Abstract
Infectious diarrhea is a leading cause of morbidity and of mortality; the burden of disease affects individuals of all ages but particularly young children, especially those living in poor regions where the disease is endemic. It is also a health concern for international travelers to these areas. Experts on vaccines and enteric infections and advocates for global health improvement gathered in Scotland from 8 to 10 July 2015 to discuss recent advances in the assessment and understanding of the burden of enteric diseases and progress in the development and implementation of strategies to prevent these infections. Highlights of the meeting included description of advances in molecular assays to estimate pathogen-specific prevalence, methods to model epidemiologic trends, novel approaches to generate broad-spectrum vaccines, new initiatives to evaluate vaccine performance where they are most needed, renewed interest in human challenge models, immunological readouts as predictors of vaccine efficacy, maternal immunization to prevent enteric infections, and the impact of maternal immunity on the vaccine take of infants. A follow-up scientific gathering to advance Shigella and enterotoxigenic Escherichia coli (ETEC) vaccine efforts will be held from 28 to 30 June 2016 in Washington, DC.
INTRODUCTION
Enteric infections and diarrhea represent a major global cause of disease burden and of death and disability, primarily in less-developed regions of the world (1). The most affected are children under 5 years of age and special groups, e.g., immunocompromised, the elderly, and pregnant women. Acute infectious diarrhea is also a health concern for travelers to areas where the disease is endemic, as it may require hospitalization and have chronic sequelae. Scientists from academia and government, vaccine producers, and public health advocates, including funding sponsors from around the world, gathered at the 8th International Conference on Vaccines for Enteric Diseases (VED) in Edinburgh, Scotland, to discuss recent progress in our understanding of enteric disease etiology, new methods of surveillance, the increasing awareness of the long-lasting health impairment associated with enteric infections, the development and evaluation of new vaccine candidates, and introduction of vaccines into existing immunization schedules and in emergency situations and in high-risk groups, among many other topics. Knowledge gaps were identified, as was the need for more frequent exchange of information and interactions within the research community to accelerate the development and implementation of preventive tools. This meeting highlight summarizes the main findings presented at the VED meeting and discussions and recommendations made, particularly the progress in vaccine development and clinical evaluation efforts.
Prevalence of Shigella and ETEC and broad effects associated with infection.
The Global Enteric Multicenter Study (GEMS) revealed that Shigella and enterotoxigenic Escherichia coli (ETEC) are among the top five major causes of moderate to severe diarrhea in children under 5 years of age in Africa and Asia (2). However, due to poor sensitivity of standard clinical microbiological methods to detect these organisms, the true burden of disease is suspected to be underestimated (3). Eric Houpt, University of Virginia (UVA), and colleagues reexamined a random sample of GEMS stool specimens from cases and matched controls using a molecular assay and showed an increased frequency of detection for these two organisms. Nucleic acid was extracted from archived GEMS stool samples and tested using a TaqMan array card (TAC), which detects 32 enteropathogens. The fractions attributable to Shigella and ETEC were substantially increased when measured by TAC, compared to classical clinical microbiology. James Platts-Mills, also from UVA, presented a poster that showed that the same was true when stools from children enrolled in the MAL-ED study (37) were retested with this method. In contrast, detection of rotavirus and cryptosporidium by TAC yielded results similar to those obtained using enzyme immunoassays in the original GEMS. This revised analysis of pathogen-specific burden suggests that by targeting Shigella and ETEC, a significant burden of diarrheal disease could be prevented in Africa and Asia. This notion was further supported by the presentation of Richard Rheingans from University of Florida, who showed compelling data demonstrating the long-lasting health impairment and economic burden caused by Shigella and ETEC diarrheal disease. ETEC episodes result in ∼2.6 million additional children with moderate stunting and 2 million additional children with severe stunting. These presentations highlight the health improvement and economic value of Shigella and ETEC preventive tools. Progress in the development and evaluation of vaccine candidates is described below.
Shigella vaccines.
Investigators from Glycovaxyn presented results from a phase 1 clinical evaluation of Flexyn2a, a vaccine comprised of Shigella flexneri 2a lipopolysaccharide (LPS) conjugated to Pseudomonas aeruginosa recombinant exoprotein A (rEPA) (4). Flexyn2a was given to groups of 12 adult volunteers intramuscularly, as a 10-μg dose, either alone or in the presence of alum; two doses were given 28 days apart. The vaccine was well tolerated, exhibiting only local site reactions (pain and tenderness) consistent with intramuscular vaccination and mainly in the group receiving adjuvant. It was also highly immunogenic, and the adjuvant did not increase the rate of responses (100% and 92% of the vaccinees seroconverted for serum anti-LPS IgG and IgA in the adjuvanted and nonadjuvanted groups, respectively). A follow-up phase 2 study will be conducted in collaboration with investigators at Johns Hopkins University (JHU).
Laura Martin, from GlaxoSmithKline (GSK), presented safety and immunogenicity results from a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB (5), which was given mucosally (intranasally) and parenterally (intramuscular and intradermally) to human adult volunteers. The GMMA are outer membrane particles from S. sonnei adsorbed to alhydrogel. The exclusion criteria for the study included subjects who had titers indicative of S. sonnei exposure and protection based on those found in Israeli studies (6, 7) (800 dilutional titers, corresponding to 121 enzyme-linked immunosorbent assay [ELISA] units in the GSK assay). Each vaccine group received multiple doses, with safety and immunogenicity evaluated 7 and 28 days after each vaccination and ∼168 days after the third vaccination. Immunogenicity was determined by LPS serum IgG and fecal secretory IgA. An interim analysis of 16 vaccine and 12 placebo recipients revealed that the 100-μg intramuscular dose was well tolerated, with serum IgG titers reaching levels (∼137 ELISA units) comparable to those measured in convalescent-phase sera. The next steps for this vaccine involve assessment of protection in a human challenge model and evaluation of immunogenicity in the target population.
Investigators from the International Vaccine Institute (IVI) in Seoul, South Korea, discussed the development of a broad-spectrum vaccine against shigellosis based on mutations in the Shigella O-antigen polymerase (wzy) which shorten the LPS length, thereby allowing exposure of Shigella outer membrane proteins (Omps) for induction of protective immunity. An example of such an Omp is the outer membrane protein IcsP, which is conserved across different species and serotypes of Shigella and is typically masked by LPS on the cell wall. IcsP is better exposed on strains with short-length LPS (e.g., Δwzy strain), without affecting IcsP expression itself. Δwzy mutant strains, either live (attenuated) or formalin inactivated, given intranasally protected mice against pulmonary infection with multiple Shigella serotypes.
