Abstract
Objective
To investigate whether eye-gaze avoidance, a striking phenotypic feature in fragile X syndrome (FXS), is associated with high levels of “hyperarousal” during social interactions with others. To date, almost all studies in this area have been confounded by inclusion of task demands in addition to social demands.
Method
We monitored the cardiovascular activity and eye-gaze avoidance of 50 boys and girls with FXS aged 5 to 20 years during a 25-minute intensive social interaction session with an unfamiliar experimenter. To control for possible family and genetic factors in cardiovascular activity, we compared each child with FXS with their same-sex typically developing biological sibling.
Results
Participants with FXS obtained significantly higher heart rates, lower vagal tone, and lower heart rate variability estimates both at baseline and during the social interaction session compared with their typically developing siblings. Although eye-gaze avoidance occurred at significantly higher levels in the children with FXS, this behavior decreased slightly over the course of the session (a “warm-up” effect) and did not seem to be associated with cardiovascular activity. In the girls with FXS, higher levels of the fragile X mental retardation protein were associated with higher (and more typical) heart rate variability.
Conclusions
These data suggest that both sympathetic and parasympathetic nervous systems are dysregulated in FXS. However, given that prolonged exposure to social demands does not inevitably lead to increased anxiety or “hyperarousal,” professionals should not be deterred from providing much needed social skills interventions for individuals with FXS.
Keywords: fragile X syndrome, hyperarousal, eye-gaze avoidance, vagal tone, heart rate
Fragile X syndrome (FXS) is the most common inherited known cause of intellectual disability occurring in as many as 1 in 4,000 male subjects and 1 in 8,000 female subjects.1 The syndrome results from a mutation to an unstable sequence of CGG nucleotides within the FMR1 gene, which is located on the long arm of the X chromosome.2 This critical sequence of CGG nucleotides within FMR1 repeats approximately 5 to 45 times in unaffected individuals; if the sequence expands to more than 200 repeats, hypermethylation of the promoter region of the gene is highly probable, and production of the “fragile X mental retardation protein” (FMRP), the protein product of the FMR1 gene, ceases to occur. The FMRP participates in the translational machinery that converts messenger RNA into protein, and thus, it is the lack of FMRP that seems to give rise to the cascade of physical, cognitive, and behavioral manifestations of the disorder.3 Given that FXS is an X-linked disorder, women are less affected by the syndrome and usually have higher levels of FMRP.
One of the most striking phenotypic features commonly seen in people with FXS is the propensity to avoid social situations, often manifested by severe eye-gaze avoidance and anxiety during social interactions, particularly in male.4–6 It has been suggested that eye-gaze avoidance displayed in people with FXS often follows a predictable pattern that is different from the eye-gaze avoidance commonly shown by children diagnosed with autism. For example, Cohen and coworkers6 observed 12 young male subjects with FXS while interacting with their mothers for 10 minutes and then while interacting with a stranger for 10 minutes. Results showed that children with FXS engaged in significantly higher levels of social avoidance behaviors compared with patients with Down syndrome and autism or with typically developing children. A further study conducted by Cohen and coworkers7 indicated that the manner in which children established eye contact with their parents differed between children with FXS and those diagnosed with autism. Specifically, children with FXS seemed to establish eye contact with the parent only when the parent was looking elsewhere, suggesting some kind of approach-avoidance conflict.8
The approach-avoidance hypothesis has been tested to some extent in a recent study in which pairs of FXS-affected and -unaffected siblings were exposed to conditions in which an experimenter sat face to face with the participant and delivered different levels of social and performance demands.5 Results showed that escape behaviors (including eye-gaze avoidance) were more likely to occur during conditions that involved high levels of social demands but not task demands. In the high social demand condition, eye-gaze avoidance occurred approximately 80% of the time in boys with FXS, and approximately 40% of the time in girls with FXS, despite repeated prompts for eye contact being made throughout the condition. More detailed analysis of the data indicated that prompts for eye contact from the experimenter were followed by a significant elevation in the probability of child eye contact, suggesting that the children were, in fact, responsive to the prompts for eye contact but that, once eye contact was established, it was maintained for only 1 or 2 seconds on average.
Murphy and coworkers9 have suggested that the cognitive processing demands typically associated with social interactions may contribute to increased eye-gaze avoidance in FXS. To test this theory, they experimentally manipulated levels of antecedent social and task demands while male participants with FXS were required to work on a computer task. In two of the conditions, the experimenter sat directly opposite the participant and delivered the task demands, whereas in two other conditions, the experimenter sat at the back of the room, and the task demands were delivered by the computer. In this study, however, eye-gaze avoidance was coded not only when the subject looked away from the experimenter (i.e., social avoidance) but also when the subject looked away from the computer screen (i.e., task avoidance), thus confounding task avoidance with social avoidance. Although Murphy et al.9 found that eye-gaze avoidance occurred in both sets of conditions, given their definition of eye-gaze avoidance, it seems that they were primarily studying task avoidance rather than social avoidance per se.
