Abstract
Huntington’s disease (HD) is a fatal neurodegenerative disease characterized by metabolic, cognitive, and motor deficits. HD is caused by an expanded CAG repeat in the first exon of the HTT gene, resulting in an expanded polyglutamine section. Dietary restriction (DR) increases lifespan and ameliorates age-related pathologies, including in a model of HD, but the mechanisms mediating these protective effects are unknown. We report metabolic and behavioral effects of DR in the full-length YAC128 HD mouse model, and associated transcriptional changes in hypothalamus and striatum. DR corrected many effects of the transgene including increased body weight, decreased blood glucose, and impaired motor function. These changes were associated with reduced striatal human (but not mouse) HTT expression, as well as alteration in gene expression regulating histone acetylation modifications, particularly Hdac2. Other mRNAs related to Huntington’s pathology in striatal tissue showed significant modulation by the transgene, dietary restriction or both. These results establish a protective role of DR in a transgenic model that contains the complete human HTT gene and for the first time suggest a role for DR in lowering HTT level, which correlates with severity of symptoms.
Keywords: Huntington’s Disease, Dietary Restriction, Creb-binding Protein, HDAC, YAC128, HTT, NF-κB
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease characterized by chorea, cognitive deficits, and mood disorders (Walker, 2007). HD is caused by extended CAG repeats in exon 1 of the huntingtin gene (HTT) resulting in an expanded polyglutamine (polyQ) region at the N-terminus. This mutation impacts disease prognosis and penetrance according to the number of repeats with full penetrance beyond 41 repeats (Duyao et al., 1993; Langbehn et al., 2004; McNeil et al., 1997; Rubinsztein et al., 1996). Disease severity further correlates with level of mutant protein in human patients and mouse models (Graham et al., 2006; Hodgson et al., 1999; Squitieri et al., 2003). Prominent neuropathology in HD patients includes the loss of striatal medium spiny neurons (Bruyn et al., 1979; Graveland et al., 1985), as well as loss of cortical neurons (Cudkowicz and Kowall, 1990; Hedreen et al., 1991). In addition, abnormalities in the hypothalamus may contribute to metabolic and other defects, e.g. hyperglycemia and disruption of circadian rhythms (Hult et al., 2011; Hurlbert et al., 1999; Morton et al., 2005; Petersén and Björkqvist, 2006).
In HD, there is considerable neuronal dysfunction leading to symptoms and ultimately neurodegeneration prior to death. A previous study in an HD fragment model using a prion promoter driven transgene, i.e. HD-N171-82Q, demonstrated later onset of symptoms and extended lifespan in animals subjected to dietary restriction (DR) (Duan et al., 2003). The present studies examined if DR would mitigate the phenotype in a full-length mouse model of HD. In addition, expanded polyglutamine impacts transcriptional regulation, in part by sequestering transcriptional machinery (Cui et al., 2006; Dunah et al., 2002; Nucifora Jr. et al., 2001), and these transcriptional changes in the context of DR have not been explored. Such analysis could implicate the expression of specific genes in the development of pathology and its protection by DR and might distinguish between specific effects on HD transcriptional dysregulation versus more general mechanisms that delay the ageing process. We deliberately chose the YAC128 model to assess these questions because it harbors human HTT including its promoter and intronic sequences (Slow, 2003). We report that DR rescues many of the metabolic and HD-associated phenotypes in the YAC128 model. In addition, we characterize striatal and hypothalamic genes associated with HD pathology and their changes by DR. Amongst other molecular changes, we found that protective effects of DR dovetail with other reports supporting histone acetylation as a potential therapeutic in HD. In addition, we report that DR specifically reduces the level of transgenic HTT mRNA in the striatum.
Materials and Methods
Mice and Diet
YAC128 transgenic HD mice and littermate wild-type (WT) controls were used in this study (Jackson labs, Bar Harbor, Maine USA). YAC128 mice express the human huntingtin protein containing 128 CAG repeats (Slow, 2003). 3–5 month old mice were separated into groups of ad lib feeding conditions, or dietary restriction (every other day feeding) using a balanced design (Goodrick et al., 1990). Dietary restricted mice had their food removed every other day 1 hour before lights out. Mice were kept under a 12-hour light: 12-hour dark cycles. These procedures were approved by the institutional Animal Care and Use Committee.
Rotarod and Locomotor Activity
Motor performance was assessed in all mice using a Rotamex Rotarod (Columbus Instruments, Columbus, OH, USA). A trial consists of placing the mouse in a slow rotating rod of 4 RPM with 1 RPM acceleration every 8 seconds. There were three daily trials per animal with one hour in between trials. For naïve animals, the first two days of trials are considered training days and are followed by 4 days of experimental trials. Rotarod assays were performed at 6 and 8 months of age. Locomotor activity was recorded using a Digiscan D-Micropro automated activity monitoring system (Accuscan, Inc., Columbus, OH). The device consists of transparent plastic boxes (45×20×20 cm) placed within metal frames that are equipped with 16 infrared light emitters and detectors. The number of photocell beam breaks is recorded by a computer interface in 5 minute intervals. Mice were placed into the activity monitors and activity was recorded for 60 minutes after one hour acclimation to the chambers.
Statistical Analysis
Data were analyzed using PRISM 5 Software. Effects of diet and transgene were examined using 2-Way ANOVA and Bonferroni post-hoc tests when appropriate. Relative contributions of diet, transgene, and body weight to average rotarod performance were analyzed using the General Linear Model (Mizuno et al., 1996).
Blood glucose and insulin assays
Tail blood was taken on the morning after DR groups were fed before sacrifice and three months into the diet. Blood glucose was measured using a Bayer Contour glucose meter (Bayer, Mountain View, CA). Diurnal blood glucose measurements were taken in 8 month-old mice, and within the fed 24-hour cycle to account for effects of intermittent fasting. Glucose tolerance test was carried out after a 4-hour fast followed by an intra-peritoneal injection of 20% glucose in saline, normalized for body weight (10μl/g). Blood insulin was measured using an enzyme-linked immunosorbent assay (ELISA) from Millipore (Billerica, MA).
Tissue Collection
Mice were sacrificed by brief exposure to carbon dioxide followed by decapitation. All animals were sacrificed between 10AM and 1PM over a period of 4 days using a balanced design. All mice subject to DR were sacrificed on fed days to match ad lib animals. Brain dissections were done in ice-cold brain blocks and tissue was immediately frozen in dry ice and placed at −80C until RNA extraction.
