Abstract
Background
Alcohol intoxication is known to impair decision making in a variety of situations. Previous neuroimaging evidence suggests that the neurofunctional system subserving controlled processing is especially vulnerable to alcohol in conflict‐evoking tasks. The present study investigated the effects of moderate alcohol intoxication on the spatiotemporal neural dynamics of event‐related total theta (4 to 7 Hz) power as a function of task difficulty.
Methods
Two variants of the Simon task manipulated incongruity via simple spatial stimulus‐response mismatch and, in a more difficult version, by combining spatial and semantic interference. Healthy social drinkers participated in both alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo conditions in a counterbalanced design. Whole‐head magnetoencephalography (MEG) signals were acquired and event‐related total theta power was calculated on each trial with Morlet wavelets. MEG sources were estimated using anatomically constrained, noise‐normalized, spectral dynamic statistical parametric mapping.
Results
Longer reaction times and lower accuracy confirmed the difficulty manipulation. Response conflict (incongruity) increased and alcohol intoxication decreased event‐related theta power overall during both tasks bilaterally in the medial and ventrolateral prefrontal cortices. However, alcohol‐induced theta suppression was selective for conflict only in the more difficult task which engaged the dorsal anterior cingulate (dAC) and anterior inferolateral prefrontal cortices. Theta power correlated negatively with drinking levels and disinhibition, suggesting that cognitive control is susceptible in more impulsive individuals with higher alcohol intake.
Conclusions
The spatiotemporal theta profile across the 2 tasks supports the concept of a rostrocaudal activity gradient in the medial prefrontal cortex that is modulated by task difficulty, with the dAC as the key node in the network subserving cognitive control. Conflict‐related theta power was selectively reduced by alcohol only under the more difficult task which is indicative of the alcohol‐induced impairment of conflict monitoring and top‐down regulation. Compromised executive control under alcohol may underlie a range of adverse effects including reduced competency in conflict‐inducing or complex situations.
Keywords: Cognitive Control, Magnetoencephalography, Anterior Cingulate, Simon Task, Stroop Task
Evidence from behavioral studies indicates that alcohol impairs performance as a function of the level of intoxication and cognitive load (Fillmore, 2007). The detrimental effects of alcohol are observed even at very low blood alcohol levels on relatively demanding tasks probing divided attention, complex tracking, or information processing (Koelega, 1995; Moskowitz and Fiorentino, 2000). The underlying mechanisms responsible for the sensitivity to task difficulty are not fully understood, but it appears that controlled processing is particularly susceptible to alcohol‐induced impairment in contrast to automatic, overlearned, and habitual responses (Fisk et al., 1987; Kovacevic et al., 2012; Marinkovic et al., 2013). Imaging studies suggest that the executive network is affected by alcohol intoxication especially under conditions that engage controlled processing. In a working memory task, alcohol attenuates activity in the lateral prefrontal and dorsal anterior cingulate (dAC) cortices under the highest memory load conditions (Gundersen et al., 2008; Paulus et al., 2006). Similarly, tasks probing response inhibition indicate decreased activity in the right frontotemporal areas under moderate alcohol intoxication (Gan et al., 2014; Kareken et al., 2013). Studies of decision conflict show that it is the dAC that is principally affected by alcohol under high‐conflict conditions (Kovacevic et al., 2012; Marinkovic et al., 2012a,b, 2013). Indeed, extensive evidence indicates that the medial prefrontal cortex (mPFC), including the dAC, is ubiquitously activated during conflict between stimulus‐response representations. Especially effective are those conditions that require override of an automatic response (Ridderinkhof et al., 2004). Furthermore, mPFC activity is modulated by task difficulty along the rostrocaudal gradient. More difficult tasks elicit activity in the more anterior regions of the dAC and less demanding ones activate slightly more posterior regions such as the supplementary motor area (Nachev et al., 2008). Taken together, it can be hypothesized that alcohol exerts greater effects on the high‐conflict conditions requiring more effort to override interference from the automatic, habitual responses. Such conditions place stronger demands on cognitive control as reflected in lower performance levels and are subserved by the more rostral areas of the mPFC (Picard and Strick, 2001), a process referred to as dAC engagement in conflict detection and error monitoring (Botvinick, 2007; Carter and van Veen, 2007).
The aims of the present study were to quantify and elucidate effects of alcohol and task difficulty during response conflict in healthy adults. Event‐related theta oscillations measured with electro‐ or magnetoencephalography (EEG, MEG) are particularly well suited to investigate these aims because theta power is known to be sensitive to cognitive effort. In studies that parametrically manipulated working memory, theta power scaled with increased memory load (Gevins et al., 1997; Maurer et al., 2015). Similarly, subdural recordings from human patients during virtual maze navigation indicated that theta was more prominent in more complex mazes (Kahana et al., 1999). More broadly, theta is sensitive to a variety of cognitive tasks probing attention, executive functions, and error processing (Mitchell et al., 2008; Wang et al., 2005) and it has been proposed as an endophenotype of vulnerability to alcoholism (Rangaswamy and Porjesz, 2014). Event‐related theta power evoked by response conflict is consistently reduced by alcohol intoxication, especially in more difficult, high‐conflict conditions (Kovacevic et al., 2012; Marinkovic et al., 2012b) in which more complex cognitive interference occurs. Human intracranial recordings (Cohen et al., 2008; Wang et al., 2005), MEG (Kovacevic et al., 2012), and scalp EEG source models (Hanslmayr et al., 2008) indicate that the dAC is the principal cortical theta generator in executive tasks.
The current study employed the time‐frequency, anatomically constrained MEG (aMEG) approach in an effort to describe the spatiotemporal dynamics of event‐related theta power during response conflict as a function of task difficulty and alcohol intoxication. The aMEG combines whole‐head MEG recordings and high‐resolution anatomical magnetic resonance imaging (MRI) images, utilizing a distributed source model to yield maps of spectral power estimates across time and cortical space (Dale et al., 2000; Lin et al., 2004). In contrast to the functional MRI (fMRI), aMEG possesses the temporal precision necessary to measure the oscillatory power in biologically relevant frequency ranges and changes in these oscillations over cognitively relevant time scales. Last, aMEG estimates are not confounded by alcohol's vasodilatory effects, as might be the case with the blood oxygenation level‐dependent (BOLD) signal (Iannetti and Wise, 2007; Rickenbacher et al., 2011).
