Abstract
Renovascular disease (RVD) can lead to hypertension and chronic kidney disease (CKD). Patients with advanced peripheral arterial disease (PAD) have a 5-year mortality of ~30%. Rate and causes of death in patients with significant RVD, who share similar risk factors with PAD patients, are not well defined. We assessed consecutive RVD patients who underwent renal artery stenting at our institution over 6 years. Specific causes of death were ascertained and the probability of survival was estimated. Cox models were fit to identify predictors of outcomes. We identified 281 RVD patients who underwent renal stenting. Follow-up was available for all patients (median 5.1 years). All-cause mortality was 24.2% at 5 years and 33.7% at 7 years (compounded annualized death rate: 5.5%). Of the 68 deaths, 36 (52.9%) were cardiovascular (13.2% acute myocardial infarction, 13.2% stroke, 11.8% sudden death and 10.3% congestive heart failure); 32 (47.1%) deaths had non-cardiovascular causes. In RVD patients undergoing stenting, cardiovascular events are the most common causes of death. Compared with patients with advanced PAD, RVD may have a lower 5-year mortality.
Keywords: Renovascular disease, peripheral arterial disease, cardiovascular mortality, myocardial infarction, stroke
INTRODUCTION
Renal artery atherosclerosis or renovascular disease (RVD) is a form of peripheral arterial disease (PAD) that is associated with renal artery stenosis, hypertension, and chronic kidney disease (CKD). In addition, atherosclerotic RVD is also recognized as an independent risk factor for coronary artery disease (CAD) and portends considerably increased mortality vs patients with CAD alone.[1–3]
Over the last several decades, the use of percutaneous renal artery interventions to treat RVD and its clinical consequences has increased in frequency, although evidence to support the efficacy of these interventions is lacking.[4–6] Although a number of clinical trials and patient registries of renal artery interventions have been published, the long term outcome of these patients remains unclear. The overall mortality rates and causes of death among those patients have not been well defined. Thus, we sought to define the long term outcomes and determine the causes of overall and cardiovascular death in a population of RVD patients who underwent renal artery stenting.
METHODS
Patient Population
The Institutional Review Boards of the University of Kentucky College of Medicine and the Veterans Affairs Medical Center in Lexington, Kentucky, approved this study. The sample consisted of all consecutive patients who underwent percutaneous treatment of RVD between 1/1/2006 and 12/31/2012. Using a chart review for procedural codes of renal angioplasty and renal artery stenting, a total of 320 procedures in 290 patients were identified. Nine patients were excluded due to: inability to locate records or confirm procedure (n=7), intervention performed at an outside hospital (n=1), or intervention performed on a transplanted kidney (n=1). The remaining 281 patients represented the study cohort. Of those, 30 patients underwent multiple renal revascularization procedures performed on separate dates. In these cases, only the data of the initial procedure was included in the analysis. All patients were treated according to the standard catheterization laboratory practice procedures in accordance with published guidelines.[7]
Outcomes
Personnel blinded to the study patient outcomes extracted pre-specified demographics and catheterization data from our institutional medical records. Data included patient demographics, medical comorbidities, baseline laboratory studies, medications at the time of the intervention, and procedural details. Peri-procedural events that were collected included: death, prolonged hospitalization, myocardial infarction, stroke or transient ischemic attack, renal failure, urgent surgery, vascular complications and transfusion or other major bleeding event. At the time of long term follow up, mortality status was determined via a search in the social security death index and the date of search was recorded. For all individuals experiencing mortality, death certificates, discharge summary, or autopsy data were used to determine cause of death. Whenever available, hospital records were obtained to clarify events leading up to death. Mortalities were classified according to the underlying cause of death. A cardiovascular death was determined if the patient suffered from an unexplained sudden death suspected to be cardiac or if death occurred in the setting of: acute myocardial infarction, refractory congestive heart failure, stroke, post-cardiovascular surgery, or vascular related complication. Death was determined to be non-cardiovascular if the patient did not fit the categories above or if death was documented to be due to progressive CKD or infections such as chronic wound infections. If unable to substantiate a plausible diagnosis related to death, it was categorized as undetermined. All events were adjudicated in duplicate by two reviewers (EW, AAL) with 100% agreement.
