Summary
Introduction
During preoperative discussions with breast reconstruction patients, questions often arise about what to expect during the recovery period. However, there is a paucity of data elucidating post breast reconstruction pain, fatigue and physical morbidity. This information is important to patient and physician understanding of reconstructive choices and the postoperative recovery process. We sought to evaluate how recovery may vary for patients based on the timing and type of reconstruction.
Materials and Methods
Patients were recruited as part of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study, a prospective, multi-centered NIH-funded study (1RO1CA152192). Here, patients completed the Numerical Pain Rating Scale (NPRS), McGill Pain Questionnaire, and Breast-Q preoperatively and at one week and three months postoperatively. Pain, fatigue, and upper body morbidity were evaluated by type and timing of reconstruction.
Results
A total of 2,013 MROC study participants had completed 3-month follow-up and therefore were included for analysis. 1,583 patients (78.6%) completed surveys at one-week post-reconstruction, 1,517 patients (75.3%) at three months post-reconstruction. Across all procedure groups, fatigue and physical well-being scores did not return to preoperative levels by three months. At three months, pain measured by the NPRS differed across procedure types (p=0.01), with tissue expander/implant (TE/I) having more pain than direct to implant (p<0.01). Similarly, at three months, chest and upper body physical morbidity, as measured by BREAST-Q, differed by procedure types (p<0.001), with generally less morbidity for autologous reconstruction as compared to TE/Is.
Conclusions
For all reconstructive procedure groups, patients have not fully recovered at three months post-surgery. Additionally, postoperative pain and upper body physical morbidity vary significantly by reconstructive procedure with patients undergoing TE/I reporting the most distress.
Keywords: breast cancer, breast reconstruction, recovery, pain, fatigue, BREAST-Q, physical distress
Introduction
Breast reconstruction after mastectomy has significantly increased in the United States after the enactment of the Women’s Health and Cancer Rights Act of 1998, which federally required health care coverage for breast reconstruction. Since this time, the volume of immediate breast reconstruction has increased an average of 5% per year, reaching almost 100,000 procedures in 2013.1,2 Decisions regarding the timing and the type of reconstruction are multifactorial including patient and physician concerns about: aesthetic outcomes, likelihood for postoperative complications, and expected course of recovery. There is abundant literature examining the outcomes of timing and types of breast reconstruction related to complications and aesthetic outcomes; however, there is little data elucidating the recovery period. Given the rising rates of breast reconstruction and complex decisions regarding reconstructive choices, it is crucial to understand the impact of reconstruction on pain, fatigue, and physical distress during the initial recovery phase.
Understanding the recovery phase of breast reconstruction is also an important component of informed, shared, medical decision making between patient and physician. During this discussion, patients frequently ask questions such as “When will I be back to normal?” and “Which operation takes longer to recover from?”. While surgeons may feel comfortable providing answers to these questions based on their own clinical experience, their knowledge may be limited by patient demographics and predisposition to a specific reconstructive method. Moreover, certain aspect of recovery, such as pain, fatigue and physical distress, may only be fully appreciated by patients. Therefore, these important aspects of recovery are potentially both underestimated and under-evaluated by clinicians.
With these challenges in mind, our objective was to describe patient-reported pain, fatigue, and upper body morbidity during the recovery phase of breast reconstruction. Specifically, we sought to understand differences between type (implant versus autologous) and timing (immediate versus delayed) of reconstruction and additionally to explore the degree to which patients have recovered at three months relative to their preoperative status. This information will be used to guide women in their decisions regarding reconstruction and to help accurately prepare them for the recovery period.
Materials and Methods
Patient Selection
Patients were recruited for this study as part of the Mastectomy Reconstruction Outcomes Consortium (MROC), a 5-year multi-centered prospective cohort study funded by National Cancer Institute (1RO1CA152192). Women undergoing primary breast reconstruction after mastectomy were eligible for this study. After obtaining local Institutional Review Board approval, patients were recruited in person from 11 sites in both the United States and Canada. Enrollment began in February of 2012 and will conclude in July 2016. Patients undergoing immediate, delayed, prophylactic, therapeutic, unilateral, or bilateral reconstructions were included for analysis. Surgeon and patient preferences determined type and timing of reconstruction. Specific reconstructions included: tissue expander/implant (TE/I), direct to implant (DTI), microsurgical flaps (transverse rectus abdominus myocutaneous (f-TRAM), muscle sparing transverse rectus abdominus myocutaneous (ms-TRAM), deep inferior epigastric artery perforator (DIEP), superficial inferior epigastric artery (SIEA), superior gluteal artery perforator (SGAP), and inferior gluteal artery perforator (IGAP)), and pedicled flaps (p-TRAM and lat dorsi). Of note, patients undergoing ms-TRAM were included in the f-TRAM cohort secondary to partial muscle harvest. Additionally, for this MROC sub-study, patients were excluded from analysis if they underwent combination bilateral procedures with respect to the type and timing of reconstruction (e.g., delayed/immediate or implant/flap).
