Real-time forest monitoring technologies could help track changes in tiger populations.
Keywords: forest loss, habitat monitoring, spatial analysis, tigers, tiger conservation landscapes, Remote Sensing, GIS, global forest watch, global forest change, FORMA alerts.
Abstract
The global population of wild tigers remains dangerously low at fewer than 3500 individuals. Habitat loss, along with poaching, can undermine the international target recovery of doubling the number of wild tigers by 2022. Using a new satellite-based monitoring system, we analyzed 14 years of forest loss data within the 76 landscapes (ranging from 278 to 269,983 km2) that have been prioritized for conservation of wild tigers. Our analysis provides an update of the status of tiger habitat and describes new applications of technology to detect precisely where forest loss is occurring in order to curb future habitat loss. Across the 76 landscapes, forest loss was far less than anticipated (79,597 ± 22,629 km2, 7.7% of remaining habitat) over the 14-year study period (2001–2014). Habitat loss was unevenly distributed within a subset of 29 landscapes deemed most critical for doubling wild tiger populations: 19 showed little change (1.5%), whereas 10 accounted for more than 98% (57,392 ± 16,316 km2) of habitat loss. Habitat loss in source population sites within 76 landscapes ranged from no loss to 435 ± 124 km2 (, SD = 89, total = 1676 ± 476 km2). Doubling the tiger population by 2022 requires moving beyond tracking annual changes in habitat. We highlight near–real-time forest monitoring technologies that provide alerts of forest loss at relevant spatial and temporal scales to prevent further erosion.
INTRODUCTION
Vast logging concessions, agricultural expansion, and infrastructure development are converting and fragmenting critical habitat for forest-dependent, area-sensitive vertebrates across much of the tropics (1). Some species, such as large carnivores and herbivores, have suffered more than others (2, 3). Over the past century, the tiger (Panthera tigris)—Asia’s largest apex predator—has been extirpated from >90% of its original range, and the remaining populations are under severe threat from habitat conversion and poaching (4). A review in 2005 identified 76 Tiger Conservation Landscapes (Fig. 1), representing <7% of the historic tiger range (5). By 2007, vastly reduced estimates of wild populations in India and other key range states raised concern in the conservation community that tigers were plummeting toward extinction in the wild. In response to the growing crisis, government officials, including four heads of state from the 13 tiger range states, convened in St. Petersburg, Russia, in 2010 to agree on a global recovery goal. The high-level meeting resulted in a commitment to double the wild tiger population by 2022 (6), adopted as the “Tx2” goal.
Fig. 1. Distribution map of 76 Tiger Conservation Landscapes (TCLs).
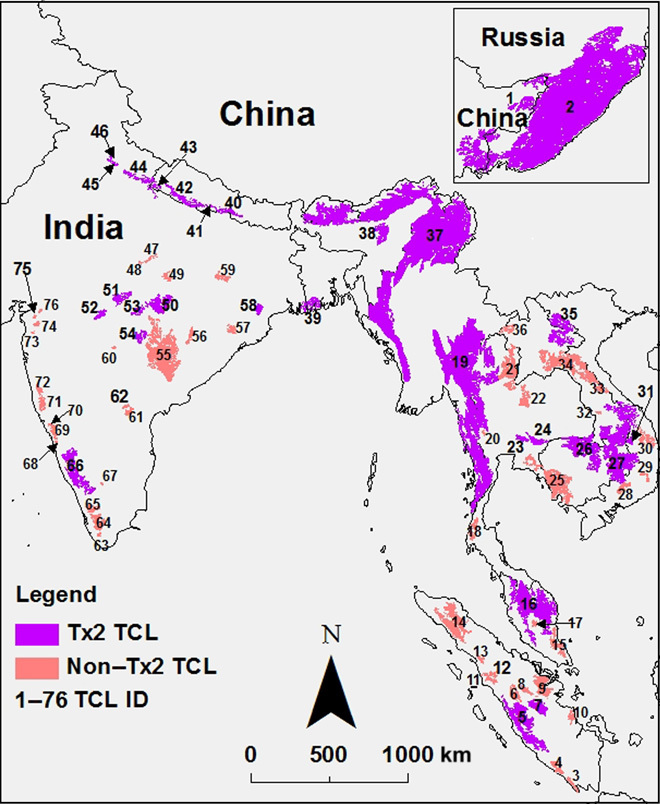
Tx2 TCLs (n = 29) are landscapes that have the potential to double the wild tiger population by 2022. One Tx2 TCL in the transboundary region of Russia and China is shown in the inset (top right corner). Each TCL has a unique ID, from 1 to 76 (table S1).
Tigers proliferate rapidly where prey and sheltered habitat are abundant, as demonstrated by tiger recovery in Panna National Park, India (7). Although generalist by nature, most tiger populations survive today in forested ecosystems but do poorly in heavily disturbed regions, restricting core breeding populations to protected areas across much of the range. However, the protected areas [International Union for Conservation of Nature (IUCN) categories I to IV] within the 76 Tiger Conservation Landscapes are too small to sustain viable populations because of the species’ solitary nature and its need for large territorial spaces generally exceeding 30 km2 in forested habitats (5). Thus, the St. Petersburg Declaration calls for halting habitat loss and maintaining connectivity among existing reserves to manage tigers as metapopulations to increase population viability and persistence (6).
A range-wide assessment determined that sufficient interconnected habitat remains to attain the population recovery goal of doubling the wild population (Tx2) within a suite of 29 priority Tiger Conservation Landscapes (8). Since the St. Petersburg meeting, population recovery in some landscapes is well underway; Nepal and India have reported an increase in resident tiger populations by 61% (9) and 31%, respectively (10), although the magnitude of this latter increase remains in dispute (11). However, achieving the Tx2 goal requires maintaining existing habitat, including ecological connectivity among source populations in the landscapes. Maintaining connectivity will require protecting existing forest corridors from degradation and loss (12). To this end, we applied two new tools, Google Earth Engine and Global Forest Watch, to analyze the Global Forest Change data set (13) and present the most recent spatially explicit results on the extent and precise location of habitat loss and fragmentation within landscapes that are home to tigers (5) and to estimate the progress made toward contributing to the Tx2 goal.