Given the selective nature of the behavioral deficits associated with alcohol and the likewise selectivity of our previous theta power observations (Kovacevic et al., 2012; Marinkovic et al., 2012b), we endeavored here to further manipulate and describe the circumstances under which the effects of intoxication are particularly evident. To accomplish these goals, 2 related tasks were administered. Both are variants of the Simon spatial interference task, and in both tasks, participants are instructed to respond to the color of the stimuli. The Simon‐simple (S‐simple) variant generates conflict between the response hand selection and the task‐irrelevant position of the stimulus on the screen. In the more difficult Simon‐Stroop (S‐Stroop) task, the spatial interference task is merged with the semantic mismatch inducing Stroop effect whereby conflict occurs when subjects cannot ignore the irrelevant semantic content of the stimulus. The resulting tasks are sufficiently similar in kind as to be comparable, yet demand distinguishably different cognitive loads. We hypothesized that the behaviorally detrimental effects of task difficulty and alcohol intoxication would dissociate in spatiotemporal event‐related theta activity patterns in a dorsomedial and lateral‐inferior prefrontal executive network. More specifically, we expected that the incongruent conditions would evoke greater event‐related theta power which would be more strongly modulated by alcohol intoxication under the more difficult task variant evoking a higher level of conflict.
Materials and Methods
Participants
Fifteen healthy, nonsmoking, right‐handed volunteers (7 men, mean [±SD] age 27.6 ± 5.4 years) completed all 4 sessions of this study. None reported alcohol‐ or drug‐related problems, head injury, or other neuropsychiatric or medical problems, and none were taking any medications at the time of the study. They did not have alcoholism‐related symptoms as assessed by the Short Michigan Alcohol Screening Test (SMAST; Selzer et al., 1975) and were negative for family history of alcoholism and drug abuse. Subjects reported light‐to‐moderate alcohol use in social situations, imbibing 2.3 ± 0.8 drinks 1.7 ± 0.9 times per week. The study's procedures were approved by the Institutional Review Board of the University of California, San Diego, and written informed consent was obtained from each subject. Subjects were provided transportation and compensated for their participation. Two additional subjects completed the study, but their data were discarded due to poor data quality.
Experimental Protocol
Subjects completed 4 scanning sessions in a within‐subject design: an MEG familiarization session, 2 recording sessions with beverage administration, and an MRI session. Over the course of a beverage‐free introductory session subjects practiced experimental tasks and were familiarized with the experimental setup. They completed a mock recording with the purpose of reducing situation‐induced arousal effects (Maltzman and Marinkovic, 1996). In addition, they completed a battery of questionnaires including the Zuckerman Sensation Seeking Scale (SSS; Zuckerman, 1971) and Eysenck Impulsiveness and Venturesomeness Scale (Eysenck and Eysenck, 1978). Subsequently, participants were scheduled for 2 MEG sessions with counterbalanced administration of alcohol and placebo that took place 17.3 ± 25.2 days apart. Prior to each MEG scan, participants were queried about their compliance with the requirement to refrain from drinking for 48 hours and from food for 3 hours before each session. All subjects tested negative on urine screens for drug use and pregnancy. Subjects imbibed either an alcohol (0.60 g/kg for men, 0.55 g/kg for women, presented as cocktail containing vodka (Grey Goose® [Bacardi, Hamilton, Bermuda] as 20% v/v in orange juice) or placebo beverage (the same volume of orange juice with the glass rim swabbed with vodka) within a 10‐minute period. Subjects’ breath alcohol concentration (BrAC) was monitored with a breathalyzer (Draeger, Lübeck, Germany) except during scanning due to electronic interference with the MEG device. Instead, a Q.E.D. Saliva Alcohol Test (OraSure Technologies, Bethlehem, PA) was used to measure BrAC.
S‐Stroop and S‐simple tasks were completed serially in a counterbalanced order, starting 45.5 ± 10 minutes after starting the beverage administration. Subjects had an average BrAC of 0.049 ± 0.009% 10 ± 8 minutes before starting the experimental tasks, and 0.06 ± 0.016% 5 ± 2 minutes upon their completion, indicating the tasks were completed on the ascending limbs of the subjects’ BrACs. High‐resolution structural MRI images were obtained from all participants in a separate session.
Structural MRI
Structural images were acquired with a 1.5 T Signa HDx whole‐body scanner (General Electric, Fairfield, CT). The acquisition protocol included a conventional 3‐plane localizer, calibration scan, and 2 high‐resolution T1‐weighted IR‐FSPGR scans (TR = 8.5 ms, TE = 3.75 ms, TI = 500 ms, flip angle = 10°, field of view = 240, matrix: 256 × 256, 166 sagittal slices, 1.2 mm slice thickness, in‐plane resolution 0.94 × 0.94 mm). Each person's cortical surface was reconstructed from these structural images with FreeSurfer (Dale et al., 1999).
Magnetoencephalography
MEG data were acquired with a whole‐head Neuromag Vectorview system (Elekta, Stockholm, Sweden). Continuous 1 kHz signal was recorded from 204 planar gradiometers. Head position indicator coils were affixed to the scalp, and numerous head points were digitized with an Isotrack II system (Polhemus, Colchester, VT) for later coregistration with MRI images.
Tasks
The S‐simple task consisted of 240 stimuli presented for 230 ms with an stimulus onset asynchrony (SOA) of 2,030 ± 100 ms jitter in 25 ms increments. As shown in Fig. 1, red or green squares were presented on the left or right side of a fixation cross within a horizontal visual angle <2.2° and a vertical visual angle <1°. Subjects were asked to respond to red stimuli with their right index finger and to green stimuli with their left index finger, ignoring stimulus position. Response conflict was induced implicitly in incongruous (Incong) trials in which the position of the stimulus on the screen did not match the laterality of the correct response. Half of the trials presented were Incong, and both congruous (Cong) and Incong conditions had equal numbers of left and right indicated trials.