Study Design
We sought to utilize the data acquired by MROC to describe the recovery period after breast reconstruction based on the timing and type of reconstructive surgery. We defined the initial recovery period as the first three months after the primary reconstructive surgery. Therefore, only patients who were at least three months postoperative at the time of data collection were included in this analysis. Patient demographic and clinical data were obtained from the medical record, and included age, body mass index (BMI), laterality, race, ethnicity, timing of reconstruction (immediate versus delayed), and axillary surgery (axillary lymph node dissection (ALND) and sentinel lymph node biopsy (SLNBx)).
Questionnaires, including various patient reported outcomes measures (PROMs), were completed electronically or on paper preoperatively, one-week postoperatively and three-months postoperatively. The PROMs employed included BREAST-Q, Numerical Pain Rating Scale (NPRS), Short Form McGill Pain Questionnaire (SF-MPQ), Brief Fatigue Inventory (BFI), and Patient Reported Outcome Measurement Information System-29 (PROMIS-29).
PRO Instruments
Pain
Pain was evaluated using two measures: NPRS and SF-MPQ, at all 3 time points (preoperatively, 1 week and 3 months postoperatively). NPRS is a widely used instrument, wherein patients rate their pain quantitatively at the time of evaluation on a Likert scale from 0 (no pain) to 10 (worst pain). The SF-MPQ provided an additional qualitative assessment of pain, distinct from the ‘intensity alone’ rating of the NPRS. This questionnaire includes 15 items, which qualitatively describe pain with words such as throbbing, shooting, heavy, and tender. These items are divided into two distinct sub-categories of qualitative pain: sensory (11 items) and affective (4 items)3 and then semi-quantitatively rated on an intensity scale of none (0), mild (1), moderate (2), and severe (3). While three distinct pain scores can derived from SF-MPQ, the affective subscale was not included in this analysis as it was considered less appropriate for our patient population and less relevant to post-surgical recovery. Therefore, SF-MPQ score represented the sensory sub-scale only, which had a score range of 0–33, with higher scores corresponding to more pain. SF-MPQ has demonstrated good internal consistency (Cronbach α = 0.73–0.89) and good test-retest reliability (interclass correlation coefficient =0.75).4,5
Fatigue
Fatigue was evaluated using two PROMs: the BFI and PROMIS-29 fatigue scale. These were both queried preoperatively and at three months. The BFI was developed at MD Anderson Cancer Center to look specifically at cancer related fatigue.6 It utilizes 10 questions to assess the severity and impact of fatigue, as evaluated on a 10-point Likert scale. Scores were totaled and averaged to generate a global fatigue score (range 0–10), greater score correlating with more fatigue. BFI has shown good internal consistency (Cronbach α = 0.82–0.97).6 PROMIS-29 is an NIH-funded, validated PRO instrument that has seven domains (Physical function, Anxiety, Depression, Fatigue, Sleep Disturbance, Satisfaction with Social Role, and Pain Interference). Here we employed the fatigue domain, which had four items scored on a scale of 1–5. Therefore the minimum raw score is 4 and maximum raw score 20, with higher scores equating to greater fatigue. The raw score can then be converted to a T score with a range of 0–100 and general population mean of 50 (standard deviation 10). A PROMIS-29 fatigue score of 40 can thus be considered one standard deviation below the general population mean, and similarly a PROMIS-29 fatigue score of 70 is two standard deviations above the general population mean.
Chest and Upper Body Physical Well-being
Chest and upper body physical well-being was evaluated by the BREAST-Q, which is a validated PROM developed at Memorial Sloan Kettering Cancer Center and University of British Columbia.7,8 The BREAST-Q consists of independent scales measuring various aspect of outcome for the patient perspective. Here, we employed the BREAST-Q Physical well-being: chest and upper body scale to evaluate physical well-being preoperatively, at one week, and at three months postoperatively. The scale was developed using the Rasch model and is scored using Q-score. Results may range from were 0–100 with higher numbers correlating to better outcomes (e.g., less chest and upper body physical morbidity).
