Abstract
Objectives
To assess whether volumetric cerebrospinal fluid (CSF) MRI can be used as a surrogate for brain atrophy assessment and to evaluate how the T2 of the CSF relates to brain atrophy.
Methods
Twenty-eight subjects [mean age 64 (sd 2) years] were included; T1-weighted and CSF MRI were performed. The first echo data of the CSF MRI sequence was used to obtain intracranial volume, CSF partial volume was measured voxel-wise to obtain CSF volume (VCSF) and the T2 of CSF (T2,CSF) was calculated. The correlation between VCSF / T2,CSF and brain atrophy scores [global cortical atrophy (GCA) and medial temporal lobe atrophy (MTA)] was evaluated.
Results
Relative total, peripheral subarachnoidal, and ventricular VCSF increased significantly with increased scores on the GCA and MTA (R = 0.83, 0.78 and 0.78 and R = 0.72, 0.62 and 0.86). Total, peripheral subarachnoidal, and ventricular T2 of the CSF increased significantly with higher scores on the GCA and MTA (R = 0.72, 0.70 and 0.49 and R = 0.60, 0.57 and 0.41).
Conclusion
A fast, fully automated CSF MRI volumetric sequence is an alternative for qualitative atrophy scales. The T2 of the CSF is related to brain atrophy and could thus be a marker of neurodegenerative disease.
Key points
• A 1:11 min CSF MRI volumetric sequence can evaluate brain atrophy.
• CSF MRI provides accurate atrophy assessment without partial volume effects.
• CSF MRI data can be processed quickly without user interaction.
• The measured T2of the CSF is related to brain atrophy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-015-3932-8) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance imaging, Cerebrospinal fluid, Brain atrophy, Imaging, Brain volume
Introduction
Brain atrophy assessment is important in clinical and research neuroradiology settings. When evaluating brain atrophy, tissue loss is assumed when the peripheral and central cerebral spinal fluid (CSF) spaces are enlarged in relation to the intracranial volume [1]. Brain atrophy is an important imaging sign in cerebral small vessel disease (SVD) [2], which is the most common cause of vascular dementia and causes one-fifth of all strokes worldwide [2, 3]. Also, brain atrophy predicts conversion to Alzheimer’s disease (AD) in patients with mild cognitive impairment (MCI) [4].
Brain atrophy can be evaluated by means of quantitative brain volume measurements, or it can be visually scored by means of rating scales such as the global cortical atrophy (GCA) scale [5] and the medial temporal lobe atrophy (MTA) scale [6, 7]. Quantitative brain volume measurements are mainly used in research, because of their relative reliability and good sensitivity compared to visual scores. Typically, brain volume is expressed as a percentage of the intracranial volume. This requires both a T1-weighted and a T2-weighted sequence [1] in which the T1-weighted sequence is used to assess brain volume, while the T2-weighted sequence is used for the measurement of intracranial volume [1]. Currently, no image segmentation method is completely accurate, as brain tissue voxels containing CSF partial volume will bring in errors in volume measurements, even with high-resolution images [8, 9]. Despite these inaccuracies, volumetric magnetic resonance imaging (MRI) can predict conversion to AD in accordance with the GCA and the MTA [10, 11]. So far, no diagnostic threshold for computerized brain atrophy evaluation has been established. Moreover, the currently available segmentation techniques are not easily implemented in daily clinical care. Therefore, visual rating scales remain the gold standard in the clinical setting. Unfortunately, the GCA [5] and the MTA scales [6, 7] suffer from subjectivity. Inter-observer agreement for the GCA scale varies from poor to substantial (kappas of 0.34 and 0.7) [5, 8], whereas the MTA scale seems more robust with inter-observer agreements between 0.82 and 0.9 [12].
Recently, Qin [9] suggested a simple approach to map and measure baseline CSF volume fraction [9] as a surrogate for brain parenchymal volume, which could thus be used to evaluate brain atrophy. The approach is based on the long transverse relaxation rate of CSF (T2,CSF) [13] as compared to other tissue types [14]. It allows for measuring the CSF volume and T2,CSF simultaneously. Apart from its potential as a surrogate for brain atrophy measurements, the latter measurement (T2,CSF) may reflect the CSF contents.
The main purpose of this study was to investigate the ability of this new, fast sequence (scan time of 1:11 min) to act as a surrogate for brain atrophy. A second purpose was to measure the T2 of the CSF and to investigate how it relates to the GCA and MTA scales.