Statistical Analysis
Dichotomous outcomes are summarized by numbers and percentages. Normally distributed outcomes are presented as means ± SD. Continuous outcomes that did not follow a normal distribution are represented as medians with interquartile ranges. The proportion of survivors was raised to the power of 1 over the median follow-up, and this number was then subtracted from 1 to determine the compounded annualized death rate. The survival function (i.e. probability of remaining alive in relation to follow-up duration) was estimated by the Kaplan-Meier method (Sigmaplot 12.3). Associations between all-cause mortality and individual patient factors were estimated and tested for significance with univariable Cox proportional hazards models. Patient factors that were tested for potential associations with future events were selected based upon previously documented associations with cardiovascular events and included: age, gender, diabetes, hypertension, CKD, known CAD, prior myocardial infarction, history of stroke, tobacco abuse, obesity (BMI > 30), and renal stent size among others.[8] These potential cardiac risk factors were included in a multivariable Cox proportional hazards model if they showed univariable associations with p< 0.10, except that gender was automatically included in the multivariable model. Results from Cox modeling were summarized by estimated hazard ratios (HR) with corresponding 95% confidence intervals (CI). The univariable and multivariate Cox modeling was also repeated for cardiovascular mortality. Due to many missing values on creatinine and hemoglobin (202 and 179 valid observations, respectively), multiple imputations were performed in conjunction with any Cox modeling involving one or both of these variables. Apart from evaluation of univariable Cox modeling results to select covariates for multivariable Cox models, a two sided p-value < 0.05 was considered significant. Version 9.3 of SAS software was used for Cox modeling.
RESULTS
Patient demographics are summarized in Table 1. Overall, cardiovascular risk factors were highly prevalent. On average, patients were on 2.7 antihypertensive medications, with 25% treated with ≥ 4 medications. Uncontrolled hypertension was the indication for renovascular intervention in 262 (93%) of patients, while impaired and/or worsening renal function was the indication in the remaining 19 (7%). Baseline blood pressure (BP) measurements were elevated on the day of the procedure with the mean systolic BP 158±28 mmHg and diastolic BP 79±15 mmHg.
Table 1.
Baseline Demographics and Clinical Characteristics
| Mean ± SD, or number (%) | |
|---|---|
| Age in years | 66.1 ± 12.6 |
| Male | 169 (60.4) |
| Hypertension | 274 (97.5) |
| Hyperlipidemia | 194 (69.5) |
| Diabetes | 106 (37.7) |
| Congestive Heart Failure | 39 (13.9) |
| Any Tobacco Use | 142 (52.0) |
| Current Tobacco Use | 84 (30.8) |
| Prior Myocardial Infarction | 37 (13.2) |
| Prior Coronary Artery Bypass Grafting | 59 (21.0) |
| Prior CVA/TIA | 42 (14.9) |
| End-Stage Renal Disease | 33 (11.7) |
| BMI | 27.9 ± 6 |
| Obesity (BMI > 30) | 89 (32.5) |
| Medications: | |
| Aspirin | 165 (64.0) |
| Clopidogrel or other P2Y12 inhibitors | 76 (29.5) |
| Statin | 162 (62.5) |
| Beta-Blocker | 194 (74.9) |
| Calcium Channel Blocker | 136 (52.7) |
| ACE-I/ARB | 154 (59.7) |
| Diuretic | 133 (51.4) |
| Average Number of Antihypertensive Agents | 2.7 ± 1.2 |
| Treatment with ≥4 Antihypertensive Agents | 64 (25.0) |
| Baseline Measurements: | |
| Systolic Blood Pressure (mmHg) | 158 ± 28 |
| Diastolic Blood Pressure (mmHg) | 79 ± 15 |
| Mean Arterial Pressure (mmHg) | 106 ± 16 |
| LDL-cholesterol (mg/dL) | 98 ± 39 |
| HDL-cholesterol (mg/dL) | 39 ± 13 |
| Creatinine (mg/dL) | 1.55 ± 0.91 |
ACE-I; Angiotensin converting enzyme inhibitor, ARB; Angiotensin receptor blocker, BMI; Body Mass index, CVA; Cerebrovascular accident, HDL; High density lipoprotein, LDL; Low density lipoprotein, TIA; transient ischemic attack.