To further understand eye-gaze avoidance in FXS, several researchers have suggested that social avoidance behavior in FXS may result from a form of “hyperarousal” in which the sympathetic and parasympathetic nervous systems of people with FXS are dysregulated.10,11 This hyperarousal hypothesis may help to explain why people with FXS often seem overly anxious or agitated during social interactions. In the first of these studies, Belser and Sudhalter12 reported that the tonic skin conductance of several male subjects with FXS was significantly elevated during conversations involving direct gaze from others. Miller and coworkers13 also found that electrodermal responses were elevated in a group of 15 male subjects with FXS during auditory, tactile, visual, and olfactory stimulation compared with a group of age- and sex-matched typically developing controls. Interestingly, Miller et al.13 also found that levels of FMRP were associated with electrodermal activity, such that people with higher levels of FMRP evidenced a more typical electrodermal response.
Several studies have since monitored physiological arousal during cognitively demanding tasks, as opposed to social tasks. For example, Roberts and coworkers10 monitored cardiovascular activity in 29 boys with FXS, aged 1 to 11 years, while they alternated between a baseline task (watching a video) and performed a series of challenging cognitive tasks (subtests from the Stanford-Binet IV). Results showed that boys with FXS had significantly elevated heart rates (a measure of sympathetic nervous system activity) and significantly lower vagal tone (a measure of parasympathetic nervous system activity) both at baseline and during completion of the cognitive tasks, in comparison to a group of age-matched typically developing children. However, in this study, no relation was found between levels of FMRP and the cardiovascular indices.
In the study by Keysor and coworkers,11 skin conductance, heart rate, vagal tone, and electromyographic activity were monitored in 13 girls with FXS aged 13 to 22 years while they performed a series of mental arithmetic, divided attention, and risk-taking tasks on a computer. Results showed that girls with FXS showed elevated levels of skin conductance at baseline in comparison to an age-matched group of control subjects. However, girls with FXS obtained similar physiological indices to a matched group of girls with Turner syndrome, suggesting that both groups of subjects were experiencing heightened arousal before the testing even began. In a further analysis of these data, Roberts and coworkers8 reported that, although heart activity in girls with FXS increased only minimally during performance of the mental arithmetic task, change in heart rate activity was associated with poorer performance on this task.
To our knowledge, no studies have directly measured the physiological correlates of social avoidance behavior in children with FXS during stressful social situations. In the present study, therefore, we exposed children with FXS to a 25-minute social interaction session with an unfamiliar experimenter. To ensure that the interaction remained stressful, eye contact prompts were presented every 30 seconds. In addition, only social demands were presented to avoid any task demands confounding the study. We simultaneously recorded measures of sympathetic and parasympathetic functioning in addition to recording observations of eye-gaze avoidance. To control for possible family and genetic factors in cardiovascular activity, we compared children with FXS to their same-sex typically developing sibling. We had two main hypotheses. First, we expected that children with FXS would show higher levels of physiological arousal and social gaze avoidance compared with their typically developing siblings. Second, we predicted that children with FXS would show greater physiological reactivity to social interaction (as evidenced by larger changes in cardiovascular indices relative to baseline) compared with their typically developing siblings. We also hypothesized that, within the group of children with FXS, low levels of FMRP (indicative of greater symptom severity) would predict increased physiological reactivity and increased eye-gaze avoidance during social interactions.
METHOD
Participants
Participants were 50 pairs of sex-matched siblings: 26 male and 24 female subjects. Each pair consisted of a child diagnosed with FXS and a typically developing biological sibling (TYP). Criteria for inclusion were as follows: one child in the household had received a diagnosis of FXS, another same-sex biological sibling also lived in the home but was unaffected by FXS (as demonstrated by DNA testing), the children were both in school or at college, and the mother of the children was a premutation carrier of the FMR1 mutation. If families had more than one typically developing same-sex sibling in the target age range, then a same-sex sibling closest in age to the child with FXS was chosen to take part in the study. Some children with FXS also had siblings with FXS, although diagnosis was not always confirmed. If families had more than one child with FXS in the target age range, then a female child with FXS closest in age to a typically developing female sibling was chosen. This was done to increase the number of female sibling pairs in the sample. Demographic characteristics of the sample are shown in Table 1. There were no significant differences in chronological age between the groups of siblings, (t49 = −0.92, p = .36). Thirty children (60%) with FXS had an older same-sex unaffected sibling (mean 3.5 years older), and 19 children (38%) with FXS had a younger same-sex unaffected sibling (mean 3.0 years younger). One pair was nonidentical twins. As expected, there were significant differences in IQ scores between the groups, with the children with FXS obtaining significantly lower IQ scores than their typically developing siblings (t49 = −17.74, p < .001).