Materials and methods
Subjects
This study contains data from two ongoing studies in our institution, which were both approved by the medical ethical review board of the University Medical Center Utrecht (Utrecht, The Netherlands). The experiments were performed according to the guidelines and regulations of the WMO (‘Wet Medisch wetenschappelijk Onderzoek’). Twenty-two subjects were included as control subjects in a study of large vessel disease (NL39070.041.11) and six subjects were included in the ‘Parelsnoer’ study, which investigates cognitive disorders. The inclusion criteria for the control subjects in the large vessel disease study were: (1) no history of cerebrovascular disease or other brain diseases, and (2) no structural lesions seen on MR imaging (conventional MRI and MR angiography). Inclusion criteria for the subjects included in the ‘Parelsnoer’ study were: (1) patients referred to a memory clinic for the evaluation of cognitive problems, and with a clinical dementia rating scale of 0, 0.5 and 1 [15], and a Mini Mental State Examination (MMSE) of 20 or higher [16]. Exclusion criteria for the subjects included in the ‘Parelsnoer’ study were: (1) Normal Pressure Hydrocephalus, (2) Morbus Huntington, (3) history of cerebrovascular disease, (4) history of schizophrenia, bipolar disorder, psychotic symptoms or major depressive disorder, (5) alcohol abuse, and (6) history of other brain diseases (brain tumour, epilepsy, encephalitis). All subjects signed informed consent. Data of subjects of both studies were combined to obtain one pool of subjects with larger age and brain atrophy variation. The mean (standard deviation, sd) age of the subjects was 65 (12) years, range: 30–80 years. Of the 28 subjects, 19 subjects were male [mean (sd) age: 67 (11) years, range: 30–80 years] and nine were female [mean (sd) age: 63 (14) years, range: 30–79 years].
MR imaging
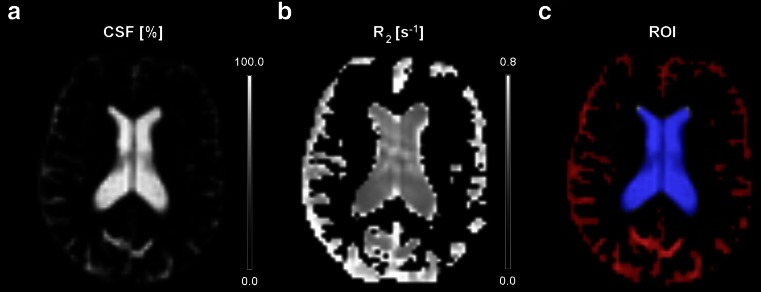
MR imaging was performed at 3 Tesla (Achieva, Philips, Best, The Netherlands). A 3D T1-weighted sequence (matrix 228x227, FOV 250 × 250 × 179 mm3, TR 7.2 ms, TE 3.5 ms, scan time 2:52 min) and CSF MRI (voxel 3 × 3 × 6 mm3, TR 7917 ms, scan time 1:11 min) [9] were performed. In the subjects who were part of the large vessel disease study, a higher resolution CSF MRI sequence (1 × 1 × 4 mm3) was performed as well. The CSF MRI sequence exploits the long T2 properties of the CSF to obtain a pure CSF map. By using a T2-preparation scheme, the T2 decay within each voxel can be fitted, from which the T2 can be estimated. Next, the fitted signal at TE = 0 [“S(0)”] is compared to the signal of pure ventricular CSF voxels, and based on this, the amount of CSF within each voxel is estimated. This yields accurate measurements of the CSF volume fraction (CSFVF) within the voxel [9]. From the obtained data, CSF volume maps and R2,CSF (= the inverse of the T2,CSF) maps are obtained (Fig. 1). More detailed information regarding the CSF sequence can be found in the online supplementary material. In Fig. 2, the sequence chart of the CSF MRI sequence is shown. The R2,CSF maps were used to calculate the T2 of the CSF. The CSF volume maps were used to estimate the CSF volume (VCSF), which was expressed as a percentage of intracranial volume. Based on the first echo (eTE = 0 ms) of the CSF MRI sequence, which includes both parenchymal and CSF signal, the intracranial volume (ICV) was measured. Region-of-interest (ROI) analyses (Fig. 1c) were performed to obtain peripheral subarachnoidal and ventricular CSF volumes and mean T2,CSF values within these regions.
Fig. 1.
A ) CSF M0 map; the signal in each voxel is scaled to pure ventricular voxels to estimate the partial volume effect from which the CSF volume can be calculated. B ) R2 (= the inverse of the T2) CSF map. C ) Regions of interest used to retrieve the peripheral subarachnoidal (red) and ventricular T2, CSF values
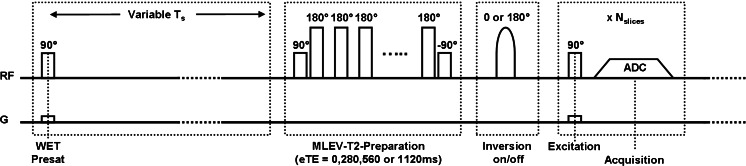
Fig. 2.
