More than 10,000-fold enhanced magnetic resonance signals with >20-min signal lifetimes on universal biomolecular markers.
Keywords: Magnetic resonance, nuclear spin states, hyperpolarization
Abstract
Conventional magnetic resonance (MR) faces serious sensitivity limitations which can be overcome by hyperpolarization methods, but the most common method (dynamic nuclear polarization) is complex and expensive, and applications are limited by short spin lifetimes (typically seconds) of biologically relevant molecules. We use a recently developed method, SABRE-SHEATH, to directly hyperpolarize 15N2 magnetization and long-lived 15N2 singlet spin order, with signal decay time constants of 5.8 and 23 minutes, respectively. We find >10,000-fold enhancements generating detectable nuclear MR signals that last for over an hour. 15N2-diazirines represent a class of particularly promising and versatile molecular tags, and can be incorporated into a wide range of biomolecules without significantly altering molecular function.
INTRODUCTION
Hyperpolarization enables real-time monitoring of in vitro and in vivo biochemistry
Conventional magnetic resonance (MR) is an unmatched tool for determining molecular structures and monitoring structural transformations. However, even very large magnetic fields only slightly magnetize samples at room temperature and sensitivity remains a fundamental challenge; for example, virtually all MR images are of water because it is the molecule at the highest concentration in vivo. Nuclear spin hyperpolarization significantly alters this perspective by boosting nuclear MR (NMR) sensitivity by four to nine orders of magnitude (1–3), giving access to detailed chemical information at low concentrations. These advances are beginning to transform biomedical in vivo applications (4–9) and structural in vitro studies (10–16).
Current hyperpolarization technology is expensive and associated with short signal lifetimes
Still, two important challenges remain. First, hyperpolarized MR is associated with high cost for the most widespread hyperpolarization technology [dissolution dynamic nuclear polarization (d-DNP), $2 million to $3 million for commercial hyperpolarizers]. Second, hyperpolarized markers typically have short signal lifetimes: typically, hyperpolarized signals may only be tracked for 1 to 2 min in the most favorable cases (6), greatly limiting this method as a probe for slower biological processes.
The presented approach is inexpensive and produces long-lived signals
Here, we demonstrate that both of these challenges can be overcome simultaneously, setting the stage for hour-long tracking of molecular markers with inexpensive equipment. Specifically, we illustrate the potential of 15N2-diazirines as uniquely powerful storage vessels for hyperpolarization. We show that diazirine can be hyperpolarized efficiently and rapidly (literally orders of magnitude cheaper and quicker than d-DNP), and that this hyperpolarization can be induced in states that maintain hyperpolarization for more than an hour.
Our approach uses parahydrogen (p-H2) to directly polarize long-lived nuclear spin states. The first demonstration of parahydrogen-induced polarization (PHIP) was performed in the late 1980s (17–19). Then, PHIP was used to rely on the addition of p-H2 to a carbon double or triple bond, incorporating highly polarized hydrogen atoms into molecules. This approach generally requires specific catalyst-substrate pairs; in addition, hydrogen atoms usually have short relaxation times (T1) that cause signal decay within a few seconds. A more recent variant, SABRE (signal amplification by reversible exchange) (20, 21), uses p-H2 to polarize 1H atoms on a substrate without hydrogenation. In SABRE, both p-H2 and substrate reversibly bind to an iridium catalyst and the hyperpolarization is transferred from p-H2 to the substrate through J-couplings established on the catalytic intermediate. Recently, we extended this method to SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei) for direct hyperpolarization of 15N molecular sites (22–24). This method has several notable features. Low-γ nuclei (13C, 15N) tend to have long relaxation times, particularly if a proton is not attached. In addition, conventional SABRE relies on small differences between four-bond proton-proton J-couplings (detailed in the Supplementary Materials), whereas SABRE-SHEATH uses larger two-bond heteronuclear J-couplings. It is extremely simple: SABRE-SHEATH requires nothing but p-H2, the catalyst, and a shield to reduce Earth’s field by about 99%. After 1 to 5 min of bubbling p-H2 into the sample in the shield, we commonly achieve 10% nitrogen polarization, many thousands of times stronger than thermal signals (22). In contrast, d-DNP typically produces such polarization levels in an hour, at much higher cost.
Diazirines are small and versatile molecular tags
A general strategy for many types of molecular imaging is the creation of molecular tags, which ideally do not alter biochemical pathways but provide background-free signatures for localization. This strategy has not been very successful in MR because of sensitivity issues. Here, we demonstrate that SABRE-SHEATH enables a MR molecular beacon strategy using diazirines  (three-membered rings containing a nitrogen-nitrogen double bond). They are highly attractive as molecular tags, primarily because of their small size. Diazirines have already been established as biocompatible molecular tags for photoaffinity labeling (25). They can be incorporated into many small molecules, metabolites, and biomolecules without drastically altering biological function. Diazirines share similarities with methylene (CH2) groups in terms of electronic and steric properties such that they can replace methylene groups without drastically distorting biochemical behavior. Furthermore, diazirines are stable at room temperature, are resistant to nucleophiles, and do not degrade under either acidic or alkaline conditions (25). With these attractive properties, diazirines have been used for the study of many signaling pathways. For example, they have been incorporated into hormones (26), epileptic drugs (27), antibiotics (28), hyperthermic drugs (29), anticancer agents (30), anesthetics (31), nucleic acids (32), amino acids (33), and lipids (34). They also have been introduced into specific molecular reporters to probe enzyme function and their binding sites such as in kinases (35), aspartic proteases (36), or metalloproteinases (37), to name a few. The nitrogen-nitrogen moiety is also intrinsically interesting, because the two atoms are usually very close in chemical shift and strongly coupled, thus suited to support a long-lived singlet state as described below.
