Abstract
Purpose
To evaluate the effect of platelet-rich plasma (PRP) eye drops in the treatment of recurrent corneal erosions (RCE).
Methods
A total of 47 eyes were included in this retrospective study. Clinical records of 20 consecutive patients with RCE who had been treated with conventional lubricant eye drops (conventional treatment group) from June 2006 to December 2008 and 27 consecutive patients treated with autologous PRP eye drops in addition to lubricant eye drops (PRP eye drops treated group) from January 2009 to September 2014 were reviewed. Major and minor recurrences were recorded and compared between two groups.
Results
This study included 31 men and 16 women. The mean age was 44.5 ± 14.5 years (range, 19 to 86 years), and the mean follow-up duration was 14.9 ± 14.4 months (range, 6 to 64 months). Of the 27 cases in the PRP eye drops treated group, there were seven major recurrences in six eyes (22.2%) and ten minor recurrences in seven eyes (25.9%). In contrast, 16 eyes (80.0%) from the 20 patients in the conventional lubricant eye drops treated group had major recurrences, and all patients in this group reported minor recurrences. The mean frequency of recurrence was 0.06 ± 0.08 per month in the PRP eye drops treated group and 0.39 ± 0.24 per month in the conventional treatment group (p = 0.003). No side effects were noted in any of the patients over the follow-up period.
Conclusions
The use of PRP eye drops for the treatment of RCE was shown to be effective in reducing the recurrence rate without any significant complications.
Keywords: Autologous serum, Corneal epithelium, Ophthalmic solutions, Platelet-rich plasma, Recurrent corneal erosions
Recurrent corneal erosion (RCE) is a chronic relapsing disease of the corneal epithelium characterized by repeated episodes of sudden onset pain, usually at night or upon waking, accompanied by redness, photophobia, and blurred vision. These symptoms are related to corneal de-epithelialization in an area in which the epithelium is weakly adherent and generally occur during the first eyelid movement in the morning.
The basic principle of treatment of RCE is to increase adhesion of the epithelium to the underlying stroma. Conservative management includes topical and systemic medication such as lubricating eye drops, hyperosmotic agents, inhibitors of matrix metalloproteinase (MMP)-9, cortico-steroids, and doxycycline [1,2]. Therapeutic contact lenses can also provide symptomatic relief and encourage healing of the corneal epithelium. Surgical management includes superficial keratectomy using a razor blade fragment, a diamond knife or a diamond burr, as well as anterior stromal puncture, Nd:YAG laser stromal puncture, and phototherapeutic keratectomy [3,4,5,6,7,8]. While the aforementioned treatment methods are useful and effective in some patients, many patients experience recurrent episodes of corneal erosions.
Autologous serum not only acts as a lubricant at the ocular surface, but also supplies several essential substances for the recovery of damaged epithelium, including vitamin A, epidermal growth factor (EGF), fibronectin and a variety of cytokines [9,10,11]. With these epitheliotrophic factors, serum facilitates the proliferation, migration, and differentiation of the ocular surface epithelium [12,13]. On the basis of these properties, serum eye drops have been effectively applied for the treatment of ocular surface diseases, severe dry eye, ocular graft-versus-host disease, persistent epithelial defects, neurotropic keratopathy, and RCE [13,14,15,16,17,18,19,20,21,22]. del Castillo et al. [23] have reported that the use of autologous serum for the treatment of patients with RCE is effective and safe in reducing the rate of recurrence.