TABLE 1.
Demographic Information
| Male Pairs | Female Pairs | |||
|---|---|---|---|---|
| FXS | Sibling | FXS | Sibling | |
| Age, y | ||||
| Mean | 14.57 | 14.60 | 13.13 | 14.03 |
| SD | (2.77) | (3.76) | (4.13) | (4.52) |
| Range | 8.2–20.4 | 7.1–21.9 | 5.0–19.6 | 6.2–23.2 |
| IQ | ||||
| Mean | 46.92 | 107.35 | 71.88 | 112.79 |
| SD | (9.99) | (9.86) | (22.12) | (12.86) |
| Range | 40–73 | 85–126 | 40–125 | 97–146 |
| Medication % | ||||
| Antidepressant | 34.6 | 7.7 | 29.2 | 0.0 |
| Stimulant | 46.2 | 7.7 | 16.7 | 0.0 |
| Antipsychotic | 7.7 | 0.0 | 0 | 0.0 |
| Antihypertensive | 11.5 | 0.0 | 8.3 | 0.0 |
| Anticonvulsant | 15.4 | 0.0 | 4.2 | 0.0 |
| More than 1 | 34.6 | 0.0 | 12.5 | 0.0 |
Note: FXS = fragile X syndrome.
Families were recruited from across the United States (west 30%, south 26%, north central 24%, and northeast 16%) and Canada (4%) through the National Fragile X Foundation, flyers distributed to special interest groups, local contacts, and our research Web site. Written informed consent was obtained from the parents of all of the participants. The ethnic distribution of the study sample was 90% white, 4% Hispanic, 2% African American, 2% Asian, and 2% Pacific Islander.
Diagnostic status of affected and unaffected children was confirmed by polymerase chain reaction and southern blot DNA analyses (Kimball Genetics, Denver, CO). All the children with FXS had CGG repeat lengths greater than 200 (full mutation range), with hypermethylated alleles. All of the unaffected children had CGG repeat lengths less than 40 (reference range); therefore, none of the siblings were carriers of the premutation or full mutation. The FMRP analyses were conducted on all blood samples from the subjects with FXS using the method devised by Willemsen and coworkers.14 Mean percentage FMRP levels were 16.7% (SD 16.1, range 2.5%–60.5%) in the boys with FXS and 52.1% (SD 18.3, range 15.0%–84.5%) in the girls with FXS. As expected, the girls with FXS had significantly higher levels of FMRP than the boys with FXS (t44 = 6.98, p < .001).
Procedures
Parental consent was obtained for all participants in the study and all procedures were approved by the local institutional review board. Two researchers arrived at the family home at 3 p.m. and conducted the experimental evaluation with each child. The order in which each pair of children received the evaluation was counterbalanced across siblings. Immediately before the session, a Polar chest belt was fitted around the child’s chest, and a heart rate and activity monitoring device (Mini-Logger 2000 system; Mini Mitter Respironics, Bend, OR) was placed on the child’s belt loop. The Mini-Logger 2000 is a battery-powered device that uses a radiotelemetry system to detect the R wave of the heart signal from a Polar chest belt. The receiver unit (measuring 4.5 × 2.5 in.) also contains a mercury-filled motion sensor mounted on a spring that can provide activity counts in 30-second epochs.
After the heart rate monitoring device had been fitted, each child sat in front of a laptop computer and watched a video of a relaxing seascape for 10 minutes (the last 5 minutes of which was used in the analysis). The laptop was then removed, and the experimenter sat in a chair directly opposite the participant, with knees almost touching. A camera operator stood several feet away from the interviewer, remaining as unobtrusive as possible. The experimenter then said: “For the next 25 minutes, we are going to have a conversation or talk to each other. The main thing I would like you to do is to maintain eye contact or look at my eyes as much as possible throughout this time.” The experimenter began this intensive interaction session by asking a question such as, “How are you doing today?,” “Have you seen any movies lately?,” “Do you have any hobbies?,” and if appropriate for the child’s age, questions such as “Where are you from?” and “How old are you?” If the participant did not respond, the experimenter asked another question. Throughout the experiment, the experimenter reminded the participant to maintain eye contact using prompts such as “Remember to look at me” or “You need to look at my eyes” approximately every 30 seconds. Each 5-minute period of the session was demarcated by the experimenter reaching forward and pressing a button on the Mini-Logger receiver unit. To provide a more naturalistic context of social interaction, proximity between the experimenter and the participant was manipulated in an ABABA design. In the “A” condition, proximity was 0 m (i.e., knees almost touching). In the “B” condition, proximity was 2 m.