Figure 1.
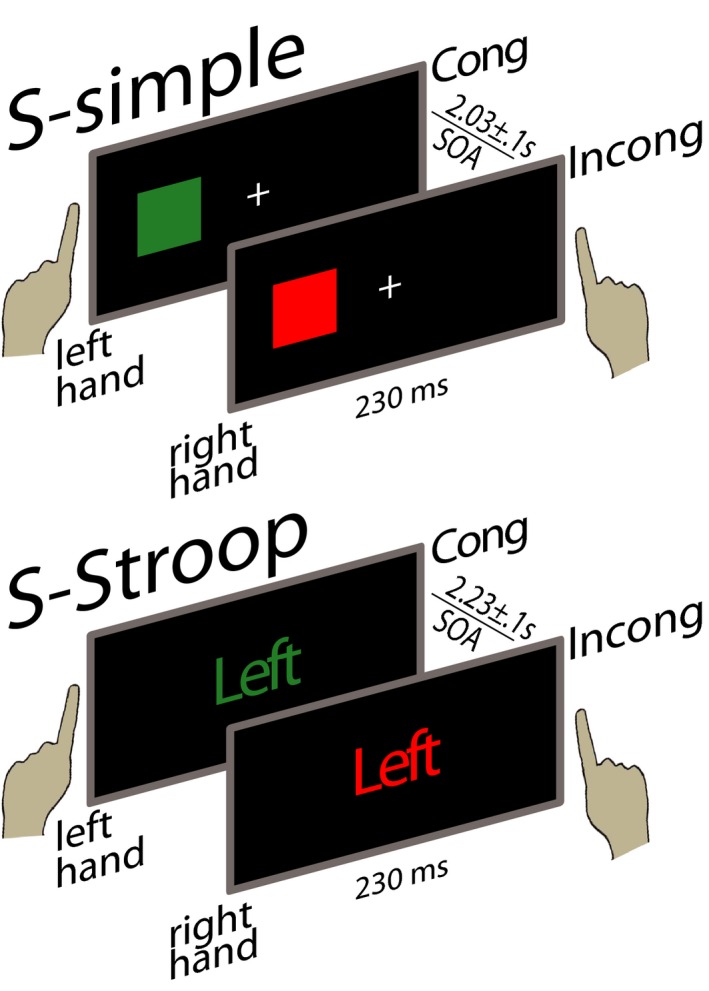
Schematic examples of the Simon‐simple (S‐simple) and Simon‐Stroop (S‐Stroop) task design. In the S‐simple task, red or green squares were presented on the left or right side of the screen. In the S‐Stroop task the word “left” or “right” was presented centrally in red or green letters. In both cases, subjects were asked to respond only to the color of the stimulus. Response conflict was induced by the mismatch between the indicated response hand and the location of the stimulus on the screen, in the case of S‐simple, or its semantic content, as in S‐Stroop. Each task contained 120 congruous (Cong) and incongruous (Incong) trials presented in a randomized sequence. The 2 tasks were counterbalanced across subjects and sessions. Timing parameters are indicated in the figure.
In the more difficult, hybrid S‐Stroop task, the word “left” or “right” was presented centrally in red or green letters on a black background (Fig. 1). About 120 stimuli of each type were presented for 230 ms with a SOA of 2,230 ± 100 ms jitter in 25 ms increments. Subjects responded to red stimuli with their right index finger and the green stimuli with their left index finger, ignoring the meaning of the word presented. Response conflict was induced in Incong trials by the mismatch between the meaning of the word presented and the laterality of the correct response. In additional 120 trials, gray words were presented as a neutral condition, requiring subjects to respond with the hand indicated by the meaning of the word presented. The purpose of this condition was to maintain reading dominance and automaticity (Marinkovic et al., 2012a).
Data Processing and Analysis
Data were analyzed with MATLAB (MathWorks, Natick, MA) routines with dependencies including FieldTrip (Oostenveld et al., 2011), MNE (Gramfort et al., 2014), and EEGLAB (Delorme and Makeig, 2004). The data were band‐pass filtered to between 0.5 and 100 Hz, then segmented into epochs extending −300 to 800 ms relative to each stimulus plus 500 ms padding on each end. These epochs were then downsampled to 250 Hz and baseline normalized to the prestimulus period. Noisy and flat channels were removed by visual inspection and epochs containing movement artifacts or other discontinuities were removed with threshold‐based rejection. Remaining epochs were subjected to independent component analysis (Delorme and Makeig, 2004), to remove the eye blink and heartbeat artifacts.
The complex power spectrum was calculated for each epoch by convolving the data with Morlet wavelets (Oostenveld et al., 2011). These wavelets were centered at 4 to 7 integer frequencies and constrained by constant standard deviations of 2 Hz and 80 ms in the spectral and temporal domains, respectively. Convolutions were computed at 4 ms intervals. Any additional artifacts were removed by visual inspection. Trials with incorrect responses were excluded from further analysis, and the number of trials in each task and beverage condition across both the S‐simple and S‐Stroop tasks was equated for each subject by removing superfluous trials at random, resulting in 86.27 trials on average per condition.
Cortically constrained source power estimates for each location on the cortical surface were computed with a spectral dynamic statistical parametric mapping approach based on minimum norm estimates (Dale et al., 2000; Lin et al., 2004). For each subject, task, and beverage condition, a map of noise‐sensitivity normalized source power was calculated by averaging across the theta band frequencies and across trials. Total event‐related theta power estimates were expressed as percent signal change from the 300 ms prestimulus period.
To examine the possible interactions of the factors of beverage and task condition, region‐of‐interest (ROI) analysis was conducted on the time courses of event‐related changes in theta power. Unbiased ROIs comprised of cortical locations with most notable source power were selected based on the group average across all task and beverage conditions and applied blindly to each subject. The ROIs primarily encompassed the cognitive control network in slightly right‐dominant frontotemporal regions including the inferior frontal cortex (iFC), the anterior insula and frontal operculum (aINS/FO), the presupplementary motor area (pre‐SMA), and the dAC cortex, as shown in Figs 3 and 4. To show early visual and late motor responses, ROIs in the lateral occipital (Occ) cortex and the primary motor (Mot) cortex are additionally included.