Platelet-rich plasma (PRP) contains more concentrated platelets than whole blood and can be obtained via centrifugation from whole blood mixed with anticoagulant. Platelets are critically important in the wound-healing process. They translocate rapidly to the wound site and adhere to the damaged tissue, initiating a healing reaction that includes the release of a variety of cytokines and growth factors. The α-granules within these platelets liberate platelet-derived growth factors, platelet factor IV, and transforming growth factor (TGF)-β. Thus, PRP is known to harbor high concentrations of growth factors and can promote effective wound healing [24,25]. PRP eye drops can be used to treat ocular surface disease based on these helpful wound-healing properties. Alio et al. [26,27] have reported that autologous PRP eye drops can be used to successfully treat corneal ulcers and ocular surface syndrome after laser-assisted in situ keratomileusis, We have previously reported improved outcomes with PRP eye drops than autologous serum eye drops in patients with persistent epithelial defect after infectious keratitis [28]. On the basis of these observations, we hypothesized that PRP eye drops are effective for corneal epithelial wound healing in RCE patients. In this study, we retrospectively evaluated the efficacy of PRP eye drops in the treatment of RCE patients.
Materials and Methods
This study was approved by the institutional review board of Kyungpook University Hospital and follows the tenets of the Declaration of Helsinki. We reviewed the charts of all consecutive patients who visited the eye clinic at Kyungpook University Hospital from June 2006 to September 2014 for the treatment of RCE and were followed for 6 months. Twenty consecutive patients with RCE who had been treated with conventional lubricant eye drops (conventional treatment group) from June 2006 to December 2008 and 27 consecutive patients treated with autologous PRP eye drops in addition to lubricant eye drops (PRP treated group) from January 2009 to September 2014 were included in this study.
All patients had suffered several relapses despite receiving different types of conservative medical treatment such as artificial tears, hyperosmotic eye drops, and therapeutic contact lenses. Patients who received PRP eye drops provided written informed consent after receiving detailed information about the nature and possible complications of autologous blood products.
The preparation of autologous PRP eye drops has been previously described in detail [28]. Briefly, 50 milliliters of whole blood was placed in five 10-mL vacutainer tubes containing anticoagulant-citrate-dextrose solution (1.4 mL) and centrifuged at 200 xg for 11 minutes. The upper two layers of the centrifuged blood, the plasma and buffy coat layer, were separated in a sterile manner and diluted to 20% (v/v) with a sterile saline solution. The final preparation was divided into 5-mL bottles wrapped in aluminum foil for protection from ultraviolet light to avoid degradation of vitamin A. The patients were instructed to store theses bottles at -20℃ until use. Bottles being used were maintained under refrigerated conditions at 4℃.
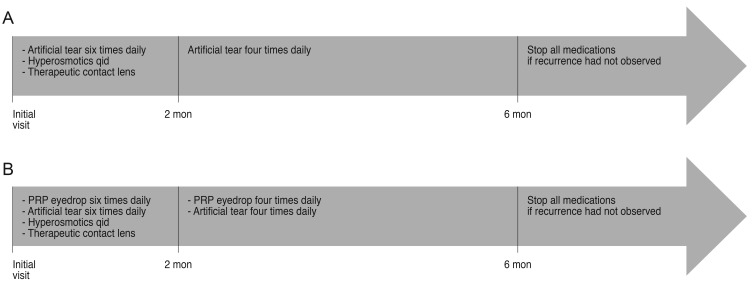
All procedures and treatments were performed by the same clinician (HKK). For all patients, the loosened epithelium was gently debrided before application of any medications. The treatment regimen for the PRP treated group was as follows: for the first 2 months, one drop each of PRP eye drops and preservative-free artificial tears was applied every 2 hours in the daytime, and hyperosmotic agents were applied four times daily. Therapeutic contact lenses were also used. After 2 months, the use of PRP eye drops and preservative-free artificial tears was reduced to four times daily for the remaining 4 months. All other medications except PRP eye drops and preservative-free artificial tears were stopped. The treatment regimen for the conventional treatment group was the same as that of the PRP treated group, with the exception that no PRP eye drops were used (Fig. 1A and 1B). Once treatment began, patients were examined after 24 hours, 72 hours, a week, and a month point. After 6 months of follow-up, patients were recommended for a visit every 2 months and whenever they experienced signs or symptoms of relapsed corneal erosions. At each follow-up session, patients underwent an ophthalmic examination (visual acuity, slit lamp biomicroscopy, intraocular pressure) and were questioned about clinical signs of recurrence with special reference to the first hours of the day.