Table 2 summarizes the procedural characteristics. In 63 patients (22.7%), both renal arteries were revascularized at the time of the index procedure. For the initial vessel, there were high rates of procedural success (99.7%) with the majority receiving stents (96.9%) while treatment with balloon angioplasty alone was rare (3.1%).
Table 2.
Angiographic and Procedural Characteristics
| Lesion Characteristics | Mean ± SD, Number (%) |
|---|---|
| Right* | 104 (37.5) |
| Left | 102 (36.8) |
| Bilateral | 63 (22.7) |
| Lesions treated per Patient | 1.2 ± 0.5 |
| Lesion #1 (n=281) | |
| Right | 145 (53.7) |
| Proximal | 206 (92.0) |
| Mid | 12 (5.5) |
| Distal** | 4 (1.8) |
| Mean % Stenosis | 77 ± 16 |
| Resting Gradient (mmHg) | 54 ± 31 |
| In-stent Restenosis | 8 (4.1) |
| Heavy Calcification | 34 (17.3) |
| Procedural Success Rate | 280 (99.7) |
| Lesion #2 (n= 63) | |
| Right | 27 (38.0) |
| Proximal | 51 (92.7) |
| Mid | 3 (5.6) |
| Distal** | 0 (0) |
| Mean % Pre-Stenosis | 76 ± 16 |
| Resting Gradient (mmHg) | 48 ± 29 |
| In-stent Restenosis | 2 (3.5) |
| Heavy Calcification | 9 (15.8) |
| Procedural Success Rate | 63 (100) |
Lesion 1 displays the lesion specifics for the first vessel, while lesion 2 is represents the lesion characteristics for those receiving intervention to the contralateral renal artery immediately following successful treatment of the first vessel.
The lesion side was not reported in 2.8% of cases.
Distal locations are not typical for atherosclerotic lesions and probably represented fibromuscular dysplasia.
Rates of procedural complications were low (vascular complications 2.1%, myocardial infarction 0.7%, peri-procedural death 0.4%). There were no cases of new renal failure requiring hemodialysis. At the time of discharge, the majority of patients were discharged home on appropriate medical therapy: aspirin (90%), clopidogrel (74%), beta-blockers (78%), angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (61%) and statin therapy (68%).
Long term follow up was completed on 100% of the sample. Follow up extended to 7.4 years (median 5.1 years, Interquartile range 3.1, 6.2). During this time, there were 68 (24.2%) deaths. The compounded annualized rate of death was 5.5%. The probability of survival following renal intervention was estimated by the Kaplan-Meier method at 75.8% at 5 years and 66.3% at 7.4 years (Figure 1). Cardiovascular causes accounted for 36 deaths (52.9% of all deaths, 12.8% of the total sample), while the remaining 32 deaths (47.1%) were non-cardiovascular. Specific causes of death were sudden cardiac death (11.8% of the overall death rate), acute myocardial infarction (13.2%), refractory congestive heart failure (10.3%) or stroke (13.2%). The remaining 3 cardiovascular deaths (4.4%) were related to a recent coronary artery bypass surgery (n=1), progressive vascular disease (n=1), or unable to classify based on limited data (n=1) (Figure 2). The non-cardiovascular deaths were mostly due to infections (11.8%) or malignancy (8.8%). Of the 6 fatal malignancies, 4 were in the lungs, 1 endometrial and 1 cervical. Only 5 deaths (7.4% of all deaths, 1.8% of the total sample) were attributable to progressive CKD. The remaining 19.1% of the deaths were secondary to progressive lung disease (2.9%), thromboembolic (2.9%), wound infections (2.9%), liver disease (2.9%), pancreatitis (2.9%), gastrointestinal bleeding (1.5%), non-vascular dementia (1.5%) and frailty (1.5%).