Event‐related theta power was averaged over time samples in task‐appropriate windows that captured group‐level peak activity (Figs 3 and 4). Those values were submitted to a repeated‐measures analysis of variance (ANOVA) with factors of beverage (alcohol, placebo) and task condition (Incong, Cong) for each ROI. A mixed‐design ANOVA including gender as a between‐group factor was performed initially but no significant effects of gender were observed. Beverage effects on conflict‐related theta power changes were assessed by comparing the difference between each condition, respectively, under alcohol and placebo for each ROI (Table 1). In addition, uncorrected baseline power estimates were submitted to the same analysis to examine the potential effects of beverage and task condition factors for the −300 to 0 ms (baseline) time window.
Table 1.
MEG Theta Results
| MEG theta results | Congruity, F(1, 14) | Beverage, F(1, 14) | Congruity × beverage, F(1, 14) | Congruity under alcohol, F(1, 14) | Congruity under placebo, F(1, 14) |
|---|---|---|---|---|---|
| S‐simple: 275 to 375 ms (T2a) | |||||
| Right iFC | 15.62** | 0.96 | 0.60 | 3.39 | 10.48** |
| Left iFC | 13.75** | 2.99 | 0.00 | 15.75** | 5.49* |
| Right dAC | 2.06 | 0.93 | 0.28 | 0.36 | 1.86 |
| Left dAC | 1.68 | 0.91 | 0.00 | 0.49 | 0.53 |
| Right pre‐SMA | 7.41* | 5.45* | 0.49 | 2.51 | 4.41 |
| Left pre‐SMA | 13.79** | 5.05* | 0.91 | 3.93 | 9.67** |
| S‐Stroop: 400 to 550 ms (T2b) | |||||
| Right aINS/FO | 5.10* | 3.38 | 2.65 | 0.60 | 5.36* |
| Left aINS/FO | 1.78 | 0.13 | 1.55 | 0.04 | 4.19 |
| Right iFC | 3.13 | 1.40 | 3.47 | 0.14 | 4.86* |
| Left iFC | 3.28 | 4.91* | 3.23 | 0.41 | 4.48 |
| Right dAC | 14.34** | 0.51 | 13.76** | 3.81 | 17.81*** |
| Left dAC | 0.24 | 0.46 | 10.59** | 3.96 | 7.92* |
| Right pre‐SMA | 4.76* | 3.39 | 6.21* | 0.01 | 7.84* |
| Left pre‐SMA | 6.15* | 0.39 | 4.42 | 0.08 | 13.61** |
iFC, inferior frontal cortex; pre‐SMA, presupplementary motor area; dAC, dorsal anterior cingulate; aINS/FO, anterior insula and frontal operculum; MEG, magnetoencephalography.
Significance level is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
For each task, included here are the results for the main effects and interactions of congruity and beverage, as well as the simple main effects of congruity under the alcohol and placebo conditions, respectively. Results are expressed as F‐values.
Results
Performance
Behavioral performance and task difficulty were assessed by measuring accuracy (% correct responses) and reaction times (RTs). A repeated‐measures ANOVA was carried out for each measure with a 2 × 2 × 2 (task × congruity × beverage) design. A significant 3‐way interaction, F(1, 14) = 5.26, p < 0.05, and an interaction between task and congruity factors, F(1, 14) = 36.42, p < 0.0001, were interrogated further (Fig. 2). Subjects responded less accurately during S‐Stroop compared to S‐simple, but only for Incong trials (87.5 vs. 94.9%), F(1, 14) = 23.35, p < 0.001. Incongruity markedly reduces accuracy in S‐Stroop (98.6 vs. 87.3%), F(1, 14) = 60.00, p < 0.0001, and barely noticeably in S‐simple (96.9 vs. 95.1%), F(1, 14) = 4.67, p < 0.05. The only effect of alcohol was a strong trend of reduced accuracy for Incong trials under S‐Stroop, 85.5% (alcohol) versus 89.5% (placebo), F(1, 14) = 4.33, p = 0.056.
Figure 2.
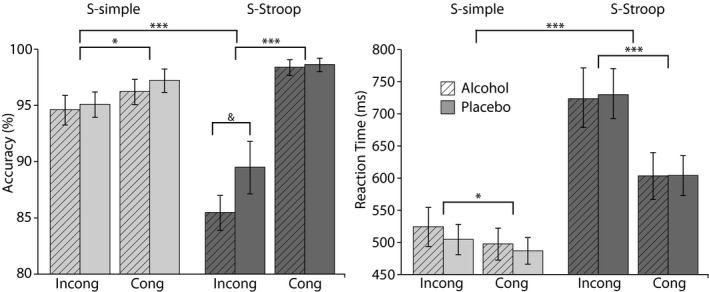
Behavioral performance measures. Accuracy and reaction times (means ± standard errors) are shown for each beverage and task condition across both tasks. The increased difficulty of the Simon‐Stroop (S‐Stroop) task is confirmed by its increased reactions times overall and reduced accuracies for incongruous (Incong) trials as compared to the Simon‐simple (S‐simple) task. Alcohol intoxication marginally reduced accuracy for Incong trials in the S‐Stroop task only. Significance levels: *p < 0.05, ***p < 0.001, & p < 0.06.
Overall, RTs were longer during the S‐Stroop task (665 ms) compared to the S‐simple task (503 ms), F(1, 14) = 103.07, p < 0.0001. Incongruity greatly slowed responses in S‐Stroop (727 vs. 604 ms), F(1, 14) = 104.89, p < 0.0001, and retarded them very slightly in S‐simple (514 vs. 492 ms), F(1, 14) = 6.29, p < 0.05. There were no effects of beverage on RTs.