Investigators from the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), and Walter Reed Army Institute of Research (WRAIR) presented results from a safety and immunogenicity study of the live attenuated oral Shigella WRSS1 vaccine performed in Bangladesh, targeting a pathogen-exposed population. The study involved adults (18 to 39 years old) and children (5 to 9 years old) who received a single immunization or three immunizations in a dose escalation fashion. Adults received one dose of 3 × 104 CFU or three doses of 3 × 105 or 3 × 106 CFU of WRSS1 or placebo. Children received one dose of 3 × 103 CFU or three doses of 3 × 104 or 3 × 105 CFU WRSS1 or placebo. WRSS1 was well tolerated both in adults and in children. Vaccine shedding was seen in 50% of the adult vaccine recipients the day after the first vaccination, while no shedding was observed in children. Adults developed an immune response evidenced by antibodies in lymphocyte supernatant (ALS) and fecal IgA, which were more pronounced in the two highest-dose cohorts. Children only showed IgA responses in stool. The immune responses, however, were less robust than in North American volunteers (8). Next steps include testing a higher dose (e.g., 106 CFU) in children to determine a threshold dose for observing a systemic response.
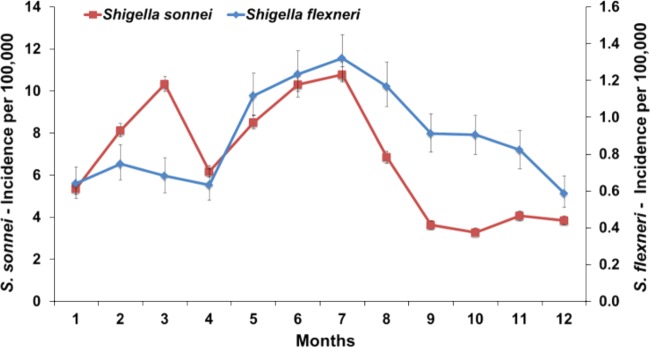
Dani Cohen, Tel Aviv University, discussed the global emergence of S. sonnei and the potential involvement of host-related biological and immunological factors. Extensive surveillance has shown that in contrast to the constant (with summer increase) incidence of S. flexneri, S. sonnei propagates cyclically (with winter outbreaks) and has higher secondary attack rates (Fig. 1). A 0.5- to 1-log-lower challenge dose is needed for S. sonnei to cause an ∼50% attack rate compared to that of S. flexneri, which may be attributable to its higher resistance to acid. This lower infective dose may in turn limit host immunity, thus allowing the prevalence of S. sonnei over other Shigella species. This hypothesis is in agreement with the observed decay in S. sonnei LPS-specific serum IgG and IgA B memory cell response after natural infection. These results highlight dose-dependent induction of protective immunity and are important to inform vaccine development.
FIG 1.
Monthly incidence of S. sonnei and S. flexneri shigellosis in Israel (more than 30,000 culture-proven cases of shigellosis, 1998 to 2012). Error bars indicate 95% confidence intervals.
Improved immunological assays to evaluate Shigella vaccines.
There is increasing interest for identification of the relevant immune markers indicative of effective vaccination. ELISA and other binding assays provide a measure of the specificity and magnitude of the antibodies induced but not of their functional antimicrobial capacity. In a poster presentation, Marcela Pasetti and colleagues from University of Maryland School of Medicine reported the development of functional assays for quantitative measurement of the opsonophagocytic antibody (OPA) and serum bactericidal antibody (SBA) activity of Shigella-specific antibodies. They demonstrated an association between the presence of serum OPA and SBA titers and reduced severity of disease in Shigella-vaccinated and -challenged individuals. The group led by Moon Nahm, University of Alabama, working in collaboration with Robert Kaminski, WRAIR, presented results from an in vitro SBA assay using different Shigella strains as targets. This assay has been successfully applied to the evaluation of bactericidal titers in serum from rabbits, as well as commercial typing and monoclonal antibodies specific for S. flexneri 2a, S. flexneri 3a, and S. sonnei LPS.
ETEC vaccines.
Unfortunately, presenters from the Naval Medical Research Center (NMRC) were unable to attend the VED meeting, and so their work is not described herein. One of the major limitations of evaluating ETEC vaccine efficacy is the lack of a suitable animal model (9). Subhra Chakraborty and colleagues from the JHU Bloomberg School of Public Health presented results from a human challenge model using the virulent ETEC strain H10407, led by the late Clayton Harro (10, 11). The lowest practical dose of H10407 that could be given to volunteers to produce a reliable attack rate was 2 × 107 CFU. This inoculum produced moderate to severe diarrhea in 71% of subjects, with a mean incubation time of 53 h (10). The bacteria were given with bicarbonate buffer following an overnight fast. Dose-dependent severity of diarrhea and immune responses against ETEC H10407 were observed. Subsequently, Michael Darsley, from the same group, reported the evaluation of a multivalent, live attenuated vaccine, ACE527, which expresses the colonization factors (CFs) CFA/I, CSI, CS2, CS3, CS5, and CS6 (12, 13) and heat-labile toxin B subunit (LTB), using the above-described challenge model. Volunteers were immunized with 1010 CFU lyophilized ACE527 with or without 25 μg double mutant heat-labile toxin (dmLT) on days 0, 28, and 56 and then challenged 6 to 7 months later with 2 × 107 CFU H10407. The primary endpoint of the study was cumulative passage of >800 g of grade 3 to 5 stool/diarrhea. When ACE527 was given with dmLT to 13 volunteers, a vaccine efficacy of 65.9% was observed (and 58.5% vaccine efficacy against diarrhea of any severity). These volunteers showed a significant reduction in the median volume and median number of stools passed compared to those from volunteers receiving placebo. ACE527 alone had a vaccine efficacy of 20.5%. The exact role of dmLT as an adjuvant or as an antigen and its contribution to protection need further understanding. This question could potentially be answered by determining protection elicited by ACE527 against an ETEC challenge strain that expresses heat-stable toxin (ST) instead of heat-labile toxin (LT).
An abstract presentation from Ann-Mari Svennerholm, University of Gothenberg Research Institute, Sahlgrenska Academy, described the induction of long-term immunological memory by an oral inactivated multivalent ETEC vaccine (ETVAX) in Swedish volunteers. This vaccine consists of four inactivated E. coli strains that overexpress CFA/I, CS3, CS5, and CS6 and the LTB-related CTB hybrid protein LCTBA (14). The vaccine was found to be safe and well tolerated when given alone or with dmLT to adult volunteers. Individuals primed with two doses of the vaccine and boosted 1 to 2 years later mounted functional mucosal immunological memory responses against the ETEC antigens (measured by ALS titers). The authors suggested that this vaccine could be given in infancy and then a booster dose given 1 year later to provide adequate protection during the first 2 to 3 years of life. This oral multivalent ETEC vaccine has previously been shown to induce IgA antibody-secreting cells (ASC) and secretory IgA responses against the colonization factors and LTB (14). Anna Lundgren presented data that describe the induction of T cell responses by this vaccine. ETVAX induced gamma interferon (IFN-γ), interleukin-17A (IL-17A), and T follicular helper cells with kinetics similar to those of the IgA ASC responses. The data suggest that the vaccine induces CD4+ T helper cells that promote IgA production and memory B cell development.
Weiping Zhang, from Kansas State University, described progress in the development and evaluation of a CFA toxoid multiepitope fusion antigen (MEFA), showing that it elicits protective antibodies against seven ETEC colonization factor antigens, CFA/I and CS1 to CS6, as well as against toxoid forms of LT and human heat-stable toxin (STa) (9). Pregnant sows were immunized 6 to 8 weeks before farrowing, and a booster was given 3 to 4 weeks later. The vaccine protected piglets against diarrhea following challenge.
