Abstract
We tested the hypothesis that the decrease in arterial pressure induced by adrenomedullin (ADM) in the hypothalamic paraventricular nucleus (PVN) is mediated by nitric oxide (NO) and/or GABA. Unilateral microinjections of ADM into the PVN of anesthetized rats caused a significant decrease in mean arterial pressure (MAP). The ADM-induced decrease in MAP was significantly attenuated by pretreatment with Nψ-nitro-L-arginine methyl ester (L-NAME, a non-selective NOS inhibitor), 7-nitroindazole sodium salt (7-NiNa, a selective neuronal NOS inhibitor), N5-(1-Iminoethyl)-L-ornithine (L-NIO, a selective endothelial NOS inhibitor) or bicuculline methiodide, but pretreatment with S-methylisothiourea (SMIT, a selective inducible NOS inhibitor) had no effect on this ADM-induced effect. In addition, coronal sections of rat brains were processed for combined NADPH-diaphorase (a marker of neuronal NOS-containing neurons) histochemistry and in situ hybridization for the receptor-activity-modifying protein 2 (a specific ADM receptor component). Double-labeled neurons were found in both parvocellular and magnocellular subdivisions of the PVN, confirming that NO-producing neurons in the PVN are capable of mediating ADM’s effects. Thus, our data provide evidence that the ADM-induced decrease in MAP in the PVN is mediated by NO from neuronal and endothelial NOS, and by GABA.
Keywords: NADPH-diaphorase, Receptor-activity-modifying protein 2, Nitric oxide synthase
1. Introduction
Adrenomedullin (ADM), a novel vasoactive peptide, was first isolated from human pheochromocytoma in 1993 [1]. This 52-amino acid peptide is a member of the calcitonin peptide family, which also includes calcitonin, calcitonin gene-related peptide (CGRP), and amylin. Both ADM and its receptor system, which is composed of a calcitonin-receptor-like receptor (CRLR) and receptor-activity-modifying protein (RAMP) 2 or 3 [2–7], have been demonstrated in peripheral tissues and in the central nervous system (CNS) [8,9]. In the CNS, high levels of ADM and its receptors have been found in the hypothalamus [10], especially in the paraventricular nucleus (PVN) [11,12].
ADM has been considered to be a potential anti-hypertensive agent because of its dramatic vasodilatory effect in the periphery of the body [1,13,14]. However, the poor understanding of ADM’s complex effects in the brain remains an impediment in its potential therapeutic application. We have shown that circulating ADM can enter the CNS [15], and ADM has different effects on arterial pressure depending on where it is applied in the brain. For instance, intracerebroventricular (i.c.v.) ADM and microinjection of ADM into the area postrema (AP) increase arterial pressure by stimulating sympathetic outflow from the brain [16–21]. Interestingly microinjection of ADM into the PVN leads to a rapid decrease in arterial pressure [22], but the mechanisms for this effect are unknown.
The PVN, composed of magnocellular and parvocellular neurons, is an important center for autonomic and neuroendocrine regulation [23]. Magnocellular neurons project to the posterior pituitary to regulate oxytocin (OXY) and arginine-vasopressin (AVP) release into the circulation [24]. Parvocellular neurons project to the anterior pituitary to modulate activity of the hypothalamic–pituitary–adrenal (HPA) axis or project to other autonomic centers in the CNS, such as the rostral ventrolateral medulla (RVLM) and the intermediolateral column of the spinal cord to regulate sympathetic activity [25–28].
This study was undertaken to identify the neurotransmitters recruited by ADM in the PVN to affect arterial pressure. Nitric oxide (NO) is a gaseous neurotransmitter in the CNS which plays an important role in regulation of cardiovascular system [29]. l.c.v. ADM stimulates NO production in the hypothalamus and activates NO-producing neurons in the PVN [19]. Microinjection of sodium nitroprusside, an NO donor, into the PVN decreases arterial pressure and heart rate [30]. These data suggest that NO in the PVN is one of the candidates to mediate the ADM-induced decrease in arterial pressure. In addition, GABA exerts a tonic inhibitory effect on the sympathetic nervous system at the level of the PVN [31,32], and a selective GABAA receptor antagonist, bicuculline, abolishes ADM-induced hyperpolarization of magnocellular neurons in the PVN [33], suggesting that GABA may also contribute to the hypotensive effect of ADM within the PVN.
To test the hypothesis that the decrease in arterial pressure elicited by microinjection of ADM into the PVN is mediated by NO and/or GABA, we first recorded mean arterial pressure (MAP) responses to microinjections of ADM into the PVN in the presence and absence of specific nitric oxide synthase (NOS) inhibitors or bicuculline. In addition, RAMP-2 in situ hybridization combined with NADPH-diaphorase (NADPH-d) histochemistry was used to further confirm that neuronal NOS-containing neurons are capable of responding to ADM.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats (250–300 g) were purchased from the Biological Animal Center, University of Alberta. They were housed in a 12:12-h light/dark cycle at 22 °C and given free access to food and water. All experimental protocols were approved by the local Animal Welfare Committee.
2.2. Intracerebral microinjection and recording of MAP
2.2.1. Surgical procedures
Rats were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine (0.5 mg/kg), and additional anesthetics were given as required. Body temperature was monitored with a rectal thermometer and maintained at 37 °C with a heating pad. The left femoral artery was cannulated using PE50 tubing (Becton Dickinson and Company, Sparks, MD, USA), which was connected to a computer-based data acquisition system D1-150 RS (DATAQ Instruments, Akron, OH, USA) via a pressure transducer. The rats were then placed in a stereotaxic apparatus. A cannula (0.2 mm OD, 0.1 mm ID; Plastics One, Wallingford, CT, USA) was angularly (8° lateral to the midline) lowered into the PVN according to the coordinates, 6.8 mm anterior, 0.1 mm lateral and 2.2 mm dorsal to the interaural zero [34,35].
