Abstract
Although axons lose some of their intrinsic capacity for growth after their developmental period, some axons retain the potential for regrowth after injury. When provided with a growth-promoting substrate such as a peripheral nerve graft (PNG), severed axons regenerate into and through the graft; however, they stop when they reach the glial scar at the distal graft-host interface that is rich with inhibitory chondroitin sulfate proteoglycans. We previously showed that treatment of a spinal cord injury site with chondroitinase (ChABC) allows axons within the graft to traverse the scar and reinnervate spinal cord, where they form functional synapses. While this improvement in outgrowth was significant, it still represented only a small percentage (<20%) of axons compared to the total number of axons that regenerated into the PNG. Here we tested whether providing exogenous brain-derived neurotrophic factor (BDNF) via lentivirus in tissue distal to the PNG would augment regeneration beyond a ChABC-treated glial interface. We found that ChABC treatment alone promoted axonal regeneration but combining ChABC with BDNF-lentivirus did not increase the number of axons that regenerated back into spinal cord. Combining BDNF with ChABC did increase the number of spinal cord neurons that were trans-synaptically activated during electrical stimulation of the graft, as indicated by c-Fos expression, suggesting that BDNF overexpression improved the functional significance of axons that did reinnervate distal spinal cord tissue.
Keywords: Spinal cord injury, Brain-derived neurotrophic factor, Chondroitinase ABC, Peripheral nerve grafting, Chronic injury, Axonal regeneration
Introduction
Although axons lose some of their intrinsic capacity for growth after their developmental period, some axons retain the potential for regrowth after injury. When provided with a growth-promoting substrate such as a peripheral nerve graft (PNG), several populations of long-injured supraspinal axons are able to regenerate into and through the PNG (Ye and Houle, 1997). However, these axons stop abruptly when they reach the distal graft-host interface when they encounter glial scar tissue. Inhibitory molecules such as chondroitin sulfate proteoglycans (CSPG) are expressed by the reactive astrocytes that mainly comprise the glial scar. When the enzyme chondroitinase ABC (ChABC), which cleaves the inhibitory sugar moiety from CSPG, is applied to a spinal cord injury site, axons are better able to regenerate and/or sprout beyond the normally inhibitory interface (Alilain et al., 2011; Barritt et al., 2006; Bradbury et al., 2002; Tom et al., 2009a). We previously demonstrated that when ChABC is administered to a chronic SCI site, significantly more axons are able to exit a PNG to reinnervate host spinal cord tissue, form functional synapses on host neurons and mediate some functional recovery (Tom et al., 2009b). While this combination treatment strategy is promising, only a small percentage (<20%) of axons present in the graft exit the distal end. It is imperative that strategies be developed to provide further impetus for axon growth out of the graft.
Neurotrophins provide both chemotrophic and chemotropic axon guidance. Several studies have demonstrated that providing sustained release of exogenous neurotrophins, either at the injury site with cellular grafts (Grill et al., 1997; Jin et al., 2000; Tuszynski et al., 2003) or distal to the injury and graft with viral vectors (Bonner et al., 2010; Taylor et al., 2006) promotes axonal regeneration. In particular, improved axonal regeneration of tracts such as the rubrospinal and reticulospinal (Sandrow et al., 2008) that extend into PNGs after injury has been observed into cellular grafts that were modified to produce brain-derived neurotrophic factor [BDNF; (Jin et al., 2002; Liu et al., 1999; Tobias et al., 2003; Ye and Houle, 1997)]. We hypothesized that overexpressing BDNF in spinal cord tissue distal to the PNG would provide more incentive for chronically injured axons that regrew into the graft to grow beyond a ChABC-treated glial scar to reinnervate the distal spinal cord. Unexpectedly, we found that combining BDNF overexpression and ChABC did not improve the ability of axons to emerge from the graft, but the combination did result in enhanced integration of those axons that did regenerate to form synapses.
Methods and materials
Surgical procedures
All procedures complied with Drexel University's Institutional Animal Care and Use Committee and National Institutes of Health guidelines for experimentation with laboratory animals. After all survival surgeries, animals were given ampicillin (200 mg/kg) and buprenorphine (0.1 mg/kg) postoperatively and placed on a thermal barrier to recover. They were returned to their cages once they became alert and responsive. All rats receiving PNGs were given cyclosporine A (CsA) (10 mg/kg, s.c., Sandimmune; Novartis Pharmaceuticals) daily starting 3 days before transplantation. After 2 weeks, these animals received CsA via their drinking water (100 mg/kg). This immunosuppression protocol has been used previously to successfully prevent against host rejection and promote long-term survival of an intraspinal graft (Houle et al., 2006; Tobias et al., 2003; Tom et al., 2009b).
C5 hemicontusion injuries
Adult female Sprague–Dawley rats (Charles River, 225–250 g) were injured as described previously (Sandrow et al., 2008; Tom et al., 2009b). Briefly, animals were injected intraperitoneally with ketamine (60 mg/kg) and xylazine (10 mg/kg). The right, dorsal surface of C5 was exposed by laminectomy. The vertebral column was stabilized by clamping the C3 and C7 vertebral bodies with forceps fixed to the base of an Infinite Horizon Impact Device (Precision Systems and Instrumentation, Lexington, KY). The animals were situated on the platform and the 1.6 mm stainless steel impactor tip was positioned over the midpoint (medial to lateral) of the right side of C5. The animals were impacted with a 200 kdyn force with displacement of tissue to a depth of 1600–1800 μm. The overlying musculature was closed using 4–0 sutures, and the skin was closed using wound clips.
Grafting a peripheral nerve “bridge”
The grafting procedure is similar to that described previously (Tom et al., 2009b) and is depicted in Fig. 1. One week before grafting (15 weeks post injury), tibial nerves of donor rats were ligated and cut as described above. One week later, donor rats were anesthetized with ketamine and xylazine and a 15 mm length of predegenerated tibial nerve was removed immediately before grafting.
