Abstract
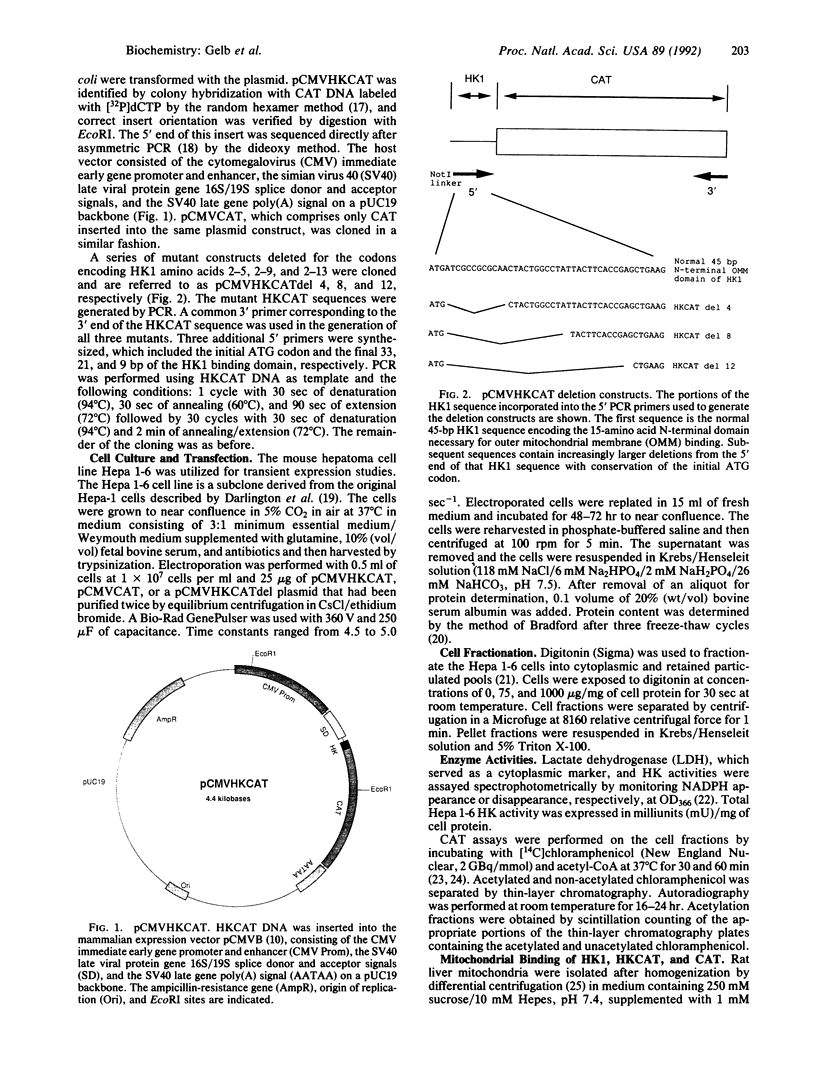
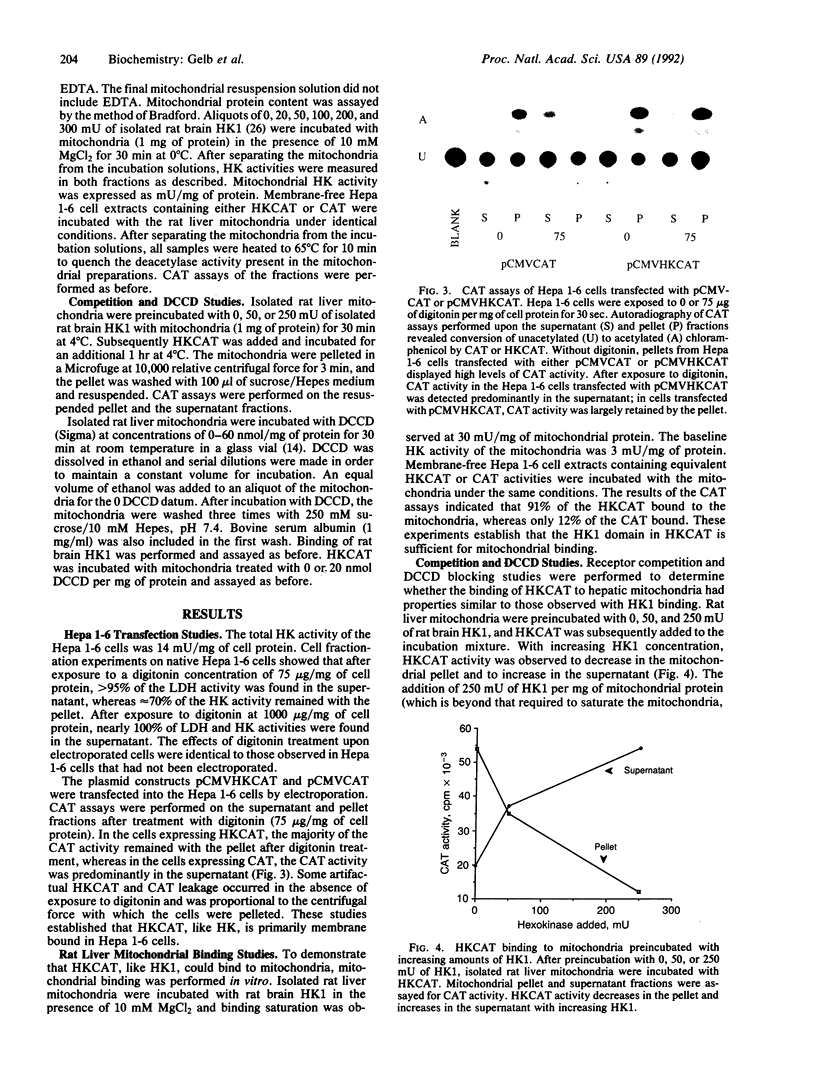
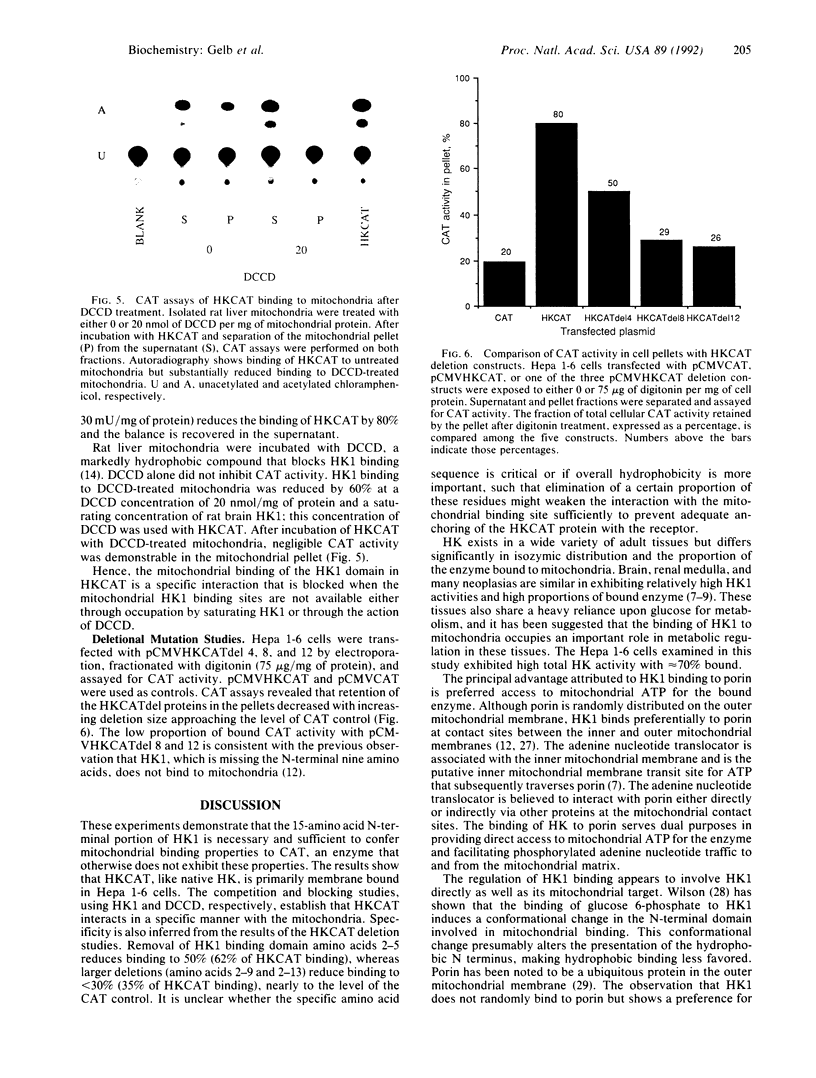
The proportion of hexokinase (HK; EC 2.7.1.1) isozyme 1 (HK1) that is bound to the outer mitochondrial membrane is tissue specific and developmentally regulated. HK activity is known to be markedly elevated in many cancer cells and a significant fraction is mitochondrial bound. This study examined the role of the 15-amino acid N-terminal domain of HK1 in binding to liver and hepatoma mitochondria. A chimeric reporter construct, pCMVHKCAT, encoding this HK1 domain coupled to the chloramphenicol acetyltransferase (CAT) gene was electroporated into mouse Hepa 1-6 hepatoma cells. After digitonin treatment, cell fractions were assayed for HK, lactate dehydrogenase, and CAT activities. Digitonin (75 micrograms/mg of protein) caused cytosolic leak but 70% of HK remained with the pellet. HKCAT, like HK, remained predominantly with the pellet; CAT form the control, pCMVCAT, remained mostly unbound. Binding of membrane-free cell extracts to rat liver mitochondria in vitro showed 91% of the HKCAT bound, whereas only 12% of CAT bound. Specificity of HKCAT binding to mitochondria was demonstrated by competition of HK1 for HKCAT binding sites on rat liver mitochondria as well as by blockage of HKCAT binding by N,N'-dicyclohexylcarbodiimide, which covalently binds to porin and blocks HK1 binding. Deletional mutant constructs of HKCAT showed reduced binding with increasing deletion size. In summary, these studies demonstrate that the 15-amino acid N-terminal domain of HK1 is necessary and sufficient to confer mitochondrial binding properties to CAT and that there is specificity for this binding to the mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V., Griffin L., Towbin J., Gelb B., Worley K., McCabe E. R. Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem Med Metab Biol. 1991 Jun;45(3):271–291. doi: 10.1016/0885-4505(91)90032-g. [DOI] [PubMed] [Google Scholar]