RT-PCR
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was measured using a Biophotometer (Eppendorf, Madison, WI). Using the manufacturer’s instructions, 500ng of RNA was used to make cDNA using RT2 First Strand Kit (SABiosciences, Frederick, MD). RT-PCR array was performed using the Mouse Huntington’s Disease RT2 Profiler™ PCR Array and RT2 SYBR Green/ROX (SABiosciences, Frederick, MD). Data were expressed as Ct (threshold cycle) values obtained from ABI SDS software package. Relative mRNA levels were determined by standard ΔΔ Ct methods and are expressed in fold change based on control group ad lib WT animals.
Results
Metabolic phenotype
Several methods of DR exert protective effects during aging; the present study entailed intermittent, or every-other-day feeding, since this approach does not result in overall caloric restriction, allowing dissociation of mechanisms from caloric intake (Anson et al., 2003). WT mice did not lose weight after 3 months of DR (Figure 1A). Body weights of YAC128 mice were higher than controls (Slow et al. 2003), and this difference was corrected by DR (Figure 1A). The association between increased body weight in relation to full length mutant HTT has been attributed to increased insulin-like growth factor (IGF-1) levels (Pouladi et al., 2010), and is independent of food consumption (Van Raamsdonk et al., 2006).
Figure 1. Dietary restriction prevents metabolic YAC128 phenotypes.

(A) Body weights of WT and HD mice under ad lib conditions or dietary restriction (started at ~4 months and continued to ~8 months of age). Values are mean ±SEM (N=12–21 per group). HD Ad lib vs. WT ad lib by Bonferroni post-tests revealed significant differences; *, p<.05. **, p<.01. ***, p<.001. (B, C) Tail blood glucose and insulin measured at 3 months of diet (after overnight feeding). Data are mean ±SEM (n=12–21). *, p<.05 transgene effect; #, p<.05, ###, p<.001 diet effect by 2Way ANOVA. (D) Glucose levels (mg/dl) measured after glucose IP injection. Values are mean ±SEM (N=7–11 per group). Main effect of diet by 2-Way ANOVA was detected, p<.01. (E) Daily blood glucose difference (measurements taken at ZT21-ZT9 in ~8 month old mice). Data are means ± SEM (N=12–21). §§, p<.01; §§§, p<.001 Bonferroni post-test. (2-column fitting image)
YAC128 mice fed ad lib were hyperglycemic relative to euglycemic WT mice. There was a reduction of baseline blood glucose on fed days in both WT and HD dietary restricted animals (Figure 2B). The blood glucose level of DR YAC128 mice was below levels in ad lib control mice, suggesting additive peripheral mechanisms. Ad lib YAC128 mice also exhibited hyperinsulinemia, which was corrected by the diet, but only to the level of ad lib fed WT mice (Figure 1C). Despite resting hyperglycemia, YAC128 mice had normal glucose tolerance tests, while improved glucose clearance was observed in dietary restricted animals of both genotypes (Figure 1D). Glucose tolerance tests were performed on fed days, after a 4-hour fast, suggesting that this short intervention is sufficient to acutely rescue the hyperglycemic effects of the transgene. We also evaluated daily fluctuations in baseline glucose levels that reflect changes in circadian rhythms (Pauly and Scheving, 1967). Diurnal blood glucose varied more dramatically in ad lib fed YAC128 mice than in other groups (Figure 1E), suggesting that the transgene disrupts circadian rhythms, a pathology corrected by DR (Morton et al., 2005; Petersén et al., 2005).
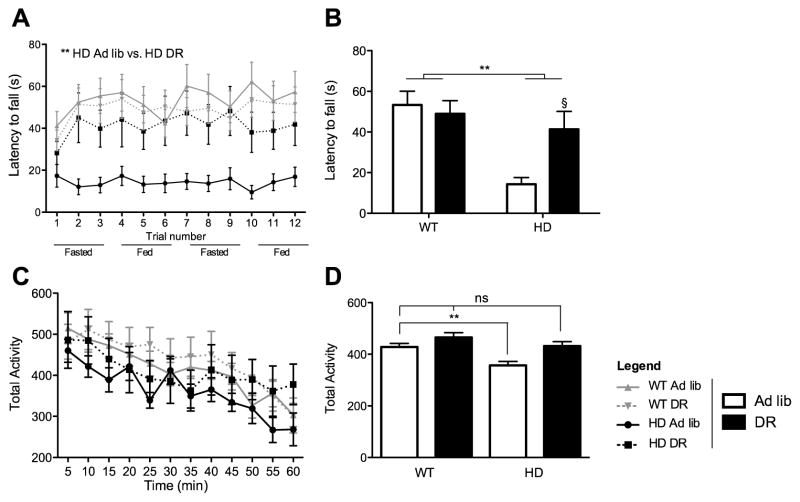
Figure 2. Dietary restriction prevents motor YAC128 phenotypes.
(A–B) Time spent on rotarod before falling of WT and HD mice under ad lib conditions or dietary restriction. Values are mean ±SEM (n=12–21 per group). **, p<.01 2-Way ANOVA transgene effect. §, p<.01 Bonferroni post test. (C) Average activity measured in 5-minute bins of a 1-hour period. (B) Average activity bins measured in 5-minute intervals for 60 minutes. Values are means ± SEM (n=12–16 per group). **, p<.01 by 1Way ANOVA followed by Dunnett’s post hoc. (2-column fitting image)
Motor phenotype
YAC128 fed ad lib exhibit motor impairments as measured by the rotarod test (Slow, 2003). DR robustly rescued rotarod performance on both fed and fasted days at 6 and 8 months (Figure 2A,B). Regression analysis (Mizuno et al., 1996) indicated that effects of the transgene, and interactions of the transgene and diet, on rotarod performance were independent of body weight (Supplementary Figure 1). Our statistical analysis agrees with results of another overweight HD full-length model, BACHD, showing that body weight only partially accounts for poor rotarod performance (Kudwa et al., 2013). In addition, 2-Way ANOVA showed statistical trends toward YAC128 hypoactivity as assayed by the open field test at seven months, and DR reversed this trend (Figure 2C). These trends showed statistical significance when all recorded discrete values were included without consideration of time factor using a 1-Way ANOVA followed by Dunnett’s post-hoc tests (Figure 2D). We also tested for changes in locomotor activity at different times of the day that would reflect differences in circadian rhythms but we did not detect any changes across groups (data not shown).