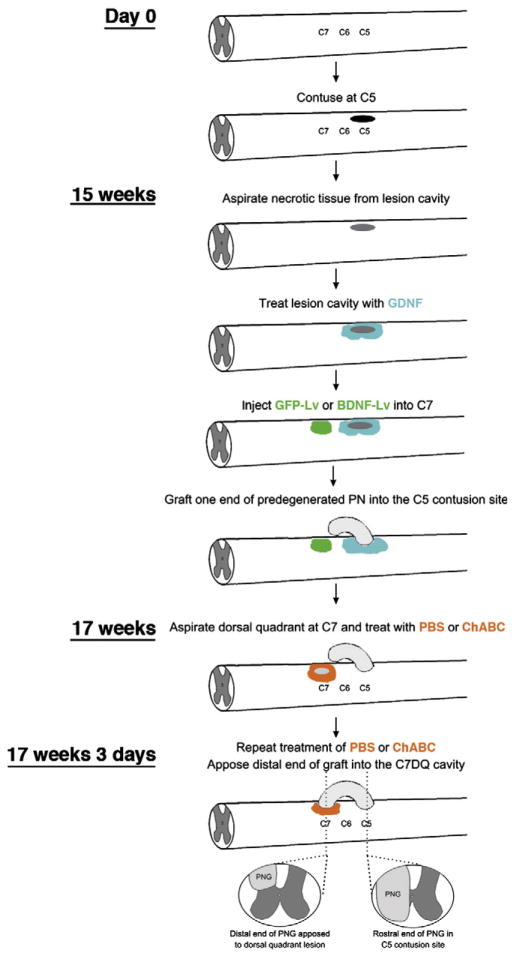
Fig. 1.
Schematic of the experimental methods and timeline.
Sixteen weeks after the C5 hemicontusion injury chronically injured rats were anesthetized with isoflurane. The C5 contusion site was exposed and a small slit was made in the overlying dura using a micro knife. The necrotic tissue within the lesion cavity was gently removed by aspiration. Great care was taken to not disturb the glial scar or underlying spinal cord that was spared from the initial injury. Although acutely injured axons readily regenerate into a predegenerated PNG, chronically injured axons need additional stimulation (Ye and Houle, 1997). Therefore, gelfoam saturated with 20 μg/ml GDNF (R&D Systems, Minneapolis, MN) in PBS was placed into the lesion cavity of all animals for 60 min (Dolbeare and Houle, 2003; Storer et al., 2003; Tom et al., 2009b). The gelfoam was replaced three times with fresh, saturated gelfoam. Additionally, 1 μl of 20 μg/ml GDNF in PBS was microinjected into ipsilateral spinal cord 1 mm rostral and 1 mm caudal to the contusion site using a pulled glass micropipette. One end of a predegenerated tibial nerve was isolated from a donor rat and ~2–3 mm of the perineurium was peeled from the proximal nerve end. This end was placed into the C5 contusion site so that it apposed the rostral cavity area and was secured by suturing perineurium to the dura. The distal end of the PNG was left free outside of the spinal column, along the C6–C8 vertebrae.
At this time, an additional laminectomy was performed at C7. One microliter of lentivirus (108 TU/ml) encoding for either BDNF or GFP was injected into C7 (Bonner et al., 2010, 2011). The overlying musculature was closed using sutures and the skin was closed using wound clips.
Three weeks later graft recipient animals were anesthetized. A laminectomy was performed on the right side of the C7 vertebral process and part of the dorsal quadrant was aspirated out, creating a cavity of ~1 mm3. This time point was chosen so that chronically injured axons had ample time to grow into and through the graft to its end. Thus, actively growing axons would be adjacent to the host spinal cord after graft apposition to C7 and maximize the potential of these axons to exit the graft soon after apposition to the treated distal interface. Animals received microinjections of 0.5 μl of ChABC (20 U/ml) or PBS immediately rostral, caudal, and ventral to the fresh DQ lesion. This pattern of microinjections was repeated three days later. Thus there were four experimental groups: distal cord treatment with GFP+PBS (n=20), GFP+ChABC (n=23), BDNF+PBS (n=17) or BDNF+ChABC (n=20). After the second series of injections, the free, distal end of the PNG was trimmed by 1 mm to help spur a regenerative response and inserted into the C7DQ cavity, where it was secured in place by suturing the perineurium to the dura. BioBrane membrane (UDL Laboratories, Rockford, IL) was placed over the graft. After the musculature was sutured, the skin was closed using wound clips.
Behavioral analyses
Animals were maintained on food and water ad libitum with a 12 h light–dark cycle. After acclimation to the testing apparatus over a 1-week period, baseline scores for forelimb open-field locomotion, walking on a grid platform, and Tread Scan (Clever Sys Inc., Reston, VA) were obtained for each animal. Animals then were subjected to the unilateral C5 contusion injury. After the final grafting procedure (i.e. apposition of the PNG into the DQ lesion site), animals were evaluated weekly for 8 weeks.
Open field locomotion
Forelimb function was evaluated in an open field measuring 2.5×3 ft. and the rats were observed for 4 min by at least two individuals blinded to the treatment condition. The forelimb locomotor scale (FLS), devised at Drexel University College of Medicine from observation of recovery patterns in cervically injured rats, is a 17-point scale that defines deficits based on range of motion, level of weight support, and whether the paw is placed parallel to the body (Cao et al., 2008; Sandrow et al., 2008). The FLS is thus similar in assessment style to the BBB rating scale for hindlimb function (Basso et al., 1995). Each animal was scored during direct observation in the open field for 4 min and videotaped for later reference if necessary. Scores were analyzed for significance using a one-way ANOVA and post-hoc Mann–Whitney tests for each time point. A p-value of <0.05 was considered significant.
Grid walking test
This test was administered as described previously (Shumsky et al., 2003). Briefly, the animals were placed on a plastic-coated wire mesh grid for 2 min, and the number of forepaw placements on the bars was counted. A correct placement was defined as a step in which the paw gripped the bar and supported body weight. The number of correct placements was expressed as a percentage of the total steps. Scores at each time point were compared for significance a one-way ANOVA followed by a two-tailed Student's t-test (SPSS). A p-value of <0.05 was considered significant.