Response Definitions, Recording Technique, and Interobserver Agreement
Child eye-gaze avoidance was defined as the child looking away from the experimenter’s face, including avoiding eye contact even when prompted; experimenter social demands was defined as any vocalization from the experimenter directed toward the participant such as giving instructions, asking questions, giving eye contact prompts, and engaging in general conversation.
Observations were recorded from DVD using software that allowed multiple behaviors to be simultaneously coded in real time.15 Behaviors were recorded as durations (i.e., the observer was required to press a specific key on the keyboard to indicate its onset and again to indicate its offset). All observational data were saved in Sequential Data Interchange Standard format for later analysis using the GSEQ version 4.2 software program (Georgia State University, Atlanta).16 During 25% of the sessions, a second observer also collected data. Agreement was calculated on a 10-second interval-by-interval basis using Cohen κ.17 The mean level of agreement for child eye-gaze avoidance and experimenter social demands was 0.71 (range 0.62–0.85) and 0.90 (range 0.84–0.96), respectively.
Data Analysis
All heart rate and activity count data were downloaded from the Mini-Logger 2000 to a personal computer using Mini-Log 2000W version 1.2 (Mini-Mitter, 2001). The data were then analyzed using MXedit version 2.21 (Delta-Biometrics, Bethesda, MD). The interbeat interval (IBI) data were first inspected to remove artifacts and to correct errors. To calculate vagal tone, the data analysis included converting the heart periods to constant sampling rates by resampling the data at 500-millisecond intervals, detrending the resampled data with a 21-point moving cubic polynomial, processing the detrended data with a digital bandpass filter (0.12 Hz for the low limit of the bandpass and 0.40 Hz for the high limit of the bandpass), and computing the natural logarithm of the bandpassed variance.
The percentage of time during which experimenter social demands and child eye-gaze avoidance occurred in each 5-minute period was calculated by dividing the number of seconds of occurrence of each target behavior by 300 and multiplying by 100. The mean percentage duration of social demands delivered by the experimenter in each 5-minute period was 58.2% (range 57.3%–59.6%) for the male subjects with FXS, 50.6% (range 47.9%–52.8%) for the TYP male subjects, 56.8% (range 54.2%–58.4%) for the female subjects with FXS, and 51.0% (range 49.0%–51.8%) for the TYP female subjects. Levels of social demands were therefore consistently applied between groups and across each 5-minute period of social interaction.
To compare FXS and TYP children, we used a mixed-effects regression model with nested random effects for child within family, using “XTREG” in STATA 10 (StataCorp, College Station, TX). We fit the effect of time by two connected straight lines, representing an initial effect between baseline and the first 5 minutes of social interaction and a subsequent trend between 5 and 25 minutes of social interaction. This model is more easily interpreted than a quadratic model in which initial and subsequent effects cannot be examined separately. In addition to time, fixed effects included group and interactions of group with each effect of time. Time-by-group interactions were tested using the 2-df Wald test to inspect both interactions simultaneously. For each response at time t, chronological age and activity counts at time t (recorded by the Mini-Logger 2000) were included as covariates. Separate analyses were conducted for the male and the female subjects.
RESULTS
Cardiovascular Indices
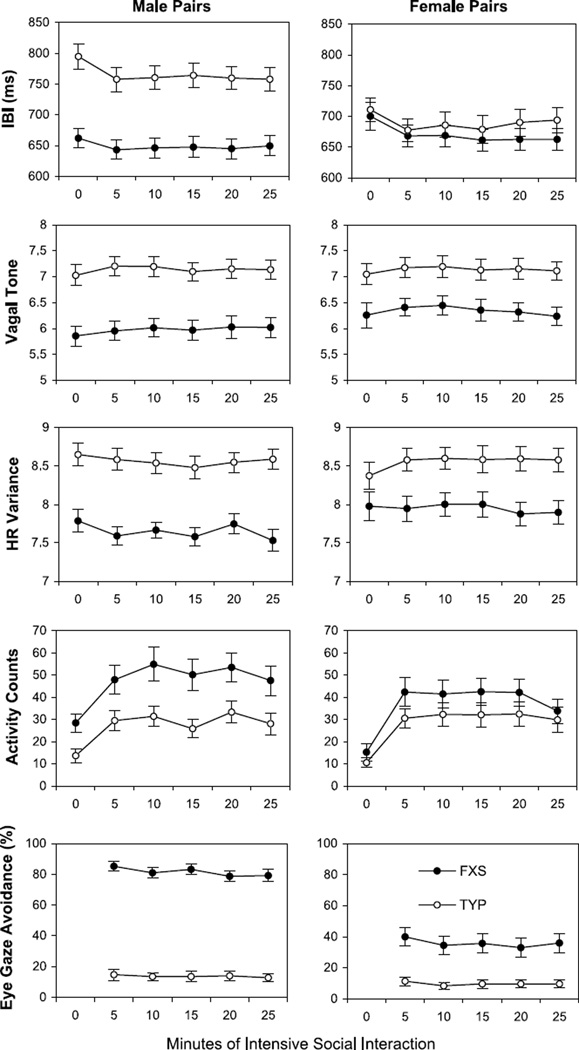
Figure 1 shows the observed means and SEs for the IBI, vagal tone, and heart rate variability measures for male and female sibling pairs at baseline and at each successive 5-minute period of social interaction.