(three-membered rings containing a nitrogen-nitrogen double bond). They are highly attractive as molecular tags, primarily because of their small size. Diazirines have already been established as biocompatible molecular tags for photoaffinity labeling (25). They can be incorporated into many small molecules, metabolites, and biomolecules without drastically altering biological function. Diazirines share similarities with methylene (CH2) groups in terms of electronic and steric properties such that they can replace methylene groups without drastically distorting biochemical behavior. Furthermore, diazirines are stable at room temperature, are resistant to nucleophiles, and do not degrade under either acidic or alkaline conditions (25). With these attractive properties, diazirines have been used for the study of many signaling pathways. For example, they have been incorporated into hormones (26), epileptic drugs (27), antibiotics (28), hyperthermic drugs (29), anticancer agents (30), anesthetics (31), nucleic acids (32), amino acids (33), and lipids (34). They also have been introduced into specific molecular reporters to probe enzyme function and their binding sites such as in kinases (35), aspartic proteases (36), or metalloproteinases (37), to name a few. The nitrogen-nitrogen moiety is also intrinsically interesting, because the two atoms are usually very close in chemical shift and strongly coupled, thus suited to support a long-lived singlet state as described below.
RESULTS AND DISCUSSION
Our first target molecule was 2-cyano-3-(D3-methyl-15N2-diazirine)-propanoic acid in methanol-d4, depicted in Fig. 1 along with the catalyst. To establish this hyperpolarization mode, we developed a new catalyst for this SABRE-SHEATH process. We synthesized [Ir(COD)(IMes)(Py)][PF6] [COD, cyclooctadiene; IMes, 1,3-bis(2,4,6-trimethylphenyl)-imidazolium; Py, pyridine] as depicted in Fig. 1A. This precursor is dissolved in a diazirine-containing methanol solution and activated by bubbling hydrogen through this solution for ~20 min. This produces the catalytically active [Ir(IMes)(H2)(Py)(Diaz)]+ species depicted in Fig. 1B.
Fig. 1. The hyperpolarization mechanism.
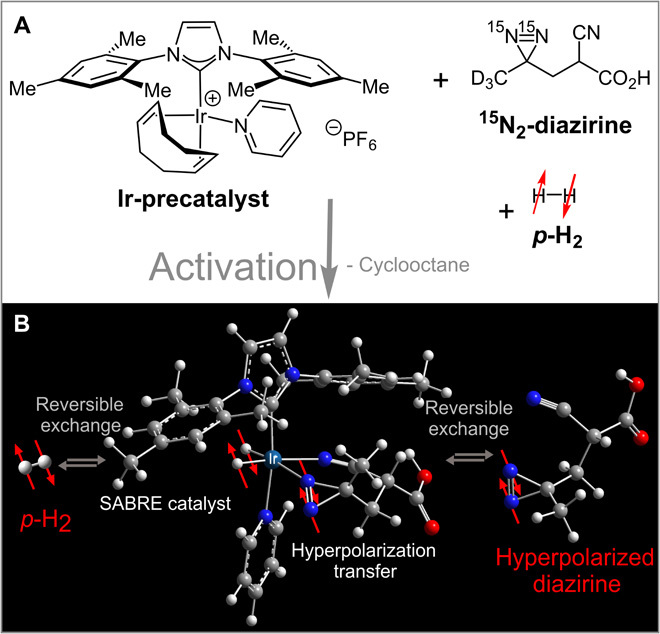
(A) The precatalyst, 15N2-diazirine substrate, and p-H2 are mixed, resulting in the activated species depicted in (B). (B) Both p-H2 and the free 15N2-diazirine [2-cyano-3-(D3 methyl-15N2-diazirine)-propanoic acid] are in reversible exchange with the catalytically active iridium complex. The catalyst axial position is occupied by IMes [1,3-bis(2,4,6-trimethylphenyl)-imidazolium] and Py (pyridine) as nonexchanging ligands. The structure shown is a local energy minimum of the potential energy surface based on all-electron DFT calculations and the dispersion-corrected PBE density functional. In the complex, hyperpolarization is transferred from the parahydrogen (p-H2)–derived hydrides to the 15N nuclei (white, hydrogen; gray, carbon; blue, nitrogen; red, oxygen).
Density functional theory calculations shed light on polarization transfer catalyst
The Ir complex conformation shown in Fig. 1B was determined by all-electron density functional theory (DFT) calculations [semilocal Perdew-Burke-Ernzerhof (PBE) functional (38), corrected for long-range many-body dispersion interactions (39), in the FHI-aims software package (40, 41); see the Supplementary Materials for details]. The calculations indicate a η1 single-sided N attachment rather than η2-N=N attachment of the diazirines. In the Ir complex, hyperpolarization is transferred from p-H2 gas (~92% para-state, 7.5 atm) to the 15N2-diazirine. Both p-H2 and substrate are in reversible exchange with the central complex, which results in continuous pumping of hyperpolarization: p-H2 is continually refreshed and hyperpolarization accumulated on the diazirine substrate.
An alternate polarization transfer catalyst is introduced
As opposed to the traditional [Ir(COD)(IMes)(Cl)] catalyst (18), the synthesized [Ir(COD)(IMes)(Py)][PF6] results in a pyridine ligand trans to IMes, improving our hyperpolarization levels by a factor of ~3 (see the Supplementary Materials). We have found that this new approach, which avoids competition from added pyridine, makes it possible to directly hyperpolarize a wide variety of different types of 15N-containing molecules (and even 13C). However, diazirines represent a particularly general and interesting class of ligands for molecular tags and are the focus here.
As depicted in Fig. 2A, the hyperpolarization proceeds outside the high-field NMR magnet at low magnetic fields, enabling SABRE-SHEATH directly targeting 15N nuclei (22). To establish the hyperpolarization, we bubble p-H2 for ~5 min at the adequate field. Then, the sample is transferred into the NMR magnet within ~10 s, and 15N2 signal detection is performed with a simple 90° pulse followed by data acquisition.
Fig. 2. Experimental and spectral distinction between magnetization and singlet spin order.
