Abstract
Patients with multiple myeloma and other B cell disorders respond poorly to pneumococcal vaccination. Vaccine responsiveness is commonly determined by measuring pneumococcal serotype-specific antibodies by enzyme-linked immunosorbent assay (ELISA), by a functional opsonophagocytosis assay (OPA), or by both assays. We compared the two methods in vaccinated elderly patients with multiple myeloma, Waldenstrom's macroglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS). Postvaccination sera from 45 patients (n = 15 from each patient group) and 15 control subjects were analyzed by multiplexed OPA for pneumococcal serotypes 4, 6B, 14, and 23F, and the results were compared to IgG and IgM antibody titers measured by ELISA. While there were significant correlations between pneumococcal OPA and IgG titers for all serotypes among the control subjects (correlation coefficients [r] between 0.51 and 0.85), no significant correlations were seen for any of the investigated serotypes in the myeloma group (r = −0.18 to 0.21) or in the group with Waldenstrom's macroglobulinemia (borderline significant correlations for 2 of 4 serotypes). The MGUS group resembled the control group by having good agreement between the two test methods for 3 of 4 serotypes (r = 0.53 to 0.80). Pneumococcal postvaccination IgM titers were very low in the myeloma patients compared to the other groups and did not correlate with the OPA results. To summarize, our data indicate that ELISA measurements may overestimate antipneumococcal immunity in elderly subjects with B cell malignancies and that a functional antibody test should be used specifically for myeloma and Waldenstrom's macroglobulinemia patients.
INTRODUCTION
Patients with multiple myeloma (MM) and other clonal B cell disorders such as Waldenstrom's macroglobulinemia (WM) and monoclonal gammopathy of undetermined significance (MGUS) are at increased risk of contracting and succumbing to pneumococcal infections (1–4). In a study of invasive pneumococcal disease, the attack rate in MM patients was 674 cases/100,000 per year compared to 11 cases/100,000 per year in an population of adults ≥18 years of age (odds ratio [OR], 62.8) (5). A recent Swedish study of MM and infection showed an increased risk of pneumonia, with a hazard ratio (HR) of 7.7 compared to matched population-based controls (6). Kristinsson et al. found an increased risk of pneumonia in MGUS patients, with a HR of 2.4 (4).
The susceptibility to pneumococcal infection among patients with B cell disorders is primarily due to an overproduction of nonfunctional immunoglobulins called M-proteins (monoclonal proteins) and, as a result, to various degrees of hypogammaglobulinemia (1, 2). Antibody-mediated opsonization of bacteria followed by phagocytosis is considered to be a critical immune defense mechanism against pneumococci. However, the immune deficiency in patients with B cell disorders encompasses not only B cell dysfunction but also defective cellular immunity, with reduced numbers and function of T cells, NK cells, and dendritic cells (2). A decreased number of CD4+ T cells is a feature of MM as well as of WM and MGUS (7, 8). This is also seen in HIV patients, another group at risk for pneumococcal infection (9).
Pneumococcal vaccination is recommended for myeloma patients even though they are known to be poor responders as defined by poor IgG responses postvaccination measured by enzyme-linked immunosorbent assay (ELISA) (1, 10–13). In a report of a previous study, we stated that pneumococcal vaccination evoked poor responses not only in MM patients but also in elderly patients with WM and MGUS compared to a control group consisting of subjects within the same age range but without hematological disorders (14).
Pneumococcal vaccine studies in high-risk populations are generally small and thus preclude determination of any clinical endpoints and measures of vaccine effectiveness. Instead, such studies are designed with immunogenicity endpoints, and serotype-specific IgG is often used as a surrogate marker for assessment of likely vaccine effectiveness. A correlate of protection of 0.35 μg/ml serotype-specific IgG (measured by ELISA) was derived as a population-based correlate in infants and has been used for comparing and licensing new pneumococcal conjugate vaccines (15, 16). This aggregate correlate for all serotypes has recently been refined, and new serotype-specific correlates for both ELISA and functional antibodies in infants have been proposed (17). The pneumococcal serotype-specific IgG ELISA has been the standard method due to its relative simplicity and cost-effectiveness, but it has its drawbacks, not least the capture of potentially nonfunctional, cross-reacting antibodies, although this problem has largely been overcome by adsorption of cell wall polysaccharide and heterologous pneumococcal polysaccharide (22F) (18). The assessment of the functionality of pneumococcal antibodies by a biological opsonophagocytosis assay (OPA) is more complicated and time-consuming but measures the functional antibody titers. It encompasses mixing various dilutions of patient sera with phagocytic HL60 cells, complement, and live pneumococci and has the actual number of surviving bacteria as the readout. It has been multiplexed for several serotypes and is regarded as the gold standard for the evaluation of pneumococcal vaccine responses (19).