Combined Shigella-ETEC vaccines.
Several groups are developing multivalent vaccines that target Shigella and ETEC simultaneously. Eileen Barry, University of Maryland School of Medicine, presented an update on her efforts to develop a combined live attenuated Shigella-ETEC vaccine. Five live attenuated Shigella strains, S. sonnei, S. dysenteriae 1, and S. flexneri 2, 3a, and 6, have been constructed and have been shown to be safe and immunogenic in preclinical studies. Further, S. flexneri 2a CVD 1208S was safe and highly immunogenic in a phase 2 clinical trial (15). ETEC antigens CFA/I and LTB have been expressed from the chromosome of CVD 1208S, and immune responses against both the ETEC and Shigella antigens were observed preclinically. Dr. Barry has further optimized ETEC antigen presentation from CVD 1208S by using the Sat autotransporter, which elicited significant anti-LTB responses in 100% of guinea pigs after a single dose.
Investigators from EveliQure Biotechnologies, Vienna, Austria, presented ShigETEC, a live attenuated vaccine that lacks LPS O antigen (due to the removal of the rfbF gene) and the ipaBC genes (involved in epithelial cell invasion) but expresses an LT-B/ST fusion protein. The vaccine induced serotype-independent protection against S. flexneri 2a and 6 and S. sonnei in the murine lethal challenge model, while inducing serum IgG and bronchoalveolar IgA against Shigella and ETEC. Antibody responses against ETEC LT and ST were detected by ELISA.
Cholera vaccines.
Dominique Legros from the World Health Organization (WHO) Global Cholera Task Force in Geneva, Switzerland, explained the creation of a global oral cholera vaccine (OCV) stockpile in 2013 and the feasibility of deployment in emergency humanitarian situations. Shanchol, Shantha Biotechnics, a killed bivalent (O1 and O139) whole-cell vaccine prequalified in 2011 as a two-dose schedule, constitutes the basis of the stockpile. As an example, this group described the outbreak OCV response in war-displaced groups in south Sudan in 2014. Their presentation emphasized the need for improved cholera surveillance systems, preparedness and control plans, and smart strategies for vaccine delivery (16). Joseph Lewnard and colleagues presented a poster with which they recounted the introduction of cholera to Haiti in 2010 by United Nations (UN) peacekeeping personnel from countries where the disease is endemic. Through mathematical modeling, they found that vaccination of UN peacekeeping forces predeployment could reduce >100-fold the number of expected cases due to an arriving cholera carrier, hence illustrating the benefit of OCV vaccination for operations that involve travel of personnel from areas of cholera endemicity to areas of nonendemicity (17). Furthermore, the use of a single-dose OCV in reactive vaccination campaigns with limited vaccine was further advocated by investigators from the JHU Bloomberg School of Public Health in Baltimore, MD, and Epicentre, Paris, France, based on their mathematical modeling of vaccine impact and simulations applied to the recent outbreaks in Haiti, Zimbabwe, and Guinea (18). These investigators also applied modeling tools to cholera incidence data from multiple sources to assess the regional distribution and burden of cholera in West Africa and reported an annual cholera incidence rate of 3.9 per 100,000 since 2009, with local incidence rates as high as nearly 5,900 per 100,000. The audience learned from Martin Mengel, from Agence Medicine Preventive, Paris, that further efforts to understand the distribution of cholera in Africa have been undertaken by AfriChol, the African Cholera Surveillance Network (19). To further understand disease transmission patterns, Amanda Debes from the JHU Bloomberg School of Public Health described a combined clinical and environmental surveillance of Vibrio cholerae for resource-limited areas that involves testing of clinical specimens with a rapid dipstick assay (crystal V. cholerae; Span Diagnostics Ltd., India), after alkaline peptone water (APW) enrichment, and blotting onto filter paper for subsequent analysis using molecular methods (20, 21). This method was successfully used in the far north region of Cameroon and enabled early detection of cases and rapid response (21).
Firdausi Qadri recapped recent efforts to introduce OCV in Bangladesh with results of a feasibility study carried out in a cluster-randomized design in collaboration with the Government of Bangladesh and using the existing Expanded Program on Immunization (EPI) infrastructure. The vaccination campaigns were accompanied by hygiene and sanitation improvement in some clusters. Overall protection against severely dehydrating cholera in the vaccine arm alone was ∼53%, whereas 57% protection was observed in the vaccine-plus-behavioral-change cohort (22). This study showed that mass vaccination campaigns with OCV are feasible and impactful. Naveena Aloysia, from Shantha Biotechnics, presented results from a phase 4 clinical study conducted in the Philippines that evaluated the safety and immunogenicity of Shanchol. This is the first study conducted in an Asian country at risk for cholera, outside India and Bangladesh, and involved over 300 subjects, including children 1 year of age and older. The vaccine was well tolerated and highly immunogenic; children up to 14 years of age had the highest conversion rates (>85%), primarily against the O1 serogroup.
Investigators from PaxVax, the University of Maryland School of Medicine, Cincinnati's Children's Hospital, and University of Vermont College of Medicine presented results of a multisite efficacy trial, based on challenge of adult volunteers immunized with a single dose of the live oral cholera vaccine CVD 103-HgR or placebo, that showed excellent safety and high efficacy. This vaccine is intended for protection of U.S. travelers going to high-risk areas and for reactive vaccination to help control disease during virgin soil outbreaks.
Investigators from Hilleman Laboratories, India, and the University of Gothenburg, Sweden, reported that they are currently developing an optimized, low-cost bivalent vaccine consisting of a single genetically engineered host strain (instead of the three strains used in current licensed vaccines) and recombinant cholera toxin B subunit (rCTB) in Bangladesh and in India. This strain is known to improve short-term protection against cholera and to confer cross protection against ETEC diarrhea. This candidate is intended as an oral vaccine in a formulation (that does not require clean drinking water following intake) designed specifically for low-income countries with a high cholera burden. In the area of cholera immunity, Nicholas Mantis, from the Wadsworth Center, showed how IgA monoclonal antibodies specific for the O antigen or the lipid A portion of the V. cholerae LPS inhibited flagellum-based motility and altered the bacterial outer membrane (Fig. 2) and proposed that secretory IgA deploys multiple effects that will ultimately impair the ability of the bacterium to colonize the intestinal epithelium (23).
FIG 2.