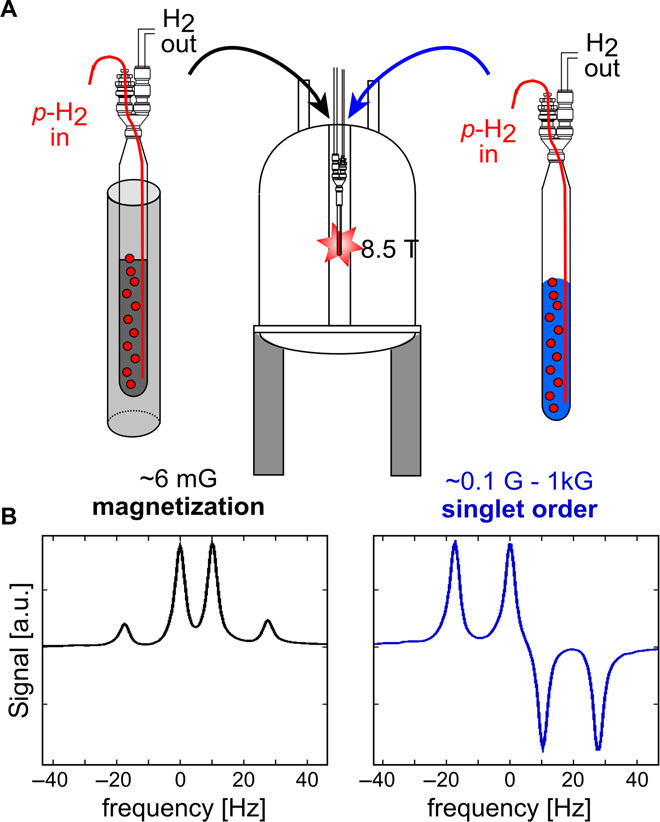
(A) Experimental procedure. The sample is hyperpolarized by bubbling parahydrogen (p-H2) through the solution in the NMR tube for 5 min and subsequently transferred into the high-field magnet for detection. If the hyperpolarization/bubbling is performed in a magnetic shield at ~6 mG, z-magnetization is created (black). If the hyperpolarization/bubbling is performed in the laboratory field anywhere between ~0.1 and ~1 kG, singlet order is created (blue). (B) Z-magnetization and singlet spin order can easily be distinguished based on their spectral appearance. a.u., arbitrary units. Z-magnetization produces an in-phase quartet (black). Singlet order gives an anti-phase quartet (blue). The spin system parameters for the 15N2 two-spin system are JNN = 17.3 Hz and Δδ = 0.58 parts per million.
Two types of hyperpolarized states can be created: Magnetization and singlet order
We create two different types of hyperpolarization on the 15N2-diazirine. We can hyperpolarize traditional z-magnetization, which corresponds to nuclear spins aligned with the applied magnetic field and is associated with pure in-phase signal as illustrated with the black trace in Fig. 2B. Alternatively, we can hyperpolarize singlet order on the 15N2-diazirine, which corresponds to an anti-aligned spin state, with both spins pointing in opposite directions, entangled in a quantum mechanically “hidden” state. This hidden singlet order is converted into a detectable state when transferred to a high magnetic field and associated with the anti-phase signal illustrated by the blue trace in Fig. 2B (see the Supplementary Materials for details). The difference in symmetry of z-magnetization and singlet order leads to differences in signal decay rates; z-magnetization is directly exposed to all NMR relaxation mechanisms and is often associated with shorter signal lifetimes, which may impede molecular tracking on biologically relevant time scales. Singlet order, on the other hand, is protected from many relaxation mechanisms because it has no angular momentum (42–52) and can therefore exhibit much longer lifetimes, enabling hour-long molecular tracking.
The type of hyperpolarized state is selected by the magnetic field
We can control which type of hyperpolarization we create by choosing the appropriate magnetic fields for the bubbling process. Z-magnetization is created in the SABRE-SHEATH mode at low magnetic fields inside a magnetic shield (22–24, 53, 54). This behavior is explained by resonance conditions for hyperpolarizing magnetization versus singlet order that we derive in the Supplementary Materials. The condition for creating magnetization, νH − νN = |JHH ± JNN|, is field-dependent in the NMR frequencies, νH and νN, and field-independent in the J-couplings. Accordingly, hyperpolarized magnetization is created at a magnetic field where the frequency difference matches the J-couplings. This magnetic field is ~6 mG, which is obtained by using JHH = −10 Hz, JNN = −17.3 Hz, γ1H = 4.2576 kHz/G, and γ15N = −0.4316 kHz/G (see the Supplementary Materials). The theoretical prediction of 6 mG matches the experimental maximum for hyperpolarized z-magnetization illustrated by the blue data in Fig. 3A.
Fig. 3. Hyperpolarization buildup in the form of magnetization or singlet order.
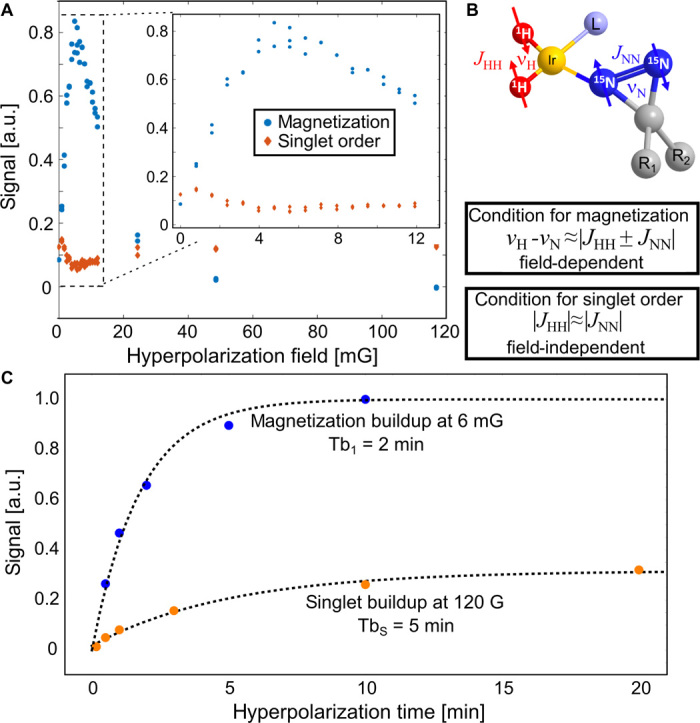
(A) Magnetization and singlet order as a function of magnetic field. The graph shows signal intensity after 5 min of bubbling. Magnetization is produced only at low magnetic fields inside the magnetic shield ideally at ~6 mG. Singlet order is obtained at any other magnetic field up to ~1 kG. (B) Spin systems and resonance conditions for forming magnetization versus singlet order. The condition for forming magnetization is field-dependent, whereas the condition for forming singlet order is not field-dependent, explaining the experimental observations of (A). (C) Polarization buildup as a function of bubbling time. Magnetization builds up faster, because the resonance condition can be matched exactly and T1 is shorter, whereas singlet order builds up slower because the matching condition is not met exactly and TS is longer. The measurements are performed with a methanol-d4 solution containing 12 mM 15N2-diazirine, 0.5 mM [IrCl(COD)(IMes)], 4 mM Py, and 960 mM D2O.