CSF MRI sequence chart, the sequence is built of three parts. (1) Part 1, a nonselective (NS) saturation pulse followed by a time delay. (2) Part two, a NS MLEV T2-preparation, based on a τCPMG of 70 ms and 0, 4, 8, and 16 refocusing pulses. From these, effective TEs of 0, 280, 560, and 1120 ms are created. (3) In between parts 2 and 3, an optional inversion pulse can be applied which can compensate for slice-timing differences in multi-slice readout. (4) Part three, a multi-slice single-shot echo planar imaging (EPI) readout
Data analysis
From the low and high resolution CSF MRI data, the following CSF volumes were obtained: VCSF (total) = total CSF volume expressed as a percentage of ICV, VCSF (PS) = peripheral subarachnoidal CSF volume expressed as a percentage of ICV, VCSF (V) = ventricular CSF volume expressed as a percentage of ICV. The following T2,CSF values were obtained: T2,CSF (total) = the mean T2,CSF value in the total CSF, T2,CSF (PS) = mean T2,CSF value measured in the peripheral subarachnoidal CSF, T2,CSF (V) = mean T2,CSF value measured in the ventricles. Qualitative imaging scales [5–7] (Table 1) were used to score (blinded) brain atrophy by two readers (J.H., experienced neuroradiologist, 10 years of experience and J.B.D.V., 4 years of experience). The first reader (J.H.) scored the images twice with an interval of one year in between. A detailed description of this is given in the online supplementary material. To perform a standard quantitative (computerized) brain atrophy evaluation, data of the 3D T1-weighted sequence were processed using FMRIB Software Library (FSL, FMRIB,Oxford, UK). For this, brain tissue voxels of the T1-weighted sequence were isolated with the brain extraction tool (BET) [17]. Subsequently, FAST (FMRIB’s Automated Segmentation Tool) was used to obtain grey matter, white matter and CSF maps, and from this, brain parenchymal volumes were calculated.
Table 1.
GCA and MTA scale
| GCA scale for global cortical atrophy | ||
| Score | ||
| 0 | No cortical atrophy | |
| 1 | Mild atrophy: opening of sulci | |
| 2 | Moderate atrophy: volume loss of gyri | |
| 3 | Severe atrophy: ‘knife blade’ atrophy’ | |
| MTA scale for medial temporal lobe atrophy | ||
| Score | ||
| 0 | No atrophy | |
| 1 | Only widening of choroid fissure | |
| 2 | Also widening of temporal horn of lateral ventricle | |
| 3 | Moderate loss of hippocampal volume (height) | |
| 4 | Severe volume loss of hippocampus | |
Statistical analysis
Statistical analysis was carried out with R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) [18] and IBM SPSS statistics version 19.0.1 (SPSS Inc., Chicago, Illinois). As an earlier study demonstrated that a quarter of brain volume variation is explained by gender [19], we first performed a Mann-Whitney U test to compare the distribution of the GCA and MTA scores between male and female subjects. Similarly, the ICV of males versus females were compared, and the relative total CSF volumes of males versus females were compared.
Cohen’s kappa statistics were used to evaluate the inter-rater and intra-rater agreement for the GCA and MTA scores. Linear regression analyses were used to correlate relative total, peripheral subarachnoidal and ventricular CSF volumes (obtained from the low resolution CSF MRI data) to the GCA and MTA scores. Similarly, linear regression analyses were used to correlate total, peripheral subarachnoidal, and ventricular T2,CSF values to the GCA and MTA scores.
Linear regression analysis was performed to compare the brain parenchymal volumes measured with the CSF MRI sequence to the brain parenchymal volumes measured with FMRIB Software (based on a T1-weighted image).
Two analyses were performed to evaluate the influence of partial volume effects on the obtained values. First, the results of the low resolution CSF MRI sequence were compared against the results of the high resolution CSF MRI sequence. For this, the values obtained for global cortical atrophy and the T2 measured within the peripheral subarachnoidal space were compared using a paired samples t-test. Second, the ventricular ROI’s were eroded, after which the mean T2,CSF values obtained from within these smaller ROIs were compared against the mean T2,CSF values obtained within the actual ventricular ROIs. To evaluate the difference between the results of both these ROIs, a paired samples t-test was used.
In our study protocol no test-retest study was included, as the earlier reproducibility test performed by Qin [9] demonstrated an excellent robustness of the CSF MRI sequence for measuring the CSF volumes and a good robustness for measuring the T2,CSF (0.7 < R2 < 0.85). Though, as an attempt to evaluate the agreement of the CSF MRI sequence within our study, we compared the results of the low resolution CSF MRI sequence against the results of the high resolution CSF MRI sequence. This was done by means of a Bland-Altman scatter plot analysis demonstrating the mean difference and limits of agreement.