Fig. 1.
Mean IBI, vagal tone, heart rate variability, activity counts, and eye-gaze avoidance for male and female sibling pairs of children plotted in successive 5-minute periods of intensive social interaction. Standard error bars are also shown. 0 min = baseline; FXS = fragile X syndrome; HR = heart rate; IBI = interbeat interval; TYP = typically developing biological sibling.
As shown in Figure 1, boys with FXS obtained significantly higher activity counts than their typically developing siblings in each 5-minute period of the experiment (p < .001). This variable was therefore included as a covariate in all subsequent analyses in both groups, as well as age. When controlling for activity counts and age of the children, baseline IBI, vagal tone, and heart rate variability indices were all significantly lower in the male subjects with FXS than in their typically developing siblings (p < .001 for each outcome). Baseline vagal tone (p = .001) and heart rate variability (p = .049) were also significantly lower in the female subjects with FXS than in their typically developing siblings. Baseline IBI was not significantly different between female sibling pairs (p = .542). There was a significant time-by-group interaction for IBI in boys (p = .027), with IBI levels in boys with FXS decreasing significantly at 5 minutes but making a slight, but non-significant, recovery at 25 minutes. The IBI levels in the TYP male subjects also decreased significantly below their baseline levels, on average, at 5 minutes and continued to decrease by 25 minutes. There was also a significant time-by-group interaction for IBI in the female subjects (p = .009). The IBI levels in the female subjects with FXS decreased below their baseline at 5 minutes and continued to decrease at 25 minutes. The IBI levels in the TYP female subjects also decreased below their baseline, on average, at 5 minutes but recovered back to baseline at 25 minutes.
For vagal tone, there was no significant time-by-diagnosis interaction in boys (p = .552) or in girls (p = .633). For heart rate variability, there was also no significant interaction in boys (p = .730). However, the girls showed a significant interaction of time and group on heart rate variability (p = .019). The girls with FXS showed little change in heart rate variability throughout the experiment. In contrast, heart rate variability in the TYP girls increased above baseline, on average, at 5 minutes and remained above baseline, on average, at 25 minutes.
Eye-Gaze Avoidance
Figure 1 (lower panel) shows the mean level of eye-gaze avoidance observed in each 5-minute period of the social interaction. As expected, the boys and girls with FXS showed significantly higher mean levels of eye-gaze avoidance compared with their typically developing siblings (p < .001 for both). The boys and girls with FXS avoided eye contact with the experimenter approximately 80% and 40% of the time, respectively, whereas typically developing siblings avoided eye contact only 10% of the time on average. Interestingly, eye-gaze avoidance decreased slightly in the male subjects with FXS over time (p < .002), but this trend was not apparent in typically developing boys (p = .588). A similar but nonsignificant trend over time was seen in the girls with FXS (p = .066) but not in typically developing girls (p = .673).
Association Between Cardiovascular Indices and Eye-Gaze Avoidance
To determine whether levels of child eye-gaze avoidance were associated with the cardiovascular indices, we reran the analyses of IBI, vagal tone, and heart rate variance at time t, adding eye-gaze avoidance at time t as a potential predictor. We also ran the analyses with eye-gaze avoidance added at time t + 5 minutes and at time t − 5 minutes to determine whether there may be a significant lead or lag effect. Results were not significant in either of these analyses.
Association Between Cardiovascular Indices and FMRP
We conducted a similar secondary analysis to that previously conducted to examine the effect of FMRP levels on IBI, vagal tone, and heart rate variability within children with FXS. First, we included FMRP and two interactions with time and used the 3-df Wald test for all three terms at once. Second, we included only a main effect of time. For this model, FMRP was not significant for the boys or the girls for all three cardiovascular measures except for heart rate variability in the girls with FXS. The 3-df test yielded p = .0099 for a time-by-FMRP interaction, such that the girls with the lowest observed level of FMRP (2.5%) were predicted to decrease in heart rate variability in the first 5 minutes of social interaction, whereas the girls with the highest level of FMRP (84.5%) were predicted to increase in heart rate variability in the first 5 minutes during the same time (when adjusted to the average age and the average activity counts). Thus, for heart rate variability, the girls with FXS who had higher (more typical) levels of FMRP had a pattern more similar to their typically developing siblings.