The condition for creating singlet order, on the other hand, JHH = ± JNN, is not dependent on the magnetic field and, for the system at hand, is fulfilled closely enough (JHH ~ 10 Hz, JNN ~ −17.3 Hz) such that singlet order can be induced virtually at any magnetic field, under the condition that the singlet state remains close to an eigenstate of the Hamiltonian, which is true for fields up to ~1 kG. As illustrated in Fig. 3C, the singlet buildup is indeed slightly slower and less efficient, as expected for an imperfectly matched resonance condition. Accordingly, the optimal magnetic field for hyperpolarizing singlet order is not tied to specific magnetic fields. Instead, the optimal magnetic field to produce singlet order is the field associated with the longest singlet lifetime, allowing for accumulation over a long time span. We find well-suited lifetimes at fields between ~0.5 and ~200 G.
Central results: 5% polarization for magnetization with 6-min decay constant, 3% singlet polarization with 23-min decay constant
For z-magnetization, we observe up to 15,000-fold signal enhancements over thermal control experiments at 8.5 T displayed in Fig. 4A. This corresponds to ~5% polarization. For singlet order, up to 5000-fold signal enhancements are observed, corresponding to ~3% singlet polarization. Notice that the singlet polarization level is not much lower than the polarization of magnetization despite the signal enhancement being three times lower. This mismatch between enhancements and polarization is attributed to the singlet readout being ~60% as efficient as the magnetization readout in our current setup (see the Supplementary Materials). Figure 4 (B and C) displays typical T1 and TS lifetime measurements. Spectra are obtained after 5-min bubbling and a varying relaxation delay at a chosen holding field. The acquired spectra are subsequently integrated and fit to mono-exponential decays shown in Fig. 4C. Under conditions of relatively low catalyst concentrations and relatively low magnetic fields, as specified in the figure caption, a T1 of 5.8 ± 0.3 min and a TS of 23 ± 3 min are observed (see the Supplementary Materials for more exhaustive data under various concentrations and magnetic fields). Typical 13C hyperpolarization studies collect data for about three times the spin lifetime, which is about 3 min (3 × 1 min) for typical 13C substrates. Here, the 23-min lifetime enables data collection for over an hour.
Fig. 4. Maximum enhancement levels and decay time constants observed for magnetization and singlet order.
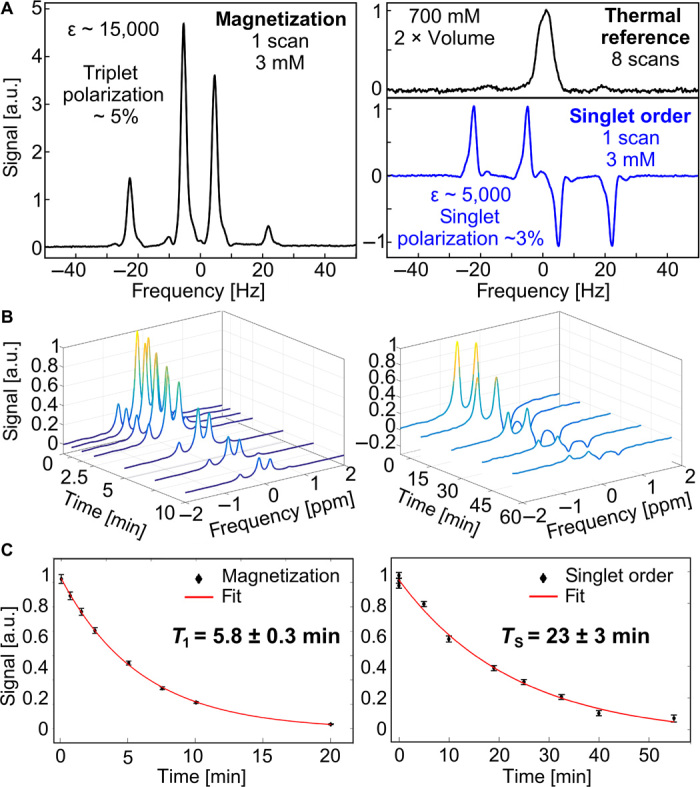
(A) Single-shot SABRE-SHEATH spectra obtained from a methanol-d4 solution containing 3 mM 15N2-diazirine, 0.125 mM [Ir(COD)(IMes)(Py)][PF6], and 240 mM D2O. Enhancements are obtained by comparison to the displayed thermal reference spectrum of the 15N2-diazirine [2-cyano-3-(D3-methyl-15N2-diazirine)-propanoic acid] in D2O. (In D2O, the chemical shift difference between the 15N nuclei is smaller than that in MeOH, and therefore, only one peak is observed.) (B) Representative T1 and TS decay measurements. T1 is measured at 120 G (12 mT) from a methanol-d4 solution containing 12 mM 15N2-diazirine, 0.125 mM [IrCl(COD)(IMes)], 1 mM Py, and 960 mM D2O. TS is measured at 3 G (0.3 mT) from a solution containing 12 mM 15N2-diazirine, 0.05 mM [IrCl(COD)(IMes)], and 0.4 mM Py. (C) The z-magnetization and singlet-order components are extracted from the data sets in (B) and fit to a single exponential (see the Supplementary Materials for further details).
CONCLUSION
The demonstrated hyperpolarization lifetimes, combined with the ease of hyperpolarization in these broadly applicable biomolecular tags, may establish a paradigm shift for biomolecular sensing and reporting in optically opaque tissue. The demonstrated lifetimes even exceed lifetimes of some common radioactive tracers used in positron emission tomography (PET) (for example, 11C, 20.3 min). However, unlike PET, MR is exquisitely sensitive to chemical transformations and does not use ionizing radiation (such that, for example, daily progression monitoring of disease is easily possible). The presented work may allow direct access to biochemical mechanisms and kinetics in optically opaque media. We therefore envision tracking subtle biochemical processes in vitro with unprecedented NMR sensitivities as well as real-time in vivo biomolecular imaging with hyperpolarized diazirines.