Results
Table 2 shows the mean, standard deviation (sd) and range of GCA and MTA scores, ICV and relative total CSF volume. No significant differences in age, GCA and MTA scores, and relative total CSF volume (18.1 % versus 16.0 %) between males and females were found. ICV was significantly different in the males compared to the females (1524 cm3 versus 1361 cm3, p < 0.01). The inter-rater agreements for the GCA and MTA scores were moderate; 0.36 and 0.52 (p < 0.01 and < 0.001). The intra-rater agreements for the GCA and MTA scores were moderate to fair; 0.58 and 0.62 (p < 0.001).
Table 2.
Subject characteristics
| All | Male | Female | |
|---|---|---|---|
| N | 28 | 19 | 9 |
| Age | |||
| Mean (sd) | 65 (12) | 67 (11) | 63 (14) |
| Range | 30 – 80 | 30 – 80 | 30 – 79 |
| GCA | |||
| Mean (sd) | 1 (1) | 1 (1) | 1 (1) |
| Range | 0 – 2 | 0 – 2 | 0 – 2 |
| MTA | |||
| Mean (sd) | 1 (1) | 1 (1) | 1 (1) |
| Range | 0 – 4 | 0 – 4 | 0 – 2 |
| ICV (cm3) | |||
| Mean (sd) | 1471 (146) | 1524 (115) | 1361 (148) |
| Range | 1081 – 1655 | 1306 – 1655 | 1081 – 1552 |
| VCSF (total) (%) | |||
| Mean (sd) | 17.4 (4.3) | 18.1 (4.0) | 16.0 (4.7) |
| Range | 11.1 – 26.1 | 12.1 – 25.5 | 11.1 – 26.1 |
GCA, global cortical atrophy; MTA, medial temporal lobe atrophy; ICV, intracranial volume; VCSF (total), relative total cerebrospinal fluid (CSF) volume (= total CSF volume expressed as a percentage of intracranial volume); sd, standard deviation
Intracranial volume was significantly different between males and females (p < 0.01). No significant differences in age, GCA and MTA score were found
Brain atrophy and whole brain CSF measurements
Table 3 shows the number of subjects for each score on the qualitative imaging scales (GCA and MTA scale) and their corresponding mean (± sd) relative total CSF volumes (total CSF volume expressed as a percentage of ICV), and mean (± sd) total T2,CSF. The correlation between the GCA and the MTA score and the relative total CSF volumes was significant (R = +0.83 and +0.72, p < 0.001). The correlation between the GCA and the MTA score and total T2,CSF was significant (R = +0.72 and +0.60, p < 0.001 and < 0.01).
Table 3.
Brain atrophy and whole brain CSF measurements
| N | VCSF (total) (%) |
T2,CSF (total) (ms) |
||
|---|---|---|---|---|
| GCA | ||||
| 0 | 5 | 12.5 ± 1.1 | 1182 ± 60 | |
| 1 | 17 | 16.7 ± 2.6 | 1339 ± 111 | |
| 2 | 6 | 23.5 ± 2.5 | 1528 ± 138 | |
| 3 | – | – | – | |
| Correlation | R = +0.83 (p < 0.001) | R = +0.72 (p < 0.001) | ||
| MTA | ||||
| 0 | 11 | 15.0 ± 3.2 | 1255 ± 109 | |
| 1 | 11 | 16.7 ± 2.7 | 1360 ± 130 | |
| 2 | 3 | 21.6 ± 4.3 | 1512 ± 211 | |
| 3 | 2 | 25.0 ± 0.7 | 1506 ± 71 | |
| 4 | 1 | 23.7 | 1522 | |
| Correlation | R = +0.72 (p < 0.001) | R = +0.60 (p < 0.01) | ||
GCA, global cortical atrophy; MTA, medial temporal lobe atrophy; VCSF (total), relative total cerebrospinal fluid (CSF) (= total CSF volume expressed as a percentage of intracranial volume); T2,CSF (total), the mean T2 value of the total cerebrospinal fluid
For each category of GCA and MTA score, the CSF volume and the T2,CSF are shown
Brain atrophy and regional CSF measurements
Figures 1 and 2 in the online supplementary material show the correlation between the GCA and the MTA scores with relative peripheral subarachnoidal and ventricular CSF volumes and T2,CSF. Table 4 shows the number of subjects for each score on the qualitative imaging scales (GCA and MTA scale) and their corresponding mean (± sd) relative peripheral subarachnoidal and relative ventricular CSF volumes. The correlations between the GCA scores and the relative peripheral subarachnoidal and the relative ventricular CSF volumes were significant (R = +0.78 and +0.78, p < 0.001). The correlations between the MTA scores and the relative peripheral subarachnoidal and relative ventricular CSF volumes were significant (R2 = +0.62 and +0.86, p < 0.001). The correlation between the GCA scores and the peripheral subarachnoidal and ventricular T2,CSF values were significant (R = +0.70 and +0.49, p < 0.001 and < 0.01). The correlation between the MTA scores and the peripheral subarachnoidal and ventricular T2,CSF values were significant (R = +0.57 and +0.41, p < 0.01 and < 0.05) (Table 5).