Transcriptional alterations in HD YAC128 model with and without DR
HD and DR entail alterations in the transcription of many genes. We previously identified a panel of genes expressed in the hypothalamus that are involved in energy balance and may be modulated by DR (Poplawski et al, 2010). The transcriptional changes induced by mutant HTT and/or DR may be divided into several groups. These include 1) genes that are dysregulated in HD and secondarily modified by DR, for which there is an interaction; 2) genes that are unaltered by HD but altered by DR at least in WT mice; 3) genes that are dysregulated in HD but not by DR in either WT or HD mice. We also used a commercial array (PAMM-123z; Qiagen) comprised of genes that are differentially expressed in various mouse and cellular HD models (e.g. Hodges et al., 2006; Kuhn et al., 2007; Luthi-Carter et al., 2000; Runne et al., 2008). Functional categories of genes dysregulated in HD and hypothesized to contribute to its pathophysiology include, but are not restricted to, histone modifications, calcium regulation, oxidative phosphorylation, cholesterol metabolism, transcription, signal transduction (e.g. neurotrophins, cAMP), calcium regulation, and synaptic transmission. Tissues were collected at 8 months, preceding the appearance of inclusions or morphological changes (Slow, 2003). Specific genes are discussed below, and Supplementary Table 1, and heat maps (Supplementary Figures 2, 3), list the hypothalamic and striatal genes that were significantly influenced by the transgene, dietary restriction, or interactions between the two as indicated by 2-Way ANOVA.
DR produces a molecular profile in the hypothalamus indicating a metabolic shift away from glucose and toward lipid metabolism independent of transgene
Many of the responses to DR appear to be mediated by neuroendocrine and autonomic responses that are largely regulated by the ventromedial hypothalamus (VMH) (Dacks et al., 2013). We characterized gene expression in the context of HD within the VMH (Figure 3), and analyzed a panel of genes that are largely responsive to energy homeostasis (Poplawski et al., 2010). DR enhanced the expression of neuropeptide genes that are regulated by nutritional state and reflect energy deficits (Belgardt et al., 2009). These transcripts were either unaffected (Pomc), or just moderately reduced (Agrp, Npy) by the transgene. DR also induced genes expected to reduce glucose metabolism (e.g., Gsk3 and Pdk1) and promote fatty acid metabolism (e.g., Crot, Crat, Acox3, and Acox1) independently of the transgene, and consistent with effects of fasting (Poplawski et al., 2010). We have previously observed changes in gene expression that indicate a switch in substrate utilization in other interventions resulting in energy deficit (Moreno et al., 2013; Poplawski et al., 2010). Yet surprisingly in the present study many of these metabolic markers (e.g. Pdk4, Cpt1a, Cpt1b, Pcx1) were not influenced by diet (data not shown). We hypothesize that this is due to the time of death, as all groups were sacrificed after a day of feeding in order to focus on chronic responses to DR, knowing that acute responses to fasting normalize within 4 hours after re-feeding (Poplawski et al., 2010).
Figure 3. Dietary restriction results in hypothalamic reprogramming towards fatty acid metabolism and away from glucose.
Quantitative real-time PCR data for murine (A) Cbp, (B) Pomc, (C) Agrp, (D) Npy, (E) Gsk3b, (F) Pdk3, (G) Pdk1, (H) Crot, (I) Crat, (J) Acox3, and (K) Acox1. Data is expressed in fold changes compared to Ad lib WT. Data are means ±SEM (n=6–11). *, p<0.05 2-Way ANOVA transgene effect. #, p<0.05; ##, p<.01; ###, p<.001 2-Way ANOVA diet effect. §, p<0.05 using Bonferroni’s post test. (2-column fitting image)
Histone modifying genes are regulated by DR in the striatum
Histone acetylation is reduced in HD and inhibition of histone deacetylase (HDAC) activity is a promising therapeutic intervention (Yeh et al., 2013). Specifically, Creb-binding-protein (CBP) is a histone acetylase whose activity is decreased in the presence of mutant HTT (Ogryzko et al., 1996; Steffan et al., 2000). A pan-cellular decrease in CBP shortens lifespan without exacerbating phenotype in HD-N171-82Q mice (Klevytska et al., 2010; Nucifora et al., 2001). Inhibition of Cbp blocks protective effects of DR to increase lifespan and reduce proteotoxicity in C. elegans, and hypothalamic expression of Cbp correlates with lifespan in mice (Zhang et al., 2009). Consistent with these observations, DR induced hypothalamic Cbp in both WT and YAC128 mice (Figure 3), suggesting that amelioration of metabolic impairments by DR in YAC128 mice might be mediated by induction of hypothalamic CBP (Hult et al., 2011). Based on hypothalamic responses, we anticipated that Cbp would also be upregulated by DR in the striatum, and thereby contribute to neuroprotection. We found a significant reduction in striatal Cbp by the transgene, and although there was a trend to correct this effect by DR it was not significant (Figure 4A). Of note, DR also did not lead to an increase in striatal Cbp mRNA in the control mice. We also examined other histone modifying genes, and it is of particular interest that striatal expression of the histone deacetylase Hdac2 was significantly reduced by DR only in the YAC128 mice (Figure 4C). In contrast, DR decreased expression of Hdac1 only in WT mice.
Figure 4. Dietary restriction is associated with histone modification transcripts in the striatum.
Expression of HD related genes in the striatum after 4 months of dietary restriction in YAC128 mice and WT controls. Quantitative real-time PCR data for murine (A) Cbp,(B) Hdac1, and (C) Hdac2. Data is expressed in fold changes compared to ad lib WT. Data are means ±SEM (n=7–9). **, p<0.01; ***, p<.001 2-Way ANOVA transgene effect. #, p<0.05 2-Way ANOVA diet effect. §, p<0.05 using Bonferroni’s post test. (single column fitting image)
DR does not affect genes involved in calcium signaling and synaptic transmission in the striatum
Figure 5 indicates striatal transcripts that were influenced by the transgene but not corrected by DR, and are therefore unlikely contributors to protective effects. The transcription of most (76%) of the genes was inhibited by the transgene and many of these proteins are associated with calcium signaling. For example Itpr1 codes for an internal calcium release channel (Yamada et al., 1994) and Ppp3ca codes for calcineurin, a phosphatase modulated by intracellular calcium (Rusnak and Mertz, 2000). Interventions that lower calcineurin activity appear to be protective in models of HD (Pardo et al., 2006; Pineda et al., 2009). The calcium transporter transcript, Atp2b2, was reduced by the transgene (Heim et al., 1992). Impairments in calcium homeostasis are evident in HD models (Bezprozvanny and Hayden, 2004), and while they may contribute to pathology, DR does not appear to provide protective effects by correcting such mechanisms. In a separate category, the transcriptional repressor Rest was significantly upregulated in HD mice, while an elevated trend by DR was observed only in the control mice. Rest activity appears to be enhanced in HD, where it represses genes such as Bdnf (Zuccato et al., 2003), but our results appear to exclude it as a potential mediator for protective effects of DR.