We also highlight habitat loss and gain within important tiger source sites (14) and corridors by applying recently developed tools that make remote sensing data more accessible and affordable. We demonstrate the scalability and preciseness of these tools by applying them to nine officially demarcated transboundary corridors of the Terai Arc Landscape in Nepal that support important tiger metapopulations along the base of the Himalayan mountains while zooming in on two of them for a closer analysis (Fig. 2, A to D, and figs. S1 and S2).
Fig. 2. Basanta and Khata corridors in the Terai Arc Landscape (TAL), Nepal.
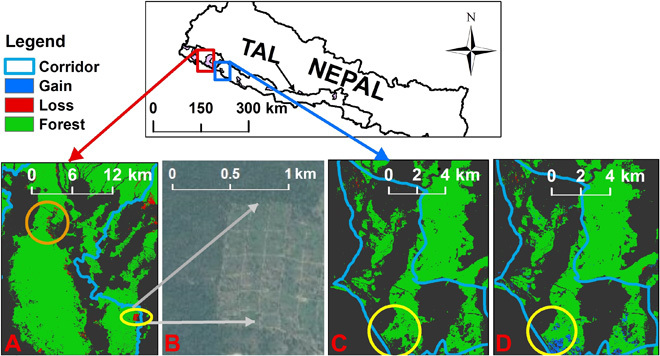
(A) Basanta corridor showing forest loss (red) between 2001 and 2014. The orange circle shows forest loss in the northern bottleneck that threatens to sever connectivity. (B) Encroached and cleared forest in the yellow circle (A) is enlarged to show details with the Digital Globe base map. (C and D) A nonforest (black) area within a yellow circle in 2000 (C) in the Khata corridor shows forest gain (blue) between 2001 and 2014 (D). Together, these four images show how spatially explicit information about when and where in the landscape forest loss is occurring enables government officials and community forest managers to take appropriate actions. The corridors are 40 km apart.
Satellite-based alert systems continue to advance in spatial and temporal resolution and may underpin future habitat conservation efforts for tigers and other wide-ranging species that are experiencing range collapse (2–4). Currently, the Global Forest Watch platform (www.globalforestwatch.org) includes a near–real-time tree cover loss alert system called FORMA (Forest Monitoring for Action). Now, park managers, biologists, and others with an interest in monitoring forest loss in a region of interest can subscribe to free alerts for that respective area. The tools include a near–real-time alert system that detects the precise location of forest loss at 500-m resolution on a 16-day cycle. We illustrate the application of this system in one of the most affected tiger landscapes, Kerinci Seblat, Sumatra.
RESULTS
Range-wide patterns of forest loss across landscapes
We detected 79,597 ± 22,629 km2 of forest loss across all 76 Tiger Conservation Landscapes (Table 1). More than 58,000 km2 of total forest loss occurred in 29 priority Tiger Conservation Landscapes (that is, required for achieving the Tx2 goal). Among the 29 priority landscapes, 98% (57,392 ± 16,316 km2) of forest loss areas were concentrated in just 10 landscapes (Fig. 3 and table S1). Three of these landscapes, Taman Negara–Belum (Fig. 1, #16) in Peninsular Malaysia and Kerinci Seblat (Fig. 1, #5) and Bukit Tigapuluh (Fig. 1, #7) in Sumatra, experienced the greatest reduction in forest cover as a percentage of the 2000 baseline, with 22, 17, and 67%, respectively (fig. S3). In Indonesia and Malaysia, 17,728 km2 of oil palm concessions (15) overlap with 14 Tiger Conservation Landscapes (table S2), and all three of the above landscapes were highly affected by the expansion of agriculture plantations.
Table 1. Forest loss (±95 % confidence interval) across TCLs and associated protected areas (PAs) between 2001 and 2014 (in square kilometers).
Tx2 TCLs are Tiger Conservation Landscapes with the potential for doubling wild tigers by 2022.
| All TCLs | Tx2 TCLs | Non-Tx2 TCLs | ||||
| TCLs (n = 76) | PAs (n = 434) | TCLs (n = 29) | PAs (n = 316) | TCLs (n = 47) | PAs (n = 118) | |
|
Forest loss (2001–2014) |
79,597 ± 22,629 (7.7%) |
22,063 ± 6273 (5.7%) |
58,245 ± 16,559 (6.9%) |
13,302 ± 3782 (4.9%) |
21,352 ± 6070 (10.8%) |
8761 ± 2491 (7.5%) |
|
Forest 2000 (baseline) |
1,040,023 | 386,770 | 842,237 | 269,584 | 197,785 | 117,186 |
Fig. 3. Cumulative forest loss (in square kilometers) in 10 Tx2 TCLs experiencing the highest forest loss between 2001 and 2014.
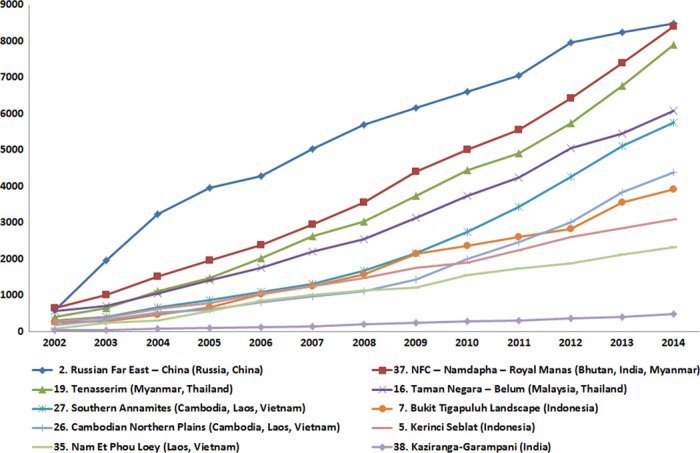
Tx2 TCLs (n = 29) are landscapes that have the potential to double the wild tiger population by 2022.