Determination of antipneumococcal IgG levels by means of later generations of serotype-specific ELISA has been shown to correlate well with opsonic activity in children but more poorly in adults, in particular, in the elderly population (19, 20). The knowledge regarding the performance of the ELISA for determination of pneumococcal vaccine efficacy in high-risk groups such as immunocompromised patients is limited. Patients with B cell malignancies have not been investigated in this aspect. In our previous study, we found unexpectedly high antipneumococcal antibody levels among some myeloma patients, possibly indicative of the presence of cross-reactive, nonfunctional antibodies (14). We therefore wished to assess the reliability of the serotype-specific pneumococcal ELISA in patients with multiple myeloma and related B cell disorders, who, due to their susceptibility to pneumococcal infections, constitute a target group for vaccination. To this end, we compared the results from serotype-specific IgG ELISA with those of a standardized OPA. Since the antibodies that mediate killing-type OPA may be of any isotype, we also investigated the postvaccination serum levels of IgM and their correlation to OPA titers.
MATERIALS AND METHODS
Study population.
In an earlier study, we investigated pneumococcal vaccine responses in 56 patients aged ≥60 years with MM (n = 24), WM (n = 15), or MGUS (n = 17) and 20 control subjects without hematological disorders but within the same age range, recruited at the Department of Hematology, Uddevalla Hospital, Uddevalla, Sweden, between May 2008 and March 2009 (14). The study persons were randomized to receive a single dose (0.5 ml) of either the 7-valent conjugated pneumococcal vaccine (PCV7) (Prevenar; Pfizer) or the 23-valent polysaccharide pneumococcal vaccine (PPV) (Pneumo 23; Sanofi Pasteur). Serum samples were collected before and 4 to 8 weeks after vaccination and were stored at −20°C until analyzed. The study was approved by the Regional Ethics Committee in Göteborg, and written informed consent was obtained from all patients.
From the above-mentioned study population, 15 patients from each disease group and 15 control subjects were randomly selected for reanalysis of their postvaccination sera by 4-fold multiplexed OPA and pneumococcal serotype-specific IgM ELISA. Both assays were performed at the World Health Organization (WHO) reference laboratory for pneumococcal serology at the Institute of Child Health, University College of London, United Kingdom. Similar proportions in each study group were immunized with conjugate or polysaccharide vaccines.
IgG pneumococcal ELISA.
The serum concentrations of IgG antibodies to the pneumococcal serotypes 4, 6B, 14, and 23F were analyzed in our previous study (14) at Statens Serum Institut, Copenhagen, Denmark, by an ELISA employing cell wall polysaccharide adsorption and were described in detail elsewhere (21). A local reference serum calibrated to the U.S. standard reference serum 89-SF was used. Antibody concentrations were expressed as micrograms per milliliter. The investigated pneumococcal serotypes are included in both vaccine types employed in the study.
OPA.
Sera were shipped frozen to the laboratory of the University College London, Institute of Child Health (UCL ICH), and analyzed for functional antibodies to pneumococcal serotypes 4, 6B, 14, and 23F by the multiplexed opsonophagocytosis assay (OPA) as previously described (22). Each serum was tested in duplicate.
Twelve OPA results from a total of 240 analyses (5%) (3 analyses each of serotype 4 and 6B, 1 analysis of serotype 14, and 5 analyses of serotype 23F) did not pass the assay acceptance criteria despite retesting and were excluded. The samples failed to pass the criteria due to incomplete (between 40% and 70%) killing of the bacteria. In the assay in use, samples are accepted only if they do not kill, i.e., show killing rates of ≤40% (negative samples) or if they display killing rates of ≥70%. Samples with values between 40% and 70% are usually “low killing” and tend to give inconsistent results and are therefore not reported.