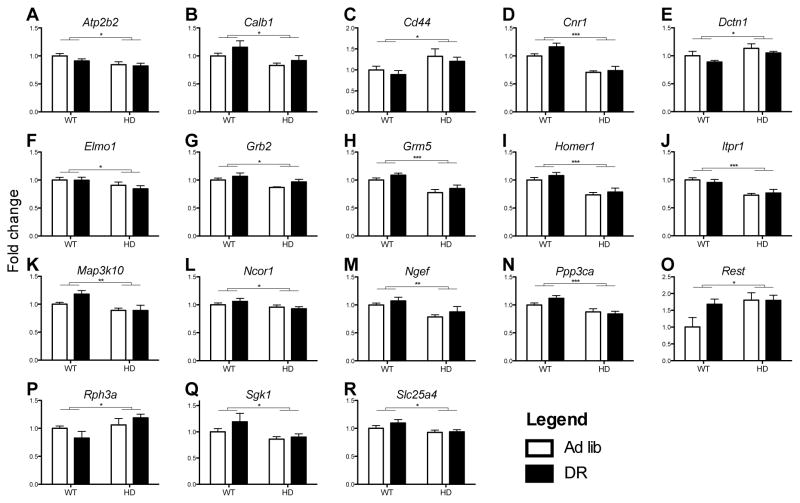
Figure 5. Many striatal genes that modulate calcium signaling were regulated by mutant HTT and unaffected by dietary restriction.
Expression of HD related genes in the striatum after 4 months of dietary restriction in the YAC128 mouse and WT controls. White bars indicate ad libitum, black bars indicate dietary restricted animals. Quantitative real-time PCR data for murine genes (A) Atp2b2, (B) Calb1, (C) Cd44, (D) Cnr1, (E) Dctn1, (F) Elmo1, (G) Grb2, (H) Grm5, (I) Homer1, (J) Itpr1, (K) Map3k10, (L) Ncor1, (M) Ngef, (N) Ppp3ca, (O) Rest, (P) Rph3a, (Q) Sgk1, and (R) Slc25a4. Data is expressed in fold changes compared to ad lib WT. Data are means ±SEM (n=7–9). *, p<0.05; **, p<.01; ***, p<.001 2-Way ANOVA transgene effect. (2-column fitting image)
DR regulates genes related to HD pathology affecting transcriptional regulation pathways
Figure 6 indicates transcripts that were unchanged in the ad lib HD mice relative to WT, but were altered by DR in both genotypes. Of these, a potential mediator of the protective effects of DR is superoxide dismutase 1 (Sod1), which plays a major role in cellular anti-oxidant defenses (Fridovich, 1995). The induction of Trp53, which codes for the p53 transcription factor, may also contribute to neuroprotection. We reported that one of the most prominent responses to fasting in the hypothalamus is the induction of p21, a major target of p53 (Poplawski et al., 2010). As with CBP, p53 is found in co-aggregates of cleaved huntingtin in the nucleus, and its activity is likely repressed in HD (Steffan et al., 2000). Sp1 codes for the transcription factor specificity protein 1, and was also induced by DR. The role of Sp1 activity in HD is unresolved, as it is inhibited in both post mortem HD tissue and some in vitro HD models (Dunah et al., 2002; Li et al., 2002) but up-regulated in others, and inhibition of Sp1 appears to be neuroprotective (Qiu et al., 2006).
Figure 6. Dietary restriction regulates striatal genes involved in transcriptional regulation.
Expression of HD related genes in the striatum after 4 months of dietary restriction in the Yac128 mouse and WT controls. White bars indicate ad libitum, black bars indicate dietary restricted animals. Quantitative real-time PCR data for murine genes (A) Bbox1, (B) Cltc, (C) Eef1a2, (D) Gabrd, (E) Gjb6, (F) Hip1, (G) Pgk1, (H) Prpf40a, (I) Sod1, (J) Sp1, (K) Sympk, (L) Tbp, (M) Trp53, and (N)Tubb5. Data is expressed in fold changes compared to ad lib WT. Data are means ±SEM (n=7–9). #, p<0.05; ##, p<.01; ###, p<.001 2-Way ANOVA diet effect. (2-column fitting image)
Finally, Figure 7 indicates genes that responded to DR in the WT mice but whose mRNA levels were regulated (or not affected) in the opposite direction in the YAC128 mice. These genes and mechanisms are therefore also unlikely to contribute to the positive response to DR. Some of these are notable in that had they responded to DR as expected, an increase in their levels would be predicted to specifically ameliorate aspects of the HD phenotype, for example Ntrk2 (Zuccato and Cattaneo, 2007). Similarly, Ppp1r1b (DARPP-32) was strongly induced by DR but this effect was greatly diminished by the transgene and it is unlikely that the induction that occurred with DR in YAC128 would significantly ameliorate phenotype. It should be noted that an analogous induction for DARPP-32 has been reported in other brain areas by DR (Yamamoto et al., 2009), and reduced striatal expression is seen in this mouse model (Metzler et al., 2010; Slow, 2003). Likewise, the dysregulation of many of these transcripts in Figure 7 by HD was corroborated in the YAC128 mouse, strengthening their status as potential disease markers (Ariano et al., 2005; Runne et al., 2008; Sorolla et al., 2008; Valenza et al., 2010).
Figure 7. Mutant HTT blocks effects of dietary restriction on mRNA levels.