The three largest landscapes, Russian Far East–China (Fig. 1, #2), Northern Forest Complex–Namdapha–Royal Manas (Fig. 1, #37), and the Tenasserims (Fig. 1, #19), lost a combined 24,798 ± 7050 km2 of forest habitat, accounting for <4% of the total landscape areas. All three are transboundary landscapes (table S1 and fig. S3).
Tracking habitat change at finer spatial scales
At a finer scale, we analyzed forest gain and loss in the Terai Arc Landscape, an important tiger habitat, in the foothills of the Himalayas, encompassing three priority Tiger Conservation Landscapes and nine corridors that link core tiger reserves (fig. S1). We detected >3 km2 of forest loss in two corridors, >2 km2 in three corridors, and >1 km2 in two corridors (figs. S1 and S2 and table S3). Forest loss in the nine corridors connecting core areas within the Terai Arc Landscape averaged 2.4 ± 0.7 km2 (SD = 1.94).
We identified important forest loss within the Basanta corridor in the Terai Arc Landscape between 2001 and 2014 (Fig. 2, A to D). The 652-km2 Basanta corridor lost 0.7% of its forest as a result of encroachment and land clearing, but, critically, it also experienced forest loss in a bottleneck area in the northern section that threatens to sever connectivity with the northern forests (Fig. 2A and fig. S2C). In contrast, our analysis identified 2.2 ± 0.40 km2 (2.7%) of forest gain in the 81.0-km2 Khata corridor, also found within the Terai Arc Landscape (Fig. 2D and fig. S2E).
The protected areas designated as source populations of tigers (14) lost 1676 ± 476 km2 of forests between 2001 and 2014 (Table 2). Ninety-one percent of all forest loss occurred within 10 protected areas. Five source populations also overlapped with oil palm concessions in Indonesia and Malaysia. Spatially explicit maps of forest loss in these source population sites and the Tiger Conservation Landscapes in which they are embedded show the heterogeneous nature of habitat erosion (Table 2 and fig. S4).
Table 2. Forest loss (± 95% confidence interval) in core tiger reserves from 2001 to 2014.
| Core tiger reserve | Tiger Conservation Landscape | Forest loss (km2) |
| Kerinci Seblat* | 5. Kerinci Seblat† | 435 ± 124 |
| Leuser National Park* | 14. Gunung Leuser | 410 ± 117 |
| Botchinsky | 2. Russian Far East–China† | 197 ± 56 |
| Bukit Barisan Selatan | 4. Bukit Balai Rejang–Selatan | 149 ± 42 |
| Endau Rompin (Johor)* | 15. Endau Rompin | 86 ± 24 |
| Belum* | 16. Taman Negara–Belum† | 66 ± 19 |
| Nam Et | 35. Nam Et Phou Louey† | 64 ± 18 |
| Bukit Barisan | 13. Sibologa | 60 ± 17 |
| Phou Louey | 35. Nam Et Phou Louey† | 39 ± 11 |
| Bukit Tigapuluh | 7. Bukit Tigapuluh Landscape† | 30 ± 8 |
| Taman Negara National Park* | 16. Taman Negara–Belum† | 26 ± 7 |
| Kaeng Krachan | 19. Tenasserims† | 20 ± 6 |
| Sikhote-Alinsky | 2. Russian Far East–China† | 16 ± 5 |
| Thung Yai Naresuan | 19. Tenasserims† | 15 ± 4 |
| Anamalai | 65. Anamalai-Parambikulam | 9 ± 2 |
| Huai Kha Khaeng | 19. Tenasserims† | 8 ± 2 |
| Periyar | 64. Shendurney | 5 ± 1 |
| Chitwan National Park | 40. Chitwan† | 5 ± 1 |
| Bardia National Park | 42. Bardia† | 4 ± 1 |
| Kuiburi | 19. Tenasserims† | 4 ± 1 |
| Bhadra | 66. Western Ghats: Bandipur–Khudrenukh–Bhadra† | 4 ± 1 |
| Mundanthurai | 63. Shendurney | 3 ± 1 |
| Shendurney | 66. Shendurney | 3 ± 1 |
| Kaziranga | 38. Kaziranga–Garampani† | 3 ± 1 |
| Kalakad | 64. Periyar–Megamala | 2 ± 1 |
| Lazovskiy | 2. Russian Far East–China† | 2 ± 1 |
| Corbett | 44. Corbett–Sonanadi† | 2 ± 1 |
| Nagarahole | 66. Western Ghats: Bandipur–Khudrenukh–Bhadra† | 2 ± 1 |
| Kanha | 50. Kanha–Phen† | 1 ± 1 |
| Parsa | 40. Chitwan† | — |
| Suklaphanta | 43. Suklaphanta† | — |
| Ussuriysky | 2. Russian Far East–China† | — |
| Katarniaghat | 42. Bardia† | — |
| Sundarbans | 39. Sundarbans† | — |
| Parambikulam | 65. Anamalai-Parambikulam | — |
| Pench | 53. Pench† | — |
| Rajaji | 45. Rajaji Minor† | — |
| Bukit Balai Rejang* | 4. Bukit Balai Rejang–Selatan | — |
| Melghat | 52. Melghat† | — |
| Mudumalai | 66. Western Ghats: Bandipur–Khudrenukh–Bhadra† | — |
| Bandipur | 66. Western Ghats: Bandipur–Khudrenukh–Bhadra† | — |
| Andhari | 54. Andhari-Tadoba† | — |
| Phen | 50. Kanha-Phen† | — |
| Bandhavgarh | 49. Bandhavgarh-Panpatha | — |
| Kishanpur WS | 44. Corbett-Sonanadi† | — |
| Kedrovaya Pad | 2. Russian Far East–China† | — |
| Ranthambhore | — | |
| Tadoba | 54. Andhari-Tadoba† | — |
| Dudhwa | 43. Suklaphanta† | — |
| Biligiri Ranga Temple | 67. Biligiri Range | — |
| Simlipal | 58. Simlipal† | — |
| Total | 1676 ± 476 |
*Overlaps with industrial plantations.