2.2.2. Experimental protocol
After stabilization for 20–30 min, saline, NOS inhibitors, or the GABAA receptor antagonist were microinjected into the PVN, and 10 min later, an injection of ADM was performed. Arterial pressure was recorded for at least 5 min after the injection of ADM. Each rat received only one combination of injections, and for each combination, the experiment was repeated in at least seven rats in which the cannula was accurately placed in the PVN, according to the histological verification described below.
2.2.3. Chemicals
All chemicals were dissolved in saline and the injections were made at a fixed volume of 0.2 μl. The chemicals included ADM (Penisula Laboratories, Belmont, CA, USA), Nψ-nitro-L-arginine methyl ester (L-NAME, a non-selective NOS inhibitor; Sigma, St. Louis, MO, USA), 7-nitroindazole monosodium salt (7-NiNa, a selective neuronal NOS inhibitor; A.G. Scientific, San Diego, CA, USA), N5-(1-Iminoethyl)-L-ornithine (L-NIO, a selective endothelial NOS inhibitor; Tocris Cookson, Ellisville, MO, USA), S-methylisothiourea (SMIT, a selective inducible NOS inhibitor; Cayman Chemical, Ann Arbor, MI, USA) and bicuculline methiodide (BIC, a GABAA receptor antagonist; Sigma). The dose for ADM injection (0.02 pmol) was chosen based on the dose response study by Smith and Ferguson [22] to evoke a maximal response in MAP. The doses of NOS inhibitors and BIC (indicated in Table 1) were carefully adjusted to ensure that the increases in MAP evoked lasted less than 10 min.
Table 1.
Effects of NOS inhibitors and bicuculline on the baseline MAP
| Treatment | Maximal changes in MAP (mm Hg) | Maximal changes in HR (bpm) |
|---|---|---|
| Saline | 3.5 ± 0.3 | 5.7 ± 1.0 |
| L-NAME (2 nmol) | 3.9 ± 0.9 | 7.6 ± 3.6 |
| L-NAME (20 nmol) | 3.2 ± 0.9 | 9.8 ± 4.1 |
| 7-NiNa (0.05 pmol) | 3.0 ± 0.8 | 8.6 ± 3.3 |
| 7-NiNa (0.1 pmol) | 3.0 ± 0.6 | 8.2 ± 3.8 |
| L-NIO (100 pmol) | 3.2 ± 0.2 | 5.9 ± 2.1 |
| L-NIO (200 pmol) | 2.9 ± 1.0 | 8.5 ± 3.8 |
| SMIT (250 pmol) | 2.8 ± 0.5 | 7.4 ± 4.6 |
| SMIT (500 pmol) | 2.6 ± 0.5 | 9.1 ± 4.8 |
| BIC (100 pmol) | 2.4 ± 0.5 | 6.3 ± 5.4 |
| BIC (200 pmol) | 2.9 ± 0.6 | 9.0 ± 4.9 |
Saline or different blockers were microinjected into the PVN, and the maximal changes in mean arterial pressure (MAP) and heart rate (HR) between 10 and 15 min after the injections were recorded. For each group, N was at least 6. There were no significant differences between the changes in MAP and HR after saline injection and those after the injections of the blockers.
2.2.4. Histology
At the end of the experiment, 1% Evans blue (Sigma) was injected into the brain for histological verification of the microinjection tracts. After the rats were sacrificed, brains were removed and fixed in 4% paraformaldehyde for 48–72 h. The brains were then frozen, and coronal hypothalamic sections (40 μm) were cut using a cryostat (−20 °C). The sections were thaw-mounted onto slides and stained with neutral red (Alled Chemical, New York, NY, USA). Evans blue within the PVN was observed under a microscope, and only data from rats with accurately placed cannulae were used for analysis and the data from rats in which cannulae were placed outside the PVN were used as site-specific controls.
2.2.5. Statistical analyses
For each rat, a mean baseline value of MAP was calculated for 120 s prior to the first injection. MAP responses to different treatments were expressed as the amplitude of decrease in MAP (differences between the minimal value of MAP within 300 s after ADM injection and the mean baseline value of MAP) and the area under the curve (AUC, the area between the baseline and each arterial pressure within 300 s after ADM injection). The amplitude and the AUC were expressed as mean ± S.E.M., and were analyzed by a one-way ANOVA followed by Newman–Keuls post hoc analysis. P < 0.05 indicated a significant difference.
2.3. Double-labeling of PVN neurons
2.3.1. Tissue preparation
Rats were deeply anesthetized with ketamine hydrochloride (100 mg/kg, Ayerst Veterinary Laboratories, Guelph, Ontario, Canada) and xylazine (0.5 mg/kg, Bayer, Toronto, Ontario, Canada), and perfused transcardially with 200 ml ice-cold saline followed by 500 ml ice-cold 4% paraformaldehyde in 0.1 M PBS (pH 7.2). The brains were removed, post fixed in half-strength fixative and 10% sucrose for 1 h, and stored in 20% sucrose overnight at 4 °C. Coronal hypothalamic sections (40 μm) were cut in a cryostat (−20 °C) and collected in PBS for the following procedures.
2.3.2. NADPH-d histochemistry
NADPH-d histochemistry was performed as described previously [36–38]. Briefly, the sections were incubated for 20 min at 45 °C in a solution containing 1 mg/ml β-NADPH (Sigma), 0.3 mg/ml nitroblue tetrazolium (Sigma) dissolved in dimethyl sulfoxide (Sigma), and 0.6% Triton X-100/PBS. The sections were mounted onto slides, air dried and stored at −70 °C for RAMP-2 in situ hybridization.