Forced locomotion
The TreadScan system (CleverSys, Reston, VA), consisting of a clear treadmill with a camera to allow for recording of step cycles from beneath the rat, was used to obtain and evaluate two aspects of gait (stride length and stride time). Forelimb and hindlimb locomotor capabilities were assessed during a 20 s period of forced locomotion. A background image was taken before each test day. The treadmill speed was 8 m/min. The recorded AVI file was converted to an MPEG file and analyzed with the CleverSys TreadScan software. Data were analyzed by two-way ANOVA between groups and time, with time taken as a repeated measure. Post hoc analysis was performed using Tukey's tests. All statistics were conducted using SPSS (version 19.0). A p-value of <0.05 was considered significant.
Electrical stimulation of the PNG
As previously described (Tom et al., 2009b), animals (n=6 per experimental group) were anesthetized with ketamine and xylazine, the graft was exposed and the middle of the PNG was lifted from surrounding tissue and placed onto a bipolar hook electrode (#PBCA6775; FHC Inc., Bowdoin, ME). The graft was bathed in warmed mineral oil while it was stimulated for 1 h (1 mA amplitude, 100 μs pulse duration, and 50 Hz frequency). Animals were killed and perfused 2 h later with 4% PFA.
Labeling of regenerated axons in PNG
To trace axons that regenerated into the PNG animals were anesthetized and the cord and graft were exposed. The graft was cut and the distal end was soaked with 10% biotinylated dextran amine (BDA, Invitrogen) to label regenerating axons by tracer diffusion though the distal end of the graft. Animals were perfused with 4% PFA two days later.
Histology
Segments of spinal cord containing the PNGs were dissected and post-fixed overnight in 4% PFA at 4 °C. The tissue then was cryoprotected in 30% sucrose before sectioning on a cryostat. Transverse sections (25 μm) through the distal apposition site were cut in a series of six sets. Sections were blocked in 5% normal goat serum, 1% bovine serum albumin, 0.1% Triton X-100 in PBS for 1 h at room temperature before incubation in the appropriate primary antibody overnight at 4 °C. The primary antibodies used were anti-TrkB (Santa Cruz, Santa Cruz, CA) and anti-c-Fos (Abcam, Cambridge, MA). Sections were rinsed in PBS and incubated in the appropriate secondary antibody overnight at 4 °C. To visualize BDA-labeled axons, sections were incubated with avidin–HRP and then reacted with diaminobenzidine (DAB; Sigma, St. Louis, MO). Sections incubated in anti-c-Fos were reacted with the appropriate secondary antibodies and then DAB. Sections were rinsed in PBS, mounted onto glass slides, and coverslipped using either VectaShield for fluorescent sections (Vector Laboratories, Burlingame, CA) or dehydrated and coverslipped using Permount (Fisher Scientific, Pittsburgh, PA) for DAB-reacted sections. Sections were imaged using a Zeiss Axioskop microscope.
To quantify BDA+ regenerating axons that emerged from PNGs, transverse C7 sections (five sections per animal) containing a PNG were used for image analysis. A virtual line was drawn 300 μm from the most ventral point of the graft–host interface and the number of axons crossing that line was counted. The mean number of axons from the 4 groups was compared statistically using a one-way ANOVA and post hoc Tukey's test (SPSS). Significance was determined if p<0.05.
To quantify the number of c-Fos+ cells after stimulation of the PNG bridge, four transverse sections containing a portion of the apposed PNG from electrophysiologically stimulated animals were imaged and montaged. A predetermined saturation threshold level (MetaMorph, Molecular Devices, Sunnyvale, CA) was applied to each section to eliminate background or non-specific staining. All images were subject to the same saturation threshold. Cells with c-Fos+ nuclei above threshold were counted in gray matter ipsilateral and contralateral to the graft. The numbers of ipsilateral c-Fos+ nuclei were statistically compared amongst groups using a one-way ANOVA and post hoc Tukey's tests (SPSS). Significance was determined if p<0.05.
Western blotting
Normal, uninjured adult rats were anesthetized with isoflurane. A laminectomy was performed at C7 and 1 μl of lentivirus (108 TU/ml) encoding for BDNF (n=3) or GFP (n=3) was microinjected using a pulled glass pipette as described above. The overlying musculature was sutured closed and the skin was closed with wound clips. Two weeks following virus injection, animals were given an overdose of Euthasol (390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin IP). Tissue encompassing the injection site was harvested and the spinal roots and meninges were stripped off. The tissue was placed in cold RIPA buffer (50 mM Tris buffer pH 7.6, 0.1% SDS, 0.25% deoxycholate, and 150 mM NaCl), in the presence of protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN), 2 mM phenyl–methyl sulfonyl-fluoride (PMSF), and 1 mM sodium fluoride. Tissue blocks were sonicated and centrifuged at 14,000 ×g for 40 min at 4 °C. The supernatants were collected and aliquots were stored at −80 °C. Protein assays were conducted to determine protein concentration for each sample. For Western blot analysis, the samples were boiled in Laemmli sample buffer for 5 min, and equal amounts of total protein were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes (BioRad, Hercules, CA). Each nitrocellulose replica was blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T), probed with primary rabbit polyclonal antibodies against BDNF (1:400; Abcam, Cambridge, MA) followed by incubation with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (IgG; Jackson ImmunoResearch Laboratories, West Grove, PA). Blots for each sample were run two or three times for each primary antibody to ensure replication of the results. To confirm equal loading of protein in each lane, the blots were stripped using buffer containing 65 mM Tris buffer (pH 6.8), 2% SDS, and 1% β-mercaptoethanol for 30 min, and re-probed with mouse monoclonal anti-actin antibody (1:8000; Sigma-Aldrich, St. Louis, MO). Immunoreactivity was detected using an enhanced chemiluminescence kit (ECL; Amersham Biosciences, Piscataway, NJ). Densitometry analyses of immunopositive bands were performed using Syngen software (Frederick, MD). To account for variability in sample loading and transfer efficiency, all data were normalized to densitometry values of actin for each sample. Values between GFP-lentivirus and BDNF-lentivirus groups were compared using Student's t-tests, with significance being indicated by a p<0.05. Final data (mean±SEM) are presented as a ratio to values from the GFP-lentivirus injected control group.