MATERIALS AND METHODS
Samples hyperpolarized with [Ir(COD)(IMes)(Py)][PF6] as precursor
We prepared a stock solution of 0.5 M [Ir(COD)(IMes)(Py)][PF6] in CD3OD. Subsequently, an appropriate aliquot of this stock solution was added into the NMR tube containing CD3OD such that a volume of 600 μl of the targeted concentration was obtained. To this solution, we then added an aliquot of a 700 mM solution of the diazirine in D2O to achieve the desired diazirine concentration.
Samples hyperpolarized with [Ir(COD)(IMes)Cl] as precursor
We prepared a stock solution of 0.5 M [Ir(COD)(IMes)Cl] plus 8 eq of pyridine, that is, 4 M in CD3OD. Subsequently, an appropriate aliquot of this stock solution was added into the NMR tube containing CD3OD such that a volume of 600 μl at the targeted concentration was obtained. To this solution, we then added an aliquot of a 700 mM solution of the diazirine in D2O to achieve the desired diazirine concentration.
Parahydrogen production
Parahydrogen (p-H2) gas (~92% para-state, 8.2 atm) was created in a Bruker parahydrogen generator (slightly modified to access higher pressures) and bubbled through the solution prepared above. For catalyst activation, bubbling was performed for 20 min before starting any experiments.
Experimental setup to obtain well-controlled milligauss fields
A set of three layers of magnetic shielding was used to obtain a low–magnetic field environment (MuMETAL Zero Gauss Chambers; product ZG-206, Magnetic Shield Corp.) The shields are degaussed with a degaussing coil wrapped around the innermost shield. In the center of the shields, we placed a solenoid with length L = 47.5 cm and N = 165 turns (see the Supplementary Materials for further details and a picture).
Supplementary Material
Funding
This work was supported by NSF grants CHE-1058727, CHE-1363008, and CHE-1416268; NIH grant 1R21EB018014; and Department of Defense Congressionally Directed Medical Research Programs Breast Cancer grant W81XWH-12-1-0159/BC112431. W.P.H., T.T., V.B., and W.S.W. also acknowledge funding from a Pratt School of Engineering Research Innovation Seed Fund Grant. Q.W. and S.J.M. acknowledge support from Duke University. G.X.O. also acknowledges support from the Burroughs Wellcome Fellowship. Furthermore, acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research. Author contributions: T.T. envisioned direct hyperpolarization of long-lived states with SABRE, designed experiments, conducted experiments, analyzed data, developed spin dynamics theory, and wrote the manuscript. G.X.O. designed and synthesized 15N2-diazirine molecules, conducted initial characterization, and edited the manuscript. A.W.J.L. conceived and synthesized SABRE catalysts, edited the manuscript, and conducted most of the experiments. K.E.C. analyzed data, developed spin dynamics theory, and assisted with experiments. Y.F. envisioned 15N2-diazirine as storage vessels for long-lived hyperpolarization and conducted initial characterization. W.P.H. designed and conducted all-electron DFT simulations. V.B. designed and advised on the all-electron DFT simulations. S.J.M. conceived and advised on the synthesis of the SABRE precatalysts and edited the manuscript. E.Y.C. advised on the experimental design and edited the manuscript. Q.W. envisioned 15N2-diazirine as a general tag for MRI molecular labeling; advised on experimental design, initial characterization, and synthesis of 15N2-diazirine; and edited the manuscript. W.S.W. envisioned 15N2-diazirine as storage vessels for long-lived hyperpolarization, advised on experimental design and development of spin dynamics theory, and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data are available from the authors upon request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501438/DC1
Synthesis of 15N2-diazirine and iridium precatalyst
Theoretical derivation of resonance conditions for hyperpolarization transfer
Spin dynamics during low- to high-field sample transfer
MD simulations of possible transitions states for hyperpolarization transfer
Sample preparation and setup to obtain well-controlled milligauss fields
Hyperpolarization buildup dynamics, lifetimes, and enhancements in detail
Appendix
Fig. S1. Synthesis of 2-cyano-3-(3′-(methyl-d3)-3′H-diazirine-3′-yl-1′,2′-15N2)propanoic acid using modified literature procedures.
Fig. S2. Spin system for polarization transfer.
Fig. S3. Eigenstates of a J-coupled two-spin system as a function of magnetic field.
Fig. S4. Simulated spectra for magnetization and singlet order.
Fig. S5. Three candidate conformations of 2-cyano-3-(D3-methyl-15N2-diazirine)-propanoic acid as identified by DFT PBE + TS calculations, as used to construct likely catalytically active structures producing hyperpolarization attached to the SABRE catalyst.
Fig. S6. First-principles derived candidate conformations of catalytic species that drive hyperpolarization transfer.
Fig. S7. Experimental SABRE-SHEATH setup.
Fig. S8. Decay time constants, buildup constants, and enhancements as a function of magnetic field and concentrations.
Fig. S9. The effect of continued singlet-polarization buildup after stopping p-H2 bubbling.
Table S1. Relative energies of the diazirine attachment modes tested in this work for the case of two different ligands that are simultaneously present: two equatorial hydrides, axial IMes, axial pyridine, one equatorial pyridine, and diazirine in an equatorial position.
Table S2. Relative energies of the diazirine attachment modes tested in this work for the case of two diazirine molecules that are simultaneously present.
Table S3. Magnetization lifetimes, T1, under varying catalyst concentrations and holding fields.
Table S4. Singlet lifetimes, TS, under varying catalyst concentrations and holding fields.
Table S5. Magnetization buildup times, Tb1, at 6 mG under varying catalyst concentrations.
Table S6. Singlet buildup times, TbS, under varying catalyst concentrations and magnetic fields.
Table S7. Enhancements, ε, under varying concentrations of catalyst [IrCl(COD)(IMes)] (1a), [Ir(COD)(IMes)(Py)][PF6] (1b), diazirine substrate (2), pyridine (3), and D2O (4).