2.3.3. RAMP-2 in situ hybridization
In situ hybridization for RAMP-2 was performed as described previously [11,39,40]. In brief, the plasmid containing RAMP-2 cDNA was made from the rat lung. Anti-sense 35S-UTP labeled probes were generated by linearizing the plasmid with SacI (Promega, Madison, WI, USA), and transcribing with T7 polymerase (Promega). Frozen sections stained for NADPH-d were brought to room temperature, then prehybridized with 4% paraformaldehyde, PBS (×2), 20 μg/ml proteinase K (Invitrogen Canada, Burlington, Ontario, Canada) in 50 mM Tris/HCl (pH 8) and 10 EDTA, PBS, 0.25% acetic anhydride in 10 mM triethanolamine (pH 8), and dehydrated in 70% and 80% ethanol/sodium acetate, then 95% ethanol (×2). Each slide was hybridized overnight at 45 °C with approximately 2 × 106 CPM of the labeled probe in 80 μl hybridization buffer (50% formamide, 10% dextran sulphate, 1 × Denhardt’s solution). The slides were rinsed twice in 2 × SSC for 10 min at room temperature, followed by RNase A in STE buffer (10 mM Tris/HCl, 250 mM NaCl, 1 mM EDTA, pH 8) for 30 min at 37 °C, 1 × SSC at 45 °C for 40 min, and 0.1 × SSC at 65 °C for 40 min. The slides were allowed to air dry, and were exposed to X-ray films (X-OMAT AR, Eastman Kodak, Rochester, NY, USA) for 96 h, dipped in NTB-2 Kodak photographic emulsion (diluted 1:1 with water), exposed for approximately 35 days, and developed for autoradiography. The slides were rinsed with water for 45 min, and dehydrated through graded ethanol. The slides were then immersed in xylene and cover-slipped for microscopy.
2.3.4. Identification of double-labeled neurons
NADPH-d-positive neurons were visualized in light-field microphotographs, and RAMP-2 mRNA signal was indicated by silver grains in dark-field microphotographs. Those NADPH-d-positive neurons that contained at least three times the density of silver grains as background were considered to be double-labeled.
3. Results
3.1. Accuracy of microinjections
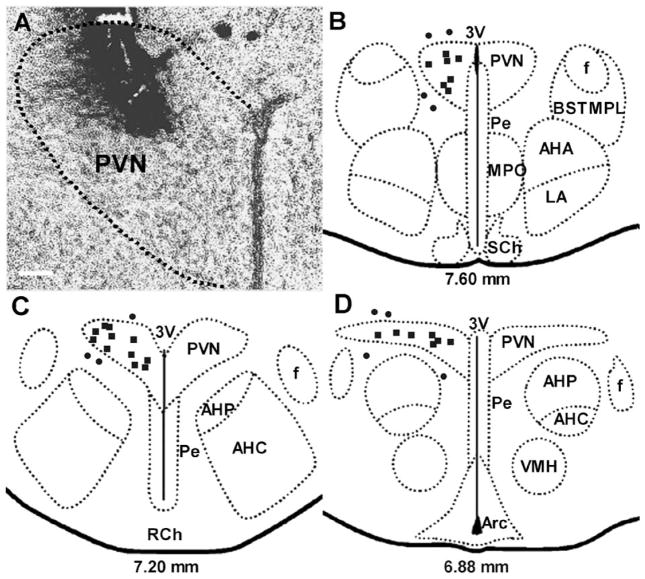
Histological verification of the microinjection sites showed that the microinjections were accurately placed within the PVN in about 70% of rats (Fig. 1).
Fig. 1.
(A) Light-field microphotograph showing the site of termination of an injection that is considered to be within the PVN. (B, C and D) Schematic representations of serial sections from 7.60 to 6.88 mm anterior to interaural zero showing the sites of termination of injection tracts in 36 rats. Each square represents a site of termination of an injection that is considered to be within the PVN, while each circle represents a site of termination of an injection that is considered to be outside of the PVN. The scale bar in (A) = 0.1 mm. 3V, third ventricle; AHA, anterior hypothalamic area, anterior part; AHC, anterior hypothalamic area, central part; AHP, anterior hypothalamic area, posterior part; Arc, arcuate hypothalamic nucleus; BSTMPL, bed nucleus of the stria terminalis, medial division, posterolateral part; f, fornix; LA, lateroanterior hypothalamic nucleus; MPO, medial preoptic nucleus; Pe, periventricular hypothalamic nucleus; PVN, paraventricular nucleus; RCh, retrochiasmatic area; VMH, ventromedial hypothalamic nucleus. Drawing is modified from Paxinos and Watson [35].
3.2. Effects of NOS inhibitors and BIC on MAP
At the doses used, all NOS inhibitors and BIC briefly increased MAP and HR after they were injected into the PVN. However, within 5–10 min, MAP and HR returned to baseline. Upon return to baseline, maximal changes in MAP and HR induced by NOS inhibitors or BIC were not significantly different to those induced by saline (Table 1).
3.3. Effects of microinjection of ADM into the PVN on MAP and HR
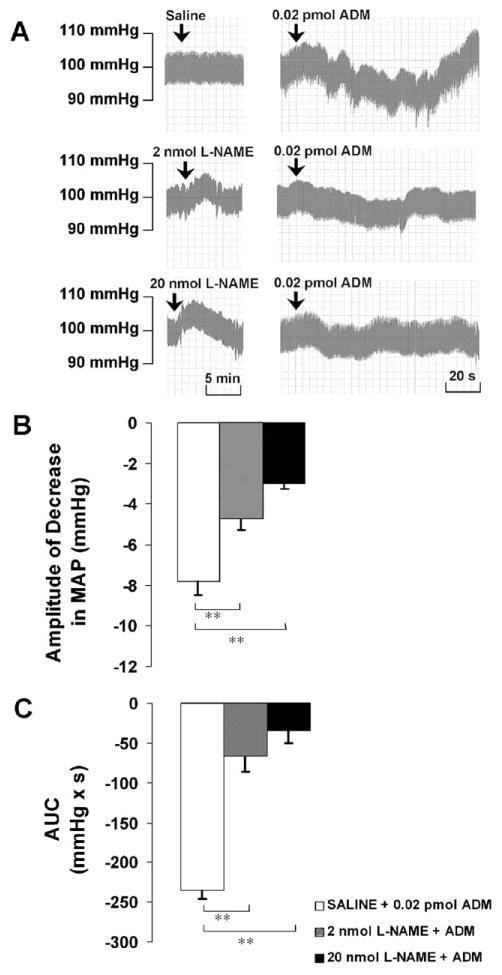
After the microinjection of saline into the PVN, the subsequent injection of ADM (0.02 pmol) induced a rapid and significant decrease in MAP with an amplitude of −7.8 ± 0.7 mm Hg and an AUC of −234.6 ± 10.6 mmHg ×s (Fig. 2). ADM injection did not cause a significant change in HR, although HR tended to be decreased (−3.0 ± 1.8 bpm, P>0.05). Injections that did not target the PVN accurately failed to cause any changes in MAP and HR.