Results
Overexpression of BDNF using lentivirus
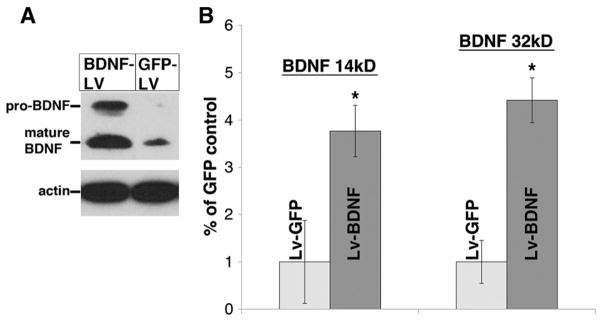
Two weeks after lentivirus encoding for GFP or BDNF was injected into normal C7 spinal cord we found that there was a basal level of mature BDNF (~14 kDa) expression in animals injected with GFP-lentivirus (Fig. 2). There was ~3.8-fold increased expression of mature BDNF at C7 in animals injected with BDNF-lentivirus (Fig. 2), compared to the GFP control. Interestingly, these animals also expressed approximately 4.4 times more of the higher molecular weight precursor to BDNF (“proBDNF”, ~28 kDa), which was virtually undetectable in the control GFP animals. This confirms previous published work using the same lentivirus (Bonner et al., 2010, 2011; Lu et al., 2012) and indicates that injecting lentivirus for BDNF into spinal cord effectively increases local expression levels of the neurotrophin.
Fig. 2.
BDNF-lentivirus increases BDNF levels within the spinal cord. Lentivirus encoding for BDNF or GFP was injected into normal C7 spinal cord tissue. (A) Western blot analysis indicates that three weeks after the injection, BDNF levels (~14 kD) were approximately 3.8 times higher in tissue in the animals that were injected with BDNF-lentivirus (dark bars in B) than GFP-lentivirus (light bars in B). Moreover, there was also an increase (~4.4×) in the expression of the immature, precursor BDNF (~32 kD) in these animals. Y-axis values in B are the ratio of the densitometric values (following normalization to actin) to those of the GFP-lentivirus control (mean±SEM). *p<0.05 between BDNF-lentivirus and GFP-lentivirus.
TrkB receptor is expressed by chronically injured axons
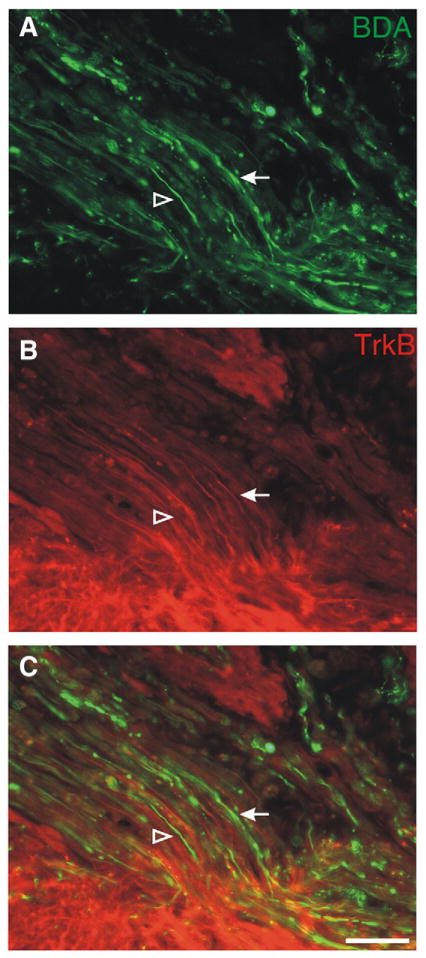
We wanted to determine if chronically injured axons that regenerated into a PNG expressed TrkB, the receptor for BDNF. At 8 weeks following grafting (~24 weeks after the initial hemicontusion), there were BDA+ axons (Figs. 3A, C, arrow) within the graft that were TrkB+ (Figs. 3B, C, arrow). However, there were other BDA+ axons (Figs. 3A, C, open arrowhead) that did not express TrkB (Figs. 3B, C, open arrowhead). Thus, some, but not all, axons within the graft maintain expression of TrkB for long post injury periods, though this does not provide information about the expression of TrkB by newly injured axons or about axons that are in an active growth phase.
Fig. 3.
TrkB expression on regenerating axons within the peripheral nerve graft. Axons that grew into the graft were labeled with BDA (green in A, C). Some of the BDA+ axons also expressed TrkB (red in B, C), as visualized by immunohistochemistry (arrow). However, there were other BDA+ axons within the graft that were not immunoreactive for TrkB (open arrowhead). Scale bar: 50 μm.
Behavioral analysis
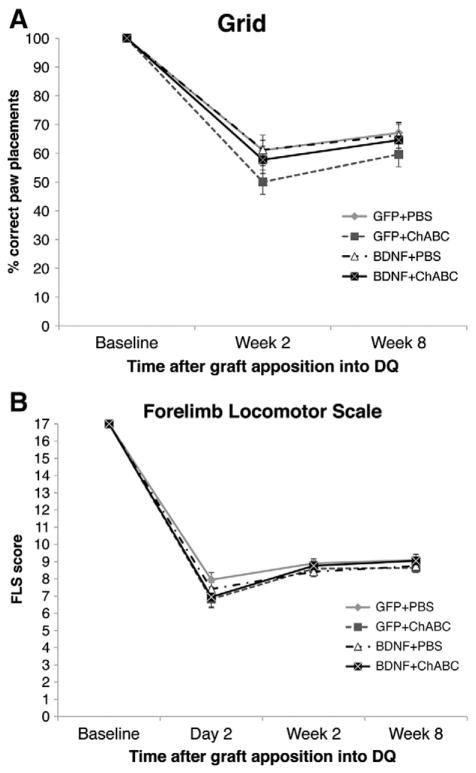
To determine if treatment with ChABC or ChABC and BDNF-lentivirus promoted any functional improvements, locomotor function of the animals was assessed using the grid walking test (Fig. 4A) and the forelimb locomotor scale (Fig. 4B). In both tests, the injuries resulted in significant impairments compared to baseline abilities. In the grid walking test, animals were only capable of correctly placing their paws on the rungs 50–61% of the time 2 weeks after apposing the PNG into the dorsal quadrant injury site. By 8 weeks, animals were placing their paws correctly on the rungs 60–66% of the time. At neither time point was there a significant difference between groups. In the FLS, there was also a significant decline in all of the animals' abilities to walk in the open-field at 2 days, 2 weeks, and 8 weeks after PNG apposition into the dorsal quadrant injury. There were no significant differences between any of the treatment groups at any time point. There were also no significant differences in stride length or stride time (as determined using the Tread Scan) between groups (data not shown).
