Abstract
Tuberculosis has a high morbidity and mortality worldwide. Mycobacterium tuberculosis (Mtb) has a complex pathophysiology; it is an aerobic bacillus capable of surviving in anaerobic conditions in a latent state for a very long time before reactivation to active disease. In the latent tuberculosis infection, the individual has no clinical evidence of active disease, but exhibits a hypersensitive response to proteins of Mtb. Only some 5–10 % of latently infected individuals appear to have reactivation of tuberculosis at any one time point after infection, and neither imaging nor immune tests have been shown to predict tuberculosis reactivation reliably. The complex pathology of the organism provides multiple molecular targets for imaging the infection and targeting therapy. Positron emission tomography (PET) integrated with computer tomography (CT) provides a unique opportunity to noninvasively image the whole body for diagnosing, staging and assessing therapy response in many infectious and inflammatory diseases. PET/CT is a powerful noninvasive tool that can rapidly provide three-dimensional views of disease deep within the body and conduct longitudinal assessment over time in one particular patient. Some PET tracers, such as 18F-fluorodeoxyglucose (18F-FDG), have been found to be useful in various infectious diseases for detection, assessing disease activity, staging and monitoring response to therapy. This tracer has also been used for imaging tuberculosis. 18F-FDG PET relies on the glucose uptake of inflammatory cells as a result of the respiratory burst that occurs with infection. Other PET tracers have also been used to image different aspects of the pathology or microbiology of Mtb. The synthesis of the complex cell membrane of the bacilli for example can be imaged with 11C-choline or 18F-fluoroethylcholine PET/CT while the uptake of amino acids during cell growth can be imaged by 3′-deoxy-3′-[18F]fluoro-l-thymidine. PET/CT provides a noninvasive and sensitive method of assessing histopathological information on different aspects of tuberculosis and is already playing a role in the management of tuberculosis. As our understanding of the pathophysiology of tuberculosis increases, the role of PET/CT in the management of this disease would become more important. In this review, we highlight the various tracers that have been used in tuberculosis and explain the underlying mechanisms for their use.
Keywords: PET/CT, Tuberculosis, 3′-Deoxy-3′-[18F]fluoro-l-thymidine, 18F-fluoroethylcholine, 68Ga-citrate
Introduction
Epidemiology
Tuberculosis (TB) remains a threat to humans with high mortality, rising incidence of multidrug resistance and HIV co-infection despite the availability of relatively cheap and effective treatment options [1–3]. TB kills 1.5 million people annually and 9.6 million develop the disease annually [2]. Most of these deaths are preventable and therefore the death toll is unacceptably high [4]. It is currently the second highest infective cause of death worldwide, only surpassed by human immunodeficiency virus (HIV). With first cases dating back 9000 years, Mycobacterium tuberculosis (Mtb), the causative agent of TB, is one of the most successful human pathogens of all time [5]. An increasing proportion of the disease is caused by organisms insensitive to first-line chemotherapeutic agents (multidrug-resistant TB [MDR-TB]), first- and some second-line agents (extensively drug-resistant TB [XDR-TB]) or even all agents (super-extensive drug-resistant TB [SXDR-TB]) [6, 7]. This is a major health-care problem worldwide, since treatment of MDR-TB and even worse XDR-TB is more challenging and expensive than drug-susceptible TB [8, 9]. In 95 % of infected individuals, the pathogen is contained as an asymptomatic latent infection. It has been estimated that a third of the world’s population harbors latent TB [10]. The perilous union of TB with HIV also represents a challenging public health priority as HIV weakens our most effective barrier against TB, our immune system, rendering infected individuals more susceptible to TB [11]. HIV causes a sharp increase in the number of LTBI patients who progress to active disease [12]. HIV co-infection also presents diagnostic challenges, potentially delaying diagnosis and treatment and thereby increasing morbidity and mortality. As a consequence of this syndetic interaction, 1.2 million patients with HIV developed TB and 400,000 people co-infected with TB and HIV died [2]. Despite the enormous burden of TB, current diagnostic methods are woefully inadequate to meet clinical and research needs [13].
Pathophysiology
Mtb is an aerobic, obligate intracellular microorganism that features an unusually complex and thick cell wall. Hallmarks are long-chain fatty acids called mycolic acids that surround the bacterial cytoplasmic membrane. The characteristic features of the Mtb include its potential to persist in host cells, slow growth, complex membrane and intracellular pathogenesis [14]. Mtb persists in host cells in a dormant, latent or persistent state using a specific genetic program to respond to stress [15, 16]. This program, formerly referred to as Dos Regulon, now DevR activity, is essential for regulon induction and hypoxic survival of Mtb [17]. Latency is defined clinically by reactive tuberculin skin test indicating delayed hypersensitivity to Mtb antigens in the absence of active disease. Persistence is used to describe to the state in which Mtb survives in host tissues under various stress conditions. Dormancy refers to a state in which Mtb remains quiescent within infected cells and is the result of metabolic and replicative shutdown of the bacillus using its DevR activity, resulting from the action of a cell-mediated response of the host that can contain but not eradicate the infection [18]. The generation time of actively replicating Mtb in synthetic medium or infected animals is about 24 and 18 h in humans [19, 20]. This contributes to the chronic nature of the disease, imposing lengthy treatment regimens and presenting a formidable obstacle for researchers. The slow growth of Mtb necessitates long antibiotic therapy rendering treatment susceptible to failure due to non-adherence [21]. The drugs used involve unpleasant side effects, and travel to treatment posts poses economic difficulties to patients. Notably, treatment failure is the major fuel for the development of drug resistance [22]. The mycobacterial cell wall is impermeable to a number of compounds, a feature in part responsible for inherent resistance to numerous drugs [23]. While mycobacteria are considered Gram positive, the second membrane executes biological functions comparable to the outer membrane of Gram-negative bacteria, such as the uptake of small hydrophilic nutrients via special membrane channels [24]. This protective outer membrane plays an important role in securing the bacillus’ integrity in the face of harsh environmental conditions [25]. This outer compartment of the cell wall consists of both lipids and proteins, some of which are linked to polysaccharides. The lipid-linked polysaccharides associated with the outer cell wall consist of lipoarabinomannan (LAM), lipomannan and phthiocerol-containing lipids such as phthiocerol dimycocerosate, dimycolyl trehalose (cord factor), sulfolipids and the phosphatidylinositol mannosides [23]. The pathogenic effects of some of the lipids include the following: LAM inhibits T cell proliferation and has bactericidal action of macrophages amidst other actions. Cord factor, another glycolipid, inhibits phagosome–lysosome fusion, contributing to the maintenance of the granuloma response. It is toxic to macrophages, killing them on contact [26, 27]. The success of Mtb as a pathogen lies in its ability to orchestrate its metabolic pathways to survive in a nutrient-deficient, acidic, oxidative, nitrosative and hypoxic environment inside granulomas or infective lesions and survive in its host for months to decades in an asymptomatic state, using DevR activity [28, 29]. The pathogenic potential of Mtb also depends largely on the type VII secretion system ESX-1, which is largely responsible for the secretion of early secreted antigenic target (ESAT-6), culture filtrate protein (CPF-10) and several other ESX-1 associated proteins. The ESX-1 governs numerous aspects of interaction between Mtb and the host cell. The ESX-1 system possesses membrane-damaging activity, allowing Mtb to escape from Mycobacterium-containing vacuole into host cell cytosol, where it polymerizes with actin and spreads from cell to cell, particularly in the later stage of the infection [30–32].
Transmission and disease progression
Mtb is transmitted as aerosol generated by the respiratory system, and in 95 % of cases in which the bacilli are inhaled, a primary infection is established [33]. The cell-mediated immunity of the host results in either the clearing of the infection or the restriction of the bacilli inside granulomas giving rise to a latent TB infection (LTBI), defined by no visible symptoms of disease, but dormant and yet alive bacilli in the host. The progress of TB can be stalled at this stage in some cases by isoniazid—or other regimens of preventive therapy [34]. This state might last for the entire life span of the individual or progress to active TB by reactivation of the existing infection with a lifetime risk of 5–10 % [35]. In the presence of HIV, this risk increases with 5 % of LTBI reactivating per year [2]. Reactivation of TB usually occurs at the upper more oxygenated lobe of the lung. This can be cured by treatment. In untreated or poorly treated cases, TB lesions develop within the lung. These lesions include caseous necrosis, fibrosis and cavities. The development of cavities close to airways allows shedding of bacilli into airways and subsequent transmission to other people as aerosol.