Figure 1.
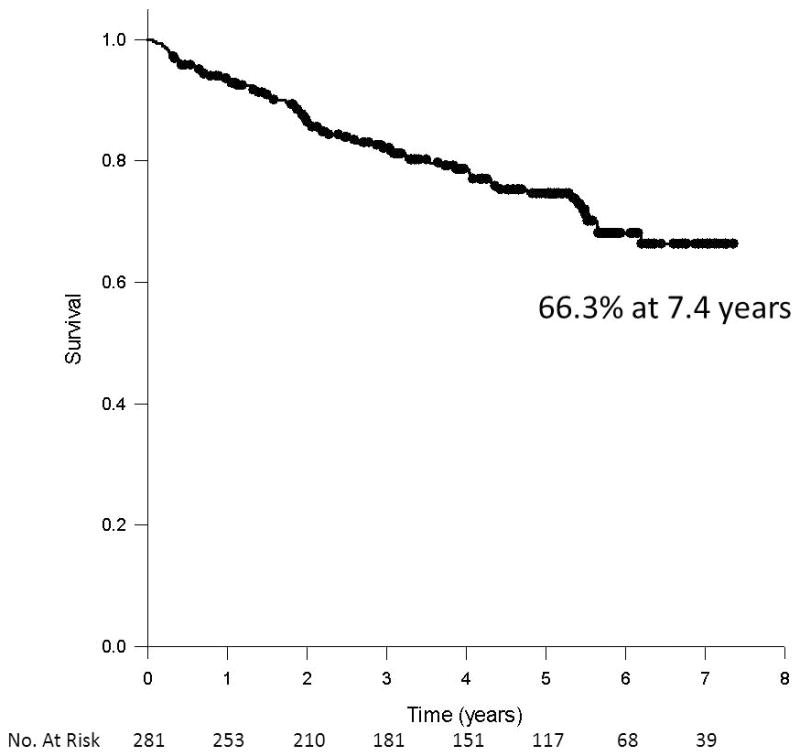
Estimate of survival following renal artery intervention by the Kaplan-Meier method.
Figure 2.
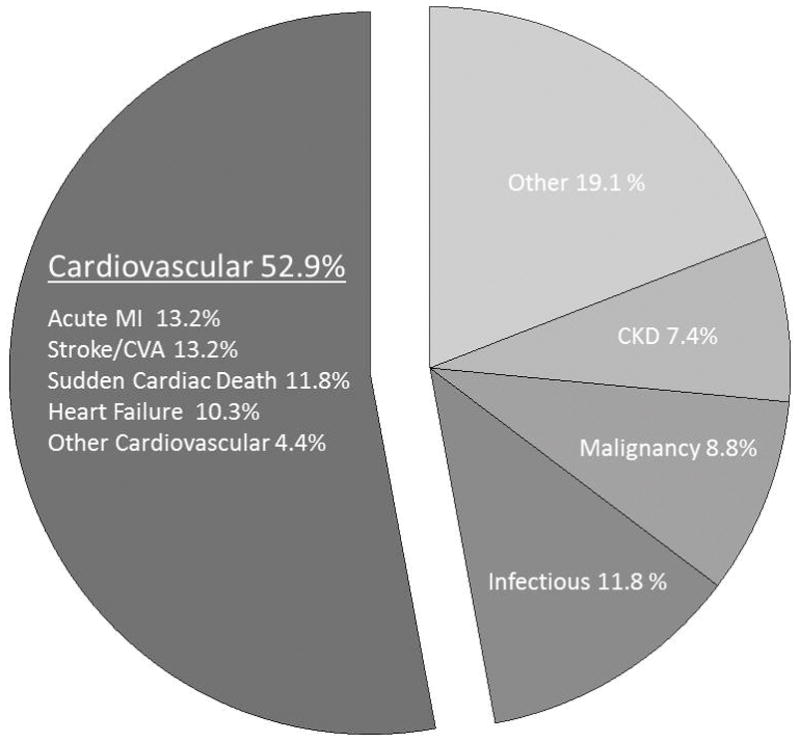
Cause of death. The majority of deaths were attributable to a cardiovascular etiologies, with each specific event type listed. Few non-cardiovascular deaths were attributable to CKD.