Fig. 4.
Combining BDNF and ChABC does not improve functional recovery. The locomotor capabilities of the animals were tested using a grid-walking test (A) and the forelimb locomotor scale (B). After injury, there was a decline in all animals' abilities to walk on a grid platform on in the open-field. There were no significant differences between groups at any of the tested time points in either test.
ChABC promotes axonal regeneration beyond the graft, but BDNF does not further enhance this outgrowth
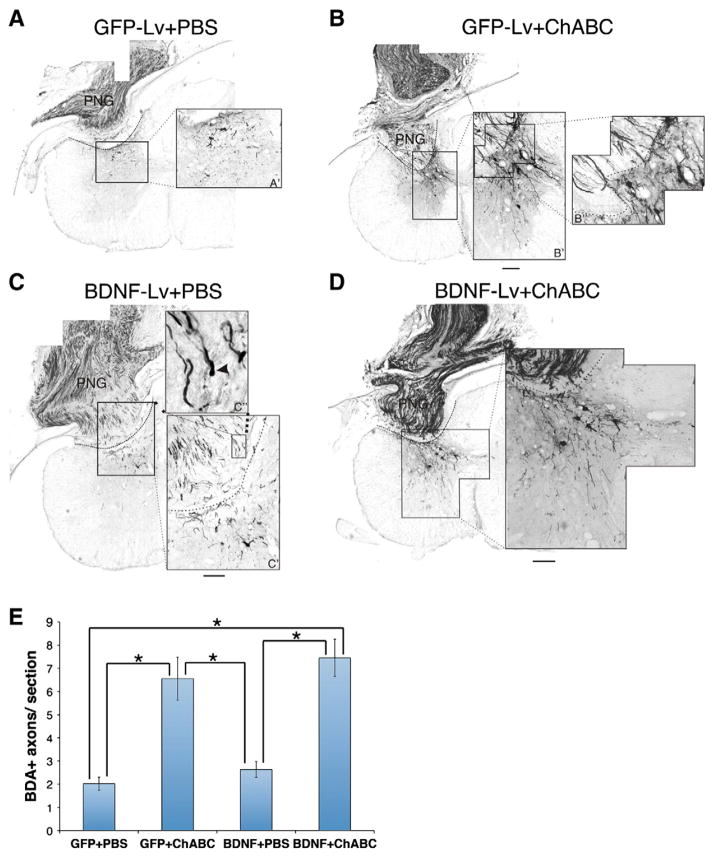
While we previously demonstrated that ChABC allows chronically injured axons to grow beyond a PNG, we tested whether exogenous BDNF distal to the graft would entice more axons to emerge from the graft. Eight weeks after grafting very few BDA+ axons that regenerated into the PNG were found to have extended beyond a PBS-treated graft-host interface into spinal cord that was injected with lentivirus for GFP (2±0.3 axons/section; Figs. 5A, E) or lentivirus for BDNF (2.6±0.4 axons/section; Figs. 5C, E). Moreover, we found examples of dystrophic, bulbous endings, a hallmark of regenerative failure, at the distal graft interface in these PBS-treated animals (Fig. 5C″, arrowhead). As shown previously, ChABC significantly increased the numbers of axons that grew out of the graft to contact host tissue (Figs. 5B, D, E). Similar numbers of BDA+ regenerating axons were found in ChABC-treated tissue injected with either lentivirus for GFP (6.6±1.0 axons/ section) or BDNF (7.5±0.8 axons/section). These data indicate that while ChABC increases axonal regeneration, the increased expression of BDNF does not further heighten this growth response.
Fig. 5.
Axonal regeneration is promoted by ChABC but is not improved by combining BDNF and ChABC. Axons within the graft were labeled with BDA. Representative images of transverse sections containing the PNG in the C7DQ were reacted with DAB. In animals treated with either GFP+PBS (A) or BDNF-lentivirus+PBS (C), few BDA+ fibers grew beyond the graft-host interface (demarked with the dashed line). Some axons that failed to grow out of the graft appeared to have dystrophic endbulbs (arrowhead in C″). On the other hand, significantly more BDA+ axons were found crossing the interface following ChABC treatment (B, B″) to reinnervate spinal cord. However, BDNF did not enhance this ChABC-facilitated regeneration. While the BDNF-lentivirus+ChABC animals had significantly more BDA+ axons distal to the PNG (D, E) than animals treated with GFP-lentivirus+PBS or BDNF-lentivirus+PBS, there was no significant difference between the BDNF-lentivirus+ChABC- and ChABC only-treated animals (E). *p<0.05; scale bar: 250 μm.