Table 4.
Brain atrophy and regional CSF volumes
| N | VCSF (PS) (%) |
VCSF (V) (%) |
||
|---|---|---|---|---|
| GCA | ||||
| 0 | 5 | 10.1 ± 0.9 | 1.0 ± 0.3 | |
| 1 | 17 | 13.2 ± 2.0 | 1.7 ± 0.7 | |
| 2 | 6 | 17.5 ± 2.3 | 3.7 ± 0.9 | |
| 3 | – | – | – | |
| Correlation | R = +0.78 (p < 0.001) | R = +0.78 (p < 0.001) | ||
| MTA | ||||
| 0 | 11 | 12.1 ± 2.6 | 1.2 ± 0.4 | |
| 1 | 11 | 13.0 ± 2.0 | 1.9 ± 0.7 | |
| 2 | 3 | 16.2 ± 3.7 | 3.3 ± 0.2 | |
| 3 | 2 | 19.1 ± 0.1 | 3.3 ± 0.7 | |
| 4 | 1 | 16.5 | 5.4 | |
| Correlation | R = +0.62 (p < 0.001) | R = +0.86 (p < 0.001) | ||
GCA, global cortical atrophy; MTA, medial temporal lobe atrophy; VCSF (PS), relative peripheral subarachnoidal cerebrospinal fluid (CSF) volume (= peripheral subarachnoidal CSF volume expressed as a percentage of intracranial volume); VCSF (V), relative ventricular CSF volume ( = ventricular CSF volume expressed as a percentage of intracranial volume).
For each category of GCA and MTA score, the VCSF (PS) and the VCSF (V) are shown
Table 5.
Brain atrophy and regional CSF T2 values
| N | T2,CSF (PS) (ms) |
T2,CSF (V) (ms) |
||
|---|---|---|---|---|
| GCA | ||||
| 0 | 5 | 1150 ± 70 | 1774 ± 217 | |
| 1 | 17 | 1305 ± 117 | 1992 ± 111 | |
| 2 | 6 | 1493 ± 142 | 2000 ± 86 | |
| 3 | – | – | – | |
| Correlation | R = +0.70 (p < 0.01) | R = +0.49 (p < 0.01) | ||
| MTA | ||||
| 0 | 11 | 1225 ± 112 | 1834 ± 176 | |
| 1 | 11 | 1324 ± 139 | 1954 ± 98 | |
| 2 | 3 | 1479 ± 218 | 1965 ± 96 | |
| 3 | 2 | 1478 ± 71 | 1930 ± 10 | |
| 4 | 1 | 1466 | 2125 | |
| Correlation | R = +0.57 (p < 0.01) | R = +0.41 (p < 0.05) | ||
GCA, global cortical atrophy; MTA, medial temporal lobe atrophy; T2,CSF (PS), the mean T2 value of the peripheral subarachnoidal cerebrospinal fluid; T2,CSF (V), the mean T2 value of the ventricular cerebrospinal fluid
For each category of GCA and MTA score, the T2,CSF (PS) and the T2,CSF (V) are shown
CSF MRI sequence versus standard computerized brain atrophy evaluation
In one subject, the computerized segmentation failed during brain extraction and this subject was thus excluded from the analysis. The mean (sd) brain volume measured using the CSF MRI sequence was 1212 (122) ml. The mean (sd) brain volume derived from the 3D-T1w MRI sequence was 1008 (106) ml. The brain parenchymal volumes (BPV), measured by means of the CSF MRI sequence and the 3D-T1w MRI sequence showed a very strong correlation: R = 0.85, BPVCSF-MRI = 232 (± 123) + 0.97 (± 0.12) · BPV3D-T1wMRI, p < 0.01.