Fig. 2.
(A) Representative arterial pressure traces of rats that received ADM injections in the PVN after the pretreatment of saline or L-NAME at doses indicated. (B and C) Amplitude and AUC of decreases in mean arterial pressure (MAP) to microinjections of ADM into the PVN after the pretreatment of saline or L-NAME. Graphs represent mean ± S.E.M. N = 8 in each group. ** P < 0.01.
3.4. Effects of NOS inhibitors on the ADM-induced decrease in MAP
After pretreatment with L-NAME (2 nmol), the amplitude of the ADM-induced decrease in MAP was significantly reduced to −4.7 ± 0.6 mm Hg, and the AUC was attenuated to −66.7 ± 19.2 mm Hg s. L-NAME at 20 nmol significantly reduced the amplitude to −3.0 ± 0.3 mm Hg and attenuated the AUC to −34.4 ± 15.2 mmHg × s (Fig. 2).
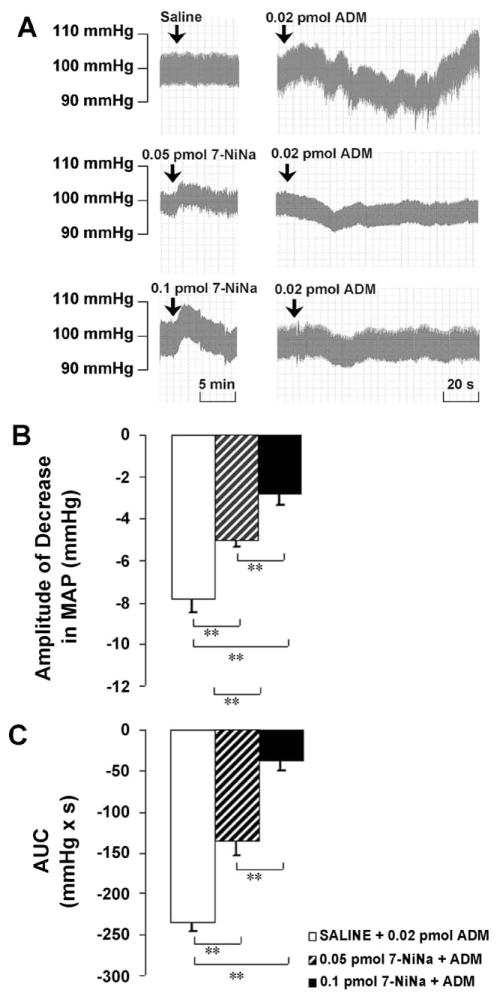
Pretreatment with 7-NiNa (0.05 pmol) significantly attenuated the amplitude of the decrease in MAP induced by ADM from −7.8 ± 0.7 to −5.0 ± 0.3 mm Hg, and attenuated the AUC from −234.6 ± 10.6 to −136.4 ± 17.0 mmHg × s. The higher dose of 7-NiNa (0.1 pmol) significantly attenuated the amplitude to −2.8 ± 0.6 mm Hg and attenuated the AUC to −37.6 ± 13.0 mmHg × s in a dose-dependent manner (Fig. 3).
Fig. 3.
(A) Representative arterial pressure traces of rats that received ADM injections in the PVN after the pretreatment of saline or 7-NiNa at doses indicated. (B and C) Amplitude and AUC of decreases in mean arterial pressure (MAP) to microinjections of ADM into the PVN after the pretreatment of saline or 7-NiNa. Graphs represent mean ± S.E.M. N = 8 in each group. **P < 0.01.
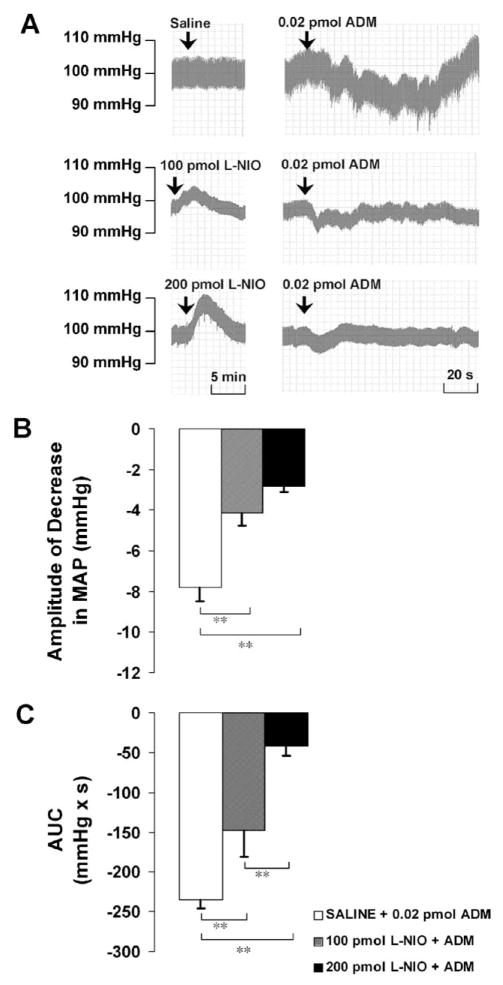
L-NIO at 100 pmol significantly attenuated the amplitude of the ADM-induced decrease in MAP from −7.8 ± 0.7 to −4.1 ± 0.7 mm Hg and attenuated the AUC from −234.6 ± 10.6 to −148.3 ± 33.3 mmHg × s. The higher dose of L-NIO (200 pmol) significantly attenuated the amplitude to −2.8 ± 0.3 mm Hg, and reduced the AUC to −41.8 ± 12.1 mmHg × s in a dose-dependent manner (Fig. 4).
Fig. 4.