Four study patients were found to have been treated with antibiotics when postvaccination sera were collected (two MM patients and one WM patient had been given co-trimoxazole in a prophylactic dosage, and one MM patient had been given cloxacillin). To exclude the possibility that residual concentrations of antibiotics had interfered with the OPA analysis, the bactericidal effects of their serum samples were tested (using test wells containing bacteria, patient serum, and buffer) and compared to the effects seen with the complement controls of the standard assay (using test wells containing bacteria, HL-60 effector cells, complement, and buffer). Since no significant titer differences were found, these samples were not excluded from the study.
IgM pneumococcal ELISA.
Serum was assayed for IgM antibodies to pneumococcal serotypes 4, 6B, 14, and 23F at the UCL ICH laboratory by adapting a previously described IgG ELISA protocol (http://www.vaccine.uab.edu/ELISA%20Protocol.pdf) (23). Each serum was tested in duplicate.
Statistical analyses.
Geometric means with 95% confidence intervals were calculated for ELISA and OPA titers. OPA titers below the detection limit of 8 were set to 4. Comparisons between study groups were done using the Mann-Whitney U test. Correlations between ELISA and OPA titers were calculated using the Spearman rank correlation test. P values were two-sided. A significance level of P of <0.05 was used. Graph Pad Prism 5.0 software was used for all statistical analyses (GraphPad, San Diego, CA).
RESULTS
Study population.
Patient characteristics are presented in Table 1. More than half (55%) of the study persons were female. The median ages were significantly higher in the MM and WM groups (80 and 75 years, respectively) than in the MGUS and the control study groups (71 and 68 years, respectively; P < 0.05). Eleven of the MM patients (73%) and only one of the WM patients were on active cancer treatment during the study (Table 1).
TABLE 1.
Study group characteristics
| Subject characteristic | Value for study group |
||||
|---|---|---|---|---|---|
| Multiple myeloma | Waldenstrom's macroglobulinemia | Monoclonal gammopathy of undetermined significance | Control subjects | All | |
| n | 15 | 15 | 15 | 15 | 60 |
| Median age, yrs (range) | 80 (65–88) | 75 (62–88) | 71 (60–77) | 68 (61–83) | 75 (60–88) |
| Female sex, n | 7 | 9 | 9 | 8 | 33 |
| M-protein median, g/liter (range)a | 24 (0.7–44) | 15 (3.0–28) | 11 (0.5–28) | 0 (0–0) | |
| Receiving indicated treatment regimen(s), n | 11 | 1 | 0 | 0 | 12 |
| Melphalan + prednisone | 4 | 0 | 0 | 0 | 4 |
| Cyclophosphamide + dexamethasone | 4 | 0 | 0 | 0 | 4 |
| Pulse steroids | 1 | 0 | 0 | 0 | 1 |
| Thalidomide | 1 | 0 | 0 | 0 | 1 |
| Bortezomib | 1 | 0 | 0 | 0 | 1 |
| Fludarabin | 0 | 1 | 0 | 0 | 1 |
| Receiving ongoing antibiotic treatment, n | 3 | 1 | 0 | 0 | 4 |
| Given pneumococcal vaccine, n | |||||
| PCV7b | 8 | 8 | 7 | 6 | 29 |
| PPV23c | 7 | 7 | 8 | 9 | 31 |
Prevaccination levels of monoclonal (M-)protein in serum.
PCV7, 7-valent pneumococcal conjugate vaccine.
PPV23, 23-valent pneumococcal polysaccharide vaccine.
Serology.
Since no significant differences in OPA IgG or IgM antibody titers/concentrations for any of the four serotypes were found between the subgroups of patients vaccinated with PCV7 or PPV, the data were pooled for the correlation analyses (data not shown).
OPA titers.