Partial volume effects
The peripheral subarachnoidal CSF volumes, measured by means of a low (12.5 ± 2.3 %) and a high resolution (11.8 ± 4.7 %) CSF MRI sequence, were not significantly different (p = 0.38). The T2 of the CSF within the peripheral subarachnoidal space as measured by means of a low (1275 ± 129 ms) and high resolution (1420 ± 183 ms) CSF MRI sequence were significantly different (p < 0.001). The mean (sd) T2,CSF in the ventricular mask was 1912 (145) ms. The mean (sd) T2,CSF in the eroded (smaller) ventricular ROI was 2060 (153) ms. These were significantly different (p < 0.001).
Agreement analysis
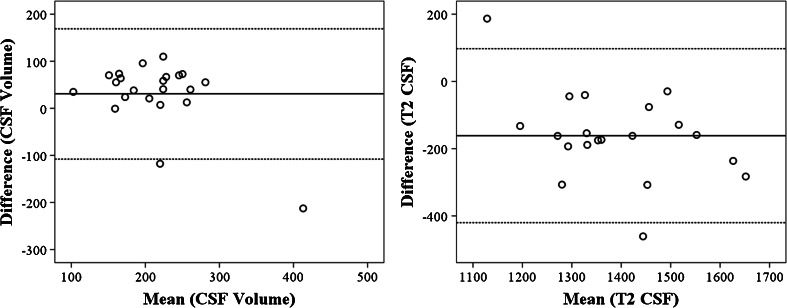
In Fig. 3, a Bland-Altman scatter plot demonstrates the agreement between the low and high resolution CSF MRI sequence for both the total CSF volume and the T2,CSF. The mean difference of the total CSF volume was 30.8 (± 70.7) ml. This difference was not significant (p = 0.054). The mean difference of the T2,CSF was -161.4 (± 132) ms (p < 0.001).
Fig. 3.
Bland-Altman plot demonstrating the agreement between the low and high resolution CSF MRI sequence performed within the same session. The coefficient of repeatability for the relative CSF volumes (figure on the left, CSF volumes expressed as a percentage of intracranial volume), was 14. The coefficient of repeatability for the T2 within the total CSF was 9.5
Discussion
We demonstrated that a fast CSF MRI sequence with an imaging time of 1:11 minutes and fast post-processing without user interaction can be used to obtain the intracranial volume, the CSF volume and the T2 relaxation time constant of the CSF. The obtained volume measurements correlated well with the qualitative atrophy scales, which show that the CSF MRI sequence can be used to evaluate brain atrophy. We found a high correlation between the T2 of the CSF and the qualitative atrophy scales. The T2 of the CSF could thus be a marker for neurodegenerative diseases.
Over the last few years, an increasing number of research groups have started to use image segmentation tools, which are used to measure the brain parenchymal volume, instead of qualitative image atrophy scores to evaluate brain atrophy. In this paper, the percentage of CSF was used as a surrogate for brain parenchymal volume. The method that we used to obtain the CSF volume exploits the long T2 of the CSF to isolate its signal. By fitting T2 decay within each voxel, and comparing the fitted signal at TE = 0 [“S(0)”] to the signal of pure ventricular CSF voxels, the amount of CSF within each voxel is estimated. This yields accurate measurements of the CSF volume fraction within the voxel [9], from which regional CSF volumes can be obtained. We limited ROI analyses to the peripheral subarachnoidal and the ventricular space. However, this could be extended to obtain measurements of, for instance, frontal, parietal or temporal CSF volume, and thus provide an assessment of regional atrophy. Regional atrophy assessment could potentially enable classification of dementia diseases based on their atrophy patterns [20]. For instance, AD and frontotemporal lobe degeneration are both associated with medial temporal lobe atrophy [21–24]. The combined presence of either temporal and posterior, or temporal and frontal atrophy may help to distinguish between both [25–27]. Additionally, regional measurements could be helpful when differentiating ventriculomegaly or hydrocephalus from brain atrophy. Enlarged ventricular CSF volume without an increase in peripheral subarachnoidal CSF volume could guide the assessor towards ventriculomegaly or hydrocephalus instead of brain atrophy. It should be noted that the CSF MRI sequence cannot differentiate between gray or white matter loss, and as such, can only supplement conventional MRI sequences but not replace them.
In this paper, we compared the results of the brain parenchymal volume measurements obtained from our CSF MRI sequence to the results of a more standard computerized quantitative brain atrophy evaluation. We demonstrated a strong correlation between both brain parenchymal volume measurements. The linear analysis showed that our CSF MRI sequence estimates the ICV as systematically larger than the T1-based segmentation method by about 230 ml. This is probably due to a slight difference, in between both methods, in the exact (subvoxel) localization of the border between CSF and tissue. As well, small amounts of variation may be explained by partial volume effects in either the 3D-T1w sequence, caused by the inclusion of brain tissue in CSF voxels, or by partial volume effects in our CSF MRI sequence, which are discussed below. More recent segmentation software may perform even better, but the scope of our comparison was to compare to standard available segmentation software.