BDNF enhances integration of regenerated axons
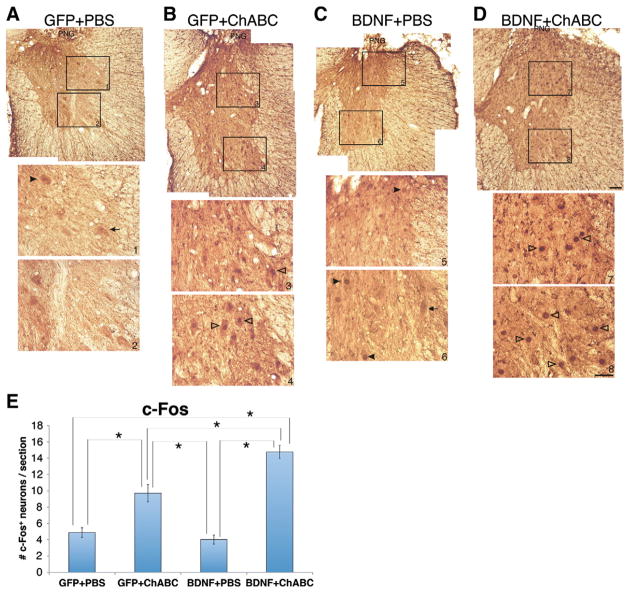
We previously showed that chronically injured axons that regenerate beyond a PNG are capable of forming functional synapses as indicated by increased c-Fos expression in host neurons after electrical stimulation of the PNG (Tom et al., 2009b). We tested whether BDNF overexpression in tissue distal to the graft affected the function of the axons that regenerated beyond a ChABC-treated interface. To test this, we specifically stimulated the PNG and examined the extent of c-Fos expression in neurons ventral and ipsilateral to the C7DQ apposition site. We found that in tissue that was treated with PBS and the GFP lentivirus, very few neurons were found to be c-Fos+ (4.9±0.6 neurons/ section; Figs. 6A, E). Similarly, there were very few c-Fos+ neurons in animals that were treated with PBS and the BDNF lentivirus (4.0±0.6 neurons/section; Figs. 6C, E). While there were some neurons that had c-Fos+ nuclei in these two groups of animals (Figs. 6A, C, E; arrowheads), there were also neurons that very clearly did not express c-Fos+ (Figs. 6A, C, E; arrows). On the other hand, there were significantly more neurons expressing c-Fos after graft stimulation in animals that were treated with ChABC and the GFP-lentivirus (9.7±1.1 neurons/section; Figs. 6B, E; open arrowheads). Overexpressing BDNF in the ChABC-treated animals resulted in an even greater number of c-Fos expressing neurons (14.8±0.8 neurons/section; Figs. 6D, E; open arrowheads) than with GFP-lentivirus and ChABC, despite there being similar numbers of axons exiting the graft in both groups of animals (Fig. 5E). Thus, BDNF appears to enhance synaptic efficacy of the axons that are able to regenerate beyond a ChABC-treated injury site.
Fig. 6.
BDNF expression distal to a graft enhances synaptic function of axons that regenerated through a ChABC-treated glial scar. The PNG was electrically stimulated for 1 h and sacrificed 2 h later. Transverse sections of tissue containing the PNG were processed for immunohistochemistry to visualize c-Fos. Animals that were treated with GFP-lentivirus+PBS (A) or BDNF-lentivirus+PBS (C) had few c-Fos+ nuclei in neurons within intermediate grey matter (inset boxes 1 and 5, respectively) or ventral horn (inset boxes 2 and 6, respectively) ipsilateral to the PNG. There were a few neurons in these sections that were positive for c-Fos (arrowheads in A, C), but most neurons were not immunoreactive for c-Fos (arrows in A, C). There were significantly more c-Fos+ neurons in grey matter ipsilateral and ventral to a PNG following GFP-lentivirus+ChABC (open arrowheads in B, E). Combining BDNF-lentivirus with ChABC treatment of the scar further and significantly increased the number of c-Fos+ neurons in spinal grey matter distal to the PNG (open arrowheads in D, E). *p<0.05; scale bar for low magnification images: 250 μm; scale bar for high magnification insets: 50 μm.
Discussion
We previously demonstrated that chronically injured axons are capable of functional regeneration into and out of a PNG if the distal interface is treated with ChABC. However, while these results were significant, a small percentage of axons that grew into the PNG actually regenerated out of the graft. Thus, we tested whether we could entice a larger percentage of axons that grow into the graft to reinnervate host tissue. We hypothesized that overexpressing BDNF using lentivirus in tissue distal to the graft in combination with ChABC-treatment of the glial scar would increase chronically injured axons' ability to grow through the graft-host interface. We found that exogenous BDNF did not augment growth of long-injured axons beyond a ChABC-treated interface. Interestingly, though, despite there being similar numbers of axons that regrew into spinal cord tissue in ChABC-treated animals that were injected with either BDNF-lentivirus or GFP-lentivirus, regenerated axons formed functional synapses on significantly more neurons in the animals that overexpressed BDNF. Thus, while BDNF did not have an effect on the number of regenerating axons beyond the PNG, it did have a significant and positive effect on the integration of axons that did regenerate.
BDNF may enhance the integration of regenerated axons by promoting synapse formation (Alsina et al., 2001; Bamji et al., 2006; Sanchez et al., 2006). Additionally, the ability of BDNF to shape the strength of existing synapses and enhance excitability is well documented. BDNF can increase the amount of neurotransmitter released into the synaptic cleft (Kang and Schuman, 1995; Lessmann, 1998; Li et al., 1998; Lohof et al., 1993) and increasing postsynaptic neuronal excitability [e.g. increasing the number of action potentials triggered in response to a single depolarizing step, decreasing voltage-gated K+ currents, and upregulating chloride co-transporters (Boulenguez et al., 2010; Gonzalez and Collins, 1997; Youssoufian and Walmsley, 2007; Zhang et al., 2008)]. While it is unclear at this point if our results stem from increased synapse formation or an increase in the efficiency of signal transduction of the regenerated axons (or perhaps a combination of both), it will be very important to determine the exact mechanism for how BDNF is affecting the function/integration of regenerated axons. Interestingly, pro-BDNF, which is also elevated in the animals that were injected with BDNF-lentivirus, has been linked to synaptic depression (Lu, 2003; Woo et al., 2005; Yang et al., 2009). However, it is unclear as to how much pro-BDNF vs. mature BDNF is secreted. Furthermore, matrix metalloproteinases, which convert pro-BDNF to mature BDNF (Ethell and Ethell, 2007; Hwang et al., 2005), are upregulated following spinal cord injury (Zhang et al., 2011). Thus, it is unclear how pro-BDNF is affecting synapse formation in this study but it would be important to elucidate this in future studies.