Association Between Cardiovascular Indices and Psychoactive Medication Status
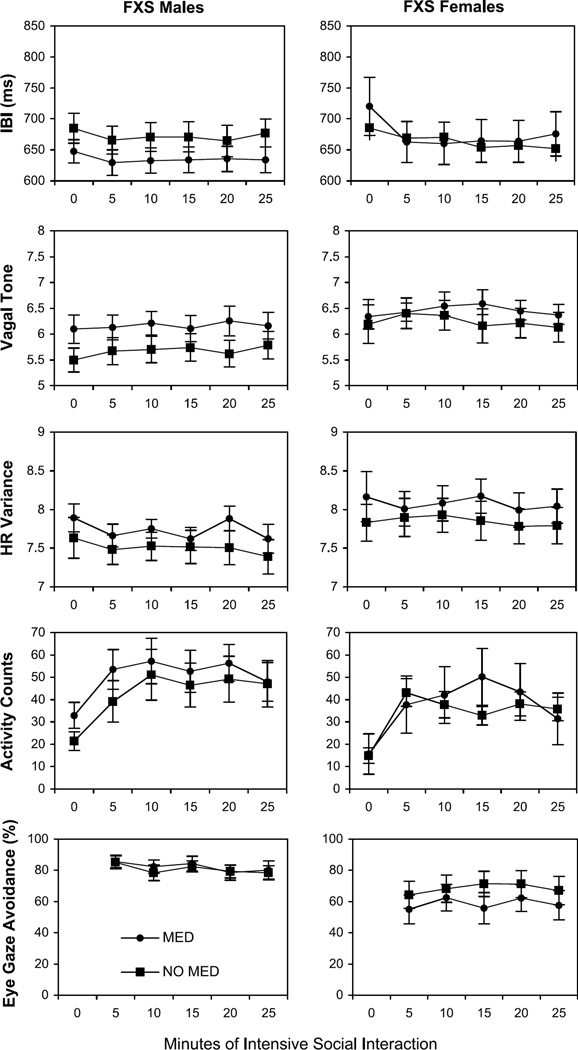
As shown in Table 1, 46.2% of the boys with FXS and 16.7% of the girls with FXS were taking stimulant medications at the time of the study. Antidepressant medications were being taken by 34.6% of the boys with FXS and 29.2% of the girls with FXS. These medications are likely to influence (directly and/or indirectly) some of the measures (including the physiological measures as well as neurobehavioral systems that could influence behavior and emotion during the study). Figure 2 shows the primary variables broken down according to whether the children with FXS were taking psychoactive medication. Although some trends can be seen in the data, statistical analysis indicated that there were no significant differences between the subjects who were taking psychoactive medication and those who were not taking psychoactive medication on any of the variables.
Fig. 2.
Mean IBI, vagal tone, heart rate variability, activity counts, and eye-gaze avoidance for boys and girls with FXS plotted in successive 5-minute periods of intensive social interaction. Data are displayed according to whether the children were taking psychoactive medication. Standard error bars are also shown. 0 min = baseline; FXS = fragile X syndrome; HR = heart rate; IBI = interbeat interval; MED = medication.
DISCUSSION
We monitored the cardiovascular activity of the 50 boys and girls with FXS during a 25-minute intensive social interaction session and compared each child with their same-sex typically developing sibling. To remove the potential confound of including task demands during the social encounter, the experimenter presented only antecedent social demands and delivered eye contact prompts every 30 seconds to ensure that the interaction was stressful. Results showed that the boys with FXS obtained significantly higher heart rates (as evidenced by lower IBIs), significantly lower vagal tone, and significantly lower heart rate variability compared with their typically developing siblings, both at baseline and throughout the 25-minute social interaction session. The girls with FXS also obtained significantly lower vagal tone and heart rate variability estimates than their typically developing siblings throughout the experiment, although their heart rates were similar to their typically developing siblings. Taken together, these results support previous literature suggesting that the children with FXS seem to have an overactive sympathetic nervous system (as evidenced by increased heart rates and low heart rate variability) and an underactive parasympathetic nervous system (as evidenced by lower vagal tone).10
It seems likely that the dysregulated cardiovascular activity found in the children with FXS may result from the increased distress of making eye contact with others. If the children with FXS find this social task somehow demanding, threatening, or novel, then engaging in eye-gaze avoidance will temporarily result in the removal of this distress. This coping strategy may, perversely, explain why eye-gaze avoidance occurs so frequently in the children with FXS because the contingent removal of aversive stimulation constitutes negative reinforcement of the behavior. As predicted, both boys and girls with FXS showed significantly higher levels of eye-gaze avoidance than their sex-matched typically developing siblings, avoiding eye contact with the experimenter a remarkable 80% and 40% of the time on average, respectively. These data support a previous study in which observations of eye-gaze avoidance were also coded from videotape in the boys and girls with FXS.5 Interestingly, however, we were unable to demonstrate an association between cardiovascular activity and eye-gaze avoidance in the present study. One possibility is that cardiovascular activity and eye-gaze avoidance in FXS occur on different temporal scales. Future studies could use time series analysis to examine whether a significant moment-to-moment lead or lag time exists between the two variables, or whether the relation is more extended in time. It is also possible that the children with FXS were already experiencing anticipatory anxiety about the social interaction, even before it had begun, so that cardiovascular activity may already have peaked in these children.