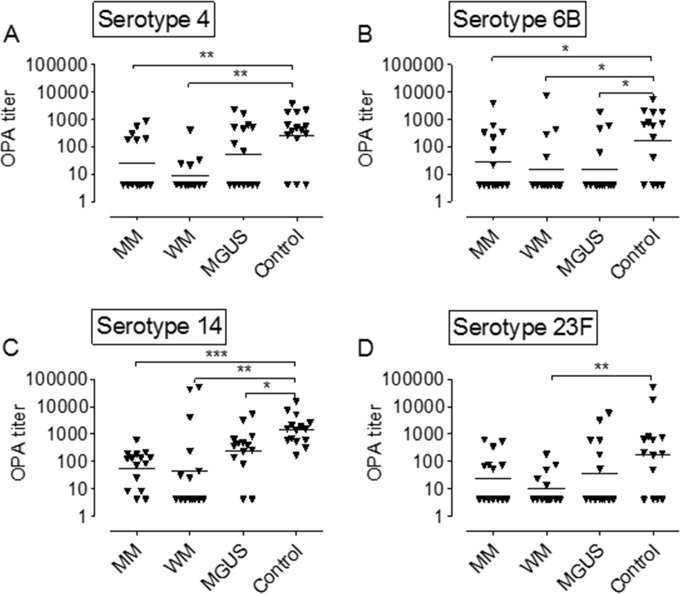
All three patient groups had lower antipneumococcal OPA titers than the control subjects (Fig. 1). This was significant for all four pneumococcal serotypes in the group of WM patients, for three (serotypes 4, 6B, and 14) in the MM group, and for two (serotypes 6B and 14) among the MGUS patients (Fig. 1). The distribution of the unreportable OPA results among the study groups was as follows: 2 results from the MM group, 5 results from the WM group, 3 results from the MGUS group, and 2 results from control subjects. Eight of the 12 unreportable results were single individual samples and individual serotypes, and 4 results were from 2 WM patients, each with two serotypes affected (serotypes 6B and 23F and serotypes 4 and 23F, respectively). There was no obvious skew regarding the unreported OPA results in favor of certain serotypes or patient groups.
FIG 1.
Opsonophagocytic antibody (OPA) titers after pneumococcal vaccination. (A) Serotype 4. (B) Serotype 6B. (C) Serotype 14. (D) Serotype 23F. Each triangle represents one study person. Horizontal bars denote the geometric mean value for each study group. Statistical comparisons have been performed for the respective patient groups versus the control group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Mann-Whitney U test).
Correlations between ELISA IgG and OPA titers.
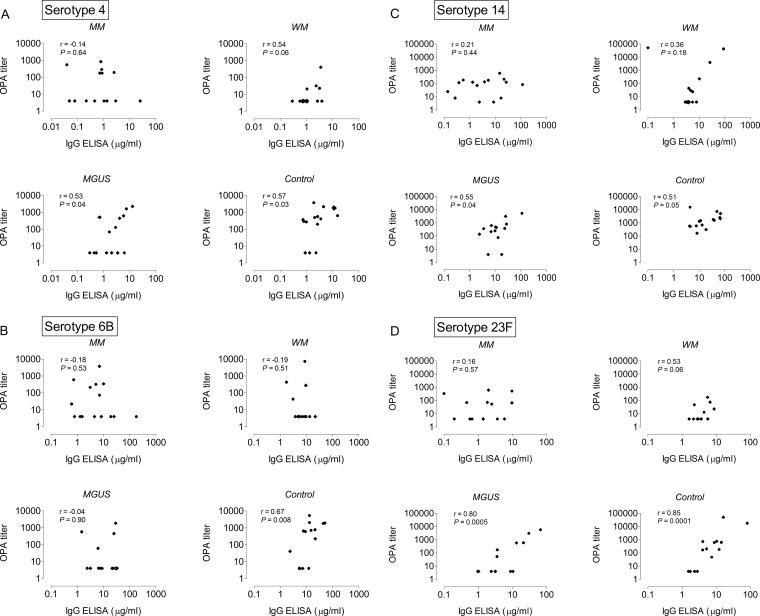
The correlations between antipneumococcal IgG concentrations and functional antibody titers as measured by OPA are presented in Fig. 2. While there were significant correlations between ELISA concentrations and OPA titers for all four pneumococcal serotypes among the control subjects (correlation coefficients [r] between 0.51 and 0.85), no correlations were found for any of the investigated serotypes in the myeloma group (r between −0.18 and 0.21). Correlations were significant for three serotypes (serotypes 4, 14, and 23F) among the MGUS patients (r between 0.53 and 0.80) and almost reached significance for serotypes 4 and 23F in the WM group (r = 0.54 and 0.53; P = 0.06) (Fig. 2).
FIG 2.
Correlations between antipneumococcal IgG titers (ELISA) and opsonophagocytic antibody titers (OPA) after pneumococcal vaccination. (A) Serotype 4. (B) Serotype 6B. (C) Serotype 14. (D) Serotype 23F. MM, multiple myeloma; WM, Waldenstrom's macroglobulinemia; MGUS, monoclonal gammopathy of unknown significance. Diamonds indicate persons vaccinated with 7-valent conjugated pneumococcal vaccine (PCV7); circles indicate persons vaccinated with 23-valent pneumococcal polysaccharide vaccine (PPV). Note that the values on the x and y axes differ between the different pneumococcal serotypes (A to D). r and P values were calculated by the Spearman rank sum test.
