Allan Reiss) and MH081998 (PI Scott Hall) from the National Institute of Mental Health, and a gift from the Lynda and Scott Canel Family Fund.
Appendix
Raw means and SDs for the ABC-C subscales and VABS age-equivalent score by age band for males and females with FXS
| Males | I. Irritability/ Agitation |
II. Lethargy/Social Withdrawal |
III. Stereotypic Behavior |
IV. Hyperactivity Noncompliance |
V. Inappropriate Speech |
VABS Age Equivalent |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Band (years) |
N | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 10 to 12 | 22 | 10.27 | 6.92 | 4.41 | 5.21 | 5.77 | 5.11 | 13.73 | 7.73 | 4.64 | 3.14 | 4.77 | 1.88 |
| 12 to 14 | 20 | 9.35 | 7.76 | 7.40 | 7.70 | 4.95 | 4.14 | 13.65 | 8.73 | 3.80 | 3.05 | 6.30 | 2.58 |
| 14 to 16 | 27 | 6.74 | 5.95 | 5.78 | 4.15 | 3.26 | 3.65 | 11.44 | 8.11 | 4.22 | 2.64 | 6.12 | 1.93 |
| 16 to 18 | 24 | 5.12 | 6.30 | 4.83 | 5.00 | 2.67 | 3.35 | 8.63 | 8.10 | 3.67 | 2.32 | 7.52 | 2.78 |
| 18 to 20 | 23 | 6.61 | 8.24 | 6.91 | 6.91 | 4.87 | 4.26 | 8.96 | 8.21 | 5.00 | 3.50 | 7.24 | 2.43 |
| Females | I. Irritability/ Agitation |
II. Lethargy/Social Withdrawal |
III. Stereotypic Behavior |
IV. Hyperactivity Noncompliance |
V. Inappropriate Speech |
VABS Age Equivalent |
|||||||
| Age Band (years) |
N | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 10 to 12 | 16 | 5.37 | 7.97 | 3.19 | 3.53 | 1.44 | 2.58 | 4.75 | 6.40 | 2.13 | 2.83 | 8.50 | 2.28 |
| 12 to 14 | 17 | 1.53 | 2.92 | 3.65 | 5.59 | 0.76 | 1.35 | 2.47 | 2.81 | 0.59 | 1.06 | 8.80 | 2.74 |
| 14 to 16 | 24 | 6.58 | 8.83 | 4.25 | 5.26 | 1.29 | 2.05 | 6.08 | 7.49 | 1.83 | 2.78 | 10.68 | 2.00 |
| 16 to 18 | 19 | 3.63 | 9.00 | 5.58 | 6.92 | 1.53 | 3.17 | 3.53 | 6.90 | 1.26 | 2.13 | 11.87 | 4.00 |
| 18 to 20 | 19 | 1.89 | 3.04 | 3.95 | 4.03 | 0.26 | 0.73 | 3.32 | 3.23 | 0.89 | 1.45 | 12.47 | 2.65 |
Note. N is the number of observations available in each age band.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data were included in the model to improve estimation.
Contributor Information
Kristin M. Hustyi, Email: khustyi@stanford.edu.
Scott S. Hall, Email: hallss@stanford.edu.
Booil Jo, Email: booil@stanford.edu.
Amy A. Lightbody, Email: aal@stanford.edu.
Allan L. Reiss, Email: areiss1@stanford.edu.
References
- Aman MG. Aberrant behavior checklist: Current identity and future developments. Clinical and Experimental Pharmacology. 2012;2(3) [Google Scholar]
- Aman MG, Burrow WH, Wolford PL. The aberrant behavior checklist-community: Factor validity and effect of subject variables for adults in group homes. American Journal on Mental Retradation. 1995;100(3):283–292. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behaviour checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89(5):485–491. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. American Journal of Mental Deficiency. 1985;89(5):492–502. [PubMed] [Google Scholar]
- Anderson DK, Maye MP, Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities. 2011;116(5):381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron K, Oliver C, Moss J, Berg K, Burbidge C. The prevalence and phenomenology of self-injurious and aggressive behaviour in genetic syndromes. Journal of Intellectual Disability Research. 2011;55(2):109–120. doi: 10.1111/j.1365-2788.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal of Mental Retardation. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Bishop E, Olmsted M, Mallya UG, Berry-Kravis E. Medication utilization for targeted symptoms in children and adults with fragile X syndrome: US survey. Journal of Developmental and Behavioral Pediatrics. 2012;22(1):62–69. doi: 10.1097/DBP.0b013e318236c0e1. [DOI] [PubMed] [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, Abrams MT. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics. 1995;95(5):744–752. [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, Urv TK Outcome measures working groups. Outcome measures for clinical trials in fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2013;34(7):508–522. doi: 10.1097/DBP.0b013e31829d1f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Lewis MH. Self-injury and comorbid behaviors in developmental, neurological, psychiatric, and genetic disorders. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-injurious behavior: Gene-brain-behavior relationships. Washington, DC: American Psychological Association; 2002. pp. 23–40. [Google Scholar]
- Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, McBride SM. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Research. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. Journal of Cognition and Development. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter KP, Dekker MC, Verhulst FC, Koot HM. Developmental course of psychopathology in youths with and without intellectual disabilities. Journal of Child Psychology and Psychiatry. 2007;48(5):498–507. doi: 10.1111/j.1469-7610.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Koot HM, van der Ende J, Verhulst FC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2002;43(8):1087–1098. doi: 10.1111/1469-7610.00235. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Psychopathology in children with intellectual disability. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41(4):407–417. [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Ort S, Finucane B, Shapiro LR, Leckman JF. The trajectory of cognitive development in males with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(3):422–426. doi: 10.1097/00004583-198905000-00020. [DOI] [PubMed] [Google Scholar]