Statistical Analysis
Comparisons in baseline characteristics, including PROMs, across procedure types were performed using chi-square tests for categorical variables and analysis of variance for continuous variables. To determine whether patient outcomes recovered to their baseline level, each PROM was compared between 3 months post-op and baseline using an intercept only mixed-effects model with change-scores as the response variable and random intercepts for study sites to account for between study site difference. Significance of the intercept in each PROM was used to test for significant recovery of the outcome to the baseline level. Using linear mixed-effect model with study site as random intercepts, outcomes by procedure types were compared by both PROM and time. For example, for the comparison of pain across procedure types at 1 week, the model was fit using one-week NPRS as the response variable, and with six indicators for seven procedure types as primary predictors. Each model was also adjusted for baseline demographic as well as clinical and surgical characteristics. Adjusted means at each follow-up time during recovery phase were calculated based on the model for each outcome measure by each procedure type. Score tests were used for the significance of the overall adjusted difference in outcomes across procedure types. P values less than 0.01 were considered statistically significant.
Results
During the study period, February 13, 2012–August 9, 2014, a total of 2,013 MROC study participants were both three months postoperative from primary reconstructive surgery and completed pre-operative surveys and therefore included for analysis. Of these patients, a total of 1,583 (78.6%) completed surveys at 1 week, and a total of 1,517 (75.4%) completed surveys at three months. The majority of patients underwent TE/I reconstruction (n=1,329), followed by DIEP flap (n=296), DTI (n=96), f-TRAM (n=91), p-TRAM (n=83), Lat dorsi (n=62), and SIEA (n=56). Demographic, clinical and surgical characteristics varied significantly across surgical procedure types. (Table 1) Additionally, the unadjusted baseline values of all outcome measures varied significantly across reconstruction types. (Table 2)
Table 1.
Baseline Demographic and Surgical Characteristics by Procedure Types (N = 2,013)
| Implant | Autologous | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | TE/I (n=1329) | DTI (n=96) | p-TRAM (n=83) | DIEP (n=296) | f-TRAM (n=91) | SIEA (n=56) | Lat dorsi (n=62) |
| Age, years | 48.1 (10.3) | 48.4 (12.4) | 52.9 (9.0) | 50.6 (8.6) | 51.8 (8.3) | 52.8 (7.8) | 53.4 (10.0) |
| Body Mass Index, kg/m2 | 25.8(5.4) | 26.0 (6.7) | 28.9 (6.0) | 29.0(5.3) | 31.0 (5.5) | 30.6 (6.9) | 24.9 (5.1) |
| Education High | |||||||
| School | 95 (7.2) | 9 (9.38) | 13 (15.7) | 48 (16.3) | 7 (7.69) | 15 (26.8) | 8 (12.9) |
| College | 705 (53.3) | 48 (50.0) | 48 (57.8) | 181 (61.6) | 48 (52.8) | 38 (67.9) | 40 (64.5) |
| Graduate | 523 (39.5) | 39 (40.6) | 22 (26.5) | 65 (22.1) | 36 (39.6) | 3 (5.4) | 14 (22.6) |
| Income | |||||||
| <$50,000 | 199 (15.4) | 13 (14.3) | 25 (30.9) | 61 (21.1) | 21 (23.9) | 23 (43.4) | 18 (30.0) |
| $50,000–$99,000 | 387 (30.0) | 27 (29.7) | 28 (34.6) | 135 (46.7) | 24 (27.3) | 13 (24.5) | 27 (45.0) |
| >$100,000 | 706 (54.6) | 51 (56.0) | 28 (34.6) | 93 (32.2) | 43 (48.9) | 17 (32.1) | 15 (25.0) |
| Race | |||||||
| American Indian | 5 (0.4) | 1 (1.0) | 2 (2.4) | 2 (0.7) | 0 (0) | 2 (3.6) | 2 (3.3) |
| Asian | 54 (4.1) | 5 (5.2) | 5 (6.0) | 14 (4.8) | 3. (3.4) | 2 (3.6) | 3 (4.9) |
| Black | 97 (7.4) | 1 (1.0) | 5 (6.0) | 13 (4.5) | 12 (13.5) | 0 (0) | 4 (6.6) |
| Native Hawaiian | 2 (0.1) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 1 (1.6) |
| White | 1156 (88.0) | 89 (92.7) | 71 (85.5) | 260 (89.7) | 74 (83.2) | 51 (92.7) | 51 (83.6) |
| Ethnicity | |||||||