Clinical symptoms and risk factors
The classic features of pulmonary TB include chronic cough, weight loss, fever, night sweats and hemoptysis [36]. The risk for development of active TB disease is governed by exogenous and endogenous factors. Exogenous factors accentuate the progression from exposure to infection. Bacillary load in the sputum of the infected person, duration and proximity to an infectious TB case are key factors. Endogenous factors, on the other hand, lead to the progression from infection to active TB disease [37]. Malnutrition, tobacco smoking and indoor air pollution from solid fuel have been documented to be most important risk factors for TB worldwide, followed by HIV infection, diabetes and excessive alcohol intake [38]. Extrapulmonary TB occurs in 10–42 % of patients. The occurrence of extrapulmonary disease depends on the age, presence or absence of underlying disease, ethnic background, immune status of the individual and the strain or lineage of Mtb [37]. The disease may occur in any part of the body and can mimic a lot of clinical diseases, which potentially delays the diagnosis. HIV co-infection with TB presents major challenges to the diagnosis and treatment of TB. The manifestation of TB varies depending on the immune status of the host. Soon after HIV infection, TB presentation is similar to HIV seronegative individuals. As the CD4 count drops, the presentation becomes atypical, with atypical pulmonary manifestations and a greater proportion of patients (more than 50 % in some cases) presenting with extrapulmonary disease. At very low CD4 counts, the pulmonary features of disease may be completely absent and disseminated TB may present as a nonspecific febrile illness with high mortality, in which clinical diagnosis may be completely missed and will only be discovered at autopsy [39–42].
Diagnosis of tuberculosis
Robert Koch first used sputum microscopy and culture to identify TB over 130 years ago. The diagnosis of active TB in many parts of the world has still remained the same [43]. Although inexpensive and accessible, the technique is operator dependent and has a poor sensitivity (45–80 %). Sputum culture has a specificity of 98 %, but it takes 2–8 weeks for results to be available depending on culture media and bacillary burden [44]. Furthermore, sputum is difficult to collect from infants and children and the sensitivity of microscopy of direct acid- and alcohol-fast stains is low, often requiring multiple samples from an individual before the diagnosis can be established [45, 46]. Sputum culture is considered the golden standard for the diagnosis; liquid media have largely replaced solid culture media, e.g., the Lowenstein–Jensen slope; liquid media have been shown to considerably improve sensitivity and speed in diagnosis of active TB [47]. The diagnosis of LTBI differs from active TB. LTBI by definition refers to a clinical state where one has Mtb infection without evidence of disease. It is diagnosed by positive immunological response to proteins of Mtb in the absence of clinical or radiological findings. Traditionally, the Mantoux tuberculin skin test (TST) has been used to assess the immunological response. This test is conducted by injecting 0.1 ml of a tuberculin-purified protein derivative by the intradermal route on the inner surface of the forearm. This should produce a discrete pale elevation of the skin of about 6–10 mm when done correctly. The test is read by measuring the diameter of the induration of the skin produced. The test is interpreted along with the clinical risk of the individual. While an induration of more than 15 mm is positive, in a person with high risk for disease such as contact with a TB patient a diameter of 10 mm is considered positive. The test has limitations of being falsely negative in particularly immunocompromised patients, while it gives false-positive results in other patients with other mycobacteriosis or people who have had previous vaccinations against TB [47].
Imaging in response to diagnostic limitations
Plain chest radiography (and in affluent settings, also CT) are the mainstays for diagnostic imaging of pulmonary TB, but are often nonspecific and unable to provide a definitive diagnosis due to the heterogeneous presentation, particularly in case of HIV co-infection when CD4 counts are low [48–50]. On radiographs, primary TB is represented by consolidation (Ghon focus), adenopathy and pleural effusion. The Ghon focus most commonly occurs in the mid and lower lung zones. When there is hilar lymphadenopathy in addition to the Ghon focus, a Ghon complex is formed. The radiological features of reactivation TB include focal patchy opacities, cavitation, fibrosis, nodal calcification and flecks of caseous material. These commonly occur in the posterior segments of the upper lobes and superior segment of the lower lobes of the lungs. These features may be completely absent in patients with severe immune deficiency. As a result of the limitations of traditional diagnostics, new approaches have been developed. The interferon gamma release assay (IGRA) is a blood test measuring cellular immune response to the TB infection similar to the traditional tuberculin skin test (TST), with similar sensitivity and improved specificity in BCG-vaccinated individuals [51]. However, neither IGRA nor TST is able to distinguish latent infection and active disease and they are both dependent on host responses, which may be compromised in immune-deficient patients and children [52, 53]. Other new diagnostic methods include urine LAM testing, which detects active TB in HIV patients with high specificity, but modest sensitivity [54]. The Xpert MTB/RIF assay rapidly detects Mtb nucleic acid in sputum, by targeting the rpoB-gene of Mtb, and at the same time genetic mutations predicting rifampicin resistance. It has a specificity of 98 %, but variable sensitivity depending on the sputum bacterial load [55, 56]. Albeit being useful for rapid initial diagnosis, it only establishes susceptibility to rifampin and is inferior to culture for monitoring treatment [57]. During treatment, subsets of Mtb within the bacterial population that reflect naturally occurring drug-resistant mutants may emerge. This usually occurs during sub-optimal treatment with inadvertent monotherapy. Most currently used diagnostic systems including liquid culture fail to detect such subsets at the start of treatment. Currently used diagnostic platforms accept that if <1 % of the bacterial population is resistant to a particular drug at one particular drug concentration (the so-called breakpoint) that is generally accepted to be achieved in blood by using the standard TB dosing regimen, that strain of Mtb should be considered susceptible to that drug. The idea behind accepting Mtb at that breakpoint is the assumption that by always using a regimen containing three to five active drugs, the treatment will not fail because the <1 % Mtb resistant to a particular drug will be covered by other components of the drug regimen. Current breakpoints recommended by EUCAST have recently been questioned. In vitro hollow fibber models mimicking plasma-drug concentration and population kinetics demonstrated that an important subset of patients might not reach target drug concentrations over time. These individuals may fail on regimes that according to the currently used EUCAST breakpoint would have drug-susceptible TB. Furthermore, very few of the currently available drugs have efficacy in slowly replicating persistent organisms [58, 59]. PET/CT may provide an important tool in the detection of this subset of Mtb population.