BDNF alone can promote plasticity that has functional implications, even in the absence of any influence from supraspinal regeneration (Boyce et al., 2007; Boyce et al., 2012; Lu et al., 2012). This is likely due to plasticity of local, existing circuitry. BDNF-induced reshaping of existing circuitry may not always be positive and can result in spasticity (Boyce et al., 2012; Lu et al., 2012). Unlike these other studies, we did not observe any ill-effects in the animals treated with BDNF lentivirus. This may be due to differences in BDNF expression levels. Both Boyce et al. (2012) and Lu et al. (2012) injected significantly more virus encoding for BDNF than we did in this study, indicating that further experiments optimizing BDNF overexpression to maximize positive effects and minimize detrimental effects is critical.
Another potential mechanism for why neurons distal to the graft were more responsive to electrical stimulation of the PNG in animals treated with BDNF+ChABC than ChABC alone is that BDNF may promote collateralization of the regenerated axons (Cohen-Cory, 1999; Cohen-Cory and Fraser, 1995; Danzer et al., 2002; Jeanneteau et al., 2010; Marler et al., 2008). However, this explanation seems less likely than BDNF enhancing synapse formation or synaptic strength since the quantification of BDA+ axons in distal tissue should have accounted for a significant increase in axon branching.
We did not observe any effects of BDNF on behavioral function in our animals. We may observe functional recovery mediated by the BDNF-mediated enhanced synaptic transmission in a more challenging complete SCI model, unlike the incomplete injury model we used where there is already a large influence of spontaneous, compensatory plasticity (Bareyre et al., 2004; Courtine et al., 2008). The lack of correlation of increased c-Fos induction and a change in behavioral data in this study suggests that either we did not promote sufficient regeneration to have a functional effect or that the regenerated axons formed synapses on inappropriate target neurons that are unlikely to affect functional outcome measures. We need to develop methods to better control synapse formation of regenerated axons on appropriate neuron pools. It should also be noted that previously, we found that ChABC treatment of the distal interface resulted in increased stride length (Tom et al., 2009b). However, in this study, we did not find that there were any differences in gait, even in the ChABC-treated animals. We believe that the two data sets cannot be accurately compared due to a technical discrepancy in how the data was collected. In the previous study, the speed of the treadmill was adjusted for individual animals so that each rat displayed a consistent gait pattern. In the present study, the speed of the treadmill was kept constant for all animals and did not take into account differences in individual rats' abilities. It could be that a wide variability in the animals' abilities to walk on a set treadmill speed precluded us from seeing any functional improvements.
While there is evidence within the literature that chronically injured axons are capable of responding to BDNF, we did not observe any additional regeneration beyond a ChABC-treated injury site. Published studies have shown that injured axons extend significantly better into lesion cavity-filling grafts of fibroblasts or mesenchymal stem cells that are engineered to express BDNF compared to unmodified fibroblast grafts (Jin et al., 2002; Tobias et al., 2003). The present study is different from the aforementioned studies because we overexpressed BDNF only distal to the cellular graft. The finding that BDNF did not enhance axonal extension beyond the graft was surprising since BDNF was shown to promote regeneration of acutely injured axons beyond grafts of mesenchymal stem cells (Lu et al., 2012) or Schwann cell-seeded channels (Bamber et al., 2001), a substrate that is similar to a PNG. However, chronically injured neurons, which were studied here, are quite different from acutely injured ones in that they may have undergone atrophy (Bregman et al., 1998; Kobayashi et al., 1997; Liu et al., 2002) and they express lower levels of regeneration associated genes (Storer and Houle, 2003; Tetzlaff et al., 1991). Thus, even though chronically injured axons can respond to BDNF, BDNF appears to have differential effects on axonal regeneration in an acute vs. a chronic injury setting. Another contributing factor is that increased levels of pro-BDNF found in the animals treated with BDNF-lentivirus may have a negative effect on outgrowth and negate positive tropic/trophic effects of mature BDNF (Koshimizu et al., 2009).
Schwann cells secrete several neurotrophins and basal lamina components (e.g. laminin and fibronectin) and express growth-promoting molecules on their cell surface (Ard et al., 1987; Kleitman et al., 1988; Martini, 1994). It is possible that while we have evidence that injecting lentivirus is an effective means of increasing spinal cord BDNF levels, this increase may not be higher than BDNF levels within the PNG, providing little incentive for axons to grow beyond the graft (i.e. “neurotrophin trapping”). Moreover, the combination of the other growth-supportive molecules associated with the PNG may make the graft more hospitable for axons than even spinal cord tissue with a high concentration of BDNF. This may be even more relevant for chronically injured axons since they have a lower intrinsic capacity for regrowth than do acutely injured axons.
There are several additional possible explanations for the absence of an additive effect of BDNF and ChABC on axonal regeneration. We found that some, but not all, axons within the graft express TrkB, the receptor that binds BDNF. Kwon et al. (2004) found that chronically injured neurons in the red nucleus express TrkB receptor on their cell bodies, but not on their axons, and respond to BDNF delivered to the cell bodies (Kwon et al., 2002), but not to the injury site (Kwon et al., 2004). While we found that some chronically injured axons do express TrkB, our results do not necessarily contradict those from Kwon et al. We treated our chronic injury site with GDNF prior to grafting. This treatment is sufficient to upregulate regeneration-associated genes and to promote extension of chronically injured axons into a PNG (Dolbeare and Houle, 2003; Storer et al., 2003). It is possible that treatment of the lesion site with GDNF also increases the expression of neurotrophin receptors, including TrkB, in injured axons.
Additionally, while rubrospinal axons are one type that extends into a PN grafted into a chronic injury site, the vast majority of regenerating axons originate from areas other than the red nucleus, such as the reticular formation (Sandrow et al., 2008; Ye and Houle, 1997). Preliminary data from the lab indicates that descending axons regenerating into a PNG express TrkB (Amin and Houle, 2011), though we do not know what chronically injured axonal population expressed the TrkB receptor in this study. It is also important to note that the immunohistochemistry for TrkB was conducted on tissue from animals in which the distal end of the graft was apposed into the C7DQ site months earlier. Thus, while we find that some axons at this very late time point express TrkB, the immunostaining likely does not accurately reflect TrkB levels at the time of graft apposition.