| Hispanic | 86(6.6) | 3 (3.2) | 1 (1.2) | 14 (4.8) | 13 (14.4) | 0 (0) | 2 (3.4) |
| Non-Hispanic | 1219(93.4) | 90 (96.8) | 82 (98.8) | 279 (95.2) | 77 (85.6) | 51 (100) | 56 (96.6) |
| Laterality | |||||||
| Unilateral | 507(38.2) | 33 (34.4) | 67 (80.7) | 172 (58.1) | 57 (62.6) | 37 (66.1) | 47 (75.8) |
| Bilateral | 822 (61.8) | 63 (65.6) | 16 (19.3) | 124 (42.0) | 34 (37.4) | 19 (33.9) | 15 (24.2) |
| Timing | |||||||
| Immediate | 1304 (98.1) | 94 (97.9) | 74 (89.2) | 239 (80.7) | 69 (75.8) | 50 (89.3) | 47 (75.8) |
| Delayed | 25 (1.9) | 2 (2.1) | 9 (10.8) | 57 (19.3) | 22 (24.2) | 6 (10.7) | 15 (24.2) |
| Axillary Surgery | |||||||
| No | 255 (19.2) | 39 (40.6) | 32 (38.5) | 120 (40.5) | 35 (38.5) | 15 (26.8) | 23 (37.1) |
| ALND | 421 (31.7) | 10 (10.4) | 11 (13.3) | 60 (20.3) | 11 (12.1) | 13 (23.2) | 15 (24.2) |
| SLNBx | 614 (46.2) | 45 (46.9) | 40 (48.2) | 115 (38.9) | 45 (49.4) | 28 (50.0) | 24 (38.7) |
| Mixed | 39 (2.9) | 2 (2.1) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) |
Note: P-values from comparing across the seven procedure types are <0.001 for all characteristics except for race, which was 0.24 (compared as White vs. non-White). Note that axillary surgery was compared across No, ALND and SLNBx.
All cell values are N (%), except for age and BMI which are mean (SD).
Abbreviations: TE/I is tissue expander/implant reconstruction; DTI is direct to implant reconstruction; p-TRAM is pedicled transverse rectus myocutaneous flap; DIEP is deep inferior epigastric artery perforator flap; f-TRAM is free transverse rectus myocutaneous flap; SIEA is superficial inferior epigastric artery flap; lat dorsi is latissimus dorsi myocutaneous flap. ALND is axilarry lymph node dissection SLNBx is sentinel lymph node biopsy
Table 2.
Unadjusted Values of the Patient-reported Outcome Measures
| Implant | Autologous | |||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Time | TE/I (n=1329) | DTI (n=96) | p-TRAM (n=83) | DIEP (n=296) | f-TRAM (n=91) | SIEA (n=56) | Lat dorsi (n=62) |
|
|
|
|||||||
| NPRS | baseline | 1.1 (1.7) | 1.1 (1.9) | 0.8 (1.4) | 1.4 (1.8) | 1.3 (1.8) | 1.5 (2.1) | 1.6 (2.3) |
| 1-week | 4.0 (2.1) | 4.0 (2.2) | 3.0 (1.9) | 3.9 (2.3) | 4.2 (2.3) | 4.3 (2.1) | 3.8 (2.3) | |
| 3-month | 2.0 (2.0) | 1.1 (1.3) | 1.6 (2.2) | 1.7 (1.9) | 1.7 (1.9) | 1.5 (1.5) | 2.0 (2.1) | |
| p-values* | 0.37 | 0.58 | 0.72 | 0.86 | 0.87 | 0.74 | 0.49 | |
|
| ||||||||
| SF-MPQ | baseline | 2.9 (4.3) | 3.0 (4.5) | 2.5 (3.9) | 3.8 (4.5) | 4.1 (5.5) | 5.0 (6.4) | 4.1 (5.0) |
| 1-week | 10.9 (6.3) | 11.0 (7.2) | 8.4 (5.2) | 9.5 (6.0) | 11.6 (6.7) | 10.5 (6.5) | 11.0 (7.5) | |
| 3-month | 5.5 (5.4) | 4.6 (4.5) | 4.3 (3.8) | 4.9 (4.7) | 6.5 (6.4) | 5.4 (5.1) | 5.3 (7.5) | |
| p-values* | 0.03 | 0.25 | 0.12 | 0.12 | 0.02 | 0.09 | 0.08 | |
|
|
|
|||||||
| BFI | baseline | 2.3 (2.1) | 2.0 (2.0) | 2.5 (2.2) | 2.7 (2.2) | 2.7 (2.3) | 3.1 (2.5) | 2.2 (1.9) |
| 3-month | 2.9 (2.3) | 2.6 (2.5) | 2.8 (2.6) | 3.0 (2.4) | 2.7 (2.4) | 3.0 (2.4) | 2.8 (2.2) | |
| p-values* | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
|
|
|
|||||||
| PROMIS-29 | baseline | 49.2(9.9) | 48.3(10.4) | 49.3(10) | 50.5(9.7) | 50.3(9.6) | 52.1(10) | 48.7(8.4) |
| 3-month | 51.7(10.3) | 48.5(11.2) | 51.7(10.4) | 52(10.6) | 50.3(10.3) | 51.5(9.8) | 49.5(9.1) | |
| p-values* | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
|
|
|
|||||||
| PWB | baseline | 80.1 (14.2) | 81.3 (14.2) | 78.6 (15.2) | 75.4 (14.5) | 75.6 (16.8) | 73.7 (17.2) | 75.2 (16.4) |
| 1-week | 56.2 (12.8) | 56.2 (13.8) | 65.8 (11.8) | 62.5 (12.7) | 58.4 (13.0) | 61.5 (12.5) | 57.3 (15.8) | |
| 3-month | 68.3 (14.0) | 71.6 (14.7) | 71.7 (14.3) | 72.0 (14.2) | 67.8 (14.8) | 71.0 (13.2) | 68.3 (15.2) | |
| P-values* | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
P-values compared 3 month and baseline values of each measurement for each procedure type and adjusted for within-study center correlation.