We found no association between FMRP levels and the cardiovascular indices in the boys with FXS, suggesting that the dysregulation of the autonomic nervous system among the children with FXS may be linked to other factors. However, it should be pointed out that, in the boys with FXS, the range of FMRP values was severely restricted, with most of the boys with FXS having FMRP levels less than 20%. Thus, we may have failed to detect an association between cardiovascular activity and FMRP levels in this group simply because of a lack of variance in FMRP. In support of this explanation, we did find an association between FMRP levels and heart rate variability in the girls with FXS. Specifically, we found that the girls who had FMRP levels that were closer to the reference range had heart rate variability levels that were similar to their typically developing siblings. These data suggest, at least in the girls with FXS, that brain FMRP levels may be associated with sympathetic nervous system regulation.
There are several advantages to the design of the study presented here over previous reports. First, children with FXS were directly compared with their sex-and age-matched typically developing sibling to reduce any systematic variance related to (other) genetic and family variables. Second, to increase the ecological validity of the study, the intensive social interaction session was conducted in the child’s natural environment, shortly after the experimenter had arrived at the child’s home. We also systematically manipulated the degree of proximity between the experimenter and the child without allowing the child to escape from the interaction. Given that previous studies to date have monitored physiological activity in laboratory settings and have used nonsocial stressors (cognitive tasks) to elicit cardiovascular activity, the present study can therefore be considered a more naturalistic test of the hyperarousal hypothesis in FXS. A further unique contribution of this study is that we also directly measured eye-gaze avoidance throughout the experiment. This allowed us to determine whether there was an association between this prominent behavioral feature of FXS and cardiovascular activity. In addition, we controlled for the potential confound of increased motor activity in children with FXS by recording activity counts from the Mini-Logger 2000 system and using this measure as a covariate in our analyses.
There are also several weaknesses to the present study. First, we did not include a comparison group of children with similar levels of developmental disability. It is possible, for example, that dysregulation of cardiovascular activity may not be specific to children with FXS and may also occur in children with other forms of developmental disability. For example, autonomic dysregulation has also been found in patients diagnosed with Turner syndrome,18 Rett syndrome,19 autism,20 and anxiety disorders.21 Second, although we were able to statistically control for differences in age and activity level of the children, future studies should attempt to directly control these factors using groups with smaller age ranges and less variance in activity level. Third, many of the children with FXS were taking medications at the time of the study that may have directly influenced cardiovascular activity. For example, 46% of the boys with FXS were taking stimulant medication, whereas 29% of the girls with FXS were taking antidepressant medication at the time of the study. Further analysis of the data showed that, although some trends emerged in the data, there were, in fact, no statistical differences in cardiovascular activity between those children with FXS taking psychoactive medications and those not taking psychoactive medications. Given that a large proportion of the patients with FXS take medications, future studies should investigate the effect of psychoactive medications on hyperarousal and social avoidance behavior in larger groups of children with FXS.
In a previous study, we exposed children with FXS to both social and performance demands and found that escape behaviors were more likely to occur during social tasks.5 However, in that study, children were exposed to social demands for only 5 minutes, leaving the question open as to what would transpire if the social interaction session was prolonged in duration. In the present study, therefore, we designed the experiment so that social demands were consistently applied for a longer period of time (25 minutes) to determine whether eye-gaze avoidance and concomitant cardiovascular indices would increase, a reflection of the so-called hyperarousal effect. Surprisingly, we found that both eye-gaze avoidance and heart rate actually decreased slightly over time in the boys with FXS. Roberts and coworkers8 also reported a “warming up” effect over the course of a research assessment period. This finding has extremely important implications for intervention. For example, some investigators have suggested that interventions to improve eye contact should not be attempted because of the potential risk for increasing levels of hyperarousal and anxiety.22 The data from the present study suggest that, on the contrary, exposure to social demands does not inevitably lead to increased anxiety and hyperarousal in FXS. Rather, it seems likely that preventing the child from escaping from the social situation and providing repeated prompts for eye contact may eventually lead to extinction of the social avoidance behaviors in FXS.