†Priority TCLs.
Assessing the impact of range-wide forest loss on tiger numbers
The protected areas in the 76 Tiger Conservation Landscapes lost a combined 22,063 ± 6273 km2 (5.7%) of forest cover range-wide, and those within the 29 priority landscapes lost 13,302 ± 3782 km2 (Table 1) of forest cover. We estimate that forest cover loss led to a decrease of about 400 tigers (table S1) in the priority landscapes, over the time of the study (2001–2014), on the basis of the potential capacity of the respective habitat types represented in the landscapes to support tigers (8). Our assumptions are as follows: (i) fractionated patches are large enough to support a single adult female tiger and (ii) the prey base in the patch was adequate to support a single female.
Near–real-time tracking of changes in tiger habitat
We mapped the FORMA alerts since 2006 for the Kerinci Seblat Tiger Conservation Landscape in Sumatra, an area of rapid forest cover change due to expansion of industrial agriculture. Between 2006 and 2007, 7848 alerts were issued for this landscape, 67% of which fall in forest loss areas in the Global Forest Watch 2014 map (Fig. 4). There were 1152 alerts issued for this landscape between January and August 2015.
Fig. 4. FORMA alerts from Global Forest Watch are issued for an area when large-scale tree cover is lost.
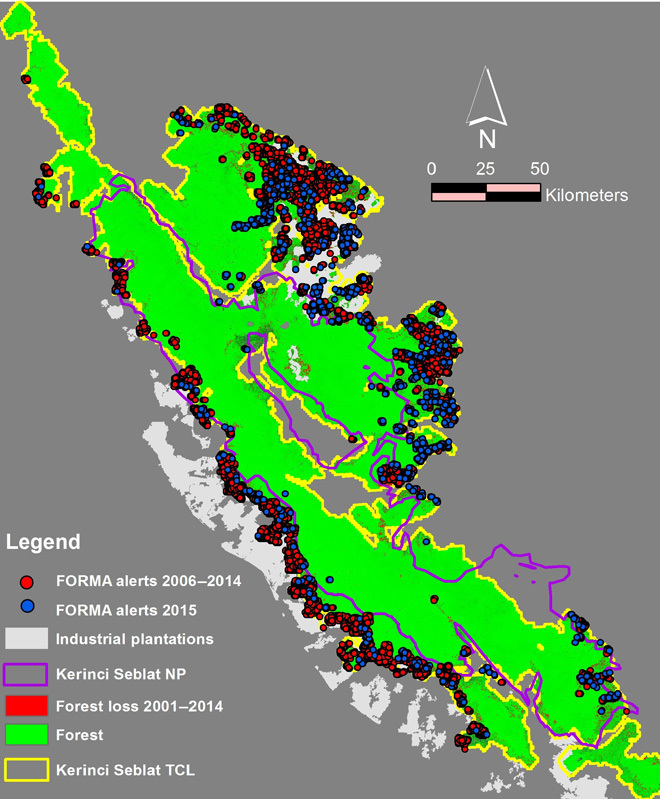
Alerts for the Kerinci Seblat TCL, Sumatra, from 2006–2014 and 2015 (January to August) were plotted over forest cover change showing that alerts from 2006–2014 fall within areas of forest loss.
DISCUSSION
Evaluating changes in tiger habitat from 2001 to 2014
The decline in tiger habitat over a 14-year period is open to several interpretations. From our perspective, it is remarkable and unexpected that only 7.7% of the range was lost to conversion over the study period. Before undertaking the analysis, we predicted habitat loss to be much higher, considering that (i) the 13 tiger range states represent some of the fastest-growing economies in the world and (ii) many of the South Asian habitats that dominate the 29 highest-priority Tiger Conservation Landscapes are surrounded by human-dominated areas supporting the highest rural population densities on Earth (5). As predicted, extensive loss among tiger landscapes occurred in areas of oil palm expansion.
Most encouraging was that loss was less than expected in the 51 tiger reserves that serve as source sites in the priority landscapes. This suggests that if future habitat loss is prevented, the tiger recovery now underway in some range states will accelerate. In these promising locales of enhanced protection, a doubling of the tiger population could be attainable by 2022.
Another interpretation is that even though extensive habitat loss was limited to 10 Tiger Conservation Landscapes, the impact in those affected areas has been devastating. The best example is the Cambodian Northern Plains landscape, which contains five large reserves of tropical dry forest characteristic of this region, which lost enough habitat to support almost 174 tigers (table S1 and fig. S4A). Likewise, habitat loss in the Southern Annamites (fig. S4B) and Bukit Tigapuluh (fig. S4C) may result in shrinking habitat for >50 tigers in each landscape.
Whichever interpretation prevails, the conservation community must remain vigilant. Among key tiger range states, as much as $750 billion annually is expected to be invested in infrastructure projects over the next decade (16). Even if only a fraction of this investment finances new road construction within tiger landscapes, the effects of new road networks can be extensive (17). One new roadway would bisect the Tenasserims landscape to permit a superhighway between Myanmar and Thailand, and another would bisect the Terai Arc Landscape with a railroad and major highway system from India. Large roads are mortality magnets for tigers (18, 19).
Tigers will often occupy forests within a conservation landscape that lie outside the boundaries of protected areas (IUCN I to VI). For instance, in the Russian Far East, most of the tigers live outside formally protected areas (20). Across the tiger’s range, governments should design smart green infrastructure (21) to reduce habitat loss in corridors and other critical areas for tiger conservation. However, some habitat degradation during the construction phase is inevitable, in which case conservation managers can monitor the extent of damage using the alert system we present below.