Abbreviations: NPRS is numeric pain rating scale; SF-MPQ is MPQ Sensory subscales; BFI is brief fatigue inventory; PWB is BREAST-Q upper body and chest wall physical well-being scale. TE/I is tissue expander/implant reconstruction; DTI is direct to implant reconstruction; p-TRAM is pedicled transverse rectus myocutaneous flap; DIEP is deep inferior epigastric artery perforator flap; f-TRAM is free transverse rectus myocutaneous flap; SIEA is superficial inferior epigastric artery flap; lat dorsi is latissimus dorsi myocutaneous flap.
Cell values are mean (SD).
Pain
Pain scores, using the NPRS and the SF-MPQ instruments, increased from the baseline to one-week postoperatively and returned to preoperative levels by three months. (Table 2; Figures 1 and 2) At one week postoperatively, adjusted mean pain scores did not vary significantly across procedures, however, adjusted mean pain scores at 3 months, as measured by NPRS, varied significantly different across procedures (p=0.01), with greater pain in patients undergoing TE/I reconstruction as compared to direct to implant (p<0.01). (Table 3) Additionally, patients who received immediate reconstructions, compared to delayed, did not show significant differences in pain at one week or at three months (results not shown).
Figure 1.
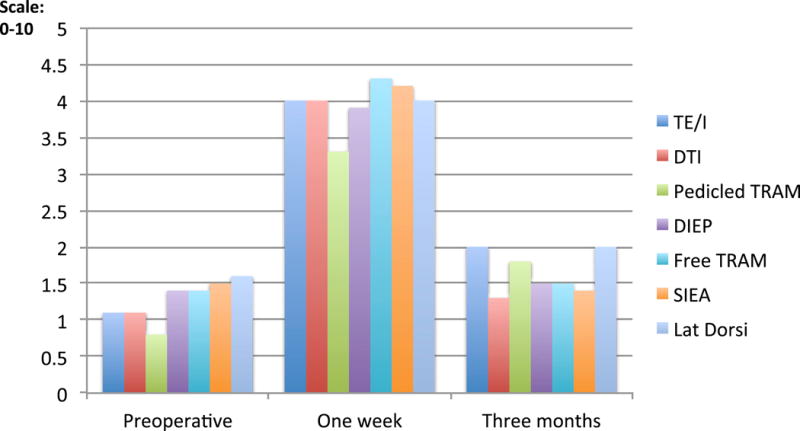
Numerical Pain Rating Score (NPRS) on a scale of 1–10 at three time points: preoperative, one week postoperative, and three months postoperative comparing types of reconstruction
Figure 2.
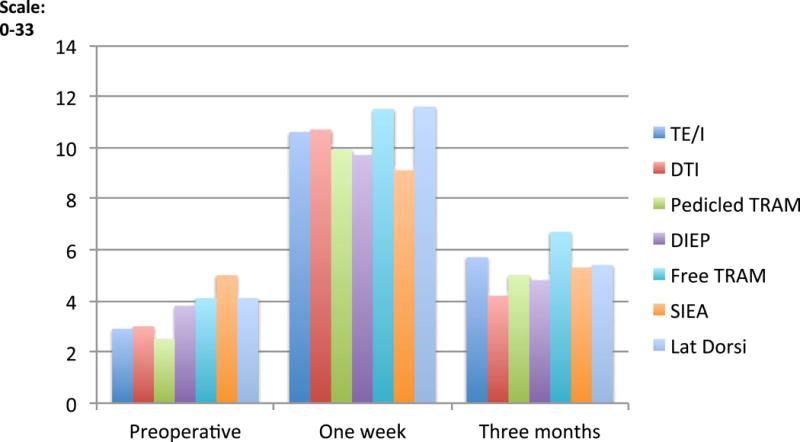
Short Form-McGill Pain Questionnaire (SF-MPQ) on a scale of 0–33 at three points: preoperative, one week postoperative, and three months postoperative comparing types of reconstruction
Table 3.