Magnetoencephalography
The S‐simple and S‐Stroop tasks evoked similar patterns of spatiotemporal activity as shown in Figs 3 and 4. Both tasks were characterized by early activity in the Occ area with theta power peaking at ~125 ms (T1) after stimulus presentation. Subsequently, event‐related theta activity emerged in a bilateral, slightly right‐dominant network consisting of the iFC and pre‐SMA, peaking from 275 to 375 ms (T2a) in S‐simple and 400 to 550 ms (T2b) in S‐Stroop. In contrast to S‐simple, the network evoked by S‐Stroop extended further anteriorly to include the aINS/FO and dAC cortex. In these time windows incongruity increased theta power in both tasks, but in S‐Stroop, this effect was suppressed by alcohol intoxication. Finally, the bilateral Mot cortices were engaged during motor preparation and execution, with estimated activity peaking at 375 to 475 ms (T3a) and 550 to 650 ms (T3b) under the S‐simple and S‐Stroop tasks, respectively.
Figure 3.
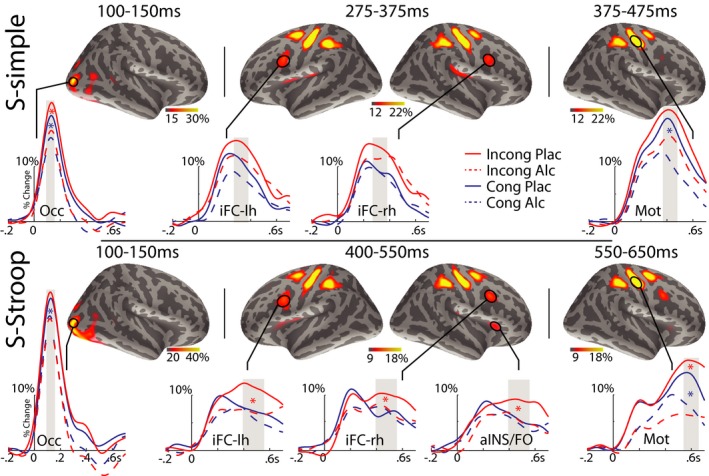
Group average maps and time courses of event‐related theta source power estimated to the lateral cortical surface. In both tasks, early activity (~125 ms) is estimated to the occipital (Occ) cortex. Subsequently, the inferior frontal cortex (iFC) is recruited and the right anterior insula/frontal operculum (aINS/FO) is additionally activated in the Simon‐Stroop (S‐Stroop) task. Last, in both tasks, theta band power reaches its peak prior to the subjects’ response in the primary motor (Mot) cortex. Maps show estimates for incongruous (Incong) placebo trials integrated across the time windows shown as gray bars on the time course traces shown for each beverage and conflict condition. These time windows were used for statistical analysis. Red and blue stars indicate significant alcohol‐induced attenuation of theta for the Incong and congruous (Cong) conditions, respectively, at p < 0.05. Alcohol selectively reduced theta power only on more difficult Incong trials in S‐Stroop. Data are presented as percent change in theta power relative to baseline.
Figure 4.
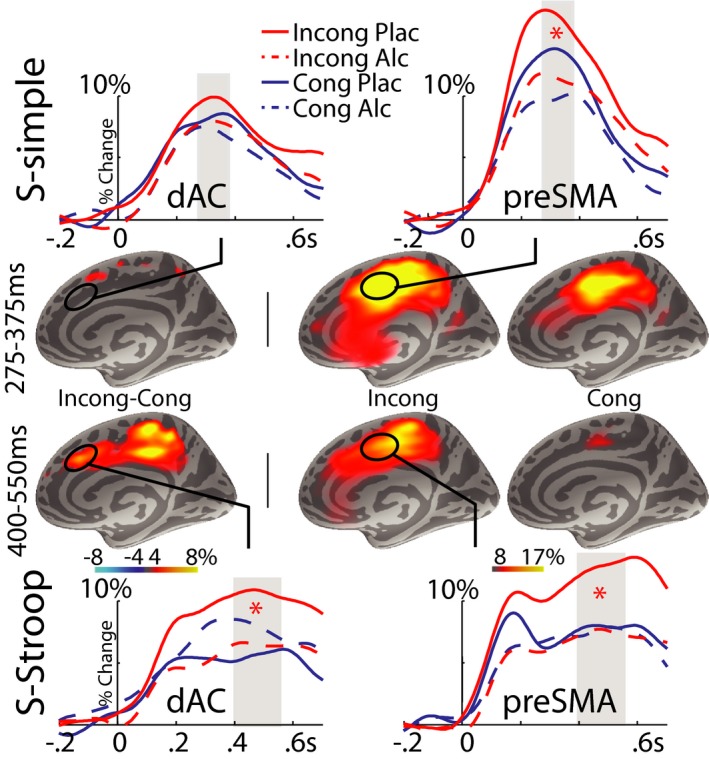
Group average maps and time courses of event‐related theta source power estimated to the right medial cortical surface. The left‐most panel shows the maps of the differential activity between incongruous (Incong) and congruous (Cong) conditions whereas the middle and right‐hand maps show the overall event‐related theta power to the Incong and Cong conditions. The maps show activity integrated across the time windows uses in statistical analysis, as indicated by gray bars overlaid on the time courses. Red stars indicate significant theta attenuation by alcohol only on the Incong trials (p < 0.05). Theta band power in the presupplementary motor area (pre‐SMA) and dorsal anterior cingulate (dAC) cortices peak in S‐simple and S‐Stroop from 257 to 375 and 400 to 550 ms, respectively. As the spatiotemporal patterns of activity were similar bilaterally, only the slightly dominant right hemisphere is shown. Data are presented as percent change relative to baseline.
In the S‐simple task, at ~125 ms (T1), event‐related theta power was reduced by alcohol in the bilateral Occ, F(1, 14) = 17.59, p < 0.001 (left), and F(1, 14) = 5.82, p < 0.05 (right). As outlined in Table 1, at ~325 ms (T2a), incongruity increased theta power in the bilateral pre‐SMA and iFC, and alcohol reduced theta power in bilateral pre‐SMA. There was no interaction between the factors of congruity and beverage, suggesting that alcohol reduced theta overall, with similar effects across both congruity conditions. At ~425 ms (T3a), theta power was increased overall to Incong stimuli, F(1, 14) = 12.87, p < 0.01, in the right Mot cortex. It was reduced by alcohol intoxication in the bilateral Mot cortices, F(1, 14) = 5.58, p < 0.05.