- Emerson E. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. Journal of Intellectual Disability Research. 2003;47(1):51–58. doi: 10.1046/j.1365-2788.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter NJ, Holden JJ, Simensen R, Howard-Peebles PN, Maddalena A, Nance W. Longitudinal assessment of adaptive and maladaptive behaviors in fragile X males: growth, development, and profiles. American Journal of Medical Genetics. 1999;83(4):257–263. doi: 10.1002/(sici)1096-8628(19990402)83:4<257::aid-ajmg5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, Habicht K. Longitudinal IQ changes in fragile X males. American Journal of Medical Genetics. 1989;33(4):513–518. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2006;36(7):935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal of Mental Retardation. 2008;113(1):44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108(2):105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin E, Jo B, Lightbody AA, Hazlett HC, Piven J, Hall SS, Reiss AL. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics. doi: 10.1542/peds.2013-3990. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz AM, Spiridigliozzi GA, Gullion CM, Ransford SN, Rao K. Aberrant behaviors of young boys with fragile X syndrome. American Journal of Mental Retardation. 1994;98(5):567–579. [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data, Second Edition. Hoboken, NJ: Wiley & Sons, Inc.; 2002. [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, McMahon D. Risperidone in children with autism and serious behavioral problems. The New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Meredith RM, de Jong R, Mansvelder HD. Functional rescue of excitatory synaptic transmission in the developing hippocampus in Fmr1-KO mouse. Neurobiology of Disease. 2011;41(1):104–110. doi: 10.1016/j.nbd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Sixth Edition. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252(5009):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Powis L, Oliver C. The prevalence of aggression in genetic syndromes: A review. Research in Developmental Disabilities. 2014 doi: 10.1016/j.ridd.2014.01.033. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods, Second Edition. Newbury Park, CA: Sage Publications Inc.; 2002. [Google Scholar]
- Reiss AL, Freund L. Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. American Journal of Medical Genetics. 1992;43(1–2):35–46. doi: 10.1002/ajmg.1320430106. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. The American Journal of Psychiatry. 2005;162(7):1361–1369. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, Hessl D. Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. Journal of Autism and Developmental Disorders. 2012;42(7):1377–1392. doi: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SR, Rojahn J, Reese RM. Brief report: reliability and validity of instruments for assessing psychotropic medication effects on self-injurious behavior in mental retardation. Journal of Autism and Developmental Disorders. 1997;27(1):89–102. doi: 10.1023/a:1025825322955. [DOI] [PubMed] [Google Scholar]
- Shedlack KJ, Hennen J, Magee C, Cheron DM. A comparison of the Aberrant Behavior Checklist and the GAF among adults with mental retardation and mental illness. Psychiatric Services. 2005;56(4):484–486. doi: 10.1176/appi.ps.56.4.484. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Smith LE, Barker ET, Seltzer MM, Abbeduto L, Greenberg JS. Behavioral phenotype of fragile X syndrome in adolescence and adulthood. American Journal on Intellectual and Developmental Disabilities. 2012;117(1):1–17. doi: 10.1352/1944-7558-117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition, Survey Form Manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sudhalter V, Cohen IL, Silverman W, Wolf-Schein EG. Conversational analyses of males with fragile X syndrome, Down syndrome, and autism: Comparison of the emergence of deviant language. American Journal on Mental Retardation. 1990;94(4):431–441. [PubMed] [Google Scholar]
- Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB., Jr Self-injurious behavior in young boys with fragile X syndrome. American Journal of Medical Genetics. Part A. 2003;118A(2):115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman R, Abbeduto L. Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities. 2014 doi: 10.1016/j.ridd.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64(1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, Bailey DB., Jr Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. American Journal of Medical Genetics. Part A. 2014;164A(1):141–155. doi: 10.1002/ajmg.a.36232. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Smits A, Mohkamsing S, van Beerendonk H, de Haan A, de Vries B, Oostra BA. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Human Genetics. 1997;99(3):308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(12):1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]