Covariate Adjusted1 Predicted Mean Scores at One Week Postop (n = 1,583) and at Three Months Postop (n = 1,517) by Procedure Types for Various Patient-reported Outcome Measures
| Implant | Autologous | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Measures | TE/I | DTI | p-TRAM | DIEP | f-TRAM | SIEA | Lat dorsi | P-values2 | |
|
|
|
||||||||
| NPRS | 1-week | 4.0 | 4.0 | 3.3 | 3.9 | 4.3 | 4.2 | 4.0 | 0.27 |
| 3-month | 2.0 | 1.3 | 1.8 | 1.5 | 1.5 | 1.4 | 2.0 | 0.01 | |
|
| |||||||||
| SF-MPQ | 1-week | 10.6 | 10.7 | 9.9 | 9.7 | 11.5 | 9.1 | 11.6 | 0.32 |
| 3-month | 5.7 | 4.2 | 5.0 | 4.8 | 6.7 | 5.3 | 5.4 | 0.05 | |
|
| |||||||||
| BFI | 3-month | 3.0 | 2.8 | 2.8 | 2.9 | 2.8 | 2.7 | 2.9 | 0.94 |
|
| |||||||||
| PROMIS-29 | 3-month | 51.9 | 49.2 | 52.5 | 51.7 | 50.5 | 50.0 | 51.2 | 0.16 |
|
|
|
||||||||
| PWB | 1-week | 56.7 | 56.6 | 62.9 | 62.3 | 57.7 | 61.3 | 56.0 | <.001 |
| 3-month | 67.5 | 70.6 | 70.9 | 72.9 | 68.3 | 73.0 | 69.3 | <.001 | |
Abbreviations: NPRS is numeric pain rating scale; SF-MPQ is MPQ Sensory subscales; BFI is brief fatigue inventory; PWB is BREAST-Q upper body and chest wall physical well-being scale. TE/I is tissue expander/implant reconstruction; DTI is direct to implant reconstruction; p-TRAM is pedicled transverse rectus myocutaneous flap; DIEP is deep inferior epigastric artery perforator flap; f-TRAM is free transverse rectus myocutaneous flap; SIEA is superficial inferior epigastric artery flap; lat dorsi is latissimus dorsi myocutaneous flap.
Adjusted for baseline values of the outcome variable, age, BMI, education (3 levels), income (3 levels), race (as White, Asian, black and other), ethnicity, laterality, timing, and axillary surgery and for within-study center correlation and predicted at mean covariate values.
Testing for differences in adjusted means across procedure types using score test (Type III test)
NOTE: For NPRS, MPQ, BFI, and PROMIS-29, a higher value corresponds to more pain or fatigue. For PWB, a higher value corresponds to better physical well-being or less physical distress.
Fatigue
Fatigue, as measured by the PROMIS-29 fatigue scale and the BFI, did not return to preoperative baseline level by the 3 months after surgery. (Table 2; Figure 3) Fatigue also did not vary significantly by procedure type at three months. (Table 3)
Figure 3.
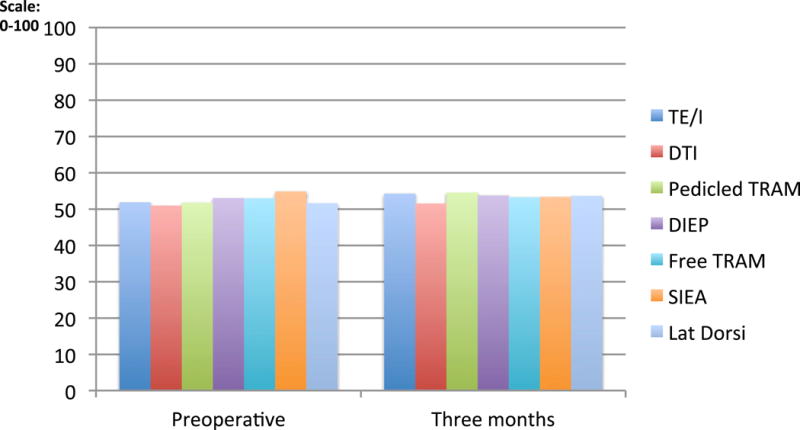
PROMIS-29 Fatigue Scale on a scale of 0–100 at two time points: preoperative and three months postoperative comparing types of reconstruction
Chest and Upper Body Physical Well-being
Upper body physical well-being, as measured by the BREAST-Q, decreased from preoperative to one week postoperatively in all sub-groups and did not return to preoperative baseline levels at three months. (Table 2; Figure 4) Upper body physical well-being varied significantly across reconstructive sub-groups at one week and at three months postoperatively (p<0.001). (Table 3) At one week, when using adjusted means to compare pairs of procedures, patients undergoing TE/I, DTI or LD reconstruction experienced greater upper body morbidity (shown in lower BREAST-Q scores) compared to patients undergoing abdominally-based autologous reconstruction (p<0.01), except f-TRAM patients. Similarly, at three months, patients undergoing TE/I reconstructions continued to experience the most distress compared to DIEP (p<0.001) and to SIEA (p=0.01). At 3 months, DTI reconstructions had less chest and upper body distress compared to TE/I reconstructions, but the difference was not significant (70.6 versus 67.5; p = 0.05). Additionally, when comparing patients undergoing delayed and immediate reconstruction there was no difference in chest and upper body physical well-being at either one week or three months after adjusting for procedure type and baseline covariates.