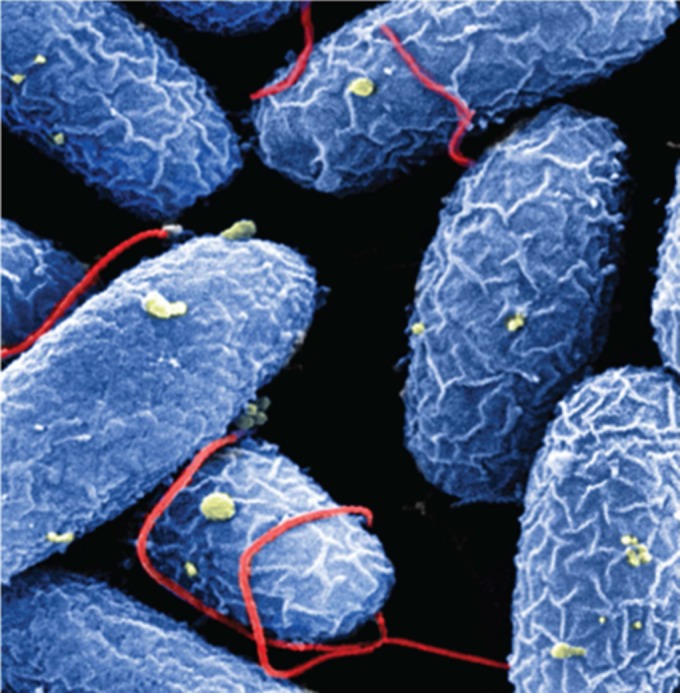
Vibrio cholerae O395 was treated with the murine monoclonal IgA antibody 2D6 for 60 min and processed for scanning electron microscopy. 2D6 binds the O-polysaccharide portion of V. cholerae lipopolysaccharide, which covers the bacterial membrane surface (blue) and single polar flagellum (red). Treatment with 2D6 IgA resulted in membrane wrinkling, shown by surface membrane blebs (yellow), flagellum-flagellum cross-linking, and flagellar entanglement with neighboring bacterial bodies. Original magnification, ×22,500. (Photo courtesy of Kara Levinson, Richard Cole, and Nicholas Mantis, reproduced with permission.)
Cholera infection and maternal immunization with an oral cholera vaccine.
Maternal immunization is gaining attention as a practical and effective means of protection for mothers and young infants. Pregnant women are a high-risk group during cholera outbreaks, and while oral cholera vaccination has been recommended for other groups, expecting mothers have been excluded due to the lack of safety data. Lise Grout and colleagues from the European Programme for Intervention Epidemiology (EPIET), Sweden, the Ministry of Health and Public Hygiene, Guinea, and Médecins Sans Frontières reviewed pregnancy outcomes in women with cholera who were hospitalized during the 2010–2011 outbreak in Haiti and investigated a retrospective cohort of women who were pregnant during or after the OCV campaign study in Guinea (24). Of 263 hospitalized pregnant women in Haiti, none died, but 21 (8%) suffered intrauterine fetal death, with the main risk factors being severe dehydration and vomiting. In Guinea, of 2,494 vaccinated women (ascertained by card or oral history) examined, no association between fetal exposure to OCV and risk of pregnancy loss or malformation was found (24). These results open the discussion for the inclusion of pregnant women in future vaccination campaigns, particularly during outbreaks or when risk of cholera infection is high.
Salmonella vaccines and live vector vaccines.
Several presentations addressed the use of the oral live attenuated Salmonella enterica serovar Typhi Ty21a strain as a live vector vaccine. Madushini Dharmasena, U.S. Food and Drug Administration (FDA)-Center for Biologics Evaluation and Research (CBER), described the expression of S. flexneri 2a and 3a O-antigen synthesis gene clusters from the viaB operon of Ty21a. High levels of O-antigen production were achieved by replacing the native promoter with the strong lpp promoter. The recombinant Ty21a vaccine strains expressing heterologous S. flexneri 2a or 3a LPS elicited significant serum antibody responses against both homologous and heterologous LPS. Furthermore, these strains were able to protect mice against challenge with S. flexneri 2a and 3a. Using this approach, the investigators hope to develop a multivalent Ty21a-based vaccine expressing LPS of S. sonnei, S. dysenteriae 1, S. flexneri 2a and 3a, and S. flexneri 6 that could protect against ∼85% of shigellosis worldwide. Yun Wu, Protein Potential LLC, presented work describing a genetically engineered Ty21a strain that expresses the S. sonnei O-antigen gene cluster as well as genes that encode glutamine-glutamate acid resistance carried on the chromosome. The latter genes enable the bacteria to survive in vitro at pH 2.5, which would allow the vaccine to survive passage through the highly acidic environment of the stomach. Preclinical studies on this candidate vaccine are ongoing. Rocky Cranenburgh described some of Prokarium Ltd.'s platforms and vaccine candidates. They have an antibiotic marker gene-free system called “operator-repressor titration for vaccines” (ORT-VAC) (25). Vaccines include Vaxonella, which has an S. Typhimurium WT05 backbone, and Typhetec, a bivalent typhoid-ETEC vaccine that uses the S. Typhi ZH9 vaccine strain as the background strain. Kevin Killeen also presented an update on progress of Matrivax Inc.'s typhoid protein capsular matrix vaccine. This vaccine consists of entrapment of Vi capsular polysaccharide in a matrix composed of cross-linked carrier proteins. If it is successful, they will consider including S. Paratyphi A, S. Enteritidis, and S. Typhimurium O antigens in this vaccine to create a broad-spectrum typhoid and nontyphoidal Salmonella (NTS) vaccine. Interestingly, there were no talks describing progress on development of Vi conjugate vaccines, the typhoid and paratyphoid challenge model, or development of NTS and paratyphoid vaccines—most likely because much of this work had been presented at the Invasive Salmonellosis Meeting held in Bali, Indonesia, earlier in 2015.
Rotavirus vaccines.
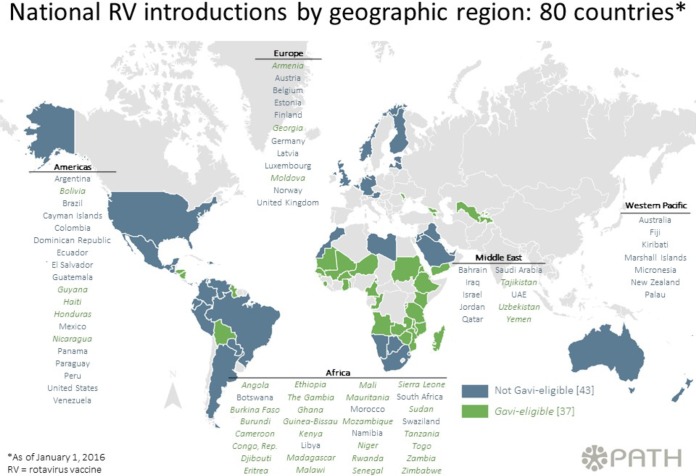
Rotavirus vaccines are widely utilized globally, and the impact of the vaccines in middle- and high-income countries is well documented. The effectiveness of the live attenuated oral rotavirus vaccines in low- and lower-middle-income countries is, however, a matter of concern due to the decreased efficacy that was observed in clinical studies in Africa and Asia (38) compared with efficacy in industrialized nations. An increasing number of African countries are introducing rotavirus vaccines into their routine immunization schedules, with GAVI (Global Alliance for Vaccines and Immunizations) support (Fig. 3). Thus, several studies documented attempts to better understand the vaccine performance in these regions. Laura Beres, from the Centre for Infectious Disease Research in Zambia (CIDRZ), presented the results of a case-control study to determine rotavirus vaccine effectiveness in Lusaka Province, Zambia. The Zambian government, in partnership with CIDRZ and Absolute Return for Kids (ARK), introduced a pilot rollout of Rotarix (GSK Biologicals, Rixensart, Belgium) in all public health facilities in Lusaka Province. Children with diarrhea were recruited from six sentinel health facilities, and the severity of each episode was estimated using the 20-point Vesikari severity scoring system. Among children who were age eligible for the vaccine and had vaccination card-confirmed vaccination status, approximately 25% were rotavirus positive, and one-fifth of these were hospitalized with severe rotavirus diarrhea (Vesikari score of >11). For children ≥6 months of age who received two doses of vaccine, the adjusted vaccine effectiveness was ∼23% against any rotavirus diarrhea and ∼68% against severe rotavirus diarrhea. This trend toward greater protection against more severe rotavirus disease is known and has been described elsewhere (39). Although this study is a small pilot assessment limited by sample size, it suggests an impact for national vaccine implementation.