ELISA IgM concentrations for pneumococci.
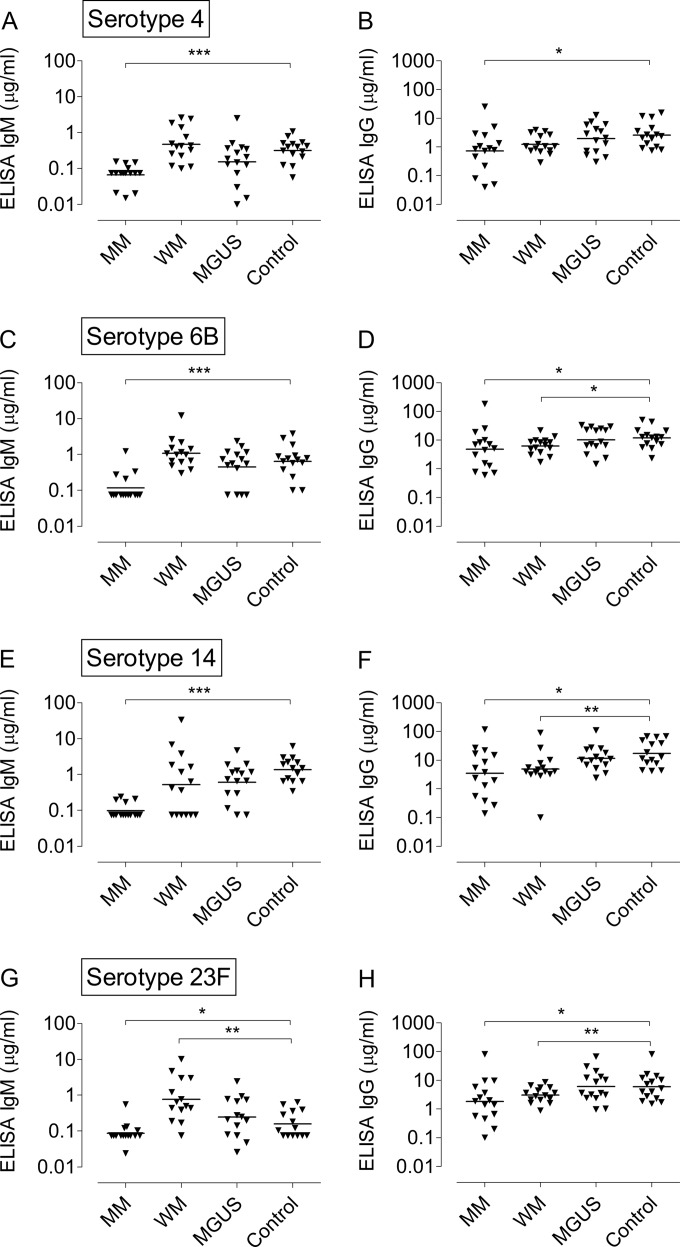
IgM antibody titers were low among the MM patients for all four pneumococcal serotypes, both in relation to the vaccine-induced IgG titers and in comparison with the IgM responses seen in the other three study groups (P < 0.001 for MM patients versus control subjects for serotypes 4, 6B, and 14; P < 0.05 for serotype 23F) (Fig. 3). The control subjects, patients with WM, and MGUS patients had comparable IgM titers after vaccination (Fig. 3).
FIG 3.
(A, C, E, and G) ELISA IgM titers after pneumococcal vaccination. (B, D, F, and H) Corresponding IgG titers are shown for comparison. Each triangle represents one study person. Horizontal bars denote the geometric mean value for each study group. (A and B) Serotype 4. (C and D) Serotype 6B. (E and F) Serotype 14. (G and H) Serotype 23F. Statistical comparisons have been performed for the respective patient groups versus the control group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Mann-Whitney U test).
Correlations between ELISA IgM and OPA titers.
The correlations between ELISA IgM and OPA titers were generally poor and inconsistent. Significant correlations were found only for WM patients regarding serotype 6B and for the control subjects regarding serotypes 4 and 14 (Table 2). No significant correlation coefficients were seen for either MM or MGUS patients (Table 2).
TABLE 2.