18F-FDG PET
PET/CT has the ability to associate the pharmacological, immunologic and microbiological aspects of TB lesions with anatomic information, allowing a holistic approach to understanding the disease [14]. The value of imaging TB with 2-[18F]fluoro-2-deoxyglucose (18F-FDG) PET/CT has been well documented [60–62]. 18F-FDG PET has been used to detect TB granulomas and assess disease activity [63, 64] and the extent of disease [65]. Efforts to use 18F-FDG PET to distinguish benign from malignant lesions have been made and results have generally not been encouraging [66, 67]. Active TB avidly takes up 18F-FDG, both in pulmonary and extrapulmonary lesions. Thus, 18F-FDG PET can be very useful to assess the extent of active TB. The detection of extrapulmonary lesions is particularly useful, as obtaining tissue or fluid for analysis may not always be possible or may be invasive. Many studies have been conducted to distinguish avid TB from malignancy and other granulomatous conditions [68–71]. Since 18F-FDG is a nonspecific tracer, it cannot reliably distinguish tuberculomas from malignant lung lesions and frequently gives rise to a false-positive diagnosis in patients evaluated for malignancy [72–74]. The overlap between the standardized uptake value (SUV) in malignant and benign lesions has led to the investigation of several dichotomization methods, such as the use of SUV cutoff thresholds, dual tracer imaging, dual time point imaging (DTPI) or delayed imaging. There is, however, no consensus about the use of 18F-FDG PET to differentiate TB from malignancy or other granulomatous or other inflammatory lesions. In a review, Cheng et al. point out that the increased specificity of dual-time point imaging depends on several factors and therefore recommend the selective use of DTPI to improve the diagnostic accuracy and interpretation confidence only in specific situations These situations include obese, overweight or poorly controlled diabetic patients who have high background uptake of 18F-FDG [75]. It is important for the interpretation of an 18F-FDG PET scan to have a high suspicion for TB, particularly in a TB-endemic area or for an immigrant from an endemic area now living in an area with low TB prevalence [76]. Other studies have evaluated the use of 18F-FDG PET in differentiating latent from active TB [77, 78]. TB has recently been shown to be more dynamic than previously thought [79, 80]. The infection occupies a more diverse spectrum rather than simply latent and active disease (Fig. 1). Thus, while high uptake in a lesion in a patient with TB may represent active disease, it may also represent a host immune system response that will eventually prevail [78]. It is therefore prudent to exercise when interpreting high 18F-FDG uptake as active TB in a patient with no known history of active disease or symptoms of TB, but only a positive tuberculin skin test or interferon gamma release assay. 18F-FDG PET has also been used to evaluate treatment response during and after therapy (Fig. 1). Treatment of TB is a lengthy process, usually taking at least 6 months, and resistant Mtb species are emerging. It is important to have an early test to predict outcome early in the course of treatment to enable timely change to appropriate therapy to prevent resistant species. 18F-FDG PET has been shown to be very useful in monitoring treatment response in this regard [81, 82]. Other studies also used 18F-FDG PET to assess response on completion of treatment and some studies, in particular in the context of MDR-TB, have shown patients remaining free of TB months after completing treatment [83–85]. 18F-FDG has also been evaluated and found to be useful for assessing TB in specific organs of the body, such as tuberculous infections of the skeleton. It has been shown to help in distinguishing acute pyogenic from chronic tuberculous spondylitis [86–88]. 18F-FDG PET has, however, not been useful in distinguishing TB from atypical TB, sarcoidosis and HIV-associated lymphadenopathy [89–91]. An overview of original publications involving 18F-FDG PET in more than one patient with TB is presented in Table 1. The articles were found by entering PET/CT and tuberculosis in medical Pubmed and all the references of those articles were reviewed for additional references. Publications including only one case were excluded.
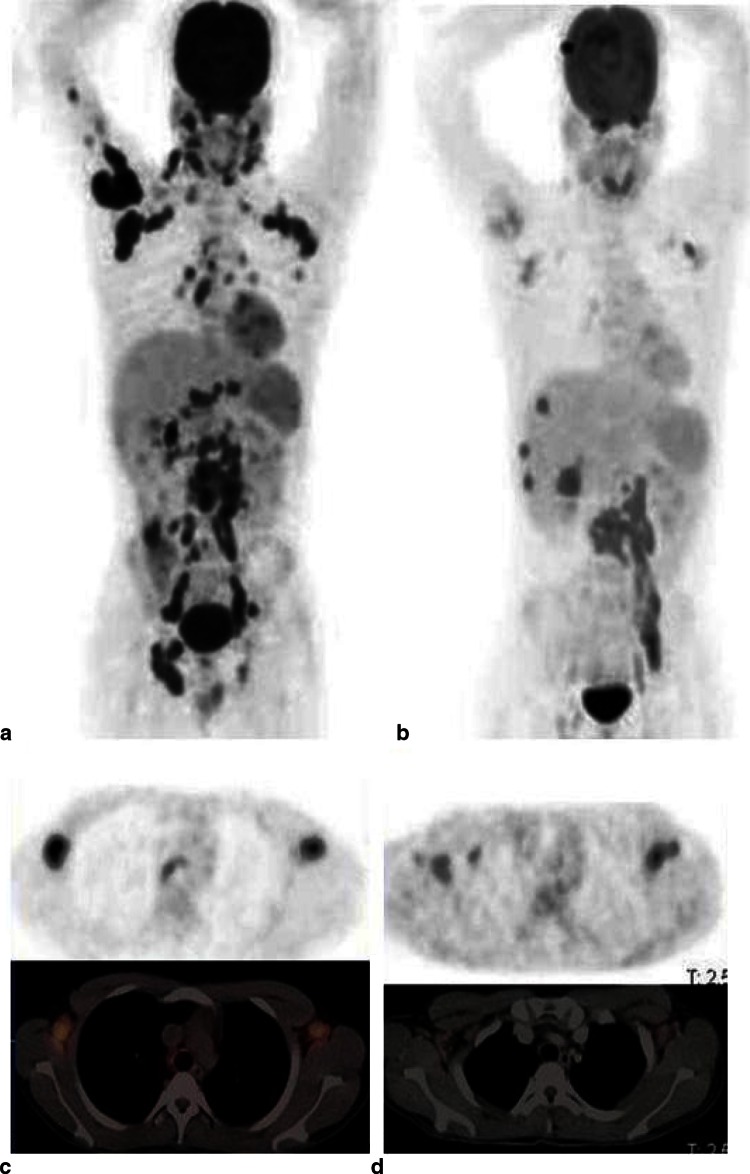
Fig. 1.
18F-FDG PET/CT scan before anti-TB treatment and 2 months after initiation of treatment for interim assessment of treatment response. a Maximum intensity projection (MIP) image before treatment (PET images only), showing extensive disease: pulmonary, cervical, axillary, mediastinal, abdominal, pelvic and inguinal lymph nodes, hepatic and skeletal metastasis to the lumbar spine and right humerus. b MIP image after 2 months of anti-TB treatment (PET images only): complete metabolic response of the pulmonary and right humeral lesions and the pelvic and inguinal lymph nodes. Good metabolic response in the mediastinal, cervical and axillary nodes. Active disease is still present in the lumber spine with progression of the hepatic lesions. c Transverse scans showing axillary nodes before treatment (PET and integrated PET/CT images). d Transverse scans showing response of axillary nodes after 2 month of anti-TB therapy; nodal uptake diminished but still present
Table 1.