Fig. 1. Diagram of the treatment protocols of the (A) conventional treatment group and (B) platelet-rich plasma (PRP) treatment group.
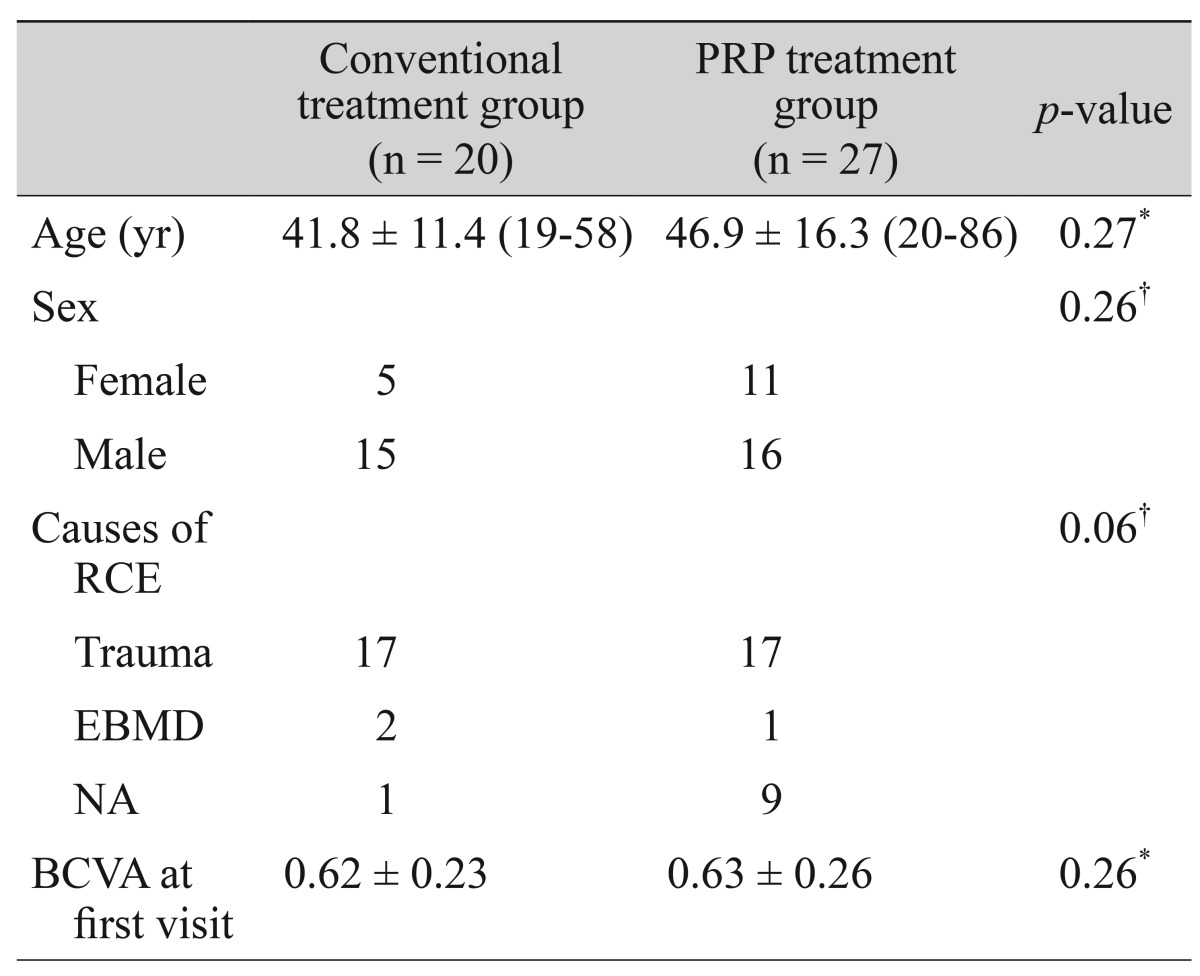
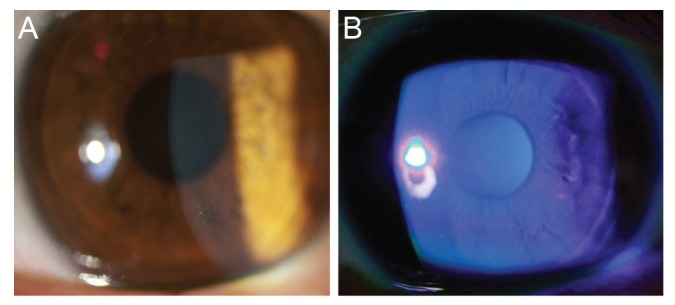
Major recurrence was defined as the presence of RCE symptoms that requires an ophthalmic consultation with the definitive presence of epithelial defect or macrocyst, resulting from loosely adherent epithelium on ophthalmic assessment (Fig. 2A and 2B). Minor recurrence was defined as the experience of pain, redness, tearing, or photophobia at nighttime or on awakening that not sufficiently severe to seek medical advice and no definitive epithelial defect or macrocyst on ophthalmic examinations. Microcystic epithelial defect can be observed in minor recurrences (Fig. 3A and 3B). During the follow-up period, if a patient presented with a major recurrence, we repeated the entire treatment protocol. For cases of minor recurrence, we maintained the treatment protocol with close observation. Mann-Whitney U-test was used to compare the frequency of recurrence between two study groups. The p-values of less than 0.05 were considered statistically significant.
Fig. 2. Slit-lamp photographs (A) and fluorescein staining (B) in a patient with major recurrence of erosions, which showed definitive loosely-adherent epithelium with macrocystic epithelial detachment.