(A) Representative arterial pressure traces of rats that received ADM injections in the PVN after the pretreatment of saline or L-NIO at doses indicated. (B and C) Amplitude and AUC of decreases in mean arterial pressure (MAP) to microinjections of ADM into the PVN after the pretreatment of saline or L-NIO. Graphs represent mean ± S.E.M. N = 8 in each group. **P < 0.01.
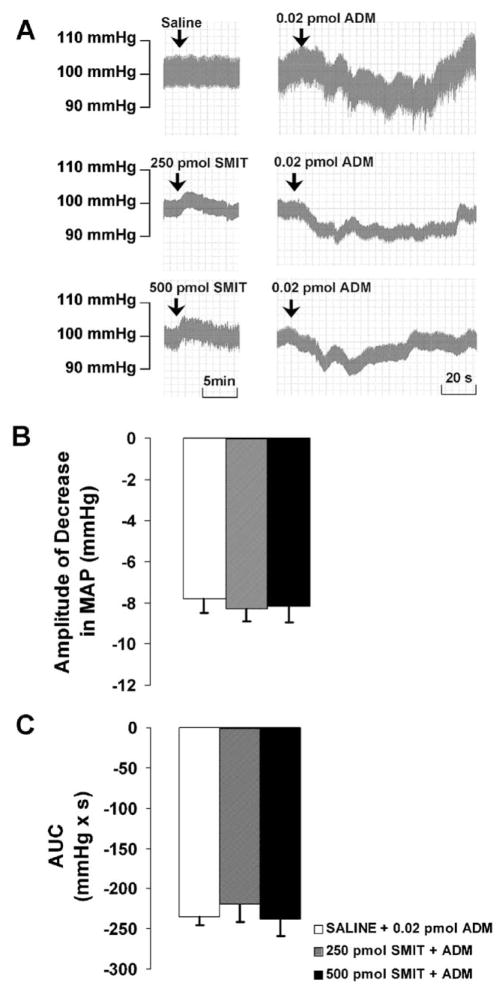
After the pretreatment with SMIT (250 or 500 pmol), microinjection of ADM into the PVN led to a decrease in MAP with an amplitude of −8.3 ± 0.6 or −8.2 ± 0.8 mm Hg, and an AUC of −219.0 ± 22.9 or −237.6 ± 20.8 mmHg × s, respectively. There were no significant differences between the responses to SMIT plus ADM and those to saline plus ADM (Fig. 5).
Fig. 5.
(A) Representative arterial pressure traces of rats that received ADM injections in the PVN after the pretreatment of saline or SMIT at doses indicated. (B and C) Amplitude and AUC of decreases in mean arterial pressure (MAP) to microinjections of ADM into the PVN after the pretreatment of saline or SMIT. Graphs represent mean ± S.E.M. N = 8 in each group.
3.5. Effects of BIC on the ADM-induced decrease in MAP
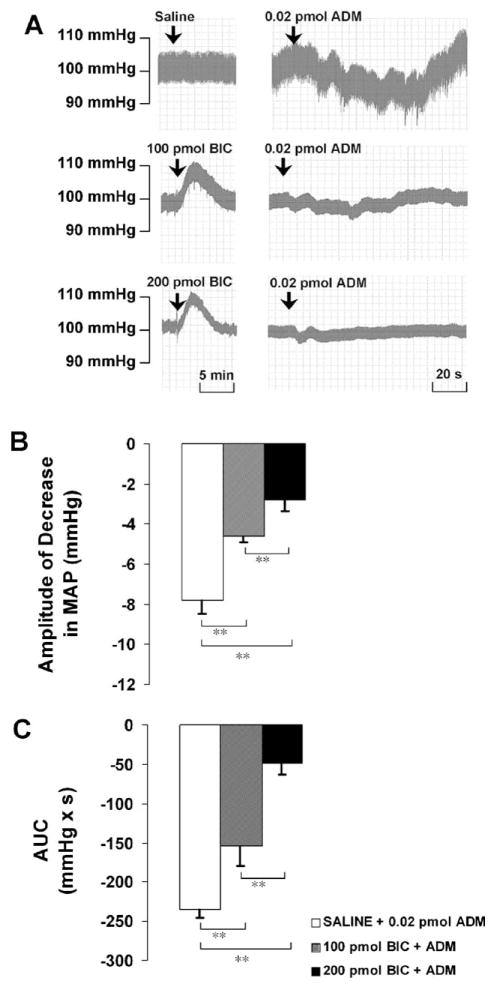
Pretreatment with BIC at 100 pmol significantly attenuated the amplitude of the decrease in MAP induced by ADM from −7.8 ± 0.7 to −4.6 ± 0.3 mm Hg and attenuated the AUC from −234.6 ± 10.6 to −153.5 ± 25.7 mmHg × s. The higher dose of BIC (200 pmol) significantly reduced the amplitude to −2.8 ± 0.6 mm Hg, and attenuated the AUC to −49.5 ± 14.1 mmHg × s in a dose-dependent manner (Fig. 6).
Fig. 6.
(A) Representative arterial pressure traces of rats that received ADM injections in the PVN after the pretreatment of saline or BIC at doses indicated. (B and C) Amplitude and AUC of decreases in mean arterial pressure (MAP) to microinjections of ADM into the PVN after the pretreatment of saline or BIC. Graphs represent mean ± S.E.M. N = 8 in each group. *P < 0.05, **P < 0.01.