Figure 4.
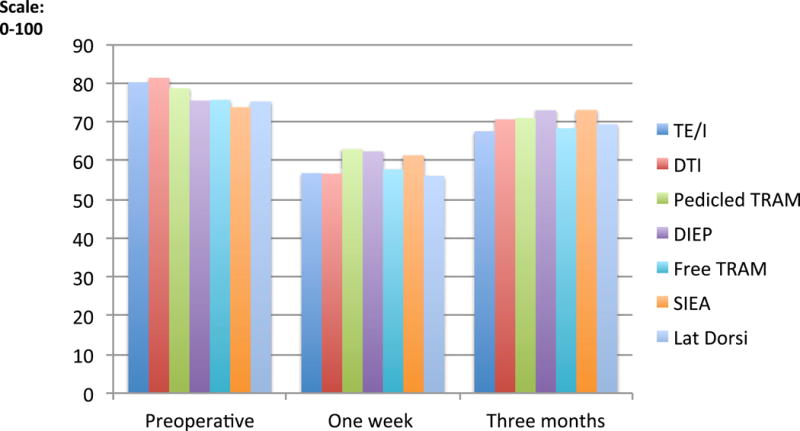
Physical Well Being (BREAST-Q) for chest and upper body on a scale of 0–100 at three time points: preoperative one week postoperative, and three months postoperative comparing types of reconstruction
Discussion
In the literature, there is little information regarding the general recovery period of breast reconstruction as it relates to both timing and type of reconstruction. Therefore, both patients and physicians struggle to make decisions about reconstructive options based on only assumptions and the personal experience of each individual surgeon. Historically, physicians have generally surmised that abdominally-based autologous reconstruction requires a longer and more arduous recovery compared to implant reconstruction; however, the evidence to support this is largely anecdotal. Previous studies have additionally shown PRO data helps both physicians and patients make informed decisions, manage expectations, and give a unique patient-centered perspective to surgical outcomes.9–13
Here, in a large prospective multi-centered observational study, using various validated PROMs, we have examined the recovery period of breast reconstruction after mastectomy and report several key findings: Firstly, for patients across all procedure types, recovery may not be complete at three months post-surgery. Secondly, patients undergoing abdominally-based autologous reconstruction generally experience less overall pain and less chest and upper body morbidity than patients undergoing two-stage implant reconstructions (TE/I). And finally, patients undergoing single stage implant reconstructions (DTI) may experience less chest and upper body morbidity than two-stage implant reconstruction (TE/I) patients at 3 months.
These conclusions have important clinical implications for both surgeons and patients. Notably, these findings debunk historical anecdotes that patients undergoing abdominally based breast reconstruction have a longer and more difficult recovery as compared to implant-based reconstructions. This investigation demonstrates, at one week, patients undergoing abdominally based breast reconstructions and implant-based breast reconstructions both have similar levels of pain as well as fatigue. Furthermore, at three months, patients undergoing traditional TE/I reconstructions have more pain and chest wall morbidity as compared to both autologous and DTI reconstructions. While these differences in procedure types were found to be statistically significant, they may not be clinically discernable. It is, however, still important to appreciate that patients undergoing more complex operations do not necessarily bear either more pain at one week and three months postoperatively. Often patients (and surgeons) assume the longer operation will convey greater time to recovery; here we see this assumption may be false.
It is also important to note, irrespective of method of reconstruction, recovery is not complete at three months. While pain has returned to preoperative baseline level, fatigue, and physical well-being of the chest wall are still significant sources of postoperative morbidity. This information is valuable for patients who seek to optimally plan their recovery and for surgeons who wish to establish realistic expectations. Patients may not appreciate fatigue and chest wall morbidity as significant factors in their recovery period. Therefore, it is important for surgeons to emphasize that pain is not the only component of postoperative recovery.