CAD: coronary artery disease, CHF: congestive heart failure, CM: Cardiomyopathy, TIA: Transient ischemic attack.
Results from univariable Cox models for all-cause and cardiovascular mortality are displayed in Table 3 with estimated Hazard Ratios (HR). Variables associated with all-cause and/or cardiovascular mortality (p<0.10) included: age, baseline hemoglobin and creatinine, BMI, diabetes, heart failure, atrial fibrillation, prior stroke/transient ischemic attack, coronary artery disease, and prior coronary artery bypass grafting. These variables were included in multivariable Cox models examining all-cause and/or cardiovascular mortality (results are summarized in Figure 3). Notably, age was the only significant multivariable predictor of all-cause mortality. Significant associations with cardiovascular death involved prior stroke (HR 2.52, CI 1.11 – 5.72, p=0.03) and preexisting heart failure (HR 3.31, CI 1.46 – 7.52, p<0.01) with higher baseline hemoglobin levels being protective (HR 0.74, CI 0.61 – 0.90, p<0.01). Body mass was also a significant multivariable predictor of cardiovascular mortality (HR 1.08, CI 1.01 – 1.15, p=0.02).
Table 3.
Univariable Predictors of All-cause and Cardiovascular Mortality*
| All-cause Death | Cardiovascular Death | |
|---|---|---|
| Age | 1.04 (1.02–1.06, p<0.001) | 1.03 (1.00 – 1.06, p = 0.02) |
| Gender | 1.50 (0.90 – 2.50, p=0.12) | 1.60 (0.79 – 3.26, p=0.19) |
| Height | 1.00 (0.98 – 1.03, p=0.92) | 0.98 (0.95 – 1.02, p=0.29) |
| Body Mass Index | 1.04 (1.00 – 1.09, p=0.03) | 1.07 (1.02 – 1.12, p=0.01) |
| Any tobacco history | 0.78 (0.49 – 1.28, p=0.34) | 1.00 (0.52 – 1.92, p=1.0) |
| Current tobacco | 0.66 (0.37 – 1.19, p=0.17) | 0.70 (0.32 – 1.54, p=0.38) |
| Obesity (BMI > 30) | 1.35 (0.82 – 2.22, p=0.24) | 1.38 (0.71 – 2.70, p=0.34) |
| Hyperlipidemia | 0.89 (0.53 – 1.48, p=0.65) | 0.95 (0.47 – 1.92, p=0.88) |
| Hypertension | 0.42 (0.13 – 1.33, p=0.14) | 0.33 (0.08 – 1.37, p=0.13) |
| Diabetes | 1.88 (1.17 – 3.03, p<0.01) | 1.87 (0.97 – 3.60, p=0.06) |
| Congestive Heart Failure | 2.61 (1.54 – 4.44, p<0.001) | 5.37 (2.78 – 10.36, p<0.001) |
| Cardiomyopathy | 2.20 (0.80 – 6.05, p=0.13) | 3.14 (0.96 –10.24, p=0.05) |
| Chronic Obstructive Pulmonary Disease | 1.07 (0.56 – 2.04, p=0.84) | 1.12 (0.47 – 2.70, p=0.80) |
| Prior Stroke/Transient Ischemic Attack | 1.56 (0.85 – 2.86, p=0.15) | 2.20 (1.04 – 4.68, p=0.04) |
| Atrial Fibrillation | 5.39 (2.31 – 12.61, p<0.001) | 6.63 (2.31 – 19.00, p<0.001) |
| Coronary Artery Disease | 2.01 (1.19 – 3.39, p<0.01) | 2.47 (1.16 – 5.26, p=0.02) |
| End-Stage Renal Disease | 1.49 (0.78 – 2.84, p=0.23) | 0.98 (0.35 – 2.77, p=0.97) |
| Prior Myocardial Infarction | 1.39 (0.74 – 2.60, p=0.30) | 1.79 (0.81 – 3.92, p=0.15) |
| Prior Coronary Artery Bypass | 1.44 (0.85 – 2.44, p=0.18) | 1.84 (0.92 – 3.67, p=0.09) |
| Known Peripheral Vascular Disease | 1.07 (0.56 – 2.04, p=0.85) | 1.47 (0.70 – 3.06, p=0.31) |
| Baseline Hemoglobin | 0.81 (0.71 – 0.92, p<0.001) | 0.73 (0.61 – 0.88, p<0.001) |
| Baseline Creatinine | 1.29 (1.04 – 1.59, p=0.02) | 1.23 (0.91 – 1.68, p=0.42) |
Shown are estimated Hazard Ratios (HR) and associated 95% Confidence intervals (CI).