An earlier study demonstrated that over half of the variation in brain volume can be explained by ICV and a quarter of the variation in brain volume can be explained by gender [19]. This was confirmed in our study, as we demonstrated a significantly different ICV in male compared to female subjects. The strength of the approach presented in this paper is that it accounts for this variation by expressing CSF volumes as a ratio to the ICV.
We found an increase in the T2,CSF with increased visual ratings of global cortical and medial temporal lobe atrophy. Qin suggested that the regional variation in T2,CSF is likely explained by variation in the composition of the CSF [9]. The CSF composition in patients can be different due to both differences in O2 partial pressure and differences in CSF protein content. Zaharchuk found a profound effect of oxygen partial pressure on the longitudinal relaxation rate (T1) of the CSF [28]. Even more so, they showed that inhalation of 100 % oxygen changed the T1 of CSF, an effect which was most pronounced in the cortical sulci [29]. This matches with our findings, which show that the T2 of the peripheral subarachnoidal CSF has a stronger relationship with the GCA and MTA scale than the T2 of the ventricular CSF. We hypothesize that subjects with more brain atrophy have a lower oxygen content of CSF. Small vessel disease could lead to decreased brain perfusion and thus decreased oxygen diffusion from the blood vessels towards the CSF. A decrease in oxygen content would lead to a longer T2, which would correspond to our observations. Unfortunately, we were not able to measure the partial oxygen pressure of CSF in our subjects and thus to investigate this phenomenon. Further research with dedicated measurements of the partial oxygen pressure in the CSF are needed to investigate this. So far, the partial oxygen pressure of the CSF within this patient population has not been investigated, as the measurements are rather difficult to perform. Besides oxygen pressure, changes in the protein content of the CSF related to pathology should be investigated as a potential explanation for the regional variation in T2 of the CSF. Moreover, though Qin [9] suggested variation in composition of the CSF as the most likely source for variation in T2 of the CSF, it may still be possible that the T2 mapping sequence yields systematic errors in the estimated T2. These might be related to partial volume effects and imperfections in the excitation and refocusing pulses (heterogeneity in the transmit RF field, B1). Thus, the reproducibility of the method and its robustness against B1 variation (due to the use of an MLEV T2 preparation scheme) should be established in future studies before firm conclusions on the origin of regional T2 variation can be drawn.
In this paper, an MLEV scheme was selected based on the initial work of Qin [9]. The advantage of the pre-pulse approach is that it can match resolution and coverage to other functional scans such ASL and functional MRI. This increases the abilities to perform partial volume corrections within these modalities, which is especially important in populations with varying degrees of atrophy.
Although the CSF MRI sequence seems promising, there are some limitations. One of them is partial volume effects, which could have potentially influenced our results and the found correlations. However, several observations demonstrate that the partial volume effects had no major influence on the results. First, the ventricles should be more or less free of partial volume effects, and we demonstrated that the correlations with the GCA and MTA scores hold true even when only the ventricular CSF volume or T2,CSF were evaluated. In addition, eroding the ventricular masks to smaller ROIs only had a minor influence on the T2,CSF values. Second, we investigated the influence of partial volume effects by comparing the results obtained with a low resolution (3 × 3 × 6 mm3) CSF MRI sequence to the results obtained with a high resolution CSF MRI sequence (1 × 1 × 4mm3) and found no significant difference between the peripheral subarachnoidal CSF volumes. However, the difference in between the T2,CSF, measured with both the low and high resolution CSF MRI sequence in the peripheral subarachnoidal space, was significant. As explained before, this may indicate that the T2 has a higher sensitivity to partial volume effects than the CSF volume measurements.
In conclusion, we demonstrated that a fast CSF MRI sequence has the potential to replace qualitative imaging atrophy scales and supplement conventional segmentation methods. The method is advantageous, as it requires little post-processing time and no user interaction. The T2,CSF should be investigated more thoroughly, as it could be a marker for neurodegenerative diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 654 kb)
Acknowledgements
This study was approved by the medical ethical review board of the University Medical Center Utrecht (Utrecht, The Netherlands). This research was supported by the Dutch Technology Foundation STW (grant number: 11047), Applied Science Division of the NWO, the technology program of the Ministry of Economic Affairs and the ZonMW Electromagnetic Fields and Health Program (grant numbers: 5300005 and 16126322). The research leading to these results has also received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n°337333 (JS and JZ). We thank the people involved in the Utrecht Vascular Cognitive Impairment memory clinic projects for patient recruitment and assessment. The guarantor of this study is Jeroen Hendrikse. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Written informed consent was obtained from all subjects (patients) in this study. None of the study subjects or cohorts have been previously reported. Methodology: prospective, diagnostic study, performed at one institution.