REFERENCES AND NOTES
- 1.Ardenkjær-Larsen J. H., Fridlund B., Gram A., Hansson G., Hansson L., Lerche M. H., Servin R., Thaning M., Golman K., Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U.S.A. 100, 10158–10163 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottberg A., Stachura M., Kowalska M., Bissell M. L., Arcisauskaite V., Blaum K., Helmke A., Johnston K., Kreim K., Larsen F. H., Neugart R., Neyens G., Garcia Ruiz R. F., Szunyogh D., Thulstrup P. W., Yordanov D. T., Hemmingsen L., Billion-fold enhancement in sensitivity of nuclear magnetic resonance spectroscopy for magnesium ions in solution. Chem. Phys. Chem. 15, 3929–3932 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Shchepin R. V., Coffey A. M., Waddell K. W., Chekmenev E. Y., Parahydrogen induced polarization of 1-13C-phospholactate-d2 for biomedical imaging with > 30,000,000-fold NMR signal enhancement in water. Anal. Chem. 86, 5601–5605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues T. B., Serrao E. M., Kennedy B. W. C., Hu D.-E., Kettunen M. I., Brindle K. M., Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat. Med. 20, 93–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshari K. R., Wilson D. M., Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 43, 1627–1659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson S. J., Kurhanewicz J., Vigneron D. B., Larson P. E. Z., Harzstark A. L., Ferrone M., van Criekinge M., Chang J. W., Bok R., Park I., Reed G., Carvajal L., Small E. J., Munster P., Weinberg V. K., Ardenkjaer-Larsen J. H., Chen A. P., Hurd R. E., Odegardstuen L.-I., Robb F. J., Tropp J., Murray J. A., Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]-pyruvate. Sci. Transl. Med. 5, 198ra108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurhanewicz J., Vigneron D. B., Brindle K., Chekmenev E. Y., Comment A., Cunningham C. H., DeBerardinis R. J., Green G. G., Leach M. O., Rajan S. S., Rizi R. R., Ross B. D., Warren W. S., Malloy C. R., Analysis of cancer metabolism by imaging hyperpolarized nuclei: Prospects for translation to clinical research. Neoplasia 13, 81–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal R., Kurhanewicz J., Prostate cancer in 2013: The changing role of imaging in clinical care. Nat. Rev. Urol. 11, 75–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Park I., Nelson S. J., Imaging tumor metabolism using in vivo magnetic resonance spectroscopy. Cancer J. 21, 123–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentine K. G., Mathies G., Bédard S., Nucci N. V., Dodevski I., Stetz M. A., Can T. V., Griffin R. G., Wand A. J., Reverse micelles as a platform for dynamic nuclear polarization in solution NMR of proteins. J. Am. Chem. Soc. 136, 2800–2807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H.-Y., Ragavan M., Hilty C., Protein folding studied by dissolution dynamic nuclear polarization. Angew. Chem. Int. Ed. 52, 9192–9195 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Smith A. N., Caporini M. A., Fanucci G. E., Long J. R., A method for dynamic nuclear polarization enhancement of membrane proteins. Angew. Chem. Int. Ed. 54, 1542–1546 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Bornet A., Ji X., Mammoli D., Vuichoud B., Milani J., Bodenhausen G., Jannin S., Long-lived states of magnetically equivalent spins populated by dissolution-DNP and revealed by enzymatic reactions. Chem. Eur. J. 20, 17113–17118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappuis Q., Milani J., Vuichoud B., Bornet A., Gossert A. D., Bodenhausen G., Jannin S., Hyperpolarized water to study protein–ligand interactions. J. Phys. Chem. Lett. 6, 1674–1678 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Akbey Ü., Franks W. T., Linden A., Lange S., Griffin R. G., van Rossum B.-J., Oschkinat H., Dynamic nuclear polarization of deuterated proteins. Angew. Chem. Int. Ed. 49, 7803–7806 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y., Hilty C., Affinity screening using competitive binding with fluorine-19 hyperpolarized ligands. Angew. Chem. Int. Ed. 54, 4941–4944 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers C. R., Weitekamp D. P., Transformation of symmetrization order to nuclear-spin magnetization by chemical reaction and nuclear magnetic resonance. Phys. Rev. Lett. 57, 2645–2648 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Bowers C. R., Weitekamp D. P., Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987). [Google Scholar]
- 19.Eisenschmid T. C., Kirss R. U., Deutsch P. P., Hommeltoft S. I., Eisenberg R., Bargon J., Lawler R. G., Balch A. L., Para hydrogen induced polarization in hydrogenation reactions. J. Am. Chem. Soc. 109, 8089–8091 (1987). [Google Scholar]
- 20.Adams R. W., Aguilar J. A., Atkinson K. D., Cowley M. J., Elliott P. I. P., Duckett S. B., Green G. G. R., Khazal I. G., López-Serrano J., Williamson D. C., Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 323, 1708–1711 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Cowley M. J. Adams R. W., Atkinson K. D., Cockett M. C. R., Duckett S. B., Green G.G. R., Lohman J. A. B., Kerssebaum R., Kilgour D., Mewis R. E., Iridium N-heterocyclic carbene complexes as efficient catalysts for magnetization transfer from para-hydrogen. J. Am. Chem. Soc. 133, 6134–6137 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theis T., Truong M. L., Coffey A. M., Shchepin R. V., Waddell K. W., Shi F., Goodson B. M., Warren W. S., Chekmenev E. Y., Microtesla SABRE enables 10% nitrogen-15 nuclear spin polarization. J. Am. Chem. Soc. 137, 1404–1407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shchepin R. V., Truong M. L., Theis T., Coffey A. M., Shi F., Waddell K. W., Warren W. S., Goodson B. M., Chekmenev E. Y., Hyperpolarization of “neat” liquids by NMR signal amplification by reversible exchange. J. Chem. Phys. Lett. 6, 1961–1967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong M. L., Theis T., Coffey A. M., Shchepin R. V., Waddell K. W., Shi F., Goodson B. M., Warren W. S., Chekmenev E. Y., 15N hyperpolarization by reversible exchange using SABRE-SHEATH. J. Phys. Chem. C 119, 8786–8797 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubinsky L., Krom B. P., Meijler M. M., Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 20, 554–570 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita T., Caño-Delgado A., Seto H., Hiranuma S., Fujioka S., Yoshida S., Chory J., Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433, 167–171 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Park K. D., Salomé C., Wilson S. M., Stables J. P., Liu R., Khanna R., Kohn H., Development and characterization of novel derivatives of the antiepileptic drug lacosamide that exhibit far greater enhancement in slow inactivation of voltage-gated sodium channels. ACS Chem. Neurosci. 