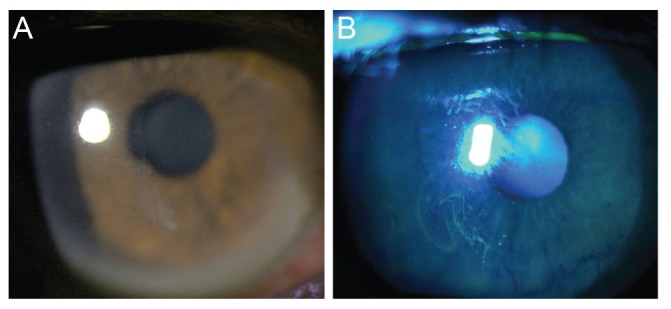
Fig. 3. Slit-lamp photographs (A) and fluorescein staining (B) in a patient with minor recurrence. Microcystic epithelial irregularities were observed without definitive epithelial defect.
Results
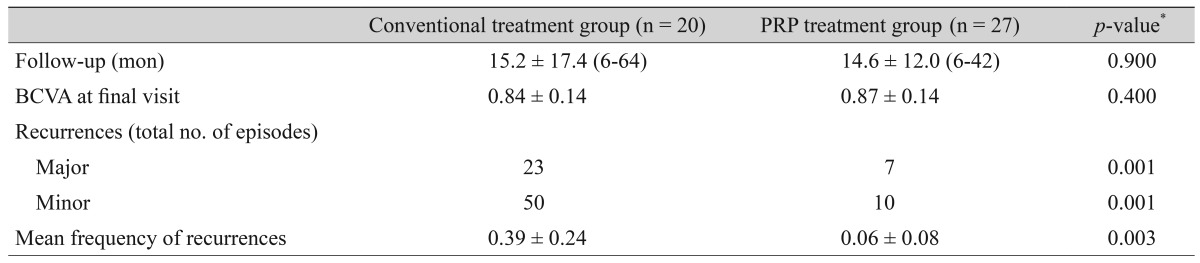
This study included 31 men and 16 women. The mean patient age was 44.5 ± 14.5 years (range, 19 to 86 years), and the mean follow-up duration was 14.9 ± 14.4 months (range, 6 to 64 months). The conventional treatment group included 20 eyes of 20 patients (15 men and 5 women), with a mean age of 41.8 ± 11.4 years (range, 19 to 58). Predisposing factors for RCE were ocular trauma in 16 eyes and epithelial basement membrane dystrophy in two eyes. The PRP treated group included 27 eyes of 27 patients (16 men and 11 women) with RCE. The mean age of the PRP treated group was 46.9 ± 16.3 years (range, 20 to 86 years). Seventeen patients in this group had a history of ocular trauma and one patient had epithelial basement membrane dystrophy, while nine patients had no identifiable factors. Characteristics and treatment results of the conventional and PRP treated groups are presented in Table 1. No significant differences in age, sex, follow-up duration, or predisposing factors were observed between the two study groups.