Tracking habitat change at finer spatial scales
Zooming into affected landscapes, we found that the Khata corridor of the Terai Arc Landscape experienced forest gain between 2001 and 2014. These results correlate with a community-managed forestry program to restore forests for tiger dispersal in this region (22). Community stewardship of the corridor has also extended to the creation of community-based antipoaching teams that patrol the forests to prevent wildlife and habitat poaching (22). Recent camera trap surveys have confirmed that tigers now use this corridor that connects Nepal’s Bardia National Park with India’s Katarniaghat Tiger Reserve (9). Tiger surveys in 2009 and 2013 estimate that adult tiger numbers in Bardia have increased from 18 to 50 animals (9). Presumably, dispersal from other areas would have contributed to such a rapid increase, especially because tigers have been confirmed in the corridor.
In contrast, recent surveys indicate that tigers are now absent from the Basanta corridor, a region in which they were frequently sighted in previous surveys (9, 22). Human encroachment into Basanta has been severe in recent years. People in search of land have begun to clear forests and establish communities, as detected through our analysis by regional experts. Consequently, the tiger dispersal bottleneck in the northern part of the corridor has been narrowed (Fig. 2A). Overall, with the exception of two corridors, there has been little forest loss in other transboundary corridors and in the protected areas of the Terai Arc Landscape. Occupancy surveys in this landscape conducted between 2009 and 2014 indicate an increase in tiger populations in four of the five embedded protected areas, with an overall increase of 61% in tiger numbers (9). This increase can be attributed, in part, to dispersal through the corridors connecting Indian and Nepalese protected areas where tigers have been recorded (23).
As more tiger landscapes are surveyed, spatial statistics on habitat loss and gain can be tested against population densities to assess the complex relationship between tigers and landscape change. We assume that tiger populations should increase where there has been little or no habitat loss over the period of the study. In contrast, we assume that where we measure significant habitat loss, tigers are negatively affected by disturbance or the resulting increase in poaching of tigers and prey. Models that predict population change in response to habitat loss are important because camera-trapping and occupancy surveys at landscape scales are expensive and labor-intensive. Habitat monitoring could become a predictive tool or proxy that can be applied between less frequent ground surveys. Infrequent ground surveys are still vital in detecting other variables that indicate tiger presence, such as availability of prey and disturbances beneath the forest canopy that cannot be detected by satellite imagery.
A “big data” approach to monitoring loss of tiger habitat
The rapid advancement of technology has removed constraints of storage capacity and processing speeds needed to automate analysis over big data sets. New tools, such as Google Earth Engine and Global Forest Watch, make complex analyses of global data accessible through a free, user-friendly interface. Their combined technology makes annual updates of forest cover change at high resolution (30 m) available to scientists and range-state wildlife officials. Before the release of Global Forest Watch in February 2014, monitoring tiger habitat across the entire range was restricted to decadal intervals, because of limited accessibility of spatial data, vast demands of computing power, and a lack of technical knowledge of remote sensing. Such longitudinal studies were often limited to a single landscape. Now, changes in tiger habitat can be calculated in a single corridor, protected areas, or any of the 76 landscapes by conservationists without technical training in remote sensing.
Remote sensing technologies are also important for strengthening environmental policy. Government officials from the tiger range states are committed to doubling the wild tiger population by 2022, a target promoted by the Global Tiger Initiative. This initiative, which was initially hosted by the World Bank and is now under the World Resources Institute, was formed in 2008, at the request of the range states, to accelerate international efforts to conserve the tiger. Range-state officials reconvened in Bhutan in 2012 to assess progress, where they advocated for improved tiger habitat monitoring. They identified maintaining habitat integrity as a Key Performance Indicator toward the Tx2 goal (24). A ministerial-level conference hosted by the government of India in April 2016 will adopt the monitoring protocols presented here as the official monitoring tool as the Global Recovery Program moves forward.
Enabling timely action to prevent further loss of tiger habitat
This study was made possible by free availability of satellite imagery, cloud computing services, and interactive web tools. We were able to analyze 14 years of high-resolution global forest loss data across 76 landscapes that span 13 countries. Although our results are critical for pinpointing areas for restoration and providing range-state officials with a global update of the state of tiger habitat, more frequent forest loss data are needed to enable preventive action. Fortunately, the technology used for this study continues to advance at a record pace. New data sets that are highly relevant to the conservation community are already accessible.
Monthly forest loss alerts are available through FORMA, Global Forest Watch. Although alerts generated from MODIS (moderate resolution imaging spectroradiometer) imagery are significantly coarser in resolution (500 m) than actual forest loss data (30 m) used for our analysis, these alerts could guide park managers to areas of concern, as we have shown in the case of the Kerinci Seblat Tiger Conservation Landscape. Monitoring these FORMA alerts would have enabled conservation managers in the field to investigate the sources of forest loss in a timely fashion.
This is just the beginning; researchers and engineers are working on systems that provide alerts at finer spatial scales that start to approach real time. Starting this year, Global Forest Watch will provide a tropical forest loss alert (with a spatial resolution of 30 m) that will be generated weekly, with plans of expanding the alert system to the global scale.
The potential for conservationists to harness alert technology is unprecedented. Park managers that control patrols now have continually improving tools at their disposal to identify the specific location of forest loss within large reserves soon after a disturbance occurs. This allows for a timely response to the first signs of forest incursions. In the context of tigers, these systems can be used to monitor critical areas including Tx2 Tiger Conservation Landscapes, protected areas within conservation landscapes, buffer zones, and corridors. Park managers in range states can simply subscribe to monthly forest loss alerts in these areas and take action as near–real-time data enter their inbox.