2, 90–106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riber D., Venkataramana M., Sanyal S., Duvold T., Synthesis and biological evaluation of photoaffinity labeled fusidic acid analogues. J. Med. Chem. 49, 1503–1505 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Hosoya T., Hiramatsu T., Ikemoto T., Aoyama H., Ohmae T., Endo M., Suzuki M., Design of dantrolene-derived probes for radioisotope-free photoaffinity labeling of proteins involved in the physiological Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Bioorg. Med. Chem. Lett. 15, 1289–1294 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Luo L., Parrish C. A., Nevins N., McNulty D. E., Chaudhari A. M., Carson J. D., Sudakin V., Shaw A. N., Lehr R., Zhao H., Sweitzer S., Lad L., Wood K. W., Sakowicz R., Annan R. S., Huang P. S., Jackson J. R., Dhanak D., Copeland R. A., Auger K. R., ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat. Chem. Biol. 3, 722–726 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Hall M. A., Xi J., Lor C., Dai S., Pearce R., Dailey W. P., Eckenhoff R. G., m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J. Med. Chem. 53, 5667–5675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebmann M., Di Pasquale F., Marx A., A new photoactive building block for investigation of DNA backbone interactions: Photoaffinity labeling of human DNA polymerase β. ChemBioChem 7, 1965–1969 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Suchanek M., Radzikowska A., Thiele C., Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat. Methods 2, 261–268 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto M., Hatanaka Y., A novel biotinylated diazirinyl ceramide analogue for photoaffinity labeling. Bioorg. Med. Chem. Lett. 18, 650–652 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Kaneda M., Masuda S., Tomohiro T., Hatanaka Y., A simple and efficient photoaffinity method for proteomics of GTP-binding proteins. ChemBioChem 8, 595–598 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Fuwa H., Takahashi Y., Konno Y., Watanabe N., Miyashita H., Sasaki M., Natsugari H., Kan T., Fukuyama T., Tomita T., Iwatsubo T., Divergent synthesis of multifunctional molecular probes to elucidate the enzyme specificity of dipeptidic γ-secretase inhibitors. ACS Chem. Biol. 2, 408–418 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Chan E. W. S., Chattopadhaya S., Panicker R. C., Huang X., Yao S. Q., Developing photoactive affinity probes for proteomic profiling: Hydroxamate-based probes for metalloproteases. J. Am. Chem. Soc. 126, 14435–14446 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Ambrosetti A., Reilly A. M., DiStasio R. A. Jr, Tkatchenko A., Long-range correlation energy calculated from coupled atomic response functions. J. Chem. Phys. 140, 18A508 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Blum V., Gehrke R., Hanke F., Havu P., Havu V., Ren X., Reuter K., Scheffler M., Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 180, 2175–2196 (2009). [Google Scholar]
- 41.Havu V., Blum V., Havu P., Scheffler M., Efficient O(N) integration for all-electron electronic structure calculation using numeric basis functions. J. Comput. Phys. 228, 8367–8379 (2009). [Google Scholar]
- 42.Carravetta M., Levitt M. H., Long-lived nuclear spin states in high-field solution NMR. J. Am. Chem. Soc. 126, 6228–6229 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Carravetta M., Johannessen O. G., Levitt M. H., Beyond the T1 limit: Singlet nuclear spin states in low magnetic fields. Phys. Rev. Lett. 92, 153003 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Warren W. S., Jenista E., Branca R. T., Chen X., Increasing hyperpolarized spin lifetimes through true singlet eigenstates. Science 323, 1711–1714 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pileio G., Carravetta M., Hughes E., Levitt M. H., The long-lived nuclear singlet state of 15N-nitrous oxide in solution. J. Am. Chem. Soc. 130, 12582–12583 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Levitt M. H., Singlet nuclear magnetic resonance. Annu. Rev. Phys. Chem. 63, 89–105 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Zhivonitko V. V., Kovtunov K. V., Chapovsky P. L., Koptyug I. V., Nuclear spin isomers of ethylene: Enrichment by chemical synthesis and application for NMR signal enhancement. Angew. Chem. Int. Ed. Engl. 52, 13251–13255 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Franzoni M. B., Buljubasich L., CSpiess H. W., Munnemann K., Long-lived 1H singlet spin states originating from para-hydrogen in Cs-symmetric molecules stored for minutes in high magnetic fields. J. Am. Chem. Soc. 134, 10393–10396 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Soon P. C., Jerschow A., Canary J. W., Long-lived 1H nuclear spin singlet in dimethyl maleate revealed by addition of thiols. Angew. Chem. Int. Ed. Engl. 53, 3396–3399 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Stevanato G. Hill-Cousins J. T., Håkansson P., Roy S. S., Brown L. J., Brown R. C., Pileio G., Levitt M. H., A nuclear singlet lifetime of more than one hour in room-temperature solution. Angew. Chem. Int. Ed. 54, 3740–3743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canet D., Bouguet-Bonnet S., Aroulanda C., Reineri F., About long-lived nuclear spin states involved in para-hydrogenated molecules. J. Am. Chem. Soc. 129, 1445–1449 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Jonischkeit T. Bommerich U., Stadler J., Woelk K., Niessen H. G., Bargon J., Generating long-lasting 1H and 13C hyperpolarization in small molecules with parahydrogen-induced polarization. J. Chem. Phys. 124, 201109 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Theis T., Ledbetter M. P., Kervern G., Blanchard J. W., Ganssle P. J., Butler M. C., Shin H. D., Budker D., Pines A., Zero-field NMR enhanced by parahydrogen in reversible exchange. J. Am. Chem. Soc. 134, 3987–3990 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Theis T., Ganssle P., Kervern G., Knappe S., Kitching J., Ledbetter M. P., Budker D., Pines A., Parahydrogen-enhanced zero-field nuclear magnetic resonance. Nat. Phys. 7, 571–575 (2011). [Google Scholar]