Original articles on the use of 18F-FDG PET in TB
| Journal/year | 1st author | Use | No. of TB pts/pts studied | Major finding of 18F-FDG and TB | Sens (%) | Spec (%) |
|---|---|---|---|---|---|---|
| Ann Nuc Med 1996 | Ichiya et al. [63] | 1, 6 | 8/24 | Detected and assessed activity in TB lesions, but was unable to distinguish TB from MAC* | na | Na |
| Radiology 2000 | Goo et al. [64] | 1, 3 | 10/10 | Active tuberculomas were 18F-FDG avid and caused false positives in cancer evaluation | na | Na |
| Chest 2003 | Hara et al. [66] | 1, 3, 6 | 14/116 | TB, atypical TB and cancer were discriminated by performing both 18F-FDG and 11C-choline PET scans | na | Na |
| Neoplasia 2005 | Mamede et al. [67] | 1, 3 | 10/60 | Uptake correlated with inflammation of TB lesions causing false-positive results in cancer | 87–97.8 | Na |
| Tuberculosis 2007 | Hofmeyr et al. [83] | 3, 5 | 2/2 | Was useful in TB diagnosis in high-risk patients and in monitoring anti-TB treatment | na | Na |
| Clin Nuc Med 2008 | Park et al. [84] | 5 | 2/2 | Was useful in assessing response to anti-TB therapy in patients with tuberculoma | na | Na |
| EJNMMI 2008 | Yen et al. [68] | 3 | 8/96 | TB was a major cause of false positives in evaluating lymph nodes in lung cancer | 73.8 | 88.9 |
| EJNMMI 2008 | Kim et al. [75] | 4 | 25/25 | Assessed TB activity by visual assessment and SUV change from early to delayed scan | 71.4–100 | 81.8–100 |
| EJNMMI 2009 | Demura et al. [81] | 1, 4, 5 | 25/47 | Distinguished latent TB from active TB and in monitoring anti-TB therapy response | na | Na |
| Nuc Med Comun 2009 | Castaigne et al. [103] | 1, 6 | 6/10 | Was useful in detecting TB as a cause of fever of unknown origin in HIV patients | na | Na |
| Pediatr Surg Int 2009 | Hadley et al. [68] | 3, 6 | 3/18 | Was a major cause of false positive for cancer in HIV children | na | Na |
| Spine 2009 | Kim et al. [86] | 5, 6 | 11/30 | Had prognostic value in anti-TB therapy of the spine and detected residual disease | 85.7–100 | 68–82.6 |
| Lung 2010 | Hahm et al. [90] | 1, 6 | 26/41 | Was unable to distinguish TB from MAC | na | Na |
| World J Gastroenterol 2010 | Tian et al. [70] | 3 | 3/3 | Was a cause of false positive in assessing abdominal malignancy | na | Na |
| Nuklearmedizin 2010 | Sathekge et al. [65] | 2, 7 | 16/16 | Detected more extensive disease when compared to contrast-enhanced CT | na | Na |
| Acta Radiol 2010 | Tian et al. [92] | 5 | 3/3 | Was useful in assessing response to treatment in non-pulmonary TB | na | Na |
| S Afr Med J 2010 | Sathekge et al. [93] | 3 | 12/30 | Was not useful in differentiating benign from malignant lesions in a TB-endemic area | 87 | 25–100 |
| QJNMMI 2010 | Sathekge et al. [89] | 3, 6 | 37/83 | Was not useful for assessing malignancy in lymph nodes in TB, HIV or TB and HIV co-infection | Na | Na |
| Nuc Med Commun 2011 | Kim et al. [87] | 6 | 8/23 | Was useful in distinguishing TB spondylitis from pyogenic spondylitis | 86.6 | 62.9 |
| Ann Nuc Med 2011 | Li et al. [94] | 3 | 8/96 | TB caused high false positives for cancer with PET only; accuracy improved with combined PET/CT | 96.7 | 75.7 |
| Ann Thoracic Med 2011 | Kumar et al. [95] | 1, 3 | 12/35 | Increased SUV cutoff improved specificity and with acceptable sensitivity in mediastinal node evaluation | 87–93 | 40–70 |
| J Korean Med Sci 2011 | Lee et al. [71] | 3, 6 | 54/54 | Found low accuracy in the evaluation of lung cancer pts with parenchymal sequelae from previous TB | 60 | 69.2 |
| J Nucl Med 2011 | Sathekge et al. [82] | 1, 2, 5 | 24/24 | Was useful to predict HIV patients who would respond to anti-TB therapy | 88 | 81 |
| Eur J Rad 2012 | Soussan et al. [96] | 2 | 16/16 | Found 2 distinct patterns of pulm TB uptake | na | Na |
| EJNMMI 2012 | Sathekge et al. [97] | 5, 7 | 20/20 | Was useful in distinguishing lymph nodes responding to anti-TB from those that did not | 88–95 | 66–85 |
| Int J Tuberc Lung dis 2012 | Martinez et al. [98] | 5 | 21/21 | Was useful in evaluating early therapeutic response to anti-TB | na | Na |
| BMC Pulm Med 2013 | Heysell et al. [78] | 2, 4 | 4/4 | Demonstrated the usefulness in the management of high-risk TB pts who are sputum negative | na | Na |
| Eur Spine J 2014 | Dureja et al. [88] | 5 | 33/33 | SUVmax was found to be a quantitative marker for response in spinal TB | na | Na |
| Sci Trans Med 2014 | Coleman et al. [99] | 5 | 18/18 | Demonstrated usefulness of assessing the response of anti-TB in macaques and pts with XDR-TB | 96 | 75 |
| J Korean Med Sci 2014 | Jeong Y-J et al. [100] | 1 | 63/63 | Found pts with old healed lesions with high SUV to be at risk for development of active TB | na | Na |
| Sci Trans Med 2014 | Chen et al. [85] | 5 | 28/28 | Demonstrated that changes at 2 months of anti-TB are early predictors of the final outcome in MDR-TB | na | Na |
| Chest 2014 | Maturu et al. [91] | 6 | 29/117 | Did not find any significant difference in the findings in TB and sarcoidosis | na | Na |
| Nuc Med Commun 2015 | Huber et al. [101] | 3, 6 | 122/207 | Found more likely to detect cancer in the evaluation of granulomatous lesions in pts > 60 years | na | Na |
| EJNMMI 2015 | Fuster D et al. [102] | 7 | 4/26 | Recommended 18F-FDG should be considered first line in the imaging of spondylodiscitis | 83 | 88 |
When an article evaluated more than one feature of TB, then the sensitivity and specificity apply to the use indicated by the number highlighted in italics and bold
Pts patients, MAC Mycobacterium avium complex
* Pulm pulmonary
1To detect TB lesions and assess disease activity
2To assess the extent of disease
3To assess the effect of TB on cancer staging or diagnosis with 18F-FDG
4To differentiate latent from active TB
5To monitor treatment response
6To assess the ability to differentiate TB from nonmalignant conditions, including atypical mycobacteria, as well as to assess the effect TB has on 18F-FDG imaging of nonmalignant conditions
7To compare the detection of TB by PET with other modalities
Other PET tracers
Besides 18F-FDG, other PET tracers have been investigated for imaging of TB, including 11C-choline, [18F]fluoroethylcholine (18F-FEC), 3′-deoxy-3′-[18F]fluoro-l-thymidine (18F-FLT), 68Ga-citrate, [18F] sodium fluoride (18F-NaF) and radiolabeled anti-TB drugs (Table 2). The wall of Mtb consists of many complex lipids. 11C-choline or 18F-FEC can image the transportation and utilization of choline in this lipid-rich envelope of Mtb. The incorporation of thymidine into the DNA of bacteria can be imaged by the thymidine analog 18F-FLT during the proliferation of Mtb. The uptake of 68Ga-citrate by TB can be extrapolated from studies with 67Ga-citrate and is dependent on specific and nonspecific uptake mechanisms. Nonspecific mechanisms include increased vascular permeability in areas of inflammation, while specific factors include binding to the siderophores the bacteria used to trap iron from hosts’ transferrin and other iron sources. 18F-NaF has a strong binding affinity for calcium and can be used to visualize micro-calcifications in old TB lesions. Drugs used for the treatment of TB have also been labeled with radioisotopes and can be used to study the biodistribution and pharmacokinetics of these drugs.
Table 2.
Mechanism of PET tracer uptake in TB
| Tracer | Clinical or pre-clinical (animal model used) | Mechanism of uptake | Use(s) |
|---|---|---|---|
| 18F-fluoro-deoxy-glucose [60–103] | Clinical | Uptake during the respiratory burst by activated inflammatory cells as by glucose transporters and is phosphorylated to FDG-6-phospate and remains trapped in the cell | Assesses disease activity, staging (especially extrapulmonary) monitoring therapy and early prediction of nonresponse |
| 18F-Fluoroethylcholine or 11C-choline [104–106] | Clinical | Uptake during the synthesis of the complex lipid layer of the cell wall | Combined with FDG, helps distinguish TB from malignancy and possible role in therapy monitoring |
| 3′-Deoxy-3′-18F-fluoro-l-thymidine [107, 108] | Clinical | Uptake during the synthesis of nucleic acids as bacteria proliferates | Combined with FDG, helps distinguish TB from malignancy |
| 68Ga-citrate [109, 110] | Clinical | Accumulates in bacterial siderophores of Mtb and in plasma lactoferrin similar to 67Ga-citrate and also accumulates by nonspecific mechanisms as increased vascular permeability | Detects TB lesions and may be better than CT in the detection of extrapulmonary lesions |
| 11F-sodium fluoride [111] | Preclinical (mice) | Binds to micro-calcification in chronic TB lesions | Potentially helps to distinguish acute from chronic TB |
| 11C-Rifampicin [112] | Preclinical (baboons) | Binds to (and inhibits) Mtbs DNA-dependent RNA polymerase | Determines whether there is adequate accumulation of drug in the infected site |
| 11C-Isoniazid [112] | Preclinical (baboons) | Binds to Mtb enzymes and generates reactive oxygen species resulting in inhibition of cell wall lipid synthesis and depletion of nucleic acid pools and metabolic depression | Determines whether there is adequate accumulation of drug in the infected site |
| 11C-Pyrazinamide [112] | Preclinical (baboons) | Binds to cell membrane proteins, disrupts membrane energetics and inhibits membrane transport functions in Mtb | Determines whether there is adequate accumulation of drug in the infected site |
11C-Choline/18F-fluoroethylcholine
11C-Choline was evaluated for differentiation of lung cancer and other lesions including active TB [66, 104, 105]. While 75 % accuracy was found when using 11C-choline alone for this differentiation, combining 11C-choline and F-FDG appeared to yield better results [105]. Both tracers displayed a high uptake in malignant lesions. In TB, however, 18F-FDG uptake was much higher than 11C-choline uptake. The use of only one tracer may miss extrapulmonary disease in areas where the tracers have a physiologically high bio-distribution such as the brain for 18F-FDG and the liver for 18F-FEC [106]. 18F-FEC has been suggested to be useful for evaluation of TB therapy.