Table 1. Demographic and clinical data of patients.
Values are presented as mean ± SD (range), number, or mean ± SD.
PRP = platelet-rich plasma; RCE = recurrent corneal erosions; EBMD = epithelial basement membrane dystrophy; NA = no identifiable factor; BCVA = best-corrected visual acuity.
*Mann-Whitney U-test for difference between two serum groups; †Chi-square test.
The mean follow-up duration of the conventional treatment group was 15.2 ± 17.4 months (range, 6 to 64 months), compared to 14.6 ± 12.0 months (range, 6 to 42 months) in the PRP treated group. Of the 27 eyes in the PRP treated group, there were seven major recurrences in six eyes (22.2%) and 10 minor recurrences in seven eyes (25.9%). In contrast, 16 of the 20 eyes in the conventional treatment group (80.0%) suffered major recurrences, and all patients in this group reported minor recurrences. The mean frequency of recurrence was 0.06 ± 0.08 per month in the PRP treated group and 0.39 ± 0.24 per month in the conventional treatment group (p = 0.003) (Table 2). No side effects were noted in any of the treated patients. In all patients, recovery of the epithelial defect was fully achieved within the first 5 days of treatment.
Table 2. Treatment outcomes of patients and comparison between groups.
Values are presented as mean ± SD (range) or mean ± SD unless otherwise indicated.
PRP = platelet-rich plasma; BCVA, best-corrected visual acuity.
*Mann-Whitney U-test for difference between two serum groups.
Discussion
Blood-derived products are known to be powerful, effective, and safe remedies for various wound-healing processes [29,30]. Similarly, eye drops derived from serum have been shown to be effective and safe for the treatment of various ocular surface diseases, such as severe dry eye syndrome, persistent epithelial defects, and neurotrophic keratitis [13,14,15,16,17,18,19,20,21,22,23]. Autologous serum has a number of epitheliotrophic factors including EGF, TGF-β, fibronectin, and vitamin A. These factors are essential for the proliferation and maintenance of corneal epithelial cells and are also useful in treating ocular surface disorders [9,10,11]. Based on these findings, autologous serum has been also successfully employed in reducing the number of recurrences experienced by patients with RCE. del Castillo et al. [23] used autologous serum for 3 months in 11 eyes of 11 patients with acute macroform corneal erosions who had suffered several relapses despite receiving several other treatments and found that the mean recurrence rate decreased from 2.2 per month before serum treatment to 0.028 per month after treatment. Holzer et al. [31] applied autologous serum eye drops for 6 weeks after transepithelial phototherapeutic keratectomy in 25 RCE patients and reported that 80% of cases recovered without further corneal erosion. In the present study, we found that the frequency of recurrence in patients who were treated with autologous PRP eye drops was significantly lower than that of the conventional treatment group. Compared to previous reports on the use of autologous serum for RCE patients, the patients in our study had substantially more favorable outcomes [23]. A prospective, randomized clinical trial is needed to establish the exact efficacy of PRP eye drops for the treatment of RCE. Until such a study is performed, we believe that the preliminary data gathered from this study is adequately convincing that the use of autologous PRP eye drops is an efficacious medical treatment modality for RCE that are recalcitrant to conventional medical therapy.