- 55.M. W. Rathke, A. A. Millard, Boranes in functionalization of olefins to amines: 3-Pinanamine [bicyclo[3.1.1]heptan-3-amine, 2,6,6-trimethyl-]. Org. Synth. 58, 32 (1978). [Google Scholar]
- 56.Rodriguez A. D., Nickon A., A convenient synthesis of vinyl sulfides. Tetrahedron 41, 4443–4448 (1985). [Google Scholar]
- 57.Davey A. E., Parsons A. F., Taylor R. J. K., Reactions of dithiane derived alcohols: Promotion of the Thorpe-Ingold effect by trimethylsilyl and t-butyl substituents. J. Chem. Soc., Perkin Trans. 1, 1853–1858 (1989). [Google Scholar]
- 58.Church R. F. R., Weiss M. J., Diazirines. II. Synthesis and properties of small functionalized diazirine molecules. Observations on the reaction of a diaziridine with the iodine-iodide ion system. J. Org. Chem. 35, 2465–2471 (1970). [Google Scholar]
- 59.Franz S. E., Watkins R. R., Wright L. A., Weaver B. A., Hartage R. C., Ghiviriga I., Gumina G., Feske B. D., Synthetic strategy toward γ-keto nitriles and their biocatalytic conversion to asymmetric γ-lactones. Synthesis 45, 2171–2178 (2013). [Google Scholar]
- 60.Burel F., Couvercelle J.-P., Bunel C., Saiter J.-M., Poly-α-n-alkyl acrylic derivatives: Synthesis, influence of molecuur weight and n-alkyl group length on physical and physicochemical properties. J. Macromol. Sci. Part A 32, 1091–1102 (1995). [Google Scholar]
- 61.Hillier A. C., Lee H. M., Stevens E. D., Nolan S. P., Cationic iridium complexes bearing imidazol-2-ylidene ligands as transfer hydrogenation catalysts. Organometallics 20, 4246–4252 (2001). [Google Scholar]
- 62.Tayler M. C. D., Levitt M. H., Singlet nuclear magnetic resonance of nearly-equivalent spins. Phys. Chem. Chem. Phys. 13, 5556–5560 (2011). [DOI] [PubMed] [Google Scholar]
- 63.DeVience S. J., Walsworth R. L., Rosen M. S., Preparation of nuclear spin singlet states using spin-lock induced crossing. Phys. Rev. Lett. 111, 173002 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Theis T., Feng Y., Wu T., Warren W. S., Composite and shaped pulses for efficient and robust pumping of disconnected eigenstates in magnetic resonance. J. Chem. Phys. 140, 014201 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Chutia S., Rossi M., Blum V., Water adsorption at two unsolvated peptides with a protonated lysine residue: From self-solvation to solvation. J. Phys. Chem. B 116, 14788–14804 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Rossi M., Chutia S., Scheffler M., Blum V., Validation challenge of density-functional theory for peptides—Example of Ac-Phe-Ala5-LysH+. J. Phys. Chem. A 118, 7349–7359 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Schubert F., Rossi M., Baldauf C., Pagel K., Warnke S., von Helden G., Filsinger F., Kupser P., Meijer G., Salwiczek M., Koksch B., Scheffler M., Blum V., Exploring the conformational preferences of 20-residue peptides in isolation: Ac-Ala19-Lys + H+ vs. Ac-Lys-Ala19 + H+ and the current reach of DFT. Phys. Chem. Chem. Phys. 17, 7373–7385 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Tkatchenko A., Scheffler M., Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Antonio Jimenez J., Claramunt R. M., Mó O., Yáñez M., Wehrmann F., Buntkowsky G., Limbach H.-H., Goddard R., Elguero J., The structure of N-aminopyrazole in the solid state and in solution: An experimental and computational study. Phys. Chem. Chem. Phys. 1, 5113–5120 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501438/DC1
Synthesis of 15N2-diazirine and iridium precatalyst
Theoretical derivation of resonance conditions for hyperpolarization transfer
Spin dynamics during low- to high-field sample transfer
MD simulations of possible transitions states for hyperpolarization transfer
Sample preparation and setup to obtain well-controlled milligauss fields
Hyperpolarization buildup dynamics, lifetimes, and enhancements in detail
Appendix
Fig. S1. Synthesis of 2-cyano-3-(3′-(methyl-d3)-3′H-diazirine-3′-yl-1′,2′-15N2)propanoic acid using modified literature procedures.
Fig. S2. Spin system for polarization transfer.
Fig. S3. Eigenstates of a J-coupled two-spin system as a function of magnetic field.
Fig. S4. Simulated spectra for magnetization and singlet order.
Fig. S5. Three candidate conformations of 2-cyano-3-(D3-methyl-15N2-diazirine)-propanoic acid as identified by DFT PBE + TS calculations, as used to construct likely catalytically active structures producing hyperpolarization attached to the SABRE catalyst.
Fig. S6. First-principles derived candidate conformations of catalytic species that drive hyperpolarization transfer.
Fig. S7. Experimental SABRE-SHEATH setup.
Fig. S8. Decay time constants, buildup constants, and enhancements as a function of magnetic field and concentrations.
Fig. S9. The effect of continued singlet-polarization buildup after stopping p-H2 bubbling.
Table S1. Relative energies of the diazirine attachment modes tested in this work for the case of two different ligands that are simultaneously present: two equatorial hydrides, axial IMes, axial pyridine, one equatorial pyridine, and diazirine in an equatorial position.
Table S2. Relative energies of the diazirine attachment modes tested in this work for the case of two diazirine molecules that are simultaneously present.
Table S3. Magnetization lifetimes, T1, under varying catalyst concentrations and holding fields.
Table S4. Singlet lifetimes, TS, under varying catalyst concentrations and holding fields.
Table S5. Magnetization buildup times, Tb1, at 6 mG under varying catalyst concentrations.
Table S6. Singlet buildup times, TbS, under varying catalyst concentrations and magnetic fields.
Table S7. Enhancements, ε, under varying concentrations of catalyst [IrCl(COD)(IMes)] (1a), [Ir(COD)(IMes)(Py)][PF6] (1b), diazirine substrate (2), pyridine (3), and D2O (4).