Expression of HD related genes in the striatum after 4 months of dietary restriction in the Yac128 mouse and WT controls. White bars indicate ad libitum, black bars indicate dietary restricted animals. Quantitative real-time PCR data for murine genes (A) Apoe, (B) Ppp1r1b, (C) Gja1, (D) Gpx1, (E) Kcnab1, (F) Ntrk2, (G) Plod2, and (H) Rgs4. Data is expressed in fold changes compared to ad lib WT. Data are means ±SEM (n=7–9). Interactions between diet and transgene were detected in these genes by 2-Way ANOVA. *, p< 0.05; **, p<.01; ***, p<.001 2-Way ANOVA transgene effect. #, p<0.05; ##, p<.01 2-Way ANOVA diet effect. §, p<.05 Bonferroni post-test. (Single column fitting image)
DR induced one marker of oxidative stress, Gpx1, in WT mice, and inhibited it in HD mice. Gpx1 induction in YAC128 relative to WT agrees with previous reports of HD models and post mortem tissue (Sorolla et al., 2008) and may reflect increased oxidative stress. DR increased Gpx1 in control restricted animals suggesting that DR may alleviate oxidative stress in control animals by increasing Gpx1 availability, an effect observed in other tissues (Omodei et al., 2013). HD ad lib animals exhibited similar high levels of Gpx1, which may represent feedback mechanisms due to increased oxidative stress in HD brain tissue. Supporting such observations, DR reduced Gpx1 mRNA levels in YAC128 striatum, which may indicate decreased oxidative stress as a result of the DR intervention.
DR specifically reduces human HTT
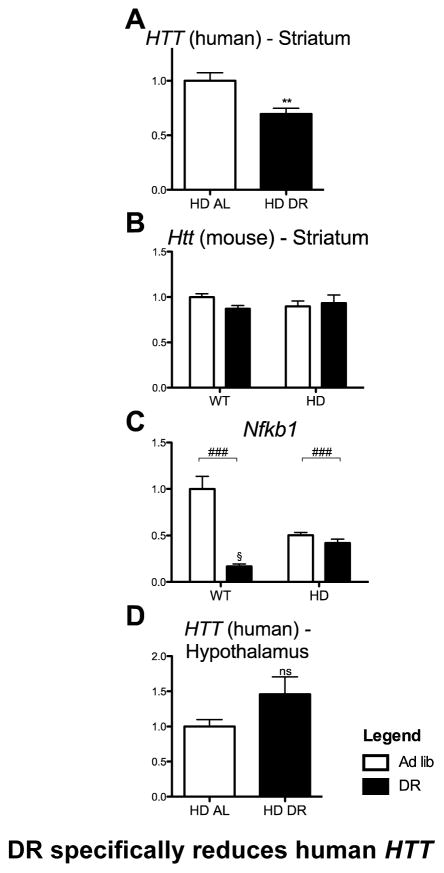
Human HTT with an expanded polyglutamine stretch is the direct molecular insult driving the behavioral and molecular phenotypes in the YAC128 model and its level correlates with phenotype and disease severity in human patients and HD mouse models (Graham et al., 2006; Slow, 2003; Squitieri et al., 2003; Van Raamsdonk et al., 2007). Sp1 up-regulates HTT transcription (Wang et al., 2012), and thus, upon the observation that DR induces Sp1 we proceeded to assay levels of mouse and human HTT transcripts in striatum and hypothalamus. Surprisingly, we found that DR specifically reduced transgene HTT mRNA levels in the striatum (Figure 8A), whereas its level was unchanged in the hypothalamus (Figure 8D). This assay could not be performed in DR HD-N171-82Q mice in which the prion promoter drives the transgene (Schilling et al., 1999). This finding indicates the presence of region-specific molecular signatures driven by DR that are capable of regulating the human HTT gene, involving either its promoter and/or regulatory sequences located in other regions of the gene. Furthermore, these effects were specific to human HTT regulatory elements, since no such changes were observed in the endogenous mouse Htt mRNA (Figure 8B). We assayed other putative regulators of HTT, such as Creb (indirect evidence based on consensus binding site) and Nfkb1, for which there is direct evidence for regulation (Bečanović et al., 2015). Creb mRNA levels were unchanged (data not shown) but the striatal level of Nfkb1 mRNA, already reduced in the YAC128, was further slightly reduced by DR, possibly contributing to the downregulation of the transgene (Figure 8C).
Figure 8. DR specifically reduces Human HTT in the Striatum.
Expression of HD related genes after 4 months of dietary restriction in the Yac128 mouse and WT controls. Quantitative real-time PCR data for striatal (A) HTT (Human), (B) Htt (Mouse), (C) Nfkb1, and hypothalamic (D) HTT (Human). Data are means ±SEM (n=7–9). **, P<.01 Student’s t-test. ###, P< 0.05 2-Way ANOVA diet effect. (Single column fitting image)
Conclusions
The present studies demonstrate that DR produced by intermittent fasting significantly ameliorates phenotypes in the YAC128 mouse model of HD. These data extend the observations of a previous study of the effects of DR on the HD-N171-82Q (Duan et al., 2003). We can conclude that DR is effective in mice carrying either the entire human HTT gene or a fragment. DR is a metabolic intervention, and obtaining comparable normalizing effects in considerably different metabolic environments suggests a specific effect of DR on reversing the negative effects of mutant huntingtin. Comparing effects of DR in these two models allows isolation (and elimination) of mechanisms likely mediating these protective effects. For example, YAC128 mice are heavier than controls and DR leads to a decrease in body weight. Conversely, HD-N171-82Q mice have low body weight and DR increases their weight. These results strongly argue against a direct role of body weight in mediating protective effects of DR in these models. On the other hand, both mouse lines are hyperglycemic, and although DR returned baseline glucose levels to normal in both, glucose tolerance tests were not similarly altered (Duan et al., 2003).
The relationship between increased body weight and full length mutant HTT is attributed to increased insulin-like growth factor (IGF-1) levels (Pouladi et al., 2010), and is independent of food consumption (Van Raamsdonk et al., 2006). It is possible that the weight loss observed in the YAC128 results from changes in IGF-1 and/or its signaling, as DR animals display decreased IGF-1 signaling (Sell, 2003).
The transcriptional profile assayed in the YAC128 mice in this study represents a snapshot of candidate mRNAs previously demonstrated to be influenced by nutritional deficit. We have shown that many of the transcriptional changes observed during fasting are not present in multiple brain areas 4 hours after re-feeding (Poplawski et al., 2010). Thus, by sacrificing animals on a fed day we aimed to distinguish chronic effects that represent the HD pathology and were not reflective of an acute fasted state. Simultaneously, however, this protocol likely decreased the number of altered metabolic genes in the hypothalamus. In future studies, comparison of the profiles of fasted and fed conditions in DR on an HD background should offer further insights into the protective mechanisms of intermittent fasting, and would address the question of how repeated fasting events lead to consistent molecular markers on fed days.