The mechanism of action of autologous PRP is likely to be the same as that of autologous serum, with the difference being a higher concentration of growth factors, which might stimulate the growth of epithelial cells and thus lead to faster healing. The high concentrations of epitheliotrophic growth factors also have an effect on tightening the epithelial adhesion complex. Alio et al. [26] concluded that autologous PRP promoted healing of dormant corneal ulcers and was accompanied by reduction in pain and inflammation. Hartwig et al. [32] reported a superior effect on cell growth in platelet releasates than in serum owing to its high content of growth factors and concluded platelet releasate could be a novel treatment option for ocular surface defects. We previously reported the effects of autologous PRP on persistent corneal epithelial defect after infectious keratitis. The platelet concentration in PRP prepared in our standardized protocol was approximately 1.5-fold higher than in whole blood, and the PRP also contained significantly higher concentrations of EGF and TGF-β1. We concluded that PRP eye drops are an effective, novel treatment option for chronic ocular surface disease, and that this might be attributable to its high concentration of platelet-contained growth factors [28]. On the basis of this observation, we have started using PRP eye drops in the treatment for patients with RCE.
Although our results were largely consistent with our expectations, our study was not without limitations. First, the sample size was small in this retrospective study. Second, between the two groups, the size of the initial epithelial erosion was not compared, which could have affected the treatment efficacy of the groups. Third, if a patient presented with a major recurrence, we repeated the whole treatment protocol. In this way, treatment duration would have been different between the groups. Fourth, the most effective concentrations of platelet and growth factors for ocular surface disease could not be determined in this retrospective clinical study. A higher concentration of growth factors could induce harmful effect. Furthermore, given the complexity of the protein content in PRP, which might have variable bioactivities, some components might have an adverse effect on the treatment of RCE. Sakimoto et al. [33] reported increased levels of MMP-2 and MMP-9 in the onset phase of RCE. The levels of MMPs might be increased with increased platelet concentration. However, with an increased level of MMP, the concentration of MMP inhibitors also increases. The action of MMPs depends on a local balance with its inhibitors [34]. Although the role of MMPs in PRP for the treatment of ocular surface disease should be elucidated, this study confirmed the positive effects of PRP on RCE and the lack of adverse effects. There were no complications reported due to PRP eye drops using. This suggests that PRP is a very safe treatment modality for RCE. There is a need for further prospective studies on larger patient series involving longer follow-up in order to confirm our findings, determine the ideal duration of treatment, and clarify the mechanisms involved in this novel treatment option. Despite these limitations, this study is the first in which the clinical effects of autologous PRP were evaluated for treatment of RCE. Based on our findings, PRP harbors a larger quantity of growth factors, which are essential to wound healing in the corneal epithelium. In conclusion, under clinical conditions, the use of autologous PRP eye drops could prove very successful in treating RCE, a fairly common ophthalmological problem. However, there is a clear need for more detailed prospective studies on a larger patient series with a longer follow-up period in order to confirm our findings and to clarify mechanisms involved in autologous PRP treatment aside from the aforementioned plentiful growth factors.