- Arora K. K., Fanciulli M., Pedersen P. L. Glucose phosphorylation in tumor cells. Cloning, sequencing, and overexpression in active form of a full-length cDNA encoding a mitochondrial bindable form of hexokinase. J Biol Chem. 1990 Apr 15;265(11):6481–6488. [PubMed] [Google Scholar]
- Arora K. K., Pedersen P. L. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988 Nov 25;263(33):17422–17428. [PubMed] [Google Scholar]
- Benz R., Kottke M., Brdiczka D. The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim Biophys Acta. 1990 Mar;1022(3):311–318. doi: 10.1016/0005-2736(90)90279-w. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Compartmentation of hexokinase and creatine phosphokinase, cellular regulation, and insulin action. Curr Top Cell Regul. 1980;16:55–86. doi: 10.1016/b978-0-12-152816-4.50007-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brdiczka D. Interaction of mitochondrial porin with cytosolic proteins. Experientia. 1990 Feb 15;46(2):161–167. doi: 10.1007/BF02027312. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Wilson J. E. Purification and properties of rat brain hexokinase. Arch Biochem Biophys. 1972 Jul;151(1):48–55. doi: 10.1016/0003-9861(72)90471-7. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Bernhard H. P., Miller R. A., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells. J Natl Cancer Inst. 1980 Apr;64(4):809–819. [PubMed] [Google Scholar]
- De Pinto V., Ludwig O., Krause J., Benz R., Palmieri F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim Biophys Acta. 1987 Nov 19;894(2):109–119. doi: 10.1016/0005-2728(87)90180-0. [DOI] [PubMed] [Google Scholar]