Correlation of ELISA IgM level and opsonophagocytosis assay results for four pneumococcal serotypes and four study groupsa
| Study group |
r value, by pneumococcal serotype |
|||
|---|---|---|---|---|
| Serotype 4 | Serotype 6B | Serotype 14 | Serotype 23F | |
| MM | 0.39 | 0.20 | 0.07 | 0.04 |
| WM | 0.54 | 0.68*** | 0.04 | 0.58 |
| MGUS | −0.16 | 0.18 | 0.44 | 0.28 |
| Controls | 0.56* | 0.40 | 0.90*** | 0.09 |
Each group contained 15 postvaccination serum samples. MM, multiple myeloma; WM, Waldenstrom's macroglobulinemia; MGUS, monoclonal gammopathy of unknown significance. *, P < 0.05; ***, P < 0.001 (Spearman rank sum test).
DISCUSSION
In a previous study of pneumococcal vaccine responses in elderly patients with multiple myeloma, Waldenstrom's macroglobulinemia, and monoclonal gammopathy of undetermined significance, we suspected falsely high ELISA IgG titers, in particular, among the myeloma patients (14). We therefore wished to assess the ELISA results by comparison with OPA findings. Excellent correlation between IgG concentrations determined by ELISA and OPA titers were seen for the control subjects for all four serotypes. In contrast, multiple myeloma patients showed no positive correlation between IgG and OPA titers for the four serotypes studied. High ELISA IgG titers with no functional activity were noted in some MM patients. Of the remaining two groups of patients studied, the MGUS patients resembled the control subjects by having good correlations between their IgG and OPA titers for 3 of 4 of the serotypes while the Waldenstrom's macroglobulinemia patients had intermediate correlations.
Discrepancies in the outcome of pneumococcal ELISA and OPA such as those described in this study have previously been noted for other immunocompromised patients with increased risk of pneumococcal infections, for example, for HIV patients (9) and renal transplant patients (24). In contrast, our results differ from those of a study of allogeneic stem cell-transplanted patients with hematological malignancies, where significant correlations between antipneumococcal IgG and OPA titers were found for all seven investigated pneumococcal serotypes (25). Of note is that the patients in that study (mean age, 37 years) were younger than ours and had different hematological diagnoses and thus may have been able to mount better IgG responses. Also, the correlations were modest, with r values ranging from 0.48 to 0.76.
Age is an important factor in evaluations of pneumococcal vaccine responses. Previous studies have shown poorer functional antibody responses after vaccination with PPV among elderly adults than among younger adults (20, 26, 27). The 13-valent conjugate pneumococcal vaccine (PCV13), which was launched after the initiation of this study, has been shown to prevent invasive pneumococcal disease and community-acquired pneumonia in adults >65 years of age (28); however, vaccine efficacy declined with increasing age (29). Since the control subjects and the MGUS patients in this study were significantly younger than the MM and WM patients, this might have influenced our results, with poorer OPA concentrations in the more diseased (and older) study groups (the MM and WM groups).
The weak correlations between ELISA-determined IgG concentrations and OPA titers among MM and WM patients in the present study could also have been affected by the advanced age of these study persons, which would be in accordance with previous studies (19, 20). Romero-Steiner et al. found poorer correlations between postvaccination IgG antibody concentrations and OPA titers in a group of elderly adults (mean age, 85 years) than in younger adults (mean age, 37 years). This was explained by lower antibody avidity with respect to capsular polysaccharides among the elderly (20) and was corroborated by showing that transfer of human serum with a high OPA titer and high avidity protected mice in an experimental model of pneumococcal infection, whereas serum with low avidity and a low OPA titer did not do so despite containing the same concentration of antipneumococcal IgG (20).
In general, higher antipneumococcal IgG levels are required to achieve the same killing capacity in elderly adults as in younger subjects (20, 26, 27, 30). Besides correlating with the differences in avidity (20), high age has been linked to loss of oligoclonality in response to pneumococcal antigens, reduced function of phagocytic cells, and decreased levels of antipneumococcal IgM as a consequence of the presence of deficient IgM memory B cells (26, 31, 32). In the present study, IgM concentrations were markedly reduced in the myeloma patients compared to the other study groups. In contrast, serum IgM levels were relatively high among the WM patients; however, a significant correlation with OPA titers was seen for only 1 of 4 tested serotypes. Our results showing poor or no correlation between IgM and OPA titers for all study groups indicate that lack of IgM was not the main reason for poor OPA function among our study subjects.