Acknowledgements
This work was supported by a BioMedical Research Institute Grant of Kyungpook National University Hospital (2011).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 2.Hope-Ross MW, Chell PB, Kervick GN, et al. Oral tetracycline in the treatment of recurrent corneal erosions. Eye (Lond) 1994;8(Pt 4):384–388. doi: 10.1038/eye.1994.91. [DOI] [PubMed] [Google Scholar]
- 3.Buxton JN, Fox ML. Superficial epithelial keratectomy in the treatment of epithelial basement membrane dystrophy: a preliminary report. Arch Ophthalmol. 1983;101:392–395. doi: 10.1001/archopht.1983.01040010392008. [DOI] [PubMed] [Google Scholar]
- 4.Soong HK, Farjo Q, Meyer RF, Sugar A. Diamond burr superficial keratectomy for recurrent corneal erosions. Br J Ophthalmol. 2002;86:296–298. doi: 10.1136/bjo.86.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean EN, MacRae SM, Rich LF. Recurrent erosion: treatment by anterior stromal puncture. Ophthalmology. 1986;93:784–788. [PubMed] [Google Scholar]
- 6.Kim SY, Ko BY. Evaluation of anterior stromal puncture using Nd:YAG laser for refractory recurrent corneal erosion. J Korean Ophthalmol Soc. 2015;56:331–338. [Google Scholar]
- 7.Choi M, Jung JW, Seo KY, et al. Comparison of Nd:YAG laser versus conservative management in the treatment of recurrent corneal erosion. J Korean Ophthalmol Soc. 2015;56:687–693. [Google Scholar]
- 8.Forster W, Grewe S, Atzler U, et al. Phototherapeutic keratectomy in corneal diseases. Refract Corneal Surg. 1993;9(2 Suppl):S85–S90. [PubMed] [Google Scholar]
- 9.Nishida T, Nakamura M, Ofuji K, et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169:159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.van Setten GB, Viinikka L, Tervo T, et al. Epidermal growth factor is a constant component of normal human tear fluid. Graefes Arch Clin Exp Ophthalmol. 1989;227:184–187. doi: 10.1007/BF02169794. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Monroy D, Ji Z, et al. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996;15:605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Hartwig D, Harloff S, et al. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005;243:706–714. doi: 10.1007/s00417-004-1106-5. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol. 1999;83:390–395. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31:579–583. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 15.Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–652. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139:242–246. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001;20:802–806. doi: 10.1097/00003226-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106:1984–1989. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 19.Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85:1188–1197. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young AL, Cheng AC, Ng HK, et al. The use of autologous serum tears in persistent corneal epithelial defects. Eye (Lond) 2004;18:609–614. doi: 10.1038/sj.eye.6700721. [DOI] [PubMed] [Google Scholar]
- 21.Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–1108. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–1120. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 23.del Castillo JM, de la Casa JM, Sardina RC, et al. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002;21:781–783. doi: 10.1097/00003226-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Knighton DR, Ciresi K, Fiegel VD, et al. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet. 1990;170:56–60. [PubMed] [Google Scholar]
- 25.Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing: single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989;84:640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alio JL, Abad M, Artola A, et al. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007;114:1286–1293.e1. doi: 10.1016/j.ophtha.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Alio JL, Pastor S, Ruiz-Colecha J, et al. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg. 2007;23:617–619. doi: 10.3928/1081-597X-20070601-13. [DOI] [PubMed] [Google Scholar]
- 28.Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012;56:544–550. doi: 10.1007/s10384-012-0175-y. [DOI] [PubMed] [Google Scholar]
- 29.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 30.Peng Y, Huang S, Wu Y, et al. Platelet rich plasma clot releasate preconditioning induced PI3K/AKT/NFκB signaling enhances survival and regenerative function of rat bone marrow mesenchymal stem cells in hostile microenvironments. Stem Cells Dev. 2013;22:3236–3251. doi: 10.1089/scd.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzer MP, Auffarth GU, Specht H, Kruse FE. Combination of transepithelial phototherapeutic keratectomy and autologous serum eyedrops for treatment of recurrent corneal erosions. J Cataract Refract Surg. 2005;31:1603–1606. doi: 10.1016/j.jcrs.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Hartwig D, Harloff S, Liu L, et al. Epitheliotrophic capacity of a growth factor preparation produced from platelet concentrates on corneal epithelial cells: a potential agent for the treatment of ocular surface defects? Transfusion. 2004;44:1724–1731. doi: 10.1111/j.0041-1132.2004.04079.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakimoto T, Shoji J, Yamada A, Sawa M. Upregulation of matrix metalloproteinase in tear fluid of patients with recurrent corneal erosion. Jpn J Ophthalmol. 2007;51:343–346. doi: 10.1007/s10384-007-0455-0. [DOI] [PubMed] [Google Scholar]
- 34.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]