Our results strengthen the hypothesis that DR would alleviate phenotypes and plausibly extend lifespan of HD patients. As such a taxing diet may render this intervention unsuitable for most patients, it is important to determine whether any of the DR-induced transcriptional alterations represent possible drug targets. The most relevant clinical target may be Hdac2, which was prominently reduced in DR HD mice. The HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) successfully alleviates HD mouse phenotypes, likely due to decreases in HDAC2 and HDAC4 proteins (Mielcarek et al., 2011). Evidence supports the idea that HDAC1 may be another potential clinical target, since it shares 83% similarity with HDAC2 (H Jia et al., 2012; Kelly and Cowley, 2013). The ketogenic diet is an alternative, potentially less toxic, clinical intervention which leads to inhibition of HDAC1/2 by generating endogenous β-hydroxybutyrate (Shimazu et al., 2013).
It is intriguing that our molecular data suggest that DR exerts positive effects through epigenetic and transcriptional mechanisms that directly decrease human HTT in the striatum. Regulation of HTT was both regionally- and species-specific, and it remains to be established how DR specifically regulates human HTT. Known transcriptional regulators of human HTT include CREB, SP1 and NF-κB (Wang et al, 2012; Leavitt 2015). In the striatum, Sp1 was increased, which might be hypothesized to be protective as Sp1 activity is decreased in some models of HD (Dunah et al., 2002; Li et al., 2002), but would be expected to paradoxically lead to an increase in HTT mRNA. On the other hand, striatal Nfκb1 mRNA was decreased, consistent with a decrease in HTT mRNA. In the hypothalamus, Nfkb1 expression did not respond to DR or the transgene (data not shown), compatible with unchanged hypothalamic HTT. The 3000 Kb of the 5′UT sequences for mouse Htt and human HTT share 91% homology, and consensus NF-κB binding sites are present in both. Interestingly, however, the recently characterized active NF-κB binding site diverges from mouse to human, although non-canonical NF-κB binding is prevalent (Bečanović et al., 2015; Wong et al., 2011). It is also possible that epigenetic changes evoked by DR contribute to a decrease in human HTT mRNA, as these can regulate Nfkb1 (Haiqun Jia et al., 2012).
In summary, we demonstrate that DR is protective in the full-length YAC128 mouse HD model. There are multiple transcriptional changes either reversed by DR or induced by DR that may contribute to the improved phenotype, and prominent amongst them is an induction of hypothalamic Cbp and a reduction in striatal Hdac2 mRNA. Moreover, much research in the HD field is currently aimed at reducing brain levels of huntingtin and all methods to date require access to the central nervous system. Importantly, this is the first report of an intervention that reduces level of human HTT mRNA without the requirement for physical access to the brain. Finally, these data highlight the need for validation of therapeutic interventions in animal models that harbor the human HTT regulatory regions in which the level of mutant HTT can be quantitated.
Supplementary Material
Highlights.
DR corrects metabolic and motor phenotypes in a full-length mouse model of HD
Markers for reduced histone acetylation are associated with dietary restriction
Dietary restriction down-regulates transcription of HTT transgene in the striatum
Acknowledgments
This research was supported by the National Institute of Aging of the National Institute of Health under award number FAG042299A. Further support came from the National Institute of Aging program “Juvenile Protective Factors” and CHDI and R01NS059936 to M.E.E. A portion of this work was used in a dissertation by CLM in partial fulfillment of requirements for the PhD degree at the Icahn School of Medicine at Mount Sinai in New York.
List of Abbreviations
- CBP
Creb binding protein
- DR
Dietary restriction
- HD
Huntington’s disease
- HTT
Mutant huntingtin protein
- IGF-1
Insulin-like growth factor
- Ppp1r1b
DARPP-32
- SAHA
Suberoylanilide hydroxamic acid
- WT
Wild-type
Footnotes
Competing interests
The authors declare that they have no competing interests
Authors’ Contributions
CLM participated in the general planning of the experiments, carried out behavioral experiments, molecular studies, data collection, analysis and interpretation, and drafting of the manuscript. MEE participated in the general planning of experiments, data interpretation, and drafting of the manuscript. CVM participated in the general planning of experiments, the interpretation and statistical analysis of data, and drafting of the manuscript.
Contributor Information
Cesar L. Moreno, Email: Cesar.moreno@mssm.edu.
Michelle E. Ehrlich, Email: Michelle.ehrlich@mssm.edu.
References
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–20. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Wagle N, Grissell AE. Neuronal vulnerability in mouse models of Huntington’s disease: membrane channel protein changes. J Neurosci Res. 2005;80:634–45. doi: 10.1002/jnr.20492. [DOI] [PubMed] [Google Scholar]
- Bečanović K, Nørremølle A, Neal SJ, Kay C, Collins JA, Arenillas D, Lilja T, Gaudenzi G, Manoharan S, Doty CN, Beck J, Lahiri N, Portales-Casamar E, Warby SC, Connolly C, De Souza RAG, Tabrizi SJ, Hermanson O, Langbehn DR, Hayden MR, Wasserman WW, Leavitt BR. A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease. Nat Neurosci. 2015;18:807–816. doi: 10.1038/nn.4014. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587:5305–14. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochem Biophys Res Commun. 2004;322:1310–7. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bruyn george willem, Bots G, RD Huntington’s chorea: current neuropathological status. Adv Neurol. 1979;23:83–93. [Google Scholar]
- Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington’s disease cortex. Ann Neurol. 1990;27:200–4. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dacks PA, Moreno CL, Kim ES, Marcellino BK, Mobbs CV. Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front Neuroendocrinol. 2013;34:95–106. doi: 10.1016/j.yfrne.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–6. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, Krainc D. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science. 2002;296:2238–43. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Genet. 1993;4:387–92. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. pii. [DOI] [PubMed] [Google Scholar]