While this was a prospective multi-centered study with a large patient cohort, our study has several limitations. First, the adjusted analysis did not include information about chemotherapy and radiation therapy. This clinical information is only gathered in the MROC cohort at the 1-year visit and therefore was unavailable to our study population. Adjuvant chemotherapy and radiation are most often initiated within 3 months of primary surgery and clearly may affect pain and fatigue;14 the impact of such adjuvant treatment on three months outcomes for each procedure type was, however, considered in our analysis. Second, the majority of patients in the TE/I cohort completed the 3-month questionnaire with their tissue expanders still in place. Therefore, this three-month assessment does not represent their long-term quality of life with breast implants, but rather their short-term quality of life with the tissue expander, which undoubtedly causes more pain and physical morbidity than the final device. Third, in the MROC study, patients do not complete the BREAST-Q Physical well-being abdomen scale until their 1-year visit. Therefore, in this investigation, we are unable to comment on potential differences in abdominal wall morbidity associated with various approaches to abdominally-based autologous reconstruction. Further, patients undergoing abdominally based reconstructions are likely experiencing more abdominal discomfort as compared to chest wall discomfort. This abdominal morbidity may distract from the discomfort/morbidity in the chest wall resulting in ‘over scored’ chest and upper body physical well-being (i.e. similar to a distracting injury in a trauma evaluation). Finally, this study only examined the acute recovery period and further studies will include one and two-year data to allow us to better understand the extent to which patients continue to recover relative to their preoperative baseline.
Conclusions
This study provides important information regarding the recovery period of breast reconstruction. Notably, for many women, recovery may not be complete at 3 months postoperatively. Additionally, patients undergoing autologous reconstruction do not have greater pain and a more difficult recovery compared to TE/I reconstructions. This information will help guide both physicians and patients in preoperative decisions regarding reconstructive choices and may help set evidence-based expectations for the recovery period.
Acknowledgments
We thank the following MROC Site Principal Investigators for overseeing patient recruitment and data collection at their respective institutions: Yoon Sun Chun, MD., Brigham and Women’s Hospital, Boston; John Barbour, MD., Georgetown University Hospital, Washington DC; Richard Greco, MD., Georgia Institute for Plastic Surgery, Savannah; Steven Kronowitz, MD., MD Anderson Cancer Center, Houston; John Kim, MD and Neil Fine, MD., Northwestern Memorial Hospital, Chicago; Gayle Gordillo, MD., Ohio State Medical Center; Nancy VanLaeken, MD., University of British Columbia, Vancouver; Daniel Sherick, MD., Saint Joseph Mercy Health System, Ann Arbor; Ed Buchel, MD., University of Manitoba, Winnipeg.
Source of Funding: This research was funded in part through the NIH/NCI via the Cancer Center Support Grant P30 CA008748 as well as the grant R01 CA152192.
Footnotes
Disclosures: The authors have no conflicts of interest or disclosures.
This work has not been previously presented and is not in consideration for publication at any other journals
References
- 1.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plastic and reconstructive surgery. 2013 Jan;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 2.Lang JE, Summers DE, Cui H, et al. Trends in post-mastectomy reconstruction: a SEER database analysis. Journal of surgical oncology. 2013 Sep;108(3):163–168. doi: 10.1002/jso.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005 Jul;103(1):199–202. doi: 10.1097/00000542-200507000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. The Clinical journal of pain. 2005 Jan-Feb;21(1):73–82. doi: 10.1097/00002508-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. European journal of pain. 2008 Oct;12(7):917–925. doi: 10.1016/j.ejpain.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999 Mar 1;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plastic and reconstructive surgery. 2009 Aug;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 8.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plastic and reconstructive surgery. 2012 Feb;129(2):293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy CM, Mehrara BJ, Long T, et al. Chest and upper body morbidity following immediate postmastectomy breast reconstruction. Annals of surgical oncology. 2014 Jan;21(1):107–112. doi: 10.1245/s10434-013-3231-z. [DOI] [PubMed] [Google Scholar]
- 10.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery. 2013 Aug;132(2):201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 11.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Annals of surgical oncology. 2013 Oct;20(11):3422–3429. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- 12.Macadam SA, Ho AL, Lennox PA, Pusic AL. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plastic and reconstructive surgery. 2013 Mar;131(3):431–441. doi: 10.1097/PRS.0b013e31827c6d55. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010 Dec 15;116(24):5584–5591. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 14.Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Annals of surgical oncology. 2014 Jul;21(7):2159–2164. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]