Habitat alert technology applies to other forest-dependent, wide-ranging taxa as well. These include some of the world’s most iconic species, such as primates, African forest elephants, and large frugivorous birds and bats. The loss of habitats occupied by these area-sensitive, forest-dependent species is well underway across the tropics and around the world (25, 26). New alert technology provides part of a solution to conserve the forested landscapes these species need to survive.
The alert system can be used to assess the status of a forest during and after a disturbance event. It can also monitor the state of logging or plantation concessions to assess how their footprint affects corridors or intrudes into protected areas. For example, in Indonesia, >4000 km2 of unbroken expanses of forests in Tiger Conservation Landscapes have been allocated for oil palm concessions. Conversion of these forests can result in significant fragmentation of forest corridors and loss of habitat in protected areas.
Conclusion
Our analysis indicates that enough wild habitat remains to allow a range-wide doubling of the wild tiger population, as indicated by Wikramanayake et al. (8). The global population could approach a trebling in the next two decades if (i) essential corridors are restored in the most deforested landscapes, (ii) source sites and priority landscapes suffer no further erosion, (iii) range-state leaders implement smart green infrastructure, and (iv) essential translocation and reintroduction programs are implemented by tiger range states now in a recovery phase. On this last point, wildlife officials can streamline such efforts by taking advantage of the recent simplification of tiger taxonomy from nine putative subspecies to two, thus removing the constraints of mixing subspecies whose designation no longer seems valid (27). Together, these new guidelines for reintroductions and near–real-time habitat monitoring to stave off continuing threats can help range states to achieve the Tx2 goal of doubling the wild tiger population by 2022.
MATERIALS AND METHODS
We used the Global Forest Change 2001–2014 data set (version 1.2) (13), freely available at Global Forest Watch (data.globalforestwatch.org), as the basis for our analysis. This data set includes tree canopy cover for year 2000, tree cover loss for 2001–2014, tree cover gain for 2001–2012, and loss year (the year in which the loss event occurred). Tree cover loss is defined as all stand-replacement disturbances of vegetation taller than 5 m, at 30-m resolution (13). Tree cover gain is when tree cover is detected in areas with no previous tree cover. We used tree canopy cover ≥25% for year 2000 to derive baseline forest cover for our study area. One of the criticisms of Global Forest Change data is that such data do not distinguish between natural forest and industrial plantations, such as oil palm. To address this issue, we used data from an industrial plantation mapping project undertaken by the World Resources Institute and Transparent World (unpublished data) to identify industrial plantations in our study area.
The boundaries of Tiger Conservation Landscapes and protected areas were demarcated to include only forest lands; this is the basis for our assumption that tree cover loss is forest loss resulting in habitat fragmentation and erosion of connectivity for tigers. Thus, in this study, we refer to tree cover loss or gain as “forest loss” or “forest gain.”
We used the geographic boundaries of Tiger Conservation Landscapes, protected areas that fall within or adjacent to the Tiger Conservation Landscapes, and, at a finer scale, nine corridors in the Terai Arc Landscape as overlays on the Global Forest Change data. We used the cloud computing functionality of Google Earth Engine to assess forest loss and gain within each Tiger Conservation Landscape. We built JavaScripts within the Google Earth Engine playground to extract forest loss pixels for each landscape, protected area, and corridor for every year from 2001 to 2014. Because forest gain data in the Global Forest Change data are available only as one cumulative layer from 2001 to 2012, we were unable to extract annual forest gain. We used forest gain only for a finer scale analysis of forest corridors in the Terai Arc Landscape in Nepal.
Industrial plantations within the Tiger Conservation Landscapes were considered forest loss, and any forest gains in these areas were excluded as forests in the analysis. We divided Tiger Conservation Landscapes and associated protected areas into two groups: (i) Tx2 Tiger Conservation Landscapes identified as having potential for contributing to doubling the wild tiger population by 2022 (n = 29) and the protected areas (n = 316) in them and (ii) the remaining 47 non-Tx2 Tiger Conservation Landscapes and their protected areas (n = 118).
We used a sample-based approach to estimate forest loss and associated uncertainty from mapped data using good practice guidance (28). We generated 200 random sampling points, at least 2 km apart, for each “forest” and “forest loss” strata within the study area as reference samples. Of these, 11 points in the forest stratum and 3 points in the forest loss stratum landed outside the boundaries of the study area and were not used. The sampling points were overlaid on the Digital Globe base map (www.digitalglobefoundation.org) with resolution ≤2.4 m and validated visually. An error matrix was constructed and 95% confidence intervals were calculated (table S4). To correct for bias due to classification error, the mapped forest and forest loss areas were adjusted by landscape using the reference data (table S5). Our area adjustment equaled that of the pan-tropical study (29), which also found that 93% of omitted forest loss in mainland tropical Asia occurred in pixels adjacent to mapped loss. On the basis of this finding, area adjustment can be expected to retain the spatial pattern of forest loss within the global map. We adjusted error for forest gain in nine corridors of the Terai Arc Landscape using the same process with field verification done by one of the authors (A.R.J.) in a different project using the Global Forest Change data (13). We present error-adjusted estimates of forest loss with an associated 95% confidence interval for forest loss mapped in all landscapes and forest gain only for nine corridors of the Terai Arc Landscape.
FORMA alerts, a feature of the Global Forest Watch platform, track large-scale tree cover loss in near-real-time. We downloaded all FORMA alerts for the Kerinci Seblat Tiger Conservation Landscape in Sumatra, which were later overlaid on the forest change map, to demonstrate how FORMA alerts coincided with forest loss.