- De Pinto V., Tommasino M., Benz R., Palmieri F. The 35 kDa DCCD-binding protein from pig heart mitochondria is the mitochondrial porin. Biochim Biophys Acta. 1985 Mar 14;813(2):230–242. doi: 10.1016/0005-2736(85)90238-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Messer J. L., Wilson J. E. Purification of a hexokinase-binding protein from the outer mitochondrial membrane. J Biol Chem. 1979 Jun 25;254(12):4946–4949. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988 Dec 5;241(1-2):105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- Jones S. N., Tibbetts C. Upstream DNA sequences determine different autoregulatory responses of the adenovirus types 5 and 3 E1A promoters. J Virol. 1989 Apr;63(4):1833–1838. doi: 10.1128/jvi.63.4.1833-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig O., De Pinto V., Palmieri F., Benz R. Pore formation by the mitochondrial porin of rat brain in lipid bilayer membranes. Biochim Biophys Acta. 1986 Aug 21;860(2):268–276. doi: 10.1016/0005-2736(86)90523-7. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe E. R. Human glycerol kinase deficiency: an inborn error of compartmental metabolism. Biochem Med. 1983 Oct;30(2):215–230. doi: 10.1016/0006-2944(83)90088-1. [DOI] [PubMed] [Google Scholar]
- Nakashima R. A., Mangan P. S., Colombini M., Pedersen P. L. Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N'-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986 Mar 11;25(5):1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- Nishi S., Seino S., Bell G. I. Human hexokinase: sequences of amino- and carboxyl-terminal halves are homologous. Biochem Biophys Res Commun. 1988 Dec 30;157(3):937–943. doi: 10.1016/s0006-291x(88)80964-1. [DOI] [PubMed] [Google Scholar]