Abbreviations
- AD
Alzheimer´s disease
- CSF
Cerebrospinal fluid
- EPI
Echo planar imaging
- eTE
Effective echo time
- GCA
Global cortical atrophy
- ICV
Intracranial volume
- MCI
Mild cognitive impairment
- M0
Equilibrium magnetization
- MLEV
Malcolm Levitt’s CPD sequence
- MRI
Magnetic resonance imaging
- MTA
Medial temporal lobe atrophy
- NS
Non selective
- ROI
Region-of-interest
- Sd
Standard deviation
- SVD
Small vessel disease
- T1
Longitudinal relaxation rate
- T2
Transverse relaxation rate
- T2, CSF
The T2 of cerebrospinal fluid
- T2-FLAIR
T2 fluid attenuation inversion recovery sequence
References
- 1.Wardlaw JM, Smith EE, Biessels GJ. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 3.Norrving B. Lacunar infarcts: no black holes in the brain are benign. Pract Neurol. 2008;8:222–28. doi: 10.1136/jnnp.2008.153601. [DOI] [PubMed] [Google Scholar]
- 4.Bouwman FH, Schoonenboom SN, van der Flier WM, et al. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol Aging. 2007;28:1070–1074. doi: 10.1016/j.neurobiolaging.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P, Pasquier F, Weerts JG, Barkhof F, et al. Qualitative assessment of cerebral atrophy on MRI: inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol. 1997;37:95–99. doi: 10.1159/000117417. [DOI] [PubMed] [Google Scholar]
- 6.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in "probable" Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheltens P, Launer LJ, Barkhof F, et al. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242:557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 8.Horska A, Calhoun VD, Bradshaw DH, et al. Rapid method for correction of CSF partial volume in quantitative proton MR spectroscopic imaging. Magn Reson Med. 2002;48:555–558. doi: 10.1002/mrm.10242. [DOI] [PubMed] [Google Scholar]
- 9.Qin Q. A simple approach for three-dimensional mapping of baseline cerebrospinal fluid volume fraction. Magn Reson Med. 2011;65:385–391. doi: 10.1002/mrm.22705. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koedam EL, Lehmann M, van der Flier WM, et al. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21:2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahue MJ, Lu H, Jones CK, et al. Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med. 2006;56:1261–1273. doi: 10.1002/mrm.21072. [DOI] [PubMed] [Google Scholar]
- 14.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC. doi:10.1176/appi.books.9780890425596
- 17.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core team. R: a language environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, url http://www.R-project.org/. 2013.
- 19.Aribisala BS, Valdes Hernandez MC, Royle NA, et al. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2013;23:1084–1092. doi: 10.1007/s00330-012-2677-x. [DOI] [PubMed] [Google Scholar]
- 20.Scheltens PH. Structural neuroimaging of Alzheimer's disease and other dementias. Aging (Milano) 2001;13:203–209. doi: 10.1007/BF03351478. [DOI] [PubMed] [Google Scholar]
- 21.Jack CR, Jr, Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/WNL.42.1.183. [DOI] [PubMed] [Google Scholar]
- 22.Barber R, Gholkar A, Scheltens P, et al. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999;52:1153–1158. doi: 10.1212/WNL.52.6.1153. [DOI] [PubMed] [Google Scholar]
- 23.Bastos-Leite AJ, van der Flier WM, van Straaten EC, et al. The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke. 2007;38:3182–3185. doi: 10.1161/STROKEAHA.107.490102. [DOI] [PubMed] [Google Scholar]
- 24.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/WNL.46.3.678. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann M, Koedam EL, Barnes J, et al. Visual ratings of atrophy in MCI: prediction of conversion and relationship with CSF biomarkers. Neurobiol Aging. 2013;34:73–82. doi: 10.1016/j.neurobiolaging.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Whitwell JL, Jack CR, Jr, Przybelski SA, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32:1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Pol LA, Hensel A, van der Flier WM, et al. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:439–442. doi: 10.1136/jnnp.2005.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaharchuk G, Martin AJ, Rosenthal G, et al. Measurement of cerebrospinal fluid oxygen partial pressure in humans using MRI. Magn Reson Med. 2005;54:113–121. doi: 10.1002/mrm.20546. [DOI] [PubMed] [Google Scholar]
- 29.Zaharchuk G, Busse RF, Rosenthal G, et al. Noninvasive oxygen partial pressure measurement of human body fluids in vivo using magnetic resonance imaging. Acad Radiol. 2006;13:1016–24. doi: 10.1016/j.acra.2006.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 654 kb)