A possible reason for the poor correlations between the ELISA and OPA analyses among our disease groups might be interference of the M-proteins with the ELISA analysis. This has been shown for various laboratory tests such as analyses of blood counts and electrolytes but also for immunological tests such as analysis of antistreptococcal antibodies (33). Unfortunately, we had too little serum left to measure the M-protein levels in the postvaccination samples and could not correctly test this hypothesis. However, this should not be the sole explanation since the correlations between IgG and OPA titers were nearly normal among the MGUS patients, who had prevaccination levels of M-protein that were almost as high as in the WM group (a median of 11 g/liter and a range of 0.5 to 28 g/liter compared to a median of 15 g/liter and a range of 3 to 28 g/liter among the WM patients). The OPA responses might also be affected by deficient cellular immunity, which has been shown to be more pronounced in MM and WM patients than in MGUS patients (8).
The major limitation of this study was the small series of patients. The age differences between 2 of the 3 patient groups (MM and WM) and the control subjects may also have affected the results. Nevertheless, an evaluation of methods for measuring pneumococcal vaccine responses has not to our knowledge previously been done for patients with B cell malignancies and related hematological disorders. Since patients with these diagnoses have a high incidence of pneumococcal infections and thus are target groups of pneumococcal vaccination, it should be of interest to find a sufficiently reliable method of measuring their vaccine responses.
Our main finding was that pneumococcal antibody measurement by ELISA in vaccinated elderly patients with multiple myeloma and Waldenstrom's macroglobulinemia did not correlate with antibody titers determined by the multiplex OPA. We suggest that ELISA measurements may overestimate antipneumococcal immunity in patients with B cell malignancies and propose the use of a functional antibody assay, in particular, for multiple myeloma and Waldenstrom's macroglobulinemia patients.
ACKNOWLEDGMENTS
The UCL ICH laboratory performs contract serology for manufacturers of pneumococcal vaccines (Merck and GSK). D.G. is an occasional consultant to or advisory board member for Merck and GSK. The other authors report no conflicts of interest.
This study was financed by grants from LUA grant 75180, the Cancer and Allergy Foundation, FoU, NU Hospital Group, Trollhättan/Uddevalla, and Göteborg Medical Society. D.G. is funded in part by the National Institute for Health Research, United Kingdom. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Bladé J, Rosiñol L. 2005. Renal, hematologic and infectious complications in multiple myeloma. Best Pract Res Clin Haematol 18:635–652. doi: 10.1016/j.beha.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Nucci M, Anaissie E. 2009. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis 49:1211–1225. doi: 10.1086/605664. [DOI] [PubMed] [Google Scholar]
- 3.García-Sanz R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A, Rodríguez-García JA, Massó P, Pérez-Aliaga A, Monteagudo MD, Navarro I, Moreno G, Toledo C, Alonso A, Besses C, Besalduch J, Jarque I, Salama P, Rivas JA, Navarro B, Bladé J, Miguel JF; Spanish Group for the Study of Waldenström Macroglobulinaemia and PETHEMA (Programme for the Study and Treatment of Haematological Malignancies). 2001. Waldenstrom macroglobulinaemia: presenting features and outcome in a series with 217 cases. Br J Haematol 115:575–582. doi: 10.1046/j.1365-2141.2001.03144.x. [DOI] [PubMed] [Google Scholar]
- 4.Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, Turesson I, Landgren O. 2012. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica 97:854–858. doi: 10.3324/haematol.2011.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ. 2010. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol Infect 138:1804–1810. doi: 10.1017/S0950268810000919. [DOI] [PubMed] [Google Scholar]
- 6.Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, Kjellander C, Turesson I, Kristinsson SY. 2015. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica 100:107–113. doi: 10.3324/haematol.2014.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt G, Goodyear O, Moss P. 2007. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 138:563–579. doi: 10.1111/j.1365-2141.2007.06705.x. [DOI] [PubMed] [Google Scholar]
- 8.Pilarski LM, Andrews EJ, Serra HM, Ledbetter JA, Ruether BA, Mant MJ. 1989. Abnormalities in lymphocyte profile and specificity repertoire of patients with Waldenstrom's macroglobulinemia, multiple myeloma, and IgM monoclonal gammopathy of undetermined significance. Am J Hematol 30:53–60. doi: 10.1002/ajh.2830300202. [DOI] [PubMed] [Google Scholar]