3.6. RAMP-2/NADPH-d double-labeling in the PVN
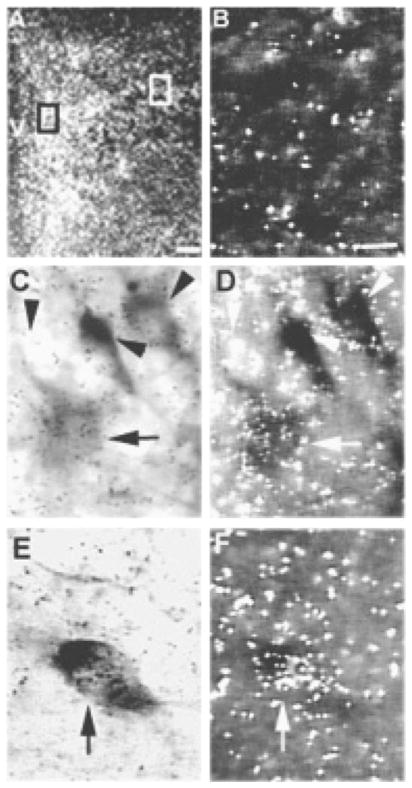
Because RAMP-2 was expressed at relatively low levels, we were unable to reproducibly quantitate numbers of NAPDH-d-positive neurons which also expressed RAMP-2. However, double-labeled neurons were consistently found in both the parvocellular and magnocellular subdivisions of the PVN (Fig. 7).
Fig. 7.
(A) Dark-field photomicrograph of the PVN showing localization of RAMP-2 mRNA with in situ hybridization. (B) Dark-field photomicrograph showing the background signal in an area near the PVN. (C and D) Light-and dark-field photomicrographs of the parvocellular subdivision at higher magnification, indicated by the black box in (A) and showing single-labeled neurons (arrowhead) and a neuron double-labeled with RAMP-2 mRNA and NADPH-d (arrow). (E and F) Light- and dark-field photomicrographs of the magnocellular subdivision at higher magnification, indicated by the white box in (A) and showing a neuron double-labeled with RAMP-2 mRNA and NADPH-d (arrow). The scale bar in (A) = 100 μm; the scale bar in (B) = 10 μm and applies in (C, D, E and F). V, third ventricle.
4. Discussion
Microinjection and double-labeling experiments were carried out in this study to investigate the neural mechanisms underlying ADM-induced effects in arterial pressure in the PVN. The microinjection experiments indicated that the ADM-induced decrease in MAP was attenuated by a non-selective NOS inhibitor, a selective neuronal NOS inhibitor and an endothelial NOS inhibitor, and a selective GABAA receptor antagonist, but was not affected by a selective inducible NOS inhibitor. These results suggest that the effect of ADM in the PVN is mediated by NO from neuronal and endothelial isoforms of NOS, and by GABA. The double-labeling experiment demonstrated that a subpopulation of neuronal NOS-containing neurons in the PVN also expresses RAMP-2, further confirming that neuronal NOS-containing neurons in the PVN can mediate the effects of ADM.
Others have shown that microinjections of ADM (0.0005–0.05 pmol) into the PVN dose-dependently decrease MAP [22]. Our data for 0.02 pmol ADM agree with these results. Because injections of ADM outside the PVN did not affect MAP, we are confident that the depressor effect of ADM was specific for the PVN.
To determine whether ADM-induced decrease in arterial pressure in the PVN is mediated by NO, we first used L-NAME to non-selectively block the production of NO in the PVN. Our results showed that L-NAME reduced both the amplitude and AUC of the ADM-induced decrease in MAP. Together with our previous study showing that i.c.v. ADM activates NO-producing neurons in the PVN and increases NO production in the hypothalamus [19], these data suggest that ADM stimulates increased production and release of NO in the PVN, and that NO, in turn, decreases arterial pressure.
To further determine which NOS isoform(s) is/are recruited by ADM to decrease arterial pressure, 7-NiNa (a selective neuronal NOS inhibitor), L-NIO (a selective endothelial NOS inhibitor) and SMIT (a selective inducible NOS inhibitor) were used to try to block the effect of ADM. Both 7-NiNa and L-NIO attenuated the decrease in arterial pressure caused by ADM, while SMIT had no effect on this response. Therefore, these data suggest that the ADM-induced decrease in arterial pressure is mediated by NO that is synthesized by neuronal and endothelial isoforms of NOS, but not by inducible NOS. It is important to note that all these NOS inhibitors themselves cause increases in MAP when they are injected into the PVN. Thus, we carefully chose doses which elicited increases in MAP that lasted less than 10 min, with a return of MAP to baseline thereafter.
With regard to the specificity of the selective NOS inhibitors, 7-NiNa, L-NIO and SMIT have been demonstrated to be selective inhibitors for neuronal, endothelial and inducible isoforms of NOS, respectively [41–43]. 7-NiNa and SMIT at even higher doses than those we used have been used as selective neuronal and inducible NOS inhibitors, respectively, in the RVLM [42]. In addition, L-NIO at much higher doses than ours has been used selectively to inhibit endothelial NOS in i.c.v. injection experiments [43]. Therefore, we assume that, at the doses we used, the NOS inhibitors we chose selectively inhibited NO production from specific NOS isoforms in the PVN.
Our results indicate that neuronal NOS and endothelial NOS are both recruited by ADM to synthesize NO, implying that at least a proportion of neuronal and/or endothelial NOS-containing cells in the PVN should be capable of responding to ADM. It has been shown that ADM activates NOS in endothelial cells of blood vessels to produce NO [13,44], and we have found that endothelial cells of blood vessels are the only endothelial NOS-positive cells in the PVN (unpublished data). Based on these data, we speculate that ADM in the PVN evokes the production and release of NO at least in part by activating endothelial NOS within the blood vessels.
To determine whether neuronal NOS-containing cells in the PVN can also respond to ADM, we used NADPH-d histochemistry and RAMP-2 in situ hybridization to double-label PVN cells. NADPH-d histochemistry has been widely used to localize neuronal NOS-containing neurons throughout the nervous system and especially in the PVN [29]. RAMP-2 mRNA was chosen as a marker of ADM-responsive neurons in the PVN because ADM binding activity has been shown to correlate with RAMP-2 mRNA levels in rats [8]. In addition, among the components of ADM receptor system (CRLR, RAMP-2 and RAMP-3), RAMP-2 is the only known specific subunit of ADM receptor, while CRLR also shows a high affinity for CGRP when combined with RAMP-1, and RAMP-3 can provide a binding site for amylin if co-expressed with the calcitonin receptor (CTR) [2–7]. Thus, we believe that the distribution of RAMP-2 mRNA most effectively represents the distribution of ADM-responsive neurons in the PVN, although we recognize that the expression pattern of mRNA does not necessarily represent that of protein. Our results show co-localization of RAMP-2 mRNA and NADPH-d in PVN neurons. Consistent with our previous study showing that i.c.v. ADM activates neuronal NOS-containing neurons in the PVN [19], these data provide anatomical evidence that a proportion of neuronal NOS-containing neurons in the PVN is capable of mediating ADM’s effects. Because these double-labeled neurons were found in both the magnocellular and parvocellular subdivisions of the PVN, NO may not only be involved in ADM’s effects on the hypothalamo-neurohypophyseal axis, but it may also mediate ADM’s effects on the autonomic nervous system and the HPA axis [19].