Figure 3.
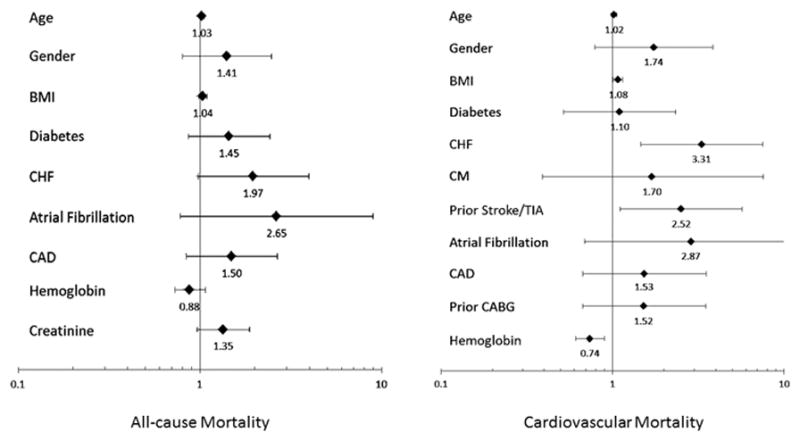
Forrest plots of the multivariable Cox models for all-cause (left) and cardiovascular mortality (right). The estimated hazard ratios (HR) and 95% confidence intervals (CI) of the included variables are shown. Hemoglobin and creatinine levels included were those obtained at the time of the stenting procedure.
BMI; Body mass index, CABG; Coronary artery bypass grafting,
DISCUSSION
In this study, we report long-term outcomes in patients with primarily atherosclerotic RVD who underwent percutaneous interventions for renal artery stenosis. This report represents one of the longest follow up of RVD patients undergoing renal stenting (exceeding 7 years with a median of 5 years) that provides detailed description of the specific causes of death in this patient population. Similar to past studies involving PAD patients, our study demonstrates the high prevalence of cardiovascular risk factors among patients with RVD and confirms cardiovascular mortality as the most common cause of death. However, when compared with historical data on PAD, patients with RVD undergoing intervention for renal artery stenosis appear to have a lower mortality over multi-year follow up. Additionally, our study demonstrates that CKD progression represents a very small fraction of the causes of death in this patient population.
Although atherosclerosis is a systemic disorder, PAD is a heterogeneous term that encompasses patients with disease in multiple vascular beds and who may not carry the same prognosis.[9] For example, patients with advanced lower extremity PAD exhibit a poor prognosis, with mortality rates as high as 30% at 5 years in some studies.[10, 11] Contemporary best medical therapy and secondary prevention may improve prognosis of such patients, though that is not clearly established. In this high-risk population, cardiovascular events are the leading cause of death and high mortality rates are due to shared cardiovascular disease risk factors and increased incidence of CAD. This has been supported by studies demonstrating that concomitant CAD prevalence may be as high as 90% in PAD patients.[12] However, observational data have also shown that PAD is a risk factor for mortality and adverse cardiovascular events, even after adjustment for CAD and traditional cardiovascular comorbidities.[13] Previous studies examining the prognosis of different vascular territories have suggested variable long term outcomes; however, these studies have not included renal artery atherosclerosis. The results of this study indicate that patients with atherosclerotic RVD undergoing percutaneous intervention exhibit a more favorable prognosis than patients with advanced lower extremity PAD for example (5-year mortality 24 vs 30%, respectively). Despite the lower overall mortality rates, these rates are still substantially higher than the rates for normal individuals > 65 years old (5-year mortality 7–10%).[14] Similar to other PAD cohorts, however, our sample did experience high rates of cardiovascular death (> 50% of all deaths).