3′-Deoxy-3′-18F-fluoro-l-thymidine
Prospective studies evaluating the diagnostic value of dual tracer PET/CT in pulmonary lesions using 18F-FLT and 18F-FDG PET noted that 18F-FLT PET is most useful when combined with 18F-FDG PET. This yielded more information than either tracer used alone. Visual inspection of images and the ratio of the maximum SUV between 18F-FLT and 18F-FDG improved the diagnostic accuracy in distinguishing malignant from benign lesions including tuberculosis [107, 108].
68Ga-citrate
68Ga-citrate was shown to have good uptake in both pulmonary and extrapulmonary TB lesions and was useful in distinguishing active lesions from inactive lesions. However, the uptake was nonspecific, as it was unable to distinguish malignant from benign lesions [109, 110]. 68Ga-citrate is potentially a very useful tracer to stage disease in cases where the diagnosis is already known. Further studies are needed to see if 68Ga-citrate will have the same use as demonstrated by 18F-FDG (Table 1). 68Ga-citrate holds promise particularly in middle income and or even developing economies where PET/CT may be available, because 68Ga is produced from a generator rather than from a cyclotron. The expense and high technical demands for running a cyclotron have been obviated and the tracer will be readily available.
18F-sodium fluoride
A study demonstrated in a murine model of chronic TB the usefulness of 18F-NaF in detecting micro-calcifications, which were not visualized by CT. This approach could potentially be applied in humans and help distinguish acute from chronic TB [111].
11C-radiolabeled drug tracers
Some chemotherapeutic agents for treatment of tuberculosis, including isoniazid, rifampicin and pyrazinamide, have been labeled with carbon-11 (11C) and their biodistribution, in particular their ability to cross the blood–brain barrier, has been evaluated in nonhuman primates. There have not been any corresponding human studies with these tracers till date. The radiolabeled chemotherapeutic agents were used to show whether the drug achieved a sufficiently high concentration in infected sites, particularly in TB meningitis and TB brain abscesses. This is an important finding, as TB of the central nervous system is usually life threatening and requires long periods of treatment (usually a year) and may sometimes need to be continued even when adverse effects develop. These radiolabeled drug tracers were not assessed for detection of TB [112].
Treatment of TB
The treatment of TB depends on whether the individual has active or LTBI. Treatment of active TB requires long-term multidrug therapy to overcome tolerance, achieve bacterial clearance and reduce the risk of transmission. Tolerance is an epigenetic drug resistance widely attributed to nonreplicating bacterial subpopulations [113]. The drugs are classified as first- and second-line agents. First-line agents—class 1, WHO—include isoniazid and rifampicin, the two most potent anti-TB agents. Resistance to these agents defines a case of MDR-TB. Second-line agents are divided by the WHO into four different classes (WHO class 2–5); class 2 includes the injectables amikacin, kanamycin and capreomycin; class 3 are the fluoroquinolones; class four includes less potent, more toxic oral agents; and class 5 includes agents with as yet unknown significance. Only if Mtb isolates are susceptible, the treatment may last 6 months. Patients with large cavitary lesions that have delayed sputum culture conversion, those with M. bovis disease that is naturally nonsusceptible to pyrazinamide, those who cannot tolerate pyrazinamide and those with meningitis TB need treatment prolongation to 9 months. The so-called “short course” of 6 months for drug-susceptible TB is a major advance, as previous therapies lasted 12–18 months. Treatment is still challenging, as adhering to a multidrug regimen for 6 months has been shown to be difficult, especially in low-resource settings [114]. Despite the presence of several new drugs under investigation, attempts to shorten the treatment still remain elusive [115]. This failure highlights the poor understanding of the tolerance of Mtb. The WHO recommends isoniazid and rifampicin for treatment of drug-susceptible infections. The treatment is in two phases: initiation and continuation. The initiation phase treatment usually contains four first-line agents including rifampicin and isoniazid for 2 months, and the continuation phase consists of isoniazid and rifampicin only for the last 4 months of treatment. The current guidelines recommend the directly observed treatment (DOTS) strategy to improve adherence. This strategy involves patients taking medication under supervision. Although this has greatly improved the success of treatment, the emergence of drug resistance could not be curbed with this strategy. There are also guidelines for drug-susceptibility testing to rapidly diagnose and appropriately treat MDR-TB [116]. Although the treatment outcome of drug-susceptible TB has been fair, with successful outcome reported by most countries at around 85 %, the outcome of MDR-TB treatment has generally been poor, with successful outcome reported under service conditions at around 50 % only [2]. The reported series do slightly better at around 60 % [117] and only few national programs attain success rates at around the target set for drug-susceptible TB [118].
In patients with LTBI, treatment is recommended for persons deemed to be at high risk of developing active disease. It is important that treatment is only initiated after active disease has been excluded by clinical and radiographic means. A failure to do so will result in inadequate treatment and development of resistant species. The preferred treatment is isoniazid daily for 9 months. The WHO recently developed the guidelines for latent TB treatment. The identification of people at risk considers factors such as the level of income of a country, prevalence of TB in a country, presence of other diseases, conditions like HIV infection and use of gamma interferon. Other guidelines combine these conditions with the size of induration after a tuberculin skin test [2, 119, 120].
Conclusions and future perspectives
18F-FDG PET/CT is a sensitive noninvasive biomarker for the detection, staging, assessing disease activity and monitoring therapy in TB. The complex and long period required for the treatment of TB makes 18F-FDG PET particularly useful, as it is able to detect at an early point in treatment drug combinations that are ineffective and lead to a change in therapy. This is not only important to reduce morbidity and mortality in the individual, but prevents the even greater public health hazard of the individual developing resistant species of Mtb and transmitting the resistant strain in the community.
PET/CT provides a unique opportunity for the in vivo histological characterization of TB lesions. This role is becoming more and more important as the molecular basis of TB is elucidated. These tracers provide an ideal platform for personalized medicine in TB treatment. PET/CT has played and continues to play a major role in the development of new drugs and therapeutic strategies like vaccines. This role will hopefully expand in the future with the development of new tracers and repurposing of existing tracers. For example, MDR-TB and LTBI have hypoxia as one of the main processes underlying their pathology. The antibiotic metronidazole that is a pro-drug activated by hypoxia has been shown to be useful in the treatment of MDR-TB [121]. Hypoxic PET tracers already validated for the management of cancer could potentially play a role in the management of TB. In conclusion, PET/CT has demonstrated its usefulness in managing different aspects of tuberculosis disease and the development of new therapeutic interventions. The role of PET/CT is likely to grow further as we aim to eradicate TB by 2015 [2].
Acknowledgments
We are grateful to Dr. Mathias I Gröschel (MD) of the Department of Internal Medicine, Pulmonary Diseases and Tuberculosis at the University Medical Center of Groningen, Groningen, The Netherlands, for his useful suggestions particularly on the pathogenesis of TB.
Authors’ contributions
Ankrah contributed to literature search, literature review, data analysis and writing; van de Werf to content planning, editing and project development; de Vries to content planning, editing and project development; Dierckx to content planning, editing and project development; Sathekge to content planning, editing and project development; Glaudemans to content planning, writing, editing and data analysis.
Compliance with ethical standards
Conflicts of interest
None.
Disclosure statement
The authors have nothing to disclose.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and the national regulations and also with the principles of the 1964 Declaration of Helsinki and its later amendments as far as they are required for this type of retrospective study.