- Pal N., Bessman S. P. Insulin effect on [14C]-valine incorporation and its relation to hexokinase activity in developing brain. Biochem Biophys Res Commun. 1988 Jul 15;154(1):450–454. doi: 10.1016/0006-291x(88)90707-3. [DOI] [PubMed] [Google Scholar]
- Polakis P. G., Wilson J. E. An intact hydrophobic N-terminal sequence is critical for binding of rat brain hexokinase to mitochondria. Arch Biochem Biophys. 1985 Jan;236(1):328–337. doi: 10.1016/0003-9861(85)90633-2. [DOI] [PubMed] [Google Scholar]
- Roos N., Benz R., Brdiczka D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. Biochim Biophys Acta. 1982 Apr 7;686(2):204–214. doi: 10.1016/0005-2736(82)90114-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schwab D. A., Wilson J. E. Complete amino acid sequence of rat brain hexokinase, deduced from the cloned cDNA, and proposed structure of a mammalian hexokinase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2563–2567. doi: 10.1073/pnas.86.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen A. P., Wilson J. E. Complete amino acid sequence of the type II isozyme of rat hexokinase, deduced from the cloned cDNA: comparison with a hexokinase from novikoff ascites tumor. Arch Biochem Biophys. 1991 May 1;286(2):645–651. doi: 10.1016/0003-9861(91)90094-y. [DOI] [PubMed] [Google Scholar]
- Tuttle J. P., Wilson J. E. Brain hexokinase: a kinetic comparison of soluble and particulate forms. Biochim Biophys Acta. 1970 Jul 15;212(1):185–188. doi: 10.1016/0005-2744(70)90195-6. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Ligand-induced conformations of rat brain hexokinase: effects of glucose-6-phosphate and inorganic phosphate. Arch Biochem Biophys. 1973 Nov;159(1):543–549. doi: 10.1016/0003-9861(73)90486-4. [DOI] [PubMed] [Google Scholar]