FIG 3.
Map showing introduction of rotavirus vaccine by geographic regions based on WHO recommendations (reproduced from the PATH website at http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet/ [accessed 7 March 2016]). Up to 80 countries have implemented rotavirus vaccination of infants as part of the national immunization programs.
Coadministration of trivalent oral poliovirus vaccine (tOPV) is known to lower the immune response to rotavirus vaccines. Bivalent and monovalent OPVs containing only poliovirus type 1 and/or 3 are increasingly used in vaccination campaigns, and bivalent OPV will soon be introduced into routine immunization schedules globally. Devy Emperador, from the U.S. Centers for Disease Control and Prevention (CDC), assessed the potential interference of coadministration of these OPV formulations on Rotarix immunogenicity through a post hoc analysis of a randomized clinical trial of OPV among healthy infants in Matlab, Bangladesh (26). Infants were randomized to receive three doses of monovalent OPV type 1 (mOPV) or bivalent OPV (bOPV) at 6, 8, and 10 weeks of age or at 6, 10, and 14 weeks of age or tOPV at 6, 10, and 14 weeks of age. Two doses of Rotarix were given at around 6 and 10 weeks of age. Rotavirus seroconversion was 56% overall. However, infants who received Rotarix and OPV concomitantly were less likely to seroconvert (47%) than those that received vaccines staggered ≥1 day from each other (63%) (P < 0.001). Coadministration of OPV, regardless of OPV type, lowers Rotarix immunogenicity to a similar extent.
Vaccine-driven strain replacement is a concern for global public health, but multiyear oscillations in the distribution of the predominant rotavirus genotypes make it difficult to determine what changes can be attributed to the impact of vaccination. Virginia Pitzer, from Yale University, developed a mathematical model to assess data on genotype-specific hospitalizations for rotavirus diarrhea in Belgium to examine the underlying dynamics driving changes in the genotype distributions before and after vaccine introduction (27). Rotavirus vaccines were introduced into the national immunization schedule of Belgium in 2006, and vaccine coverage, primarily with the monovalent Rotarix vaccine (based on an attenuated G1P1a[8] strain), has been high (>85%), resulting in a dramatic drop in the incidence of hospitalization for rotavirus gastroenteritis. Dr. Pitzer estimated that natural and vaccine-derived immunity was strongest against completely homotypic Wa-like G1P1a[8] strains and weakest against heterotypic DS-1-like G2P1b[4] strains. The predominance of G2P1b[4] infections after vaccine introduction can be explained by a combination of natural genotype fluctuations and slightly weaker natural and vaccine-induced immunity against strains heterotypic to the vaccine. However, the incidence of rotavirus gastroenteritis is predicted to remain low, despite possible changes in the relative distribution of genotypes.
Additional studies in Zambia and South Africa explored the role of maternal antibodies to rotavirus and their impact on vaccine take. Roma Chilengi and colleagues, from the Centre for Infectious Disease Research in Zambia (CIDRZ), assessed the role of maternal breast milk (BM) components (i.e., IgA), transplacentally acquired IgG, and HIV status on seroconversion in Zambian infants routinely immunized with Rotarix. Mother-child pairs were recruited when the child was 6 to 12 weeks of age. Infants were immunized with all EPI vaccines, including the rotavirus vaccine, and a month after the second dose, blood was obtained for determination of serum IgA and IgG. Breast milk was also obtained from the mothers. Seroconversion rates appeared to decrease with increasing breast milk anti-rotavirus IgA titers. The low immunogenicity of rotavirus vaccine could be explained in part by exposure to high BM antibodies and placentally acquired IgG. Sung-Sil Moon, U.S. CDC, Atlanta, GA, described similar results from a study in Soweto, South Africa (28). Mother-infant pairs were enrolled at presentation for their routine immunization visit, and maternal serum and BM samples were obtained prior to each vaccination. Again, preexisting rotavirus IgA and IgG titers were generally higher in sera of infants who failed to seroconvert than in sera of infants who did seroconvert. Highlighting the importance of early exposure to infection, Aisleen Bennet, from the Malawi College of Medicine/Liverpool Medical School, described the age-adjusted prevalence and concentration of serum anti-rotavirus IgA as a measure of rotavirus exposure in a cohort of rural children from Karonga, Malawi, prior to rotavirus vaccine introduction in October 2012. Serum samples were collected at 6, 26, and 52 weeks of life from children enrolled in a birth cohort study and examined for anti-rotavirus IgA. Children in this community were exposed to rotavirus at a young age, the majority within the first year of life. Crowding and number of children in the household were found to be risk factors for exposure.
Interestingly, Michelo Simuyandi, from CIDRZ, explored the concept that the seasonal pattern of rotavirus infection would strengthen the anti-rotavirus IgA levels in Zambian mothers and thus impact the vaccine responses in their infants. There was a significant difference in BM IgA titers between seasons, which was negatively correlated with the infant prevaccination IgA titers. These observed variations in BM IgA content across seasons may abrogate vaccine immunogenicity. The Performance of Oral Vaccines in Developing Countries (PROVIDE) study was performed to understand the biologic basis for oral polio and rotavirus vaccine underperformance in developing countries (29). Variables of interest studied in this birth cohort included measures of environmental enteropathy, malnutrition, maternal or antibody interference, and enteric coinfections. Three presentations reported the preliminary results from this study. Beth Kirkpatrick, from University of Vermont, described the Bangladeshi cohort of infants, representative of children for which oral vaccines underperform. One-third of children were premature (gestational age of ≤36 weeks); children had a median of three episodes of diarrhea in the first year of life. By 52 weeks of age, >25% of children were stunted (height-for-age Z-score of <−2) and ∼22% wasted (weight-for-age Z-score of <−2). Eric Houpt from UVA described the evaluation of a broad range of enteropathogens to test the hypothesis that reduced efficacy is partly due to misattribution of diarrhea due to nonrotavirus enteropathogens. Vaccine efficacy against severe rotavirus monoinfections was higher than that against severe rotavirus with coinfections, indicating that copathogen infections may impact estimates of vaccine efficacy against rotavirus diarrhea. Ross Colgate, University of Vermont, confirmed that IgA seropositivity after vaccination was significantly associated with clinical protection.