References
- 1.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(5):183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (2015) Global tuberculosis report 2015 (20th edition): www.who.int/tb/publications/global_report. Assessed 23 Nov 2015
- 3.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368(8):745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 4.Zumla A, George A, Sharma V, Herbett RH, Baroness Masham of Ilton. Oxley A, Oliver M. The WHO 2014 global tuberculosis report—further to go. Lancet Global Health. 2015;3(1):e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 5.Hershkovitz I, Donoghue HD, Minnikin DE, Besra GS, Lee OY, Gernaey AM, et al. Detection and molecular characterization of 9,000-year old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS ONE. 2008;3(10):e3426. doi: 10.1371/journal.pone.0003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F, Van Deun A, et al. Epidermiology of antituberculous drug resistance (The Global Project on Anti-tuberculous drug resistance): an updated analysis. Lancet. 2006;368(9553):2142–2154. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 7.Zignol M, van Gemert W, Falzon D, Sismondi’s C, Glaziou P, Floyd K, Raviglione M. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ. 2012;90(2):111D–119D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9(8):E1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resch SC, Salomon JA, Murray M, Weinstein MC. Cost-effectiveness of treating multidrug-resistant tuberculosis. PLoS Med. 2006;3(7):e241. doi: 10.1371/journal.pmed.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 11.Corbett EL, Watt CJ, Walker N, Mahar D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 12.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horshburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54(6):784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanrahan CF, Shah M. Economic challenges associated with tuberculosis diagnostic development. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):499–510. doi: 10.1586/14737167.2014.914438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson DH, Via LE, Kim P, Laddy D, Lau CY, Weinstein EA, Jain S. Nuclear imaging: a powerful novel approach for tuberculosis. Nuc Med Biol. 2014;41(10):777–784. doi: 10.1016/j.nucmedbio.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Altena R, Duggirala S, Gröschel MI, van der Werf TS. Immunology in tuberculosis: challenges in monitoring of disease activity and identifying correlates of protection. Curr Pharm Des. 2011;17(27):2853–2862. doi: 10.2174/138161211797470228. [DOI] [PubMed] [Google Scholar]
- 16.Prabowo SA, Gröschel MI, Schmidt ED, Skrahina A, Mihaescu T, Hastürk S, et al. Targeting multidrug-resistant tuberculosis (MDR-TB) by therapeutic vaccines. Med Microbiol Immunol. 2013;202(2):95–104. doi: 10.1007/s00430-012-0278-6. [DOI] [PubMed] [Google Scholar]
- 17.Gautam US, Sikri K, Vashit A, Singh V, Tyagi JS. Essentiality of DevR/DosR interaction with SigA for the dormancy survival program in Mycobacterium tuberculosis. J Bacteriol. 2014;196(4):790–799. doi: 10.1128/JB.01270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutta NK, Karakousis PC. Latent tuberculosis infection: myths, models and molecular mechanisms. Microbiol Mol Biol Rev. 2014;78(3):342–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 20.North RJ, Izzo AA. Virulent strains of Mycobacteria have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177(6):1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Kourbatova EV. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11(1):46–53. [PubMed] [Google Scholar]
- 22.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–163. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hett EC, Rubin EJ. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev. 2008;72(1):126–156. doi: 10.1128/MMBR.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faller M, Niederweis M, Schulz GE. The structure of a mycobacterial outer-membrane channel. Science. 2004;303(5661):1189–1192. doi: 10.1126/science.1094114. [DOI] [PubMed] [Google Scholar]
- 25.Houben EN, Korotkov KV, Bitter W. Take five—Type VII secretion systems of Mycobacteria. Biochim Biophys Acta. 2014;1843(8):1707–1716. doi: 10.1016/j.bbamcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Rajni Rao N, Meena LS. Biosynthesis and virulent behavior of lipids produced by Mycobacterium tuberculosis: LAM and cord factor: an overview. Biotechnol Res Int. 2011;2011:27469. doi: 10.4061/2011/274693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003;83(1–3):91–97. doi: 10.1016/S1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi A, Singh N, Bhat SA, Gupta P, Kumar A. Redox biology of tuberculosis pathogenesis. Adv Microb Physiol. 2012;60:234–263. doi: 10.1016/B978-0-12-398264-3.00004-8. [DOI] [PubMed] [Google Scholar]
- 29.Mehra S, Foreman TW, Didier PJ, Ahsan MH, Hudock TA, Kissee R, et al. The DosR Regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am J Respir Crit Care Med. 2015;191(10):1185–1196. doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simeone R, Sayes F, Song O, Gröschel MI, Brodin P, Brosch R, Majlessi L. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 2015;11(2):e1004650. doi: 10.1371/journal.ppat.1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion–mycobacteria show the way. Nat Rev Microbiol. 2007;5(11):883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 33.Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1969;99(1):109–111. doi: 10.1164/arrd.1969.99.1.109. [DOI] [PubMed] [Google Scholar]
- 34.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Exp Rev Mol Med. 2011;13:e39. doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawn SD, Zumla AL. Tuberculosis. Lancet. 2011;378(9785):57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 37.Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis—a systemic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4(3):e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Reyn CF. Optimal treatment of Codisease due to HIV and tuberculosis. J Infect Dis. 2011;204(6):817–819. doi: 10.1093/infdis/jir418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Reyn CF, Kimambo S, Mtei L, Arbeit RD, Maro I, Bakari M, et al. Disseminated tuberculosis in human immunodeficiency virus infection: ineffective immunity, polyclonal disease and high mortality. Int J Tuberc Lung Dis. 2011;15(8):1087–1092. doi: 10.5588/ijtld.10.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mudenda V, Lucas S, Shibemba A, O’Grady J, Bates M, Kapata N, et al. Tuberculosis and tuberculosis/HIV/AIDS-associated mortality in Africa: the urgent need to expand and invest in routine research autopsies. J Infect Dis. 2012;205(Suppl 2):s340–s346. doi: 10.1093/infdis/jir859. [DOI] [PubMed] [Google Scholar]
- 43.Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 2003;3(3):141–147. doi: 10.1016/S1473-3099(03)00544-9. [DOI] [PubMed] [Google Scholar]
- 44.Ichyiama S, Shimokata K, Takeuchi J. Comparative study of a biphasic culture system (Roche MB Check system) with a conventional egg media for recovery of mycobacteria. Aichi Mycobacteriosis research Group. Tuber Lung Dis. 1993;74(5):338–341. doi: 10.1016/0962-8479(93)90109-B. [DOI] [PubMed] [Google Scholar]
- 45.Zar HJ, Cornell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther. 2010;8(3):277–288. doi: 10.1586/eri.10.9. [DOI] [PubMed] [Google Scholar]
- 46.Jain SK, Ordonez A, Klinkar A, Gupte N, Thakar M, Mave V, et al. Pediatric tuberculosis in young children in India: a prospective study. Biomed Res Int. 2013;2013:783698. doi: 10.1155/2013/783698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchison DA. The diagnosis and therapy of tuberculosis during the past 100 years. Am J Respir Crit Care Med. 2005;171(7):699–706. doi: 10.1164/rccm.200411-1603OE. [DOI] [PubMed] [Google Scholar]
- 48.van Dyck P, Vanhoenacker FM, Van de Brande P, De Schepper AM. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13:1771–1785. doi: 10.1007/s00330-002-1612-y. [DOI] [PubMed] [Google Scholar]