- Graham RK, Slow EJ, Deng Y, Bissada N, Lu G, Pearson J, Shehadeh J, Leavitt BR, Raymond LA, Hayden MR. Levels of mutant huntingtin influence the phenotypic severity of Huntington disease in YAC128 mouse models. Neurobiol Dis. 2006;21:444–55. doi: 10.1016/j.nbd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Graveland G, Williams R, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science (80- ) 1985;227:770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neurosci Lett. 1991;133:257–61. doi: 10.1016/0304-3940(91)90583-f. [DOI] [PubMed] [Google Scholar]
- Heim R, Hug M, Iwata T, Strehler EE, Carafoli E. Microdiversity of human-plasma-membrane calcium-pump isoform 2 generated by alternative RNA splicing in the N-terminal coding region. Eur J Biochem. 1992;205:333–40. doi: 10.1111/j.1432-1033.1992.tb16784.x. [DOI] [PubMed] [Google Scholar]
- Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, Elliston LA, Hartog C, Goldstein DR, Thu D, Hollingsworth ZR, Collin F, Synek B, Holmans PA, Young AB, Wexler NS, Delorenzi M, Kooperberg C, Augood SJ, Faull RLM, Olson JM, Jones L, Luthi-Carter R. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15:965–77. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–92. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Hult S, Soylu R, Björklund T, Belgardt BF, Mauer J, Brüning JC, Kirik D, Petersén Å. Mutant huntingtin causes metabolic imbalance by disruption of hypothalamic neurocircuits. Cell Metab. 2011;13:428–39. doi: 10.1016/j.cmet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR. Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes. 1999;48:649–651. doi: 10.2337/diabetes.48.3.649. [DOI] [PubMed] [Google Scholar]
- Jia H, Kast RJ, Steffan JS, Thomas EA. Selective histone deacetylase (HDAC) inhibition imparts beneficial effects in Huntington’s disease mice: implications for the ubiquitin-proteasomal and autophagy systems. Hum Mol Genet. 2012;21:5280–5293. doi: 10.1093/hmg/dds379. dds379 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Pallos J, Jacques V, Lau A, Tang B, Cooper A, Syed A, Purcell J, Chen Y, Sharma S, Sangrey GR, Darnell SB, Plasterer H, Sadri-Vakili G, Gottesfeld JM, Thompson LM, Rusche JR, Marsh JL, Thomas EA. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol Dis. 2012;46:351–361. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RDW, Cowley SM. The physiological roles of histone deacetylase (HDAC) 1 and 2: complex co-stars with multiple leading parts. Biochem Soc Trans. 2013;41:741–9. doi: 10.1042/BST20130010. [DOI] [PubMed] [Google Scholar]
- Klevytska AM, Tebbenkamp AT, Savonenko AV, Borchelt DR. Partial depletion of CREB-binding protein reduces life expectancy in a mouse model of Huntington disease. J Neuropathol Exp Neurol. 2010;69:396–404. doi: 10.1097/NEN.0b013e3181d6c436. pii. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Menalled LB, Oakeshott S, Murphy C, Mushlin R, Fitzpatrick J, Miller SF, McConnell K, Port R, Torello J, Howland D, Ramboz S, Brunner D. Increased Body Weight of the BAC HD Transgenic Mouse Model of Huntington’s Disease Accounts for Some but Not All of the Observed HD-like Motor Deficits. PLoS Curr. 2013;5 doi: 10.1371/currents.hd.0ab4f3645aff523c56ecc8ccbe41a198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A, Goldstein DR, Hodges A, Strand AD, Sengstag T, Kooperberg C, Becanovic K, Pouladi MA, Sathasivam K, Cha JHJ, Hannan AJ, Hayden MR, Leavitt BR, Dunnett SB, Ferrante RJ, Albin R, Shelbourne P, Delorenzi M, Augood SJ, Faull RLM, Olson JM, Bates GP, Jones L, Luthi-Carter R. Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum Mol Genet. 2007;16:1845–61. doi: 10.1093/hmg/ddm133. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Li SH, Cheng AL, Zhou H, Lam S, Rao M, Li H, Li XJ. Interaction of Huntington disease protein with transcriptional activator Sp1. Mol Cell Biol. 2002;22:1277–87. doi: 10.1128/mcb.22.5.1277-1287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C, Ribeiro M, Duarte AI, Humbert S, Saudou F, Pereira de Almeida L, Hayden M, Rego AC. IGF-1 intranasal administration rescues Huntington’s disease phenotypes in YAC128 mice. Mol Neurobiol. 2014;49:1126–42. doi: 10.1007/s12035-013-8585-5. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM. Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Hum Mol Genet. 2000;9:1259–71. doi: 10.1093/hmg/9.9.1259. [DOI] [PubMed] [Google Scholar]
- Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab. 2002;283:E1266–71. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- McNeil SM, Novelletto A, Srinidhi J, Barnes G, Kornbluth I, Altherr MR, Wasmuth JJ, Gusella JF, MacDonald ME, Myers RH. Reduced penetrance of the Huntington’s disease mutation. Hum Mol Genet. 1997;6:775–9. doi: 10.1093/hmg/6.5.775. [DOI] [PubMed] [Google Scholar]
- Metzler M, Gan L, Mazarei G, Graham RK, Liu L, Bissada N, Lu G, Leavitt BR, Hayden MR. Phosphorylation of huntingtin at Ser421 in YAC128 neurons is associated with protection of YAC128 neurons from NMDA-mediated excitotoxicity and is modulated by PP1 and PP2A. J Neurosci. 2010;30:14318–29. doi: 10.1523/JNEUROSCI.1589-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Benn CL, Franklin SA, Smith DL, Woodman B, Marks PA, Bates GP. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington’s disease. PLoS One. 2011;6:e27746. doi: 10.1371/journal.pone.0027746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Bergen H, Kleopoulos S, Bauman WA, Mobbs CV. Effects of nutritional status and aging on leptin gene expression in mice: importance of glucose. Horm Metab Res. 1996;28:679–84. doi: 10.1055/s-2007-979877. [DOI] [PubMed] [Google Scholar]
- Moreno C, Yang L, Dacks P, Isoda F, Poplawski M, Mobbs CV. Regulation of Peripheral Metabolism by Substrate Partitioning in the Brain. Endocrinol Metab Clin North Am. 2013 doi: 10.1016/j.ecl.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–63. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora FC, Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science (80- ) 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- Nucifora FC, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–8. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–9. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Omodei D, Licastro D, Salvatore F, Crosby SD, Fontana L. Serum from humans on long-term calorie restriction enhances stress resistance in cell culture. Aging (Albany NY) 2013;5:599–606. doi: 10.18632/aging.100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo R, Colin E, Régulier E, Aebischer P, Déglon N, Humbert S, Saudou F. Inhibition of calcineurin by FK506 protects against polyglutamine-huntingtin toxicity through an increase of huntingtin phosphorylation at S421. J Neurosci. 2006;26:1635–45. doi: 10.1523/JNEUROSCI.3706-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JE, Scheving LE. Circadian rhythms in blood glucose and the effect of different lighting schedules, hypophysectomy, adrenal medullectomy and starvation. Am J Anat. 1967;120:627–36. doi: 10.1002/aja.1001200312. [DOI] [PubMed] [Google Scholar]