Because of the lower immunogenicity and efficacy of live oral rotavirus vaccines in resource-limited regions, nonreplicating vaccines have been pursued as an alternative strategy. The P2-V8* rotavirus vaccine candidate consists of an E. coli-expressed VP8* subunit of VP4 from the Wa strain of human rotavirus (G1P1a[8]) fused to the P2 epitope from tetanus toxin and expressed as a chimeric protein. The vaccine was found to be well tolerated and immunogenic in a phase 1 study conducted in adults in the United States (30). Michelle Groome, from the National Institute for Communicable Diseases (NICD), South Africa, reported the robust preliminary immunogenicity results of a double-blind, randomized, placebo-controlled dose escalation study in Soweto, South Africa, evaluating the safety, tolerability, and immunogenicity of the monovalent P2-VP8* vaccine, first in toddlers and then in infants. Three different dose levels of vaccine, adsorbed to aluminum hydroxide and administered intramuscularly, were first evaluated for safety in toddlers (aged 24 to 35 months) who received a single injection of vaccine or placebo. After safety review, cohorts of infants received three vaccine injections at 4-week intervals, per dose level concomitant with routine EPI vaccinations. The two highest dose levels tolerated in the infant cohort are being assessed in an expanded infant cohort. Thus far, the vaccine has been well tolerated, without vaccine-related safety concerns, at all dose levels in both toddlers and infants and shows robust immune responses similar to those seen in the adult study.
Norovirus vaccines.
Norovirus has increasingly been recognized for its predominant role as a cause of acute gastroenteritis (AGE) in infants, in the elderly, and in outbreaks. Several presentations highlighted the importance of norovirus infection in these different populations. Kayoko Shioda, from the U.S. CDC, described the global age distribution of pediatric norovirus AGE cases (defined as children aged <5 years) to investigate differences by income level and disease severity (31). A systematic review of the literature from January 2001 to August 2014 included detailed data on age distribution among patients <5 years of age with laboratory-confirmed norovirus AGE. Approximately 70% of AGE cases occurred between 6 and 23 months of age; interestingly, a younger age distribution was observed in lower-income countries and in inpatient settings. The younger age of pediatric norovirus cases in low-income settings and among inpatients implies a higher force of infection and more frequent severe disease among the youngest children, respectively. These findings suggest that a potential future immunization schedule that complies with current EPI schedules would be optimal and potentially could protect up to ∼85% of infants.
Two studies in developing countries also emphasized the burden of norovirus in these populations. Innocent Mwape, CIDRZ, described a study enrolling children <5 years of age presenting with AGE to health care facilities in Lusaka Province, Zambia, who were assessed by PCR for the presence of norovirus. Norovirus was associated with 10% of cases, and both genotype I (GI) (28%) and GII (72%) strains were identified by sequence analysis. Sylvia Becker-Drepps, University of North Carolina at Chapel Hill, described norovirus burden in a population-based sample of Nicaraguan children <5 years of age after the introduction of rotavirus vaccine (32). AGE stool samples were assayed for 14 enteropathogens; norovirus was identified as the most common pathogen among diarrheal stools in the study (20%) but was also commonly detected among healthy stools (13%). Norovirus was more commonly detected among children <2 years of age with diarrhea than among older children (26% versus 16%, P = 0.02). In addition, episodes in which norovirus was detected were more likely to be associated with vomiting than episodes in which norovirus was not detected (41% versus 24%, P = 0.008).
Three reports described the epidemiology and burden of norovirus infection in various U.S. cohorts. Jan Vinje, U.S. CDC, described an investigation in long-term care facilities (LTCF) in the United States that prospectively studied the epidemiology, virology, and genetic host factors of naturally occurring norovirus outbreaks (33). Acute- and convalescent-phase stool, serum, and saliva samples from cases, exposed individuals, and nonexposed controls were collected. Norovirus infection was confirmed using quantitative PCR in stool or 4-fold increase in serum antibody titers. The presence of histoblood group antigens (HBGAs) (secretor, ABO, and Lewis type) in saliva was determined. Almost half of all positive symptomatic individuals shed virus for at least 21 days. Viral load was highest in newly emerging GII.4 viruses, and these viruses also infect the nonsecretor population. These findings should help to guide development of targeted prevention and control measures in the elderly. Aron Hall, U.S. CDC, reported the results of a year-long investigation assessing “medically-attended acute gastroenteritis” episodes among children and adults. Overall, 11% of cases were norovirus positive, although infection was highest among children <5 years of age (18%) and lowest among the elderly ≥65 years of age. A poster presentation from Brian Rha, U.S. CDC, reported similar results from a review of the diagnostic codes of gastroenteritis-associated medical encounters of active-duty U.S. military personnel and their dependents, in which approximately one-third of all-cause gastroenteritis encounters were norovirus associated. Highest rates occurred in children <5 years of age.
Takeda Vaccines, Inc., is developing a multivalent, adjuvanted candidate norovirus vaccine based on virus-like particles (VLPs) from a GI.1 strain and a consensus GII.4 sequence (Fig. 4), which is derived from three natural GII.4 variants, to induce a broad heterotypic immunity in adults and children (34). This vaccine is alum based and administered by intramuscular injection. Robert Bargatze from Takeda Vaccines, Bozeman, MT, presented an overview of this vaccine program, reporting that investigational vaccine formulations with dosages ranging from 5 to 150 μg of the two VLPs, with and without monophosphoryl lipid A (MPL), have been evaluated in phase 1 and 2 trials, which have enrolled over 1,100 adults to date. The vaccine formulations are generally well tolerated, with no vaccine-related serious adverse effects reported. High immune responses have been observed in terms of serum IgG and IgA and the capacity of antibodies to block histoblood group antigen (HBGA) VLP binding. The latter has been implicated as a potential serologic correlate of protection in human challenge studies (35). Jakob Cramer, from Takeda Pharmaceuticals International, Switzerland, reported the results of a phase 2 randomized, placebo-controlled double-blind study of two formulations of the alum and MPL-adjuvanted VLP candidate in healthy adults. The two candidate VLP formulations with various ratios of GI.1 to GII.4 were well tolerated and elicited rapid and robust immune responses with acceptable safety profiles. No severe adverse events were reported. Molly Steele, from Emory University, described a deterministic, age-structured compartmental model developed to track norovirus transmission and immunity in the U.S. population. Three age-specific transmission parameters were estimated using maximum likelihood and fitting the model to age-specific monthly U.S. hospitalizations between 1996 and 2007. Two vaccine strategies were simulated under the assumption that immunization provides the same duration of protection as natural infection: routine immunization at around the time of birth and of individuals turning 65 years old, followed by revaccination every 5 years. In initial simulations, vaccine efficacy and coverage were both assumed to be 50%. The model outputs achieved good fit to the U.S. hospitalization data, and results indicated that 0 to 4 year olds were much more important in transmission than subjects in older age groups. The modeling analysis demonstrated that population-level impacts of norovirus vaccination may be maximized by vaccinating young children, due to their importance in transmission.
FIG 4.