In the S‐Stroop task, alcohol intoxication reduced event‐related theta power in the right lateral Occ cortex, F(1, 14) = 4.84, p < 0.05, at ~125 ms (T1). In 400 to 550 ms time interval (T2b), theta power was increased by incongruity but, in contrast with S‐simple, only on placebo trials. Interaction between the factors of beverage and congruity was particularly prominent in the dAC bilaterally (Table 1). The conflict‐related difference between Incong and Cong trials was significant in most ROIs under placebo, but it was completely abolished by alcohol (Table 1). Finally, during motor preparation and response execution at ~600 ms (T3b), theta power was reduced by alcohol intoxication in the Mot cortex bilaterally, F(1, 14) = 12.88, p < 0.01.
In addition to the above time windows, we also tested for effects in the prestimulus baseline (−300 to 0 ms) in both tasks. There were no effects of either beverage or task conditions on theta estimates in the uncorrected baseline interval confirming that all the observed effects were due to changes in event‐related theta.
In summary, overall activity patterns were similar for both tasks, but the S‐Stroop task engaged a more extensive anterior prefrontal network including the bilateral dAC and right aINS/FO consistent with a difficulty‐related “anteriorization” gradient. Even though alcohol intoxication reduced theta power overall, activity was selectively reduced by alcohol only on more difficult Incong trials in S‐Stroop.
Postexperimental Questionnaire
In a postexperimental questionnaire, participants rated the beverage content and other variables on the Likert scale ranging from 1 (definitely not alcohol) to 5 (definitely alcohol). They discerned the content with ratings at 4.7 ± 0.5 under alcohol and 1.7 ± 0.8 under placebo, χ 2 = 15.0, p < 0.0001. On the scale ranging from 1 (not at all) to 5 (very much), participants reported feeling moderately intoxicated under alcohol (2.6 ± 0.8) and not at all intoxicated under placebo (1.1 ± 0.3), χ 2 = 13.0, p < 0.001. Finally, subjects rated the S‐Stroop task as being more difficult (2.5 ± 0.7) than S‐simple (1.7 ± 0.6), χ 2 = 11.0, p < 0.001.
Discussion
This study utilized an aMEG approach to examine how the spatiotemporal dynamics of conflict‐related theta band (4 to 7 Hz) activity are modulated by alcohol intoxication in 2 variants of the Simon task differing in difficulty. Our main findings are that (i) both tasks activate a distributed executive control network comprising the medial and inferolateral frontal cortices, (ii) alcohol intoxication generally reduces event‐related theta power in this network and decision conflict increases theta power, but (iii) alcohol‐induced theta suppression is selective for conflict in the more difficult task, which (iv) engages more anterior areas of the medial (i.e., dAC) and lateral prefrontal cortex (i.e., aINS/FO). The selectivity of alcohol‐induced deficits to higher levels of conflict is indicative of alcohol's role in disrupting the engagement of the top‐down regulation that facilitates decision making. Compromised executive control in difficult situations may be at the heart of various negative effects of alcohol, such as reduced competency in complex or response conflict‐inducing environments and the inability to disengage from counter productive behaviors.
Both Simon interference task variants employed here include a response conflict‐inducing scenario in which the correct response is contradicted by an automatic response representation. In the case of the traditional Simon task (S‐simple), the spatial location of the stimulus is irrelevant to the task as subjects respond only to its color. Nevertheless, response conflict is evoked by a mismatch between stimulus laterality and the responding hand. In the present study, only a very weak behavioral effect was observed in S‐simple with slightly lower accuracy and longer RTs on Incong trials. In contrast, the S‐Stroop combined semantic and spatial aspects of the mismatch resulting in a more salient conflict and a much more pronounced conflict‐related decline in performance accuracy and speed. The RT differential between the S‐simple and S‐Stroop tasks was mirrored in peak theta power latency. This not only confirms the tasks’ selectivity for theta oscillations but also provides evidence that these oscillations are germane to behavioral outcomes. In addition, the S‐Stroop task was subjectively rated as being significantly more difficult than the S‐simple version. Taken together, these results indicate that the Incong condition in the S‐Stroop task imposed a significantly higher degree of selection conflict between response representations, thereby engaging additional, more anterior brain areas.
The aMEG approach employed in the present study estimated the generation of event‐related theta power to the bilateral pre‐SMA and iFC for both tasks in addition to primary visual and motor areas. This distributed cognitive control network is in concordance with those reported in fMRI studies (van Veen and Carter, 2002) and previous aMEG estimates (Kovacevic et al., 2012). Under conditions of increased difficulty in S‐Stroop, the network extended anteriorly into the prefrontal cortex to encompass the dAC and right‐dominant iFC and aINS/FO. Indeed, dAC activation by tasks evoking response competition is a ubiquitous finding and yet the functional neuroanatomy of the mPFC is a matter of debate. Some accounts place an emphasis on detecting conflict (Botvinick, 2007; Carter and van Veen, 2007) and others conceptualize the dAC as playing a key role in response selection and evaluation (Picard and Strick, 1996). On that view, the dAC has a regulative role and can exert top‐down control of motor actions (Paus et al., 1993). Our previous studies relying on temporal precision of the aMEG approach indicate that the dAC is involved in both early conflict detection and late stages of response preparation and execution (Kovacevic et al., 2012). This integrated view is consistent with other accounts suggesting a more complex role of the dAC whereby the dAC contributes to aligning our actions with intents and goals by participating in a range of functions from conflict monitoring to response selection and regulation within contextual constraints (Nachev, 2006; Rushworth et al., 2004). dAC has widespread anatomical connections with different levels of the neuraxis, making it suitable for this multifaceted role in self‐regulation (Barbas, 2000; Devinsky et al., 1995).