To test whether GABAergic neurons in the PVN also contribute to the ADM-induced decrease in arterial pressure, we observed ADM-induced responses in MAP after the pretreatment of BIC, a blocker of GABAA receptors. Again the dose of BIC was carefully chosen so that the increases in MAP caused by BIC at the doses we used were short-lived (< 10 min). Our results show that blockade of GABAA receptors in the PVN attenuated the ADM-induced decrease in arterial pressure, suggesting that the ADM-induced decrease in MAP is also mediated by GABA.
By showing that both NO and GABA are recruited by ADM in the PVN to decrease arterial pressure, our results suggest three different but not exclusive cascades for the ADM-induced effect. The most likely cascade is an ADM-NO-GABA cascade. In support of this suggestion, endothelial cells have been shown to produce and release NO in response to ADM [13,44]. In addition, our data provide anatomical support for the idea that PVN neurons can produce NO upon stimulation by ADM. Furthermore, we have shown that ADM increases NO production in SK-N-SH neuroblastoma cells (data unpublished). Taken together, these data suggest that NO, either from endothelial NOS or neuronal NOS, is likely the first downstream neurotransmitter affected by ADM in the PVN. Furthermore, the NO-GABA cascade in the PVN is well established. It has been shown that microinjection of sodium nitroprusside into the PVN locally increases GABA levels [45]. In addition, enhanced activity of GABAergic neurons in the PVN mediates not only the inhibitory effect of NO on the sympathetic outflow from parvocellular PVN neurons [46–48], but also the inhibitory regulation by NO on the release of AVP from magnocellular PVN neurons [49]. Based on the above evidence, we suggest that ADM in the PVN activates NO-producing cells (including neurons and endothelial cells) which in turn produce and release NO. NO then enhances GABAergic neurotransmission within the PVN and leads to a decrease in arterial pressure.
Alternatively, ADM may simultaneously activate NO-producing cells and GABAergic neurons, or ADM may first stimulate GABAergic neurons which in turn lead to increased production and release of NO. Although we cannot rule out these possibilities, no evidence has been shown to support a direct effect of ADM on GABA neurotransmission.
In conclusion, we have shown that the ADM-induced decrease in arterial pressure is attenuated by inhibition of NO production and by blockade of GABAA receptors, suggesting that the decrease in arterial pressure induced by ADM in the PVN is likely mediated through an NO-GABA pathway. In addition, the co-localization of RAMP-2 mRNA and NADPH-d provides anatomical evidence for ADM-NO neurotransmission within the PVN. These results shed light on the mechanisms for ADM’s effects on arterial pressure in an important autonomic center in the brain and contribute to the knowledge required before ADM or its analogs can be used therapeutically.
Acknowledgments
This work was supported by the Heart and Stroke Foundation of Alberta/Northwest Territories/Nunuvut.
References
- 1.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 2.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–42. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 3.Flahaut M, Rossier BC, Firsov D. Respective roles of calcitonin receptor-like receptor (crlr) and receptor activity-modifying proteins (ramp) in cell surface expression of crlr/ramp heterodimeric receptors. J Biol Chem. 2002;277:14731–7. doi: 10.1074/jbc.M112084200. [DOI] [PubMed] [Google Scholar]
- 4.Fraser NJ, Wise A, Brown J, McLatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol. 1999;55:1054–9. doi: 10.1124/mol.55.6.1054. [DOI] [PubMed] [Google Scholar]
- 5.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. Ramps regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 6.Miret JJ, Rakhilina L, Silverman L, Oehlen B. Functional expression of heteromeric calcitonin gene-related peptide and adrenomedullin receptors in yeast. J Biol Chem. 2002;277:6881–7. doi: 10.1074/jbc.M107384200. [DOI] [PubMed] [Google Scholar]
- 7.Sexton PM, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, et al. Cgrp and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (crlr) and receptor activity modifying proteins (ramps) in rat tissues. Br J Pharmacol. 2000;130:189–95. doi: 10.1038/sj.bjp.0702975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jougasaki M, Burnett JC., Jr Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66:855–72. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 10.Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS, et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci. 2001;14:618–28. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- 11.Stachniak TJ, Krukoff TL. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. J Neuroendocrinol. 2003;15:840–50. doi: 10.1046/j.1365-2826.2003.01064.x. [DOI] [PubMed] [Google Scholar]
- 12.Ueta Y, Kitamura K, Isse T, Shibuya I, Kabashima N, Yamamoto S, et al. Adrenomedullin-immunoreactive neurons in the paraventricular and supraoptic nuclei of the rat. Neurosci Lett. 1995;202:37–40. doi: 10.1016/0304-3940(95)12204-4. [DOI] [PubMed] [Google Scholar]
- 13.Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, et al. Adrenomedullin stimulates two signal transduction pathways, camp accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem. 1995;270:4412–7. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- 14.Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and k(+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H2620–6. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- 15.Shan J, Krukoff TL. Area postrema ablation attenuates activation of neurones in the paraventricular nucleus in response to systemic adrenomedullin. J Neuroendocrinol. 2000;12:802–10. doi: 10.1046/j.1365-2826.2000.00524.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura K, Abe I, Tsuchihashi T, Fujishima M. Central adrenomedullin augments the baroreceptor reflex in conscious rabbits. Hypertension. 1999;33:992–7. doi: 10.1161/01.hyp.33.4.992. [DOI] [PubMed] [Google Scholar]
- 17.Saita M, Shimokawa A, Kunitake T, Kato K, Hanamori T, Kitamura K, et al. Central actions of adrenomedullin on cardiovascular parameters and sympathetic outflow in conscious rats. Am J Physiol. 1998;274:R979–84. doi: 10.1152/ajpregu.1998.274.4.R979. [DOI] [PubMed] [Google Scholar]