In a recent study, we showed that it is possible to improve eye contact duration in boys with FXS using a basic behavioral shaping technique.23 In that study, we sat face to face with the participant and made systematic prompts for longer and longer durations of eye contact, reinforcing each criterion response with an edible or desired item without allowing the children the opportunity to escape. Data showed that, over several hundred of these discrete trials, eye-contact duration improved significantly in four of the six boys with FXS, without concomitant increases in hyperarousal. Although further research will be needed to determine whether these gains in eye contact can be generalized and maintained over time, these data suggest that professionals should not be deterred from providing much needed social skills interventions for patients with FXS.
Acknowledgments
This research was supported by NIMH grants MH50047 and MH01142 and the Canel Fragile X Family Fund.
The authors thank the families of children with fragile X syndrome for their participation in this project. The authors also thank Jane Roberts, Rosemary Reidy, and Natalee Maynes for help with data analysis.
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AK, Safanda JF, Fall MZ, et al. Molecular predictors of cognitive involvement in female carriers of fragile X syndrome. JAMA. 1994;271:507–514. [PubMed] [Google Scholar]
- 4.Wolff PH, Gardner J, Paccla J, Lappen J. The greeting behavior of fragile X males. Am J Ment Retard. 1989;93(4):406–411. [PubMed] [Google Scholar]
- 5.Hall SS, DeBernardis GM, Reiss AL. Social escape behaviors in children with fragile X syndrome. J Autism Dev Disord. 2006;36:935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- 6.Cohen IL, Fisch GS, Sudhalter V, et al. Social gaze, social avoidance, and repetitive behavior in ragile X males: a controlled study. Am J Ment Retard. 1988;92(5):436–446. [PubMed] [Google Scholar]
- 7.Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. Am J Med Genet. 1991;38(2–3):498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- 8.Roberts J, Mazzocco MM, Murphy MM, Hoehn-Saric R. Arousal modulation in females with fragile X or Turner syndrome. J Autism Dev Disord. 2008;38(1):20–27. doi: 10.1007/s10803-007-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy MM, Abbeduto L, Schroeder S, Serlin R. Contribution of social and information-processing factors to eye-gaze avoidance in fragile X syndrome. Am J Ment Retard. 2007;112(5):349–360. doi: 10.1352/0895-8017(2007)112[0349:COSAIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JE, Boccia ML, Bailey DB, Jr, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Dev Psychobiol. 2001;39(2):107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- 11.Keysor CS, Mazzocco MM, McLeod DR, Hoehn-Saric R. Physiological arousal in females with fragile X or Turner syndrome. Dev Psychobiol. 2002;41(2):133–146. doi: 10.1002/dev.10060. [DOI] [PubMed] [Google Scholar]
- 12.Belser RC, Sudhalter V. Arousal difficulties in males with fragile X syndrome: a preliminary report. Dev Brain Dysfunct. 1995;8:270–279. [Google Scholar]
- 13.Miller LJ, McIntosh DN, McGrath J, et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83(4):268–279. [PubMed] [Google Scholar]
- 14.Willemsen R, Smits A, Mohkamsing S, et al. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Hum Genet. 1997;99:308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- 15.Obswin: Software for the collection and analysis of observational data [computer program]. Version. Birmingham: University of Birmingham; 1998. [Google Scholar]
- 16.Bakeman R, Quera V. Analyzing Interaction: Sequential Analysis with SDIS and GSEQ. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 17.Hartmann DP. Considerations in the choice of interobserver reliability estimates. J Appl Behav Anal. 1977;10:103–116. doi: 10.1901/jaba.1977.10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravholt CH, Hansen KW, Erlandsen M, Ebbehoj E, Christiansen JS. Nocturnal hypertension and impaired sympathovagal tone in Turner syndrome. J Hypertens. 2006;24(2):353–360. doi: 10.1097/01.hjh.0000200509.17947.0f. [DOI] [PubMed] [Google Scholar]
- 19.Julu PO, Kerr AM, Apartopoulos F, et al. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85(1):29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005;27(7):509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69(9):935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 22.Dykens EM, Hodapp RM, Finucane BM. Genetics and Mental Retardation Syndromes: A New Look at Behavior and Interventions. Baltimore: Paul H. Brookes Publishing; 2000. [Google Scholar]
- 23.Hall SS, Maynes NP, Reiss AL. Using percentile schedules to increase eye contact duration in children with fragile X syndrome. J Appl Behav Anal. doi: 10.1901/jaba.2009.42-171. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]