Even though dAC activity is selectively modulated by task difficulty as suggested by the present results and other evidence (van Veen and Carter, 2005), the prevailing view of the functionality of the anterior mPFC is that it is not modular. Instead, it is conceptualized as following an anterior‐to‐posterior gradient as a function of task complexity and the resulting level of controlled processing (Nachev, 2006; Picard and Strick, 2001). Our data fully support such a view inasmuch as only the SMA/pre‐SMA area is activated by S‐simple. In contrast, the S‐Stroop engages cognitive control to a higher degree to reduce interference from habitual responses, which activates dAC during conflict detection as well as during response override. The temporally distinct processing stages present in S‐Stroop under placebo are made distinguishable by the aMEG method (Figs 3 and 4). An executive network comprised of the dAC, pre‐SMA, iFC, and aINS/FO initially engages at ~200 ms poststimulus, but at this early stage, Incong and Cong trials differentiate only in the dAC, consistent with its key role in early conflict detection (Kovacevic et al., 2012; van Veen and Carter, 2002). By ~475 ms, the conflict having been successfully detected, differentiation between the conflict and nonconflict conditions spreads throughout the executive network. While the network's theta band response to Cong trials diminishes shortly thereafter, the theta band response to Incong trials is sustained and, at ~600 ms, is extended to the Mot cortex when a correct response is executed.
In human scalp recordings, event‐related theta oscillations are prominent over the frontal midline and are modulated by cognitive load, memory demands, and errors (Mitchell et al., 2008). Intracranial laminar recordings in humans indicate that the dAC is a major generator of theta oscillations across a variety of cognitive tasks (Wang et al., 2005). Theta recorded in superficial cingulate layers co‐oscillates with distributed frontal and temporal areas, suggesting its key role in integrating cognitive representations within the relevant context and with memory circuitry in a global manner (Halgren et al., 2015; Mitchell et al., 2008).
The results of the present study confirm the sensitivity of theta oscillations to alcohol and cognitive load by extending the concept to the effects of acute intoxication. Even though event‐related total theta power was reduced by alcohol overall, alcohol interacted with conflict only in the S‐Stroop task, most prominently in the dAC. As shown in Fig. 4, the earliest conflict‐related theta power was recorded only in the dAC under placebo at ~200 ms. This spatiotemporal signature is consistent with the dAC's involvement in the early conflict detection stage. Increased theta magnitude was associated with incongruity on high‐conflict trials, but only under placebo. The conflict‐related theta increase in the dAC was significantly reduced by alcohol, in agreement with previous evidence of its detrimental effects on conflict detection (Kovacevic et al., 2012). This effect is sustained for the duration of the trial across most ROIs (Table 1), indicating that alcohol affects theta power during early, conflict detection, and later, response preparation stages. Inferolateral prefrontal and frontal operculum/anterior insula aINS/FO regions were similarly affected by incongruity and alcohol, suggesting that they are a part of the neurofunctional system that subserves controlled processing during conditions of increased difficulty and conflict. Indeed, the inferolateral prefrontal cortex may activate and sustain relevant task representations (Brass et al., 2005) that are integrated with the dAC activity. A resulting engagement of this network subserves top‐down controlled processing which relies on the capacity to inhibit automatic responses that interfere with task performance (Ridderinkhof et al., 2004).
Behavioral studies of acute intoxication have clearly shown that the degree of impairment depends on the task complexity and BrAC. Performance on more complex tasks that impose a higher processing load across attentional, cognitive, or working memory domains is impaired at very low BrAC (Koelega, 1995; Moskowitz and Fiorentino, 2000). Because this effect is not task specific, it must be mediated by a neural system at the global level. Previous imaging evidence indicates that a moderate level of intoxication selectively blunts activity in the dAC across tasks probing response conflict across different cognitive domains and across the manual motor and oculomotor systems (Kovacevic et al., 2012; Marinkovic et al., 2012a,b, 2013). The current study extends these observations by showing that alcohol‐induced attenuation of the conflict‐related theta is present only under more difficult conditions and is principally generated in the dAC. This finding is in overall agreement with other studies that parametrically manipulated difficulty levels in an n‐back task and showed alcohol‐induced behavioral impairment only under high load conditions (Casbon et al., 2003). In a similar task and using fMRI, Gundersen and colleagues (2008) reported a decrease in the BOLD signal in the dAC only under the highest cognitive load. Therefore, acute alcohol intoxication exerts its effects particularly under conditions that engage cognitive control as subserved by the dAC, including parsing complex situations, suppressing automatic responses, and processing novel events (Kovacevic et al., 2012; Marinkovic et al., 2001). Even though previous investigations using a fully crossed balanced placebo paradigm were unable to detect expectancy effects on central and peripheral physiological measures noting only pharmacological effects (Marinkovic et al., 2001), a mixture of both influences may exert an impact within the realistic context of social settings.
In the present study, conflict‐related theta under alcohol correlated negatively with the weekly alcohol consumption in both the S‐simple (T2a, right pre‐SMA, r = −0.69, p < 0.01) and the S‐Stroop tasks (T2b, right iFC, r = −0.54, p < 0.05) such that less incongruity‐evoked theta was observed in heavier drinkers, indicating a diminished capacity to engage executive circuitry. In addition, theta power measured in the right iFC correlated negatively with 2 disinhibition scales: Venturesomeness on Eysenck's Impulsiveness and Venturesomeness Scale (EIS) (r = −0.61, p < 0.05; r = −0.53, p < 0.05) and thrill and adventure seeking scale of Zuckerman SSS (r = −0.53, p < 0.05; r = −0.51, p = 0.05), suggesting that alcohol‐induced theta suppression is magnified in those predisposed to impulsive behavior. Subjects in the current study were at low risk of alcoholism and were screened for symptoms of alcohol abuse and dependence and for levels of their alcohol consumption. However, both of these impulsivity measures from the EIS and SSS scales tended to correlate to alcoholism risk as measured by the SMAST (Spearman's ρ = 0.50, p = 0.06; rs = 0.55, p < 0.05, respectively), with more impulsive subjects reporting more symptoms. This is broadly consistent with findings of lower theta power and higher impulsivity in individuals at risk for alcoholism (Kamarajan et al., 2015).