As previously stated, this represents one of the longest follow-up reports for RVD patients after renal stenting in a reasonably sized dataset. Moreover, our study included “real world’ patients providing valuable information for clinical decision making and offering widely applicable results. In the ASTRAL trial, 403 patients underwent renal artery intervention, but the median follow up was < 3 years.[5] In that study, the reported mortality reached 25.6%, which is similar to that reported in our study, albeit over a shorter period of time. In particular, the compounded annualized death rate in patients undergoing renal intervention was 10.2% in ASTRAL vs 5.5% in this study. Detailed causes of death were not reported by the ASTRAL investigators. In the more recently published CORAL trial, 459 patients underwent renal artery stenting,[15] with a median follow up of 3.6 years, compared with 5.1 years in our study. The mortality rate in CORAL was 13.7% over the shorter follow up, resulting in a compounded annualized death rate of 4.0%, suggesting a more advanced atherosclerotic disease state in the “real-world” patient cohort of this study. In the CORAL study, and in our report, cardiovascular etiologies were the predominant causes of death (65% of all deaths in CORAL and 53% in our study).
Well established risk factors for patient mortality such as age, CAD, diabetes, and body mass were prevalent in our sample and associated with all-cause and cardiovascular mortality in univariable analysis (Table 3). Increasing age remained a strong predictor of all-cause mortality. With regard to cardiovascular mortality, age remained predictive, but other predictors were history of preexisting congestive heart failure and prior stroke.
Our study exhibited low rates of death attributable to end-stage renal disease (7.4% of all deaths, 1.8% of the total sample). Importantly, there is consistency in the low rate of death attributed to renal causes among reports of renal artery stenting (0.4% of the stented patients in CORAL and 1.8% in our report).[15] It is important to note that the indication for renal stenting was uncontrolled hypertension, not impaired renal function, in the majority or all of the patients in these studies. Deaths were considered CKD-related if patients had any complications related to dialysis access, sudden cardiac death suspected to be electrolyte related or death related to withdrawal of hemodialysis. The wide discrepancy between the deaths attributable to cardiovascular and renal causes emphasizes the need for aggressive primary and secondary prevention of traditional cardiovascular risk factors in this population.[16]
Other findings merit further discussion. First, although women are generally underrepresented in cardiovascular studies, they represented 40% of our sample. However, although the hazard ratio estimates appeared to favor women, gender was not significantly associated with cardiovascular mortality in either univariable or multivariable analysis. Secondly, tobacco use, which is a well-established risk factor for mortality in other PAD populations, was not significantly associated with either cardiovascular or overall mortality in our sample. [17] Thirdly, malignancy represented approximately 9% of causes of deaths, of which lung cancer represented the majority of cases. This has previously been reported with other subsets of atherosclerotic patients.[18]
Although we examined a consecutive group of patients followed for an extended period of time and with 100% follow up data, there are several limitations to our study. First, the sample size may have limited the statistical power to detect associations of modest strength. Secondly, similar to most studies on RVD, only patients undergoing renal revascularization were included, not all patients with renovascular disease. Thus, our results are not generalizable to patients with milder degrees or medically treated RVD (such as those in whom revascularization was not attempted due to total occlusion and/or renal cortical atrophy). In a recent observational study comparing two groups of patients with clinically significant renal artery stenosis, not surprisingly, there were significant differences in baseline characteristics between those allocated to medical therapy vs those undergoing renal artery revascularization. That study demonstrated significant reductions in mortality and morbidity in those undergoing renal artery intervention compared with medical therapy.[19] Thirdly, formal left ventricular (LV) functional assessment and the presence or absence of LV hypertrophy was not assessed, although both predict cardiovascular mortality. Fourth, the medications listed at the time of the procedure do not accurately correspond to subsequent compliance with key medications that may have affect cardiovascular mortality, namely statins and antiplatelet therapy. Lastly, although death certificates were examined in all cases, events leading up to death cannot be fully discerned or accurately classified. To mitigate this limitation, we were able to obtain all discharge summaries and completed a thorough review of hospitalization records when available in the 74% of deaths when the patients expired in a medical facility.