- Petersén A, Björkqvist M. Hypothalamic-endocrine aspects in Huntington’s disease. Eur J Neurosci. 2006;24:961–7. doi: 10.1111/j.1460-9568.2006.04985.x. [DOI] [PubMed] [Google Scholar]
- Petersén A, Gil J, Maat-Schieman MLC, Björkqvist M, Tanila H, Araújo IM, Smith R, Popovic N, Wierup N, Norlén P, Li JY, Roos RAC, Sundler F, Mulder H, Brundin P. Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- Pineda JR, Pardo R, Zala D, Yu H, Humbert S, Saudou F. Genetic and pharmacological inhibition of calcineurin corrects the BDNF transport defect in Huntington’s disease. Mol Brain. 2009;2:33. doi: 10.1186/1756-6606-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawski MM, Mastaitis JW, Yang XJ, Mobbs CV. Hypothalamic responses to fasting indicate metabolic reprogramming away from glycolysis toward lipid oxidation. Endocrinology. 2010;151:5206–17. doi: 10.1210/en.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouladi MA, Xie Y, Skotte NH, Ehrnhoefer DE, Graham RK, Kim JE, Bissada N, Yang XW, Paganetti P, Friedlander RM, Leavitt BR, Hayden MR. Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression. Hum Mol Genet. 2010;19:1528–38. doi: 10.1093/hmg/ddq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Norflus F, Singh B, Swindell MK, Buzescu R, Bejarano M, Chopra R, Zucker B, Benn CL, DiRocco DP, Cha JHJ, Ferrante RJ, Hersch SM. Sp1 is up-regulated in cellular and transgenic models of Huntington disease, and its reduction is neuroprotective. J Biol Chem. 2006;281:16672–80. doi: 10.1074/jbc.M511648200. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Leggo J, Coles R, Almqvist E, Biancalana V, Cassiman JJ, Chotai K, Connarty M, Crauford D, Curtis A, Curtis D, Davidson MJ, Differ AM, Dode C, Dodge A, Frontali M, Ranen NG, Stine OC, Sherr M, Abbott MH, Franz ML, Graham CA, Harper PS, Hedreen JC, Hayden MR. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. Am J Hum Genet. 1996;59:16–22. [PMC free article] [PubMed] [Google Scholar]
- Runne H, Régulier E, Kuhn A, Zala D, Gokce O, Perrin V, Sick B, Aebischer P, Déglon N, Luthi-Carter R. Dysregulation of gene expression in primary neuron models of Huntington’s disease shows that polyglutamine-related effects on the striatal transcriptome may not be dependent on brain circuitry. J Neurosci. 2008;28:9723–31. doi: 10.1523/JNEUROSCI.3044-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Sell C. Caloric restriction and insulin-like growth factors in aging and cancer. Horm Metab Res. 2003;35:705–11. doi: 10.1055/s-2004-814156. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–4. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med. 2008;45:667–78. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Gellera C, Cannella M, Mariotti C, Cislaghi G, Rubinsztein DC, Almqvist EW, Turner D, Bachoud-Lévi AC, Simpson SA, Delatycki M, Maglione V, Hayden MR, Di Donato S. Homozygosity for CAG mutation in Huntington disease is associated with a more severe clinical course. Brain. 2003;126:946–55. doi: 10.1093/brain/awg077. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu YZ, Gohler H, Wanker EE, Bates GP, Housman DE, Thompson LM. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci U S A. 2000;97:6763–8. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Leoni V, Karasinska JM, Petricca L, Fan J, Carroll J, Pouladi MA, Fossale E, Nguyen HP, Riess O, MacDonald M, Wellington C, DiDonato S, Hayden M, Cattaneo E. Cholesterol defect is marked across multiple rodent models of Huntington’s disease and is manifest in astrocytes. J Neurosci. 2010;30:10844–50. doi: 10.1523/JNEUROSCI.0917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Gibson WT, Pearson J, Murphy Z, Lu G, Leavitt BR, Hayden MR. Body weight is modulated by levels of full-length huntingtin. Hum Mol Genet. 2006;15:1513–23. doi: 10.1093/hmg/ddl072. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Metzler M, Slow E, Pearson J, Schwab C, Carroll J, Graham RK, Leavitt BR, Hayden MR. Phenotypic abnormalities in the YAC128 mouse model of Huntington disease are penetrant on multiple genetic backgrounds and modulated by strain. Neurobiol Dis. 2007;26:189–200. doi: 10.1016/j.nbd.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Luo Y, Ly PTT, Cai F, Zhou W, Zou H, Song W. Sp1 regulates human huntingtin gene expression. J Mol Neurosci. 2012;47:311–21. doi: 10.1007/s12031-012-9739-z. [DOI] [PubMed] [Google Scholar]
- Wong D, Teixeira A, Oikonomopoulos S, Humburg P, Lone IN, Saliba D, Siggers T, Bulyk M, Angelov D, Dimitrov S, Udalova IA, Ragoussis J. Extensive characterization of NF-κB binding uncovers non-canonical motifs and advances the interpretation of genetic functional traits. Genome Biol. 2011;12:R70. doi: 10.1186/gb-2011-12-7-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Makino Y, Clark RA, Pearson DW, Mattei MG, Guénet JL, Ohama E, Fujino I, Miyawaki A, Furuichi T. Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem J. 1994;302(Pt 3):781–90. doi: 10.1042/bj3020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Tanahashi T, Kawai T, Chikahisa S, Katsuura S, Nishida K, Teshima-Kondo S, Sei H, Rokutan K. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics. 2009;39:227–35. doi: 10.1152/physiolgenomics.00082.2009. [DOI] [PubMed] [Google Scholar]
- Yeh HH, Young D, Gelovani JG, Robinson A, Davidson Y, Herholz K, Mann DMA. Histone deacetylase class II and acetylated core histone immunohistochemistry in human brains with Huntington’s disease. Brain Res. 2013;1504:16–24. doi: 10.1016/j.brainres.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Poplawski M, Yen K, Cheng H, Bloss E, Zhu X, Patel H, Mobbs CV. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 2009;7:e1000245. doi: 10.1371/journal.pbio.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.