- 49.Khan MA, Kovnat DM, Bachus B, Whitcomb ME, Broody JS, Snider GL. Clinical and roentgenographic spectrum of pulmonary tuberculosis in the adult. Am J Med. 1977;62(1):31–38. doi: 10.1016/0002-9343(77)90346-1. [DOI] [PubMed] [Google Scholar]
- 50.Perlman DC, el-Sadr WM, Nelson ET, Matts JP, Telzek EE, Salomon N et al (1997) Variation of chest radiograph patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on Aids (CPCRA). The AIDS Clinical Trial Group (ACTG). Clin Infect Dis 25(2):242–246 [DOI] [PubMed]
- 51.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, Snell L, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pai M, Zwerling A, Menzies D. Systemic review: T cell based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3):177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. Interferon- γ release assays for active pulmonary tuberculosis diagnosis in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl. 4):S1120–S1129. doi: 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12(3):201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64(6):580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Sohn H, Aero AD, Menzies D, Behr M, Schwartzman K, Alvarez GG, et al. Xpert MTB/RIF testing in low TB incidence, high resource setting: limitation s in accuracy and clinical impact. Clin Infect Dis. 2014;58(7):970–976. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 57.Kayigire XA, Friedrich SO, Venter A, Dawson R, Gillespie SH, Boeree MJ, et al. Direct comparison of Xpert MTB/RIF assay with liquid and solid mycobacterial culture for quantification of early bacterial activity. J Clin Microbiol. 2013;51(6):1894–1898. doi: 10.1128/JCM.03290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother. 2014;58(10):6111–6115. doi: 10.1128/AAC.03549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis. 2015;211(Suppl 3):S96–S106. doi: 10.1093/infdis/jiu610. [DOI] [PubMed] [Google Scholar]
- 60.Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis. 2015;32:87–93. doi: 10.1016/j.ijid.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Sathekge M, Maes A, Van De Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med. 2013;43(5):349–366. doi: 10.1053/j.semnuclmed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Vorster M, Sathekge MM, Bomanji J. Advances in imaging tuberculosis: the role of 18F-FDG PET and PET/CT Curr Opin Pulm Med 20(3):287–93. doi:10.1097/MCP.0000000000000043 [DOI] [PubMed]
- 63.Ichiya Y, Kuwabara Y, Sasaki M, Yoshida T, Akashi Y, Murayama S, et al. FDG-PET in infectious lesions: the detection and assessment of lesion activity. Ann Nucl Med. 1996;10(2):185–191. doi: 10.1007/BF03165391. [DOI] [PubMed] [Google Scholar]
- 64.Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, Chung JK. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology. 2000;216(1):117–121. doi: 10.1148/radiology.216.1.r00jl19117. [DOI] [PubMed] [Google Scholar]
- 65.Sathekge M, Maes A, Kgomo M, Stoltz A, Pottel H, Van de Wiele C. Impact of FDG PET on the management of TBC treatment. A pilot study. Nuklearmedizin. 2010;49(1):35–40. doi: 10.3413/nukmed-0270. [DOI] [PubMed] [Google Scholar]
- 66.Hara T, Kosaka N, Suzuki T, Kudo K, Niino H. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron tomography study. Chest. 2003;124(3):893–901. doi: 10.1378/chest.124.3.893. [DOI] [PubMed] [Google Scholar]
- 67.Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancer cells and inflammatory lesions of the lung. Neoplasia. 2005;7(4):369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yen RF, Chen KC, Lee JM, Chang YC, Wang J, Cheng MF, et al. F-FDG PET for lymph node staging of non-small cell cancer in a tuberculous-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008;35(7):1305–1315. doi: 10.1007/s00259-008-0733-1. [DOI] [PubMed] [Google Scholar]
- 69.Tian G, Xiao Y, Chen B, Guan H, Deng QY. Multi-site abdominal tuberculosis mimics malignancy on 18F-FDG PET/CT: report of three cases. World J Gastroenterol. 2010;16(33):4237–4242. doi: 10.3748/wjg.v16.i33.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadley GP, Naude F. Malignant solid tumor, HIV and tuberculosis in children: an unholy triad. Pediatr Surg Int. 2009;25(8):697–701. doi: 10.1007/s00383-009-2409-8. [DOI] [PubMed] [Google Scholar]
- 71.Lee SH, Min JW, Lee CH, Park CM, Goo JM, Chung DH, et al. Impact of parenchymal tuberculosis sequelae on mediastinal lymph node staging in patients with lung cancer. J Korean Med Sci. 2011;26(1):67–70. doi: 10.3346/jkms.2011.26.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med. 1998;28(4):352–358. doi: 10.1016/S0001-2998(98)80038-X. [DOI] [PubMed] [Google Scholar]
- 73.Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, Im JM. False positive and false negative FDG-PET scan in various thoracic disease. Korean J Radiol. 2006;7(1):57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cloran FJ, BamksKP Song WS, Kim Y, Bradley YC. Limitation of dual time point PET in the assessment of lung nodules with low FDG avidity. Lung Cancer. 2010;68(1):66–71. doi: 10.1016/j.lungcan.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Cheng G, Torigan DA, Zhuang H, Alavi A. When should we recommend use of dual time-point and delayed time point imaging techniques in FDG PET. Eur J Nucl Med Mol Imaging. 2013;40(5):779–787. doi: 10.1007/s00259-013-2343-9. [DOI] [PubMed] [Google Scholar]
- 76.Kim DW, Park SA, Kim CG. Dual-time-point positron emission tomography findings of benign mediastinal fluorine-18-fluorodeoxyglucose uptake in tuberculosis endemic region. Indian J Nucl Med. 2011;26(1):3–6. doi: 10.4103/0972-3919.84586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, et al. Double-phase F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging. 2008;35(4):808–814. doi: 10.1007/s00259-007-0585-0. [DOI] [PubMed] [Google Scholar]
- 78.Heysell SK, Thomas TA, Sirifi CD, Rehm PK, Houpt ER. 18-Fluorodeoxyglucose positron emission tomography for tuberculosis diagnosis and management: a case series. BMC Pulm Med. 2013;13:14. doi: 10.1186/1471-2466-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin P, Flyn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185(1):15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Demura Y, Tsuchida T, Uesaka D, Umeda Y, Morikawa M, Ameshima S, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging. 2009;36(4):632–639. doi: 10.1007/s00259-008-1009-5. [DOI] [PubMed] [Google Scholar]
- 82.Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med. 2011;52(6):880–885. doi: 10.2967/jnumed.110.083709. [DOI] [PubMed] [Google Scholar]
- 83.Hofmeyr A, Lau WF, Slavin MA. Mycobacterium tuberculosis infection in patients with cancer, the role of 18-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring disease response. Tuberculosis. 2007;87(5):459–463. doi: 10.1016/j.tube.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008;33(1):1–3. doi: 10.1097/RLU.0b013e31815c5128. [DOI] [PubMed] [Google Scholar]
- 85.Chen RY, Dodd LE, Lee M, Paripati P, Hammoud DA, Mountz JM et al (2014) PET/CT imaging correlates with treatment outcome in patients with multidrug resistant tuberculosis. Sci Transl Med 6(256):265ra166. doi:10.1126/scitranslmed.3009501 [DOI] [PMC free article] [PubMed]
- 86.Kim SJ, Kim IJ, Suh KT, Kim YK, Lee JS. Prediction of residual disease of spine infection using F-18 FDG PET/CT. Spine. 2009;34(22):2424–2430. doi: 10.1097/BRS.0b013e3181b1fd33. [DOI] [PubMed] [Google Scholar]
- 87.Kim K, Kim SJ, Kim IJ, Kim BS, Pak K, Kim H. Diffuse increased splenic F-18 fluorodeoxyglucose uptake may be an indirect sign of acute pyogenic cause rather than tuberculous in patients with infectious spondylitis. Nucl Med Commun. 2011;32(12):1155–1161. doi: 10.1097/MNM.0b013e32834bbdf1. [DOI] [PubMed] [Google Scholar]