CONCLUSIONS
In patients with RVD undergoing percutaneous intervention, long term follow up reveals that cardiovascular events are the most common cause of death, with low rates attributable to progressive CKD. Overall, such patients seem to exhibit favorable mid- to long-term prognosis compared with other PAD patients. Age was the strongest predictor of overall mortality, while preexisting congestive heart failure and stroke exhibited the greatest influence on cardiovascular mortality.
Acknowledgments
Dr. Abdel-Latif is supported by the University of Kentucky Clinical and Translational Science Pilot Award (UL1TR000117), the UK COBRE Early Career Program (P20 GM103527) and the NIH Grant R56 HL124266.
Footnotes
Conflict of Interest
The authors have no financial disclosures to declare.
Institutional Review Board Approval
The Institutional Review Boards at the University Of Kentucky College Of Medicine and the Veterans Affairs Medical Center in Lexington, Kentucky reviewed this study. A waiver of consent was approved given the no additional risk conferred on the subjects of research due to the retrospective nature of the study. All identifiable information were maintained on password secure computers and none was included in the submitted manuscript.
Author Contribution Details
All authors substantially contributed to acquisition and interpretation of data, drafting of the article and final approval of the published version. EW, RC, AAL and KZ substantially contributed to conception and design, analysis and interpretation of data, critical review of the manuscript for intellectual content and final approval of the manuscript.
References
- 1.Wollenweber J, Sheps SG, Davis GD. Clinical course of atherosclerotic renovascular disease. Am J Cardiol. 1968;21(1):60–71. doi: 10.1016/0002-9149(68)90014-3. [DOI] [PubMed] [Google Scholar]
- 2.Isles C, Main J, O’Connell J, Brown I, Findlay J, Stewart R, Wilkinson R. Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scottish Med J. 1990;35(3):70–73. doi: 10.1177/003693309003500303. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68(1):293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Textor SC. Atherosclerotic renal artery stenosis: overtreated but underrated? J Am Soc Nephrol. 2008;19(4):656–659. doi: 10.1681/ASN.2007111204. [DOI] [PubMed] [Google Scholar]
- 5.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361(20):1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 6.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150(12):840–848. W150–841. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 7.Bashore TM, Balter S, Barac A, Byrne JG, Cavendish JJ, Chambers CE, et al. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. JAm CollCardiol. 2012;59(24):2221–2305. doi: 10.1016/j.jacc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Creager MA, Belkin M, Bluth EI, Casey DE, Jr, Chaturvedi S, Dake MD, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease) Circulation. 2012;125(2):395–467. doi: 10.1161/CIR.0b013e31823299a1. [DOI] [PubMed] [Google Scholar]
- 9.Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51(16):1588–1596. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 10.Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94(11):3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 12.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 13.van Kuijk JP, Flu WJ, Welten GM, Hoeks SE, Chonchol M, Vidakovic R, et al. Long-term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010;31(8):992–999. doi: 10.1093/eurheartj/ehp553. [DOI] [PubMed] [Google Scholar]
- 14.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J General Intern Med. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014;100(5):414–423. doi: 10.1136/heartjnl-2013-304082. [DOI] [PubMed] [Google Scholar]
- 18.Paraskevas KI, Mikhailidis DP, Veith FJ. Patients with peripheral arterial disease, abdominal aortic aneurysms and carotid artery stenosis are at increased risk for developing lung and other cancers. Int Angiol. 2012;31(4):404–405. [PubMed] [Google Scholar]
- 19.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63(2):186–197. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]