In sum, the present study used an aMEG approach and 2 variants of the Simon task to investigate the effects of moderate alcohol intoxication on event‐related total theta power as a function of task difficulty. Even though both tasks activate a distributed frontal network, alcohol‐induced theta suppression is selective for conflict only in the more difficult task which engages dAC and more anterior inferolateral prefrontal cortices. This extends and refines previously reported evidence of alcohol's selective effects on conflict monitoring and top‐down regulatory networks. The inability to cognitively override automatic responses is one of the most consequential effects of alcohol intoxication. Better understanding of the vulnerabilities of specific cognitive functions to alcohol can inform task design for future investigations and is a necessary part of current models explaining the role of executive control in both addictive and nonaddictive alcohol use.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by funds from the National Institutes of Health: R01‐AA016624 (to KM). The data were collected at the Radiology Imaging Laboratory, University of California San Diego, San Diego, CA, USA. We thank Sanja Kovacevic, PhD for her very important contributions to the analysis stream and data collection, and to Travis Wood, Jason Sherfey, Andrew Schulman, and Sarah Sheldon for their assistance.
References
- Barbas H (2000) Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 52:319–330. [DOI] [PubMed] [Google Scholar]
- Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7:356–366. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY (2005) The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9:314–316. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V (2007) Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci 7:367–379. [DOI] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ (2003) Deleterious effects of alcohol intoxication: diminished cognitive control and its behavioral consequences. J Abnorm Psychol 112:476–487. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J (2008) Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res 1238:127–142. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999) Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E (2000) Dynamic statistical parametric mapping: combining fMRI and MEG for high‐resolution imaging of cortical activity. Neuron 26:55–67. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995) Contributions of anterior cingulate cortex to behaviour. Brain 118(Pt 1):279–306. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ (1978) Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychol Rep 43:1247–1255. [DOI] [PubMed] [Google Scholar]
- Fillmore MT (2007) Acute alcohol‐induced impairment of cognitive functions: past and present findings. Int J Disabil Hum Dev 6:115–125. [Google Scholar]
- Fisk AD, Ackerman PL, Schneider W (1987) Automatic and controlled processing theory and its applications to human factors problems, in Human Factors Psychology (Hancock PA. ed), pp 159–197. North Holland, Amsterdam. [Google Scholar]
- Gan G, Guevara A, Marxen M, Neumann M, Junger E, Kobiella A, Mennigen E, Pilhatsch M, Schwarz D, Zimmermann US, Smolka MN (2014) Alcohol‐induced impairment of inhibitory control is linked to attenuated brain responses in right fronto‐temporal cortex. Biol Psychiatry 76:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D (1997) High‐resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7:374–385. [DOI] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Parkkonen L, Hamalainen MS (2014) MNE software for processing MEG and EEG data. NeuroImage 86:446–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K (2008) The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J 2:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Kaestner E, Marinkovic K, Cash SS, Wang C, Schomer DL, Madsen JR, Ulbert I (2015) Laminar profile of spontaneous and evoked theta: rhythmic modulation of cortical processing during word integration. Neuropsychologia 76:108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W (2008) The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci 20:215–225. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG (2007) BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging 25:978–988. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR (1999) Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399:781–784. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Manz N, Stimus AT, Anokhin AP, Bauer LO, Kuperman S, Kramer J, Bucholz KK, Schuckit MA, Hesselbrock VM, Porjesz B (2015) Deficient event‐related theta oscillations in individuals at risk for alcoholism: a study of reward processing and impulsivity features. PLoS One 10:e0142659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W II, Oberlin BG, Harezlak J, Wang Y, O'Connor SJ (2013) Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology 228:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelega HS (1995) Alcohol and vigilance performance: a review. Psychopharmacology 118:233–249. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K (2012) Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One 7:e43957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FH, Witzel T, Hamalainen MS, Dale AM, Belliveau JW, Stufflebeam SM (2004) Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage 23:582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman I, Marinkovic K (1996) Alcohol, alcoholism, and the autonomic nervous system: a critical account, in The Pharmacology of Alcohol and Alcohol Dependence (Begleiter H, Kissin B. eds), pp 248–306. Oxford University Press, New York, NY. [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I (2001) Arousal‐related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol Alcohol 36:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E (2012a) Acute alcohol intoxication impairs top‐down regulation of Stroop incongruity as revealed by blood oxygen level‐dependent functional magnetic resonance imaging. Hum Brain Mapp 33:319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E, Lee AK (2013) Effects of acute alcohol intoxication on saccadic conflict and error processing. Psychopharmacology 230:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, Kovacevic S (2012b) Event‐related theta power during lexical‐semantic retrieval and decision conflict is modulated by alcohol intoxication: anatomically constrained MEG. Front Psychol 3:Article 121. doi: 10.3389/fpsyg.2012.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brem S, Liechti M, Maurizio S, Michels L, Brandeis D (2015) Frontal midline theta reflects individual task performance in a working memory task. Brain Topogr 28:127–134. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ (2008) Frontal‐midline theta from the perspective of hippocampal “theta”. Prog Neurobiol 86:156–185. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Fiorentino D (2000) A Review of the Literature on the Effects of Low Doses of Alcohol on Driving‐Related Skills. DOT HS 809 028. US Department of Transportation National Highway Traffic Safety Administration, Washington, DC. [Google Scholar]
- Nachev P (2006) Cognition and medial frontal cortex in health and disease. Curr Opin Neurol 19:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011) FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA (2006) Alcohol attenuates load‐related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res 30:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E (1993) Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70:453–469. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996) Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6:342–353. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001) Imaging the premotor areas. Curr Opin Neurobiol 11:663–672. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B (2014) Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol 125:383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K (2011) Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol 45:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (2004) Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L (1975) A self‐administered Short Michigan Alcoholism Screening Test (SMAST). J Stud Alcohol 36:117–126. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2002) The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77:477–482. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2005) Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. NeuroImage 27:497–504. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E (2005) Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus‐response mapping, familiarity, and orienting. J Neurosci 25:604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M (1971) Dimensions of sensation seeking. J Consult Clin Psychol 36:45–52. [Google Scholar]