- 88.Dureja S, Sen IS, Acharya S. Potential role of F18 FDG PET-CT as an imaging biomarker for noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur Spine J. 2014;23(11):2449–2454. doi: 10.1007/s00586-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 89.Sathekge M, Maes A, Kgomo M, Pottel H, Stolz A, Van De Wiele C. FDG uptake in lymph nodes in HIV+ and tuberculosis patients: implications for cancer staging. Q J Nucl Med Mol Imaging. 2010;54(6):698–703. [PubMed] [Google Scholar]
- 90.Hahm CR, Park HY, Jeon K, Um SW, Suh GY, Chung MP, et al. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung. 2010;188(1):25–31. doi: 10.1007/s00408-009-9203-1. [DOI] [PubMed] [Google Scholar]
- 91.Maturu VN, Agarwal R, Aggarwal AN, Mittal BR, Bal A, Gupta N, Gupta D. Dual-time point whole-body 18F-fluorodeoxyglucose PET/CT imaging in undiagnosed mediastinal lymphadenopathy: a prospective study of 117 patients with sarcoidosis and TB. Chest. 2014;146(6):e216–e220. doi: 10.1378/chest.14-1827. [DOI] [PubMed] [Google Scholar]
- 92.Tian G, Xiao Y, Chen B, Xia J, Guan H, Deng Q. FDG PET/CT for therapeutic response monitoring in multi-site non-respiratory tuberculosis Acta Radiol. 2010;51(9):1002–1006. doi: 10.3109/02841851.2010.504744. [DOI] [PubMed] [Google Scholar]
- 93.Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J. 2010;100(9):598–601. doi: 10.7196/samj.4082. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Su M, Li F, Kuang A, Tian R. The value of 18F-FDG-PET/CT in the differential diagnosis of solitary pulmonary nodules in areas with high incidence of tuberculosis. Ann Nucl Med. 2011;25(10):804–811. doi: 10.1007/s12149-011-0530-y. [DOI] [PubMed] [Google Scholar]
- 95.Kumar A, Dutta R, Kannan U, Kumar R, Khilnani GC, Gupta SD. Evaluation of mediastinal lymph nodes using 18F-FDG PET-CT scan and its histopathologic correlation. Ann Thoracic Med. 2011;6(1):11–16. doi: 10.4103/1817-1737.74270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soussan M, Brillet PY, Mekinian A, Khafagy A, Nicolas P, Vessieres A, Brauner M. Patterns in pulmonary tuberculosis on FDG-PET/CT. Eur J Radiol. 2012;81(10):2872–2876. doi: 10.1016/j.ejrad.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Sathekge M, Maes A, D’Asseler Y, Vorster M, Gongxeka H, Van de Wiele C. Tuberculous lymphadenitis: FDG PET and CT findings in responsive and nonresponsive disease. Eur J Nucl Med Mol Imaging. 2012;39(7):1184–1190. doi: 10.1007/s00259-012-2115-y. [DOI] [PubMed] [Google Scholar]
- 98.Martinez V, Castilla-Lievre MA, Guillet-Caruba C, Grenier G, Fior R, Desarnaud S, et al. Int J Tuberc Lung Dis. 2012;16(9):1180–1185. doi: 10.5588/ijtld.12.0010. [DOI] [PubMed] [Google Scholar]
- 99.Coleman MT, Chen RY, Lee M, Lin PL, Dodd LE, Maiello P et al (2014) PET/CT imaging reveals a therapeutic response to oxalidiones in macques and humans with tuberculosis Sci Trans Med 6(265):265ra167. doi:10.1126/scitranslmed.3009500 [DOI] [PMC free article] [PubMed]
- 100.Jeong YJ, Paen JC, Nam HY, Lee JS, Lee SM, Yoo CG, et al. (18)F-FDG positron emission tomography/computer tomography findings of radiographic lesions suggesting old healed tuberculosis. J Korean Med Sci. 2014;29(3):386–391. doi: 10.3346/jkms.2014.29.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huber H, Hodolic M, Stelzmüller I, Wunn R, Hatzl M, Fellner F, et al. Malignant disease as an incidental finding at 18F-FDG-PET-CT scanning in patients with granulomatous disease. Nucl Med Commun. 2015;36(5):430–437. doi: 10.1097/MNM.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 102.Fuster D, Tomás X, Mayoral M, Soriano A, Manchón F, Cardenal C, et al. Prospective comparison of whole-body 18F-FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2015;42(2):246–271. doi: 10.1007/s00259-014-2898-0. [DOI] [PubMed] [Google Scholar]
- 103.Castaigne C, Tondeur M, de Wit S, Hildebrand M, Clumeck N, Dusart M. Clinical value of FDG-PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: a retrospective study. Nucl Med Commun. 2009;30(1):41–47. doi: 10.1097/MNM.0b013e328310b38d. [DOI] [PubMed] [Google Scholar]
- 104.Liu Q, Peng ZM, Liu QW, Yao SZ, Zhang L, Meng L, Chen JH. The role of 11C-choline positron emission tomography–computed tomography and videomediastinoscopy in the evaluation of diseases of middle mediastinum. Chin Med J. 2006;119(8):634–639. [PubMed] [Google Scholar]
- 105.Hara T, Inagaki K, Kosaka N, Morita T. Sensitive detection of mediastinal lymph node metastasis of lung cancer with 11C-choline PET. J Nuc Med. 2000;41(9):1507–1513. [PubMed] [Google Scholar]
- 106.Vorster M, Stoltz A, Jacobs AG, Sathekge MM. Imaging of pulmonary tuberculosis with 18F-fluoro-deoxy-glucose and 18F-ethylcholine. Open Nucl Med J. 2014;6:17–21. doi: 10.2174/1876388X01406010017. [DOI] [Google Scholar]
- 107.Tian J, Yang X, Yu L, Chen P, Xin J, Ma L, et al. A Multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3′-deoxy-3′-18F-fluorothymidine and 18F-FDG. J Nucl Med. 2008;49(2):186–194. doi: 10.2967/jnumed.107.044966. [DOI] [PubMed] [Google Scholar]
- 108.Xu B, Guan Z, Liu C, Wang R, Yin D, Zhang J, et al. Can multimodality imaging using 18F-FDG/F-FLT PET/CT benefit the diagnosis and management of patients with pulmonary lesions? Eur J Nucl Med Mol Imaging. 2011;38(2):285–292. doi: 10.1007/s00259-010-1625-8. [DOI] [PubMed] [Google Scholar]
- 109.Vorster M, Maes A, Jacobs A, Malefohlo S, Pottel H, Van de Wiele C, Sathkge MM. Evaluating the possible role of 68Ga-citrate PET/CT in the characterization of indeterminate lung lesions. Ann Nucl Med. 2014;28(6):523–530. doi: 10.1007/s12149-014-0842-9. [DOI] [PubMed] [Google Scholar]
- 110.Vorster M, Maes A, Van de Wiele C, Sathekge MM. 68 Ga-citrate PET/CT in tuberculosis: A pilot study. Q J Nucl Med Mol Imaging (Epub ahead of print) [DOI] [PubMed]
- 111.Ordenez AA, DeMarco VP, Klunk MH, Pokkali S, Jain SK. Imaging chronic tuberculosis lesions using sodium [18F]Fluoride positron emmission tomography in mice. Mol Imaging Biol. 2015;17(5):609–614. doi: 10.1007/s11307-015-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu L, Xu Y, Shea C, Fowler JS, Hooker JM, Tonge PJ. Radiosynthesis and bioimaging of the tuberculosis chemotherapeutics isoniazid, rifampicin and pyrazinamide in baboons. J Med Chem. 2010;53(7):2882–2891. doi: 10.1021/jm901858n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, et al. Drug tolerance in replicating mediated by macrophage-induced efflux mechanism. Cell. 2011;145(1):39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van der Werf TS, Dade GK, van der Mark TW. Patient compliance with tuberculosis treatment in Ghana: factors influencing adherence to therapy in a rural service programme. Tubercle. 1990;71(4):247–252. doi: 10.1016/0041-3879(90)90036-8. [DOI] [PubMed] [Google Scholar]
- 115.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371(17):1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guidelines for the treatment of tuberculosis (4thed) 2010: Geneva World Health Organization. www.who.int/tb/publications/2010/9789241547833/en. Assessed on 23 Nov 2015
- 117.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4(9):e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Altena R, de Vries G, Haar CH, de Lange WC, Magis-Escurra C, van den Hof S, van Soolingen D, Boeree MJ, van der Werf TS. Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000–2009. Int J Tuberc Lung Dis. 2015;19(4):406–412. doi: 10.5588/ijtld.14.0838. [DOI] [PubMed] [Google Scholar]
- 119.Sia I, Wieland M. Current concept in the management of tuberculosis. Mayo Clin Proc. 2011;86(4):348–361. doi: 10.4065/mcp.2010.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guidelines on the management of latent tuberculosis infection 2015: Geneva World Health Organization. www.who.int/tb/publications/ltbi_document_page/en/. Assessed on the 23 Nov 2015 [PubMed]
- 121.Alsaad N, Wilffert B, van Altena R, de Lange WC, van der Werf TS, Kosterink JG, Alffenaar JW. Potential antimicrobial agents for the treatment of multidrug-resistant tuberculosis. Eur Respir J. 2014;43(3):884–897. doi: 10.1183/09031936.00113713. [DOI] [PubMed] [Google Scholar]