Another possible explanation is that virtually all TrkB+ axons regenerate out of the PNG after just ChABC treatment, thus making the overexpression of BDNF in distal tissue moot. This would suggest that overexpression of other neurotrophins in the distal spinal cord may prove to be more effective. Various groups have promoted some regeneration of long-injured axons using NT-3 (Coumans et al., 2001; Kadoya et al., 2009; Lu et al., 2007; Tuszynski et al., 2003). However, these studies have largely targeted either sensory or corticospinal tract axon regeneration. Overexpressing NT-3 in distal tissue would likely have little effect on enhancing regeneration of descending axons beyond the graft in this instance because corticospinal tract axons, which are responsive to NT-3, do not readily extend axons into the grafts (Ye and Houle, 1997). GDNF may be another candidate neurotrophin to overexpress, especially since GDNF has a clear role in axonal pathfinding in vivo (Dudanova et al., 2010; Kramer et al., 2006). However, very little is known about the expression levels of GDNF receptors (GFRα and RET) by regenerating axons.
In conclusion, we found that overexpression of BDNF did not promote axonal regeneration beyond what we previously defined after ChABC only treatment, but it did enhance the integration of these axons with spinal neurons. These data suggest that increasing BDNF levels can have benefits irrespective of any tropic effects. BDNF's ability to potentiate synapse formation or synaptic transmission indicates that it can be used to enhance the effects of even a small number of regenerated fibers (thereby possibly better maximizing the effects of a limited number of regenerating axons) and that it should be considered as a future treatment strategy to promote functional recovery after SCI.
Acknowledgments
Support for this study was provided by the Craig H. Neilsen Foundation (V.J.T.), the Paralyzed Veterans of America Research Foundation (V.J.T.), and NIH/NINDS NS26380 (J.D.H.). We would like to thank Itzhak Fischer for generously providing the lentiviral vectors.
References
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- Amin AA, Houle JD. 2011 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. Will Neurotrophic Factors Promote Descending (Motor) and/or Ascending (Sensory) Axonal Outgrowth from Peripheral Nerve Grafts after Spinal Cord Injury. Program No. 893.23. Online. [Google Scholar]
- Ard MD, Bunge RP, Bunge MB. Comparison of the Schwann cell surface and Schwann cell extracellular matrix as promoters of neurite growth. J Neurocytol. 1987;16:539–555. doi: 10.1007/BF01668507. [DOI] [PubMed] [Google Scholar]
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13:257–268. [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–beta-atenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of BDNF and NT-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35:221–232. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Broude E, McAtee M, Kelley MS. Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp Neurol. 1998;149:13–27. doi: 10.1006/exnr.1997.6669. [DOI] [PubMed] [Google Scholar]
- Cao Y, Shumsky JS, Sabol MA, Kushner RA, Strittmatter S, Hamers FP, Lee DH, Rabacchi SA, Murray M. Nogo-66 receptor antagonist peptide (NEP1–40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J Neurosci Off J Soc Neurosci. 1999;19:9996–10003. doi: 10.1523/JNEUROSCI.19-22-09996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci Off J Soc Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbeare D, Houle JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J Neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Gatto G, Klein R. GDNF acts as a chemoattractant to support ephrinA-induced repulsion of limb motor axons. Curr Biol. 2010;20:2150–2156. doi: 10.1016/j.cub.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF., III Modulation of motoneuron excitability by brain-derived neurotrophic factor. J Neurophysiol. 1997;77:502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JJ, Park MH, Choi SY, Koh JY. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J Biol Chem. 2005;280:11995–12001. doi: 10.1074/jbc.M403172200. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tessler A, Fischer I, Houle JD. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil Neural Repair. 2000;14:311–317. doi: 10.1177/154596830001400407. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Wood P, Johnson MI, Bunge RP. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J Neurosci Off J Soc Neurosci. 1988;8:653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Knott L, Su F, Dessaud E, Krull CE, Helmbacher F, Klein R. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor–axon pathway selection in the limb. Neuron. 2006;50:35–47. doi: 10.1016/j.neuron.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Oschipok L, Teh J, Liu ZW, Tetzlaff W. Rubrospinal neurons fail to respond to brain-derived neurotrophic factor applied to the spinal cord injury site 2 months after cervical axotomy. Exp Neurol. 2004;189:45–57. doi: 10.1016/j.expneurol.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci Off J Soc Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Murray M, Tessler A, Fischer I. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178:150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Graham L, Wang Y, Samara R, Banos K, Haringer V, Havton L, Weishaupt N, Bennett D, Fouad K, Tuszynski MH. Motor axonal regeneration after partial and complete spinal cord transection. J Neurosci Off J Soc Neurosci. 2012;32:8208–8218. doi: 10.1523/JNEUROSCI.0308-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci Off J Soc Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- Sanchez AL, Matthews BJ, Meynard MM, Hu B, Javed S, Cohen Cory S. Development. Cambridge, England: 2006. BDNF increases synapse density in dendrites of developing tectal neurons in vivo; pp. 133pp. 2477–2486. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Storer PD, Houle JD. betaII-tubulin and GAP 43 mRNA expression in chronically injured neurons of the red nucleus after a second spinal cord injury. Exp Neurol. 2003;183:537–547. doi: 10.1016/s0014-4886(03)00181-x. [DOI] [PubMed] [Google Scholar]
- Storer PD, Dolbeare D, Houle JD. Treatment of chronically injured spinal cord with neurotrophic factors stimulates betaII-tubulin and GAP-43 expression in rubrospinal tract neurons. J Neurosci Res. 2003;74:502–511. doi: 10.1002/jnr.10787. [DOI] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Kadakia R, Santi L, Houle JD. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J Neurotrauma. 2009a;26:2323–2333. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009b;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Walmsley B. Brain-derived neurotrophic factor modulates cell excitability in the mouse medial nucleus of the trapezoid body. Eur J Neurosci. 2007;25:1647–1652. doi: 10.1111/j.1460-9568.2007.05428.x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol. 2008;586:3113–3127. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurother: J Am Soc Exp Neurother. 2011;8:206–220. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]