- 9.Feikin DR, Elie CM, Goetz MB, Lennox JL, Carlone GM, Romero-Steiner S, Holder PF, O'Brien WA, Whitney CG, Butler JC, Breiman RF. 2001. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 20:545–553. doi: 10.1016/S0264-410X(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2012. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 61:816–819. [PubMed] [Google Scholar]
- 11.Robertson JD, Nagesh K, Jowitt SN, Dougal M, Anderson H, Mutton K, Zambon M, Scarffe JH. 2000. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer 82:1261–1265. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves RM, Lea JR, Griffiths H, Faux JA, Holt JM, Reid C, Bunch C, Lee M, Chapel HM. 1995. Immunological factors and risk of infection in plateau phase myeloma. J Clin Pathol 48:260–266. doi: 10.1136/jcp.48.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinge M, Ingels HA, Slotved HC, Molle I. 2012. Serologic response to a 23-valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS 120:935–940. doi: 10.1111/j.1600-0463.2012.02922.x. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson J, Hogevik H, Andersson K, Roshani L, Andréasson B, Wennerås C. 2013. Pneumococcal vaccine responses in elderly patients with multiple myeloma, Waldenstrom's macroglobulinemia, and monoclonal gammopathy of undetermined significance. Trials Vaccinol 2:31–38. doi: 10.1016/j.trivac.2013.09.001. [DOI] [Google Scholar]
- 15.World Health Organization Expert Committee on Biological Standardization. 2003. Pneumococcal conjugate vaccine: recommendations for production and quality control of pneumococcal conjugate vaccines. http://www.who.int/biologicals/pneumococcal_conjugate_Vaccines_Recomm_Nov_2003 Accessed 9 January 2003.
- 16.Henckaerts I, Goldblatt D, Ashton L, Poolman J. 2006. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 13:356–360. doi: 10.1128/CVI.13.3.356-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. 2014. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 14:839–846. doi: 10.1016/S1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
- 18.Concepcion NF, Frasch CE. 2001. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 8:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JY, Moseley MA, Burton RL, Nahm MH. 2013. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 19:412–425. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 21.Konradsen HB, Sorensen UB, Henrichsen J. 1993. A modified enzyme-linked immunosorbent assay for measuring type-specific anti-pneumococcal capsular polysaccharide antibodies. J Immunol Methods 164:13–20. doi: 10.1016/0022-1759(93)90270-H. [DOI] [PubMed] [Google Scholar]
- 22.Burton RL, Nahm MH. 2012. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol 19:835–841. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose CE, Romero-Steiner S, Burton RL, Carlone GM, Goldblatt D, Nahm MH, Ashton L, Haston M, Ekstrom N, Haikala R, Kayhty H, Henckaerts I, Durant N, Poolman JT, Fernsten P, Yu X, Hu BT, Jansen KU, Blake M, Simonetti ER, Hermans PW, Plikaytis BD. 2011. Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera. Clin Vaccine Immunol 18:135–142. doi: 10.1128/CVI.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. 2003. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis 187:1639–1645. doi: 10.1086/374784. [DOI] [PubMed] [Google Scholar]
- 25.Cordonnier C, Labopin M, Jansen KU, Pride M, Chesnel V, Bonnet E, Einsele H, Ljungman P. 2010. Relationship between IgG titers and opsonocytophagocytic activity of anti-pneumococcal antibodies after immunization with the 7-valent conjugate vaccine in allogeneic stem cell transplant. Bone Marrow Transplant 45:1423–1426. doi: 10.1038/bmt.2009.364. [DOI] [PubMed] [Google Scholar]
- 26.Westerink MA, Schroeder HW Jr, Nahm MH. 2012. Immune responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis 3:51–67. [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkein JG, Park S, Nahm MH. 2008. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 26:5521–5526. doi: 10.1016/j.vaccine.2008.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. 2015. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 29.van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. 2015. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin Infect Dis 61:1835–1838. doi: 10.1093/cid/civ686. [DOI] [PubMed] [Google Scholar]
- 30.Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S, Kayhty H, Vakevainen M. 2011. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 29:1929–1934. doi: 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 31.Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun 73:7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S, Nahm MH. 2011. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun 79:314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy V. 2009. Artifactual laboratory abnormalities in patients with paraproteinemia. South Med J 102:167–179. doi: 10.1097/SMJ.0b013e3181831f6a. [DOI] [PubMed] [Google Scholar]