- 18.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol. 1998;274:R1505–9. doi: 10.1152/ajpregu.1998.274.5.R1505. [DOI] [PubMed] [Google Scholar]
- 19.Shan J, Krukoff TL. Intracerebroventricular adrenomedullin stimulates the hypothalamic – pituitary – adrenal axis, the sympathetic nervous system and production of hypothalamic nitric oxide. J Neuroendocrinol. 2001;13:975–84. doi: 10.1046/j.1365-2826.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Watanabe TX, Nishimura M, Nakanishi T, Sakamoto M, Yoshimura M, et al. Centrally induced vasopressor and sympathetic responses to a novel endogenous peptide, adrenomedullin, in anesthetized rats. Am J Hypertens. 1994;7:478–82. doi: 10.1093/ajh/7.5.478. [DOI] [PubMed] [Google Scholar]
- 21.Allen MA, Smith PM, Ferguson AV. Adrenomedullin microinjection into the area postrema increases blood pressure. Am J Physiol. 1997;272:R1698–703. doi: 10.1152/ajpregu.1997.272.6.R1698. [DOI] [PubMed] [Google Scholar]
- 22.Smith PM, Ferguson AV. Adrenomedullin acts in the rat paraventricular nucleus to decrease blood pressure. J Neuroendocrinol. 2001;13:467–71. doi: 10.1046/j.1365-2826.2001.00657.x. [DOI] [PubMed] [Google Scholar]
- 23.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–7. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 25.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–9. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 26.Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand. 2003;177:7–15. doi: 10.1046/j.1365-201X.2003.01042.x. [DOI] [PubMed] [Google Scholar]
- 27.Krukoff TL, Harris KH, Linetsky E, Jhamandas JH. Expression of c-fos protein in rat brain elicited by electrical and chemical stimulation of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1994;59:590–602. doi: 10.1159/000126709. [DOI] [PubMed] [Google Scholar]
- 28.Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–8. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 29.Krukoff TL. Central actions of nitric oxide in regulation of autonomic functions. Brain Res Brain Res Rev. 1999;30:52–65. doi: 10.1016/s0165-0173(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–72. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 31.Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension. 1991;18:48–55. doi: 10.1161/01.hyp.18.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by pvn-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–31. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- 33.Follwell MJ, Ferguson AV. Adrenomedullin influences magnocellular and parvocellular neurons of paraventricular nucleus via separate mechanisms. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1293–302. doi: 10.1152/ajpregu.00191.2002. [DOI] [PubMed] [Google Scholar]
- 34.Greenshaw AJ. A simple technique for determining stereotaxic coordinates for brain implantation of probes at rotated angles in one or two planes. J Neurosci Methods. 1997;78:169–72. doi: 10.1016/s0165-0270(97)00148-9. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Orlando: Academic Press; 1986. [Google Scholar]
- 36.Krukoff TL, Khalili P. Stress-induced activation of nitric oxide-producing neurons in the rat brain. J Comp Neurol. 1997;377:509–19. doi: 10.1002/(sici)1096-9861(19970127)377:4<509::aid-cne3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Petrov T, Harris KH, MacTavish D, Krukoff TL, Jhamandas JH. Hypotension induces fos immunoreactivity in nadph-diaphorase positive neurons in the paraventricular and supraoptic hypothalamic nuclei of the rat. Neuropharmacology. 1995;34:509–14. doi: 10.1016/0028-3908(95)00002-n. [DOI] [PubMed] [Google Scholar]
- 38.Yang W, Oskin O, Krukoff TL. Immune stress activates putative nitric oxide-producing neurons in rat brain: cumulative effects with restraint. J Comp Neurol. 1999;405:380–7. doi: 10.1002/(sici)1096-9861(19990315)405:3<380::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Shan J, Krukoff TL. Distribution of preproadrenomedullin mrna in the rat central nervous system and its modulation by physiological stressors. J Comp Neurol. 2001;432:88–100. doi: 10.1002/cne.1090. [DOI] [PubMed] [Google Scholar]
- 40.Stachniak TJEKT. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. Neuroendocrinology. doi: 10.1046/j.1365-2826.2003.01064.x. [DOI] [PubMed] [Google Scholar]
- 41.Moore PK, Handy RL. Selective inhibitors of neuronal nitric oxide synthase—is no nos really good nos for the nervous system? Trends Pharmacol Sci. 1997;18:204–11. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- 42.Chan SH, Wang LL, Chan JY. Differential engagements of glutamate and gaba receptors in cardiovascular actions of endogenous nnos or inos at rostral ventrolateral medulla of rats. Br J Pharmacol. 2003;138:584–93. doi: 10.1038/sj.bjp.0705081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira J, Santos AR, Calixto JB. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cgmp pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999;38:835–42. doi: 10.1016/s0028-3908(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 44.Champion HC, Bivalacqua TJ, Pierce RL, Murphy WA, Coy DH, Hyman AL, et al. Responses to human cgrp, adm, and pamp in human thymic arteries. Am J Physiol Regul Integr Comp Physiol. 2003;284:R531–7. doi: 10.1152/ajpregu.00337.2002. [DOI] [PubMed] [Google Scholar]
- 45.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, et al. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–13. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- 46.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic gaba release. J Neurophysiol. 2002;88:2664–74. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of gaba. Neuroscience. 2003;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 48.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of gaba. Am J Physiol. 1998;275:R728–34. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 49.Chiodera P, Volpi R, Capretti L, Coiro V. Gamma-aminobutyric acid mediation of the inhibitory effect of nitric oxide on the arginine vasopressin and oxytocin responses to insulin-induced hypoglycemia. Regul Pept. 1996;67:21–5. doi: 10.1016/s0167-0115(96)00098-5. [DOI] [PubMed] [Google Scholar]