Supplementary Material
Acknowledgments
We thank the Global Tiger Initiative for their continuing support in developing the habitat monitoring protocol. This effort benefited from stimulating discussions with B. Pandav and K. Varma, and the enthusiastic support of A. Steer and R. B. Zoellick while the latter was president of the World Bank. We also thank U. Karanth, W. Jetz, and H. Rainier for comments that greatly improved the manuscript. Funding: We acknowledge funding from the Global Forest Watch program at the World Resources Institute (grant no. 00534). Author contributions: A.R.J., E.D., E.W., M.L.A., D.O., B.S.J., J.S., and N.C.S. conceived the study; A.R.J., E.W., and M.C.H. developed the methodology and performed data analysis; A.R.J., E.D., E.W., and S.L. wrote the manuscript; B.S.J., J.S., C.L.D., S.P., and N.R.H. improved the manuscript by providing helpful revisions to several drafts. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501675/DC1
Fig. S1. Nine forest corridors connecting core tiger reserves across four Tiger Conservation Landscapes (TCLs) in the Terai Arc Landscape, Nepal.
Fig. S2. Zoomed-in images that show forest loss and gain in nine forest corridors connecting core tiger reserves in the Terai Arc Landscape, Nepal.
Fig. S3. Forest loss (in square kilometers) and percentage of forest loss between 2001 and 2014 in 15 Tiger Conservation Landscapes with highest forest loss, including nine priority landscapes for doubling wild tiger populations.
Fig. S4. Forest loss in priority Tiger Conservation Landscapes for doubling wild tiger populations, between 2001 and 2014.
Table S1. Forest loss in Tiger Conservation Landscapes (n = 76) and associated protected areas (n = 434).
Table S2. Tiger Conservation Landscapes overlapping with industrial plantations.
Table S3. Forest loss and gain in the forest corridors of the Terai Arc Landscape between 2001 and 2014.
Table S4. Error matrices (in terms of sample counts) for forest and forest loss maps [Hansen et al. (13), version 1.2].
Table S5. Difference between mapped area and sample estimates of forest and forest loss between 2001 and 2014 for Tiger Conservation Landscapes.
REFERENCES AND NOTES
- 1.Haddad N. M., Brudvig L. A., Clobert J., Davies K. F., Gonzalez A., Holt R. D., Lovejoy T. E., Sexton J. O., Austin M. P., Collins C. D., Cook W. M., Damschen E. I., Ewers R. M., Foster B. L., Jenkins C. N., King A. J., Laurance W. F., Levey D. J., Margules C. R., Melbourne B. A., Nicholls A. O., Orrock J. L., Song D. X., Townshend J. R., Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 1, e1500052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M. P., Schmitz O. J., Smith D. W., Wallach A. D., Wirsing A. J., Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Ripple W. J., Newsome T. M., Wolf C., Dirzo R., Everatt K. T., Galetti M., Hayward M. W., Kerley G. I. H., Levi T., Lindsey P. A., Macdonald D. W., Malhi Y., Painter L. E., Sandom C. J., Terborgh J., Van Valkenburgh B., Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinerstein E., Loucks C., Wikramanayake E., Ginsberg J., Sanderson E., Seidensticker J., Forrest J., Bryja G., Heydlauff A., Klenzendorf S., Leimgruber P., Mills J., O’Brein T. G., Shrestha M., Simons R., Songer M., The fate of wild tigers. BioScience 57, 508–514 (2007). [Google Scholar]
- 5.E. Sanderson, J. Forrest, C. Loucks, J. Ginsberg, E. Dinerstein, J. Seidensticker, P. Leimgruber, M. Songer, A. Heydlauff, T. O’Brien, G. Bryja, S. Klenzendorf, E. Wikramanayake, Setting Priorities for the Conservation and Recovery of Wild Tigers: 2005–2015. The Technical Assessment (Wildlife Conservation Society, World Wildlife Fund, Smithsonian, and Save the Tiger Fund, Washington, DC, 2006). [Google Scholar]
- 6.St. Petersburg Declaration on Tiger Conservation. Retrieved on 13 May 2015 from Global Tiger Initiative http://globaltigerinitiative.org/publication/st-petersburg-declaration-on-tiger-conservation/ [accessed 4 March 2016] (2010).
- 7.K. Ramesh, J. A. Johnson, S. Sen, R. S. Murthy, M. S. Sarkar, M. Malviya, S. Bhardwaj, M. Naveen, S. Roamin, V. S. Parihar, S. Gupta, “Status of tiger and prey species in Panna Tiger Reserve, Madhya Pradesh: Capture-recapture and distance sampling estimates” (Technical Report, Wildlife Institute of India, Dehradun, and Panna Tiger Reserve, Madhya Pradesh, 2013), p. 39. [Google Scholar]
- 8.Wikramanayake E., Dinerstein E., Seidensticker J., Lumpkin S., Pandav B., Shrestha M., Mishra H., Ballou J., Johnsingh A. J. T., Chestin I., Sunarto S., Thinley P., Thapa K., Jiang G., Elagupillay S., Kafley H., Pradhan N. M. B., Jigme K., Teak S., Cutter P., Aziz Md. A., Than U., A landscape-based conservation strategy to double the wild tiger population. Conserv. Lett. 4, 219–227 (2011). [Google Scholar]
- 9.M. Dhakal, M. Karki (Thapa), S. R. Jnawali, N. Subedi, N. M. B. Pradhan, S. Malla, B. R. Lamichhane, C. P. Pokheral, G. J. Thapa, J. Oglethorpe, S. A. Subba, P. R. Bajracharya, H. Yadav, Status of Tigers and Prey in Nepal (Department of National Parks and Wildlife Conservation, Kathmandu, Nepal, 2014). [Google Scholar]
- 10.Y. V. Jhala, Q. Qureshi, R. Gopal, Eds., The Status of Tigers in India 2014 (National Tiger Conservation Authority, New Delhi and The Wildlife Institute of India, Dehradun, 2014). [Google Scholar]
- 11.Gopalaswamy A. M., Delampady M., Karanth K. U., Kumar N. S., Macdonald D. W., An examination of index-calibration experiments: Counting tigers at macroecological scales. Methods Ecol. Evol. 6, 1055–2066 (2015). [Google Scholar]
- 12.P. Chanchani, B. Noon, L. L. Bailey, R. A. Warrier. Conserving tigers in working landscapes. Conserv. Biol. 10.1111/cobi.12633 (in press). [DOI] [PubMed]
- 13.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Walston J., Robinson J. G., Bennett E. L., Breitenmoser U., da Fonseca G. A. B., Goodrich J., Guma M., Hunter L., Johnson A., Karanth K. U., Leader-Williams N., MacKinnon K., Miquelle D., Pattanavibool A., Poole C., Rabinowitz A., Smith J. L. D., Stokes E. J., Stuart S. N., Vongkhamheng C., Wibisono H., Bringing the Tiger back from the brink—The six percent solution. PLOS Biol. 8, e1000485 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.“Oil Palm.” World Resources Institute. Accessed through Global Forest Watch on 27 September 2014. www.globalforestwatch.org.