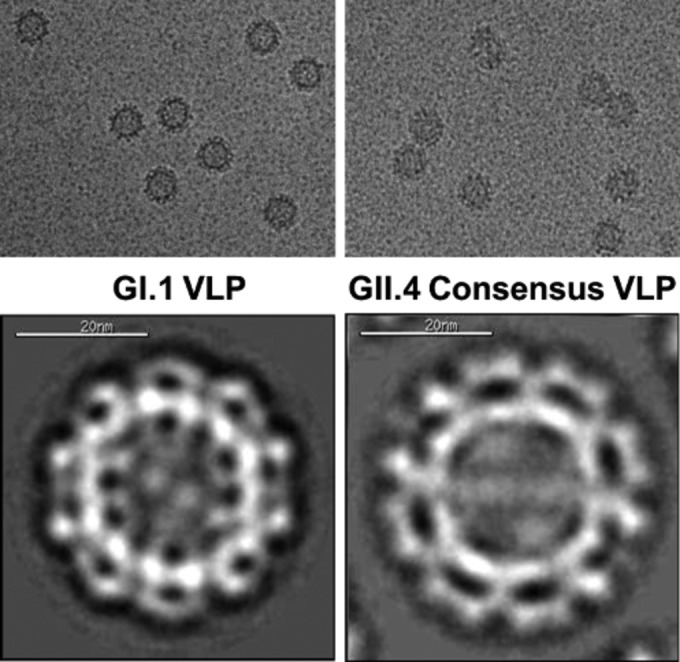
Cryo-transmission electron microscopy (cryo-TEM) micrographs of norovirus VLP antigens. (Photo courtesy of Takeda Vaccines, reproduced with permission.)
Helicobacter pylori infections.
Helicobacter pylori infection is acquired in early childhood, and yet its role in children's health remains unclear. Khitam Muhsen, from Tel Aviv University, reported on a literature analysis of the association between H. pylori infection and children's growth (36). Original studies that addressed the association between H. pylori infection or eradication and children's growth were reviewed, and the risk of bias of each study was assessed. The existing evidence, based on 48 observational studies, suggests that H. pylori infection might adversely influence children's growth; findings were more consistent across studies with low risk of bias. Regarding linear growth, observational studies have repeatedly linked H. pylori infection and slower or diminished linear growth; yet, it is not known whether this association is causal. Additional studies, especially clinical trials, are needed to elucidate the role of H. pylori eradication in children's growth and the mechanisms involved.
Advocacy for use of vaccines to improve public health.
The Defeat Diarrheal Disease (DefeatDD; http://www.defeatdd.org/) initiative presented an abstract describing their efforts to raise awareness and procure policy and financial resources to facilitate a broader distribution of newly developed vaccines, particularly for those most vulnerable, for the highest possible improvement in public health. The group has succeeded in calling attention to diarrheal disease at the global health level by proposing a coordinated approach that involves the use of vaccines, water sanitation and hygiene (WASH), nutrition, oral rehydration solution (ORS), and zinc. The DefeatDD team has helped position phase 3 trial results of the ROTAVAC vaccine in India and leads a global campaign in support of the WHO/UNICEF Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhea (GAPPD). DefeatDD advocates stressed the importance of researchers communicating the progress of their research through data-based evidence to inform the health decision-making process and have a direct life-saving impact.
Summary.
The VED meeting recounted the most recent efforts and advances worldwide to address the burden of enteric diseases. Significant progress has been made in understanding the etiology of diarrheal illnesses in the most affected areas and the impact of these illnesses on child health and development. Improved molecular methods are now available to enhance disease surveillance and to identify situations that may require reactive vaccination. Innovative vaccine approaches that use new antigens and antigen combinations to achieve broad protection are emerging. More clinical studies are being conducted in areas where disease is endemic. Further research is needed to identify host factors (e.g., maternal immunity, nutrition, age, gender) that affect disease susceptibility and responses to vaccines and the mechanisms that underlie those factors. Models that recapitulate the gut of pathogen-exposed children are needed to inform vaccine development efforts and to assess interventions. The importance of defining immune correlates of protection and further developing transferable assays that can predict vaccine efficacy was stressed. In the coming years, we expect to see success from current vaccine approaches, broader use of existing vaccines, and more innovative technology being applied to improve global health. The first International Conference on Vaccines against Shigella and ETEC (VASE), organized by PATH, will be held in June 2016 in Washington, DC, for scientists, public health professionals, and other experts to share research breakthroughs and ideas that can advance global health through enteric vaccines.
ACKNOWLEDGMENTS
We thank Robert Bargatze, Justin Lessler, Andrew Azman, Firdausi Qadri, Dani Cohen, and Nick Mantis for reviewing sections of the manuscript and for providing pictures.
S.M.T. and M.F.P. are supported, in part, by NIH, NIAID awards U19 AI109776-01 and R01 AI117734-01, respectively.
REFERENCES
- 1.WHO. 2015. WHO estimates of the global burden of foodborne diseases. Foodborne Disease Burden Epidemiology Reference Group 2007-2015. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, Morris CR, Juma J, Fields BS, Dione M, Malle D, Becker SM, Houpt ER, Nataro JP, Sommerfelt H, Pop M, Oundo J, Antonio M, Hossain A, Tamboura B, Stine OC. 2013. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 51:1740–1746. doi: 10.1128/JCM.02713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kampf MM, Braun M, Sirena D, Ihssen J, Thony-Meyer L, Ren Q. 2015. In vivo production of a novel glycoconjugate vaccine against Shigella flexneri 2a in recombinant Escherichia coli: identification of stimulating factors for in vivo glycosylation. Microb Cell Fact 14:12. doi: 10.1186/s12934-015-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di CV, Saul A. 2015. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One 10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen D, Green MS, Block C, Rouach T, Ofek I. 1988. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis 157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D, Block C, Green MS, Lowell G, Ofek I. 1989. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J Clin Microbiol 27:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotloff KL, Taylor DN, Sztein MB, Wasserman SS, Losonsky GA, Nataro JP, Venkatesan M, Hartman A, Picking WD, Katz DE, Campbell JD, Levine MM, Hale TL. 2002. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect Immun 70:2016–2021. doi: 10.1128/IAI.70.4.2016-2021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Sack DA. 2015. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli-associated diarrhea. Clin Vaccine Immunol 22:983–991. doi: 10.1128/CVI.00224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harro C, Chakraborty S, Feller A, DeNearing B, Cage A, Ram M, Lundgren A, Svennerholm AM, Bourgeois AL, Walker RI, Sack DA. 2011. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin Vaccine Immunol 18:1719–1727. doi: 10.1128/CVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty S, Harro C, DeNearing B, Ram M, Feller A, Cage A, Bauers N, Bourgeois AL, Walker R, Sack DA. 2015. Characterization of mucosal immune responses to enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: response profiles after primary infection and homologous rechallenge with strain H10407. Clin Vaccine Immunol 23:55–64. doi: 10.1128/CVI.00617-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harro C, Sack D, Bourgeois AL, Walker R, DeNearing B, Feller A, Chakraborty S, Buchwaldt C, Darsley MJ. 2011. A combination vaccine consisting of three live attenuated enterotoxigenic Escherichia coli strains expressing a range of colonization factors and heat-labile toxin subunit B is well tolerated and immunogenic in a placebo-controlled double-blind phase I trial in healthy adults. Clin Vaccine Immunol 18:2118–2127. doi: 10.1128/CVI.05342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S, Nataro JP, Blackwelder WC, Barry EM, Picking W, Levine MM. 2007. Safety and immunogenicity of CVD 1208S, a live, oral ΔguaBA Δsen Δset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccin 3:268–275. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 16.Abubakar A, Azman AS, Rumunu J, Ciglenecki I, Helderman T, West H, Lessler J, Sack DA, Martin S, Perea W, Legros D, Luquero FJ. 2015. The first use of the global oral cholera vaccine emergency stockpile: lessons from South Sudan. PLoS Med 12:e1001901. doi: 10.1371/journal.pmed.1001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewnard JA, Antillon M, Gonsalves G, Miller AM, Ko AI, Pitzer VE. 2016. Strategies to prevent cholera introduction during international personnel deployments: a computational modeling analysis based on the 2010 Haiti outbreak. PLoS Med 13:e1001947. doi: 10.1371/journal.pmed.1001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack DA, Lessler J. 2015. The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: a modeling study. PLoS Med 12:e1001867. doi: 10.1371/journal.pmed.1001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengel MA. 2014. Cholera in Africa: new momentum in fighting an old problem. Trans R Soc Trop Med Hyg 108:391–392. doi: 10.1093/trstmh/tru077. [DOI] [PubMed] [Google Scholar]
- 20.Debes AK, Ateudjieu J, Guenou E, Lopez AL, Bugayong MP, Retiban PJ, Garrine M, Mandomando I, Li S, Stine OC, Sack DA. 2016. Evaluation in Cameroon of a novel, simplified methodology to assist molecular microbiological analysis of V. cholerae in resource-limited settings. PLoS Negl Trop Dis 10:e0004307. doi: 10.1371/journal.pntd.0004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debes AK, Ateudjieu J, Guenou E, Ebile W, Sonkoua IT, Njimbia AC, Steinwald P, Ram M, Sack DA. 11 January 2016. Clinical and environmental surveillance for Vibrio cholerae in resource constrained areas: application during a 1-year surveillance in the far north region of Cameroon. Am J Trop Med Hyg doi: 10.4269/ajtmh.15-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri F, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Begum YA, Bhuiyan TR, Chowdhury MI, Uddin MJ, Khan JA, Chowdhury AI, Rahman A, Siddique SA, Asaduzzaman M, Akter A, Khan A, Ae You Y, Siddik AU, Saha NC, Kabir A, Riaz BK, Biswas SK, Begum F, Unicomb L, Luby SP, Cravioto A, Clemens JD. 2015. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet 386:1362–1371. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 23.Levinson KJ, De Jesus M, Mantis NJ. 2015. Rapid effects of a protective O-polysaccharide-specific monoclonal IgA on Vibrio cholerae agglutination, motility, and surface morphology. Infect Immun 83:1674–1683. doi: 10.1128/IAI.02856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grout L, Martinez-Pino I, Ciglenecki I, Keita S, Diallo AA, Traore B, Delamou D, Toure O, Nicholas S, Rusch B, Staderini N, Serafini M, Grais RF, Luquero FJ. 2015. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine in Guinea: a retrospective cohort study. PLoS Negl Trop Dis 9:e0004274. doi: 10.1371/journal.pntd.0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garmory HS, Leckenby MW, Griffin KF, Elvin SJ, Taylor RR, Hartley MG, Hanak JA, Williamson ED, Cranenburgh RM. 2005. Antibiotic-free plasmid stabilization by operator-repressor titration for vaccine delivery by using live Salmonella enterica serovar Typhimurium. Infect Immun 73:2005–2011. doi: 10.1128/IAI.73.4.2005-2011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, Anand A, Zaman K. 2016. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin Infect Dis 62:150–156. doi: 10.1093/cid/civ807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitzer VE, Bilcke J, Heylen E, Crawford FW, Callens M, De Smet F, Van Ranst M, Zeller M, Matthijnssens J. 2015. Did large-scale vaccination drive changes in the circulating rotavirus population in Belgium? Sci Rep 5:18585. doi: 10.1038/srep18585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, van Niekerk N, Jiang B, Madhi SA. 2016. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 62:157–165. doi: 10.1093/cid/civ828. [DOI] [PubMed] [Google Scholar]
- 29.Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, Nayak U, Taniuchi M, Naylor C, Qadri F, Ma JZ, Alam M, Walsh MC, Diehl SA, Petri WA Jr. 2015. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg 92:744–751. doi: 10.4269/ajtmh.14-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fix AD, Harro C, McNeal M, Dally L, Flores J, Robertson G, Boslego JW, Cryz S. 2015. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine 33:3766–3772. doi: 10.1016/j.vaccine.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Shioda K, Kambhampati A, Hall AJ, Lopman BA. 2015. Global age distribution of pediatric norovirus cases. Vaccine 33:4065–4068. doi: 10.1016/j.vaccine.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker-Dreps S, Bucardo F, Vilchez S, Zambrana LE, Liu L, Weber DJ, Pena R, Barclay L, Vinje J, Hudgens MG, Nordgren J, Svensson L, Morgan DR, Espinoza F, Paniagua M. 2014. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J 33:1156–1163. doi: 10.1097/INF.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini VP, Cooper EM, Hardaker HL, Lee LE, Bierhoff M, Biggs C, Cieslak PR, Hall AJ, Vinje J. 2016. Epidemiologic, virologic, and host genetic factors of norovirus outbreaks in long-term care facilities. Clin Infect Dis 62:1–10. doi: 10.1093/cid/civ747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinje J, Gregoricus N, Frenck RW Jr, Moe CL, Al-Ibrahim MS, Barrett J, Ferreira J, Estes MK, Graham DY, Goodwin R, Borkowski A, Clemens R, Mendelman PM. 2015. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 211:870–878. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atmar RL, Bernstein DI, Lyon GM, Treanor JJ, Al-Ibrahim MS, Graham DY, Vinje J, Jiang X, Gregoricus N, Frenck RW, Moe CL, Chen WH, Ferreira J, Barrett J, Opekun AR, Estes MK, Borkowski A, Baehner F, Goodwin R, Edmonds A, Mendelman PM. 2015. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol 22:923–929. doi: 10.1128/CVI.00196-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dror G, Muhsen K. 24 November 2015. Helicobacter pylori infection and children's growth—an overview. J Pediatr Gastroenterol Nutr Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.MAL-ED Network Investigators. 2014. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 59(Suppl 4):S193–S206. doi: 10.1093/cid/ciu65. [DOI] [PubMed] [Google Scholar]
- 38.WHO. 2009. Rotavirus vaccines: an update. Wkly Epidemiol Rec 84:533–540. [PubMed] [Google Scholar]
- 39.Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, Hille D, Ciarlet M, Neuzil KM. 2012. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy studies of the oral pentavalent rotavirus vaccine. Vaccine 30(Suppl 1):A24–A29. doi: 10.1016/j.vaccine.2011.08.124. [DOI] [PubMed] [Google Scholar]