- 16.ADB and ADBI, Infrastructure for a Seamless Asia (Asian Development Bank Institute, Tokyo, 2009). [Google Scholar]
- 17.Laurance W. F., Clements G. R., Sloan S., O’Connell C. S., Mueller N. D., Goosem M., Venter O., Edwards D. P., Phalan B., Balmford A., Van Der Ree R., Arrea I. B., A global strategy for road building. Nature 513, 229–232 (2014). [DOI] [PubMed] [Google Scholar]
- 18.L. Fahrig, T. Rytwinski, Effects of roads on animal abundance: An empirical review and synthesis. Ecol. Soc. 14, 21 (2009); www.ecologyandsociety.org/vol14/iss1/art21/
- 19.Kerley L. L., Goodrich J. M., Miquelle D. G., Smirnov E. N., Quigley H. B., Hornocker M. G., Effects of roads and human disturbance on Amur tigers. Conserv. Biol. 16, 97–108 (2002). [DOI] [PubMed] [Google Scholar]
- 20.S. Seker, Logging of Russian Far East damaging tiger habitat, few intact forests protected (Part I), retrieved 13 May 2015 from www.mongabay.com (2014).
- 21.J. D. Quintero, A. Morgan, R. Roca, A. Mathur, Smart Green Infrastructure in Tiger Range Countries: A Multi-level Approach (The World Bank, Washington, DC, 2010). [Google Scholar]
- 22.E. Wikramanayake, A. Manandhar, S. Bajimaya, S. Nepal, G. Thapa, K. Thapa, The Terai Arc Landscape: A tiger conservation success story in a human-dominated landscape, in Tigers of the World: The Science, Politics, and Conservation of Panthera tigris, R. Tilson, P. Nyhus, Eds. (Elsevier/Academic Press, Oxford, 2nd ed., 2010), pp. 161–172. [Google Scholar]
- 23.P. Chanchani, B. R. Lamichhane, S. Malla, K. Maurya, A. Bista, R. Warrier, S. Nair, M. Almeida, R. Ravi, R. Sharma, M. Dhakal, S. P. Yadav, M. Thapa, S. R. Jnawali, N. M. B. Pradhan, N. Subedi, G. J. Thapa, H. Yadav, Y. V. Jhala, Q. Qureshi, J. Vattakaven and J. Borah. Tigers of the transboundary Terai Arc Landscape: Status, distribution and movement in the Terai of India and Nepal (National Tiger Conservation Authority, Government of India and Department of National Park and Wildlife Conservation, Government of Nepal, 2014). [Google Scholar]
- 24.Global Tiger Initiative Secretariat, Global Tiger Recovery Program Implementation Plan: 2013–2014 (The World Bank, Washington, DC, 2014). [Google Scholar]
- 25.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B., Defaunation in the anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Piel A. K., Cohen N., Kamenya S., Ndimuligo S. A., Pintea L., and Stewart F. A., Population status of chimpanzees in the Masito-Ugalla ecosystem, Tanzania. Am. J. Primatol. 77, 1027–1035 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Wilting A., Courtiol A., Christiansen P., Niedballa J., Scharf A. K., Orlando L., Balkenhol N., Hofer H., Kramer-Schadt S., Fickel J., Kitchener A. C., Planning tiger recovery: Understanding intraspecific variation for effective conservation. Sci. Adv. 1, e1400175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olofsson P., Foody G. M., Herold M., Stehman S. V., Woodcock C. E., Wulder M. A., Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 148, 42–57 (2014). [Google Scholar]
- 29.Tyukavina A., Baccini A., Hansen M. C., Potapov P. V., Stehman S. V., Houghton R. A., Krylow A. M., Turubanova S., Goetz S. J., Aboveground carbon loss in natural and managed tropical forests from 2000 to 2012. Environ. Res. Lett. 10, 074002 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/4/e1501675/DC1
Fig. S1. Nine forest corridors connecting core tiger reserves across four Tiger Conservation Landscapes (TCLs) in the Terai Arc Landscape, Nepal.
Fig. S2. Zoomed-in images that show forest loss and gain in nine forest corridors connecting core tiger reserves in the Terai Arc Landscape, Nepal.
Fig. S3. Forest loss (in square kilometers) and percentage of forest loss between 2001 and 2014 in 15 Tiger Conservation Landscapes with highest forest loss, including nine priority landscapes for doubling wild tiger populations.
Fig. S4. Forest loss in priority Tiger Conservation Landscapes for doubling wild tiger populations, between 2001 and 2014.
Table S1. Forest loss in Tiger Conservation Landscapes (n = 76) and associated protected areas (n = 434).
Table S2. Tiger Conservation Landscapes overlapping with industrial plantations.
Table S3. Forest loss and gain in the forest corridors of the Terai Arc Landscape between 2001 and 2014.
Table S4. Error matrices (in terms of sample counts) for forest and forest loss maps [Hansen et al. (13), version 1.2].
Table S5. Difference between mapped area and sample estimates of forest and forest loss between 2001 and 2014 for Tiger Conservation Landscapes.


