Abstract
Purpose
Our study aimed to determine whether obstructive sleep apnea (OSA) is common among branch retinal vein occlusion (BRVO) patients without systemic risk factors using a Watch PAT-100 portable monitoring device.
Methods
The study participants included consecutive patients with BRVO of less than 3 months duration without any risk factors known to be associated with OSA (diabetes, coronary artery disease, stroke, hematologic diseases, autoimmune disease, etc.) except for hypertension. All patients underwent full-night unattended polysomnography by means of a portable monitor Watch PAT-100 device. The apnea-hypopnea index (AHI) was calculated as the average number of apnea and hypopnea events per hour of sleep, and an AHI score of five or more events was diagnosed as OSA.
Results
Among 19 patients (6 males and 13 females), 42.1% (8 of 19) had an AHI reflective of OSA. In the 13 patients who had no concurrent illness, including hypertension, 30.8% (4 of 13) had positive test results for OSA; three of these patients were ranked as mild OSA, while one had moderate OSA. The OSA group had an average AHI of 12.3 ± 7.8, and the average AHI was 2.0 ± 0.9 in the non-OSA group. Although it was not statistically proven, we found that OSA patients experienced a more severe form of BRVO.
Conclusions
We found a higher than expected rate of OSA in BRVO patients lacking concomitant diseases typically associated with OSA. Our findings suggest that OSA could be an additional risk factor in the pathogenesis of BRVO or at least a frequently associated condition that could function as a triggering factor.
Keywords: Branch retinal vein occlusion, Hypertension, Obstructive sleep apnea
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy and is an important cause of blindness and visual morbidity [1]. This condition affects males and females equally and occurs most frequently between the ages of 60 and 70. The prevalence of RVO is 0.7%, and the 5-year incidence is 0.8% [2]. The most common risk factors associated with branch retinal vein occlusion (BRVO) are systemic hypertension, diabetes, hyperlipidemia, smoking, and age-related atherosclerosis [3]. The interruption of venous flow in the eyes of BRVO patients afflicted with the aforementioned risk factors almost always occurs at a retinal arteriovenous intersection, where a retinal artery crosses a retinal vein [4].
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder, with an estimated prevalence of 2% for women and 4% for men in the middle-aged population [5]. It is characterized by the repetitive complete or partial collapse of the upper airway during sleep [6], which causes the cessation (obstructive apnea) or significant reduction (obstructive hypopnea) of airflow. These respiratory events result in intermittent hypoxemia and hypercapnia, cortical arousals and surges of sympathetic activity [7]. OSA is an insidious disorder, and most patients are unaware of its symptoms; it is becoming increasingly recognized as an important cause of medical morbidity and mortality. The occurrence of OSA has been linked to increased cardiovascular morbidity and mortality, hypertension, diabetes mellitus, atherosclerosis, and stroke, most of which are also common risk factors for BRVO [7,8,9,10].
Previous studies have shown an association between RVO and OSA [11,12]. However, these studies included both branch and central RVO patients, and there were not any exclusion criteria. In the present study, we aimed to determine whether OSA is common among patients with BRVO who do not have any systemic risk factors.
Materials and Methods
This study was carried out in the ophthalmology clinic of the hospital of the Yonsei University College of Medicine in Seoul, Korea. The institutional review board approved the ethical and methodological aspects of the investigation, and patients were required to provide signed written informed consent as a prerequisite for their participation. This study is also registered at ClinicalTrials.gov, number NCT01291862. Patients with BRVO of less than 3 months duration were consecutively enrolled between April 2010 and March 2011. BRVO was assessed with an indirect ophthalmoscope by a retinal specialist and confirmed by fluorescein angiography (FA). Patients with insulin-dependent diabetes, insulin-independent diabetes, coronary artery disease, stroke, hematologic diseases, autoimmune diseases, or any other condition reported to be associated with OSA were excluded, with the exception of patients with well-controlled hypertension. Further, any ocular disease other than retinal vascular occlusion and conditions compromising the capability of patients to understand or participate in the study were considered exclusionary.
All patients underwent full-night unattended level III polysomnography by means of a portable monitor Watch PAT-100 device (WP100; Itamar Medical, Caesarea, Israel). The WP100 is a Food and Drug Administration-approved portable diagnostic device for sleep apnea and is easy to use because the testing is done in the patient's own bedroom. This device is typically worn on the patient's wrist, and there are two non-invasive, finger-mounted probes: a peripheral arterial tone (PAT) probe and a pulse oximeter (http://www.itamar-medical.com/). This device continuously records on four channels: the PAT signal via a finger-mounted PAT probe, arterial oxygen saturation via finger-mounted pulse oximeter, sleep-wake state from the build-in actigraphic signal, and heart rate (derived from the PAT signal) [13]. The PAT signal measures the arterial pulsatile volume changes in the finger, which are regulated by α-adrenergic innervation of the smooth muscles of its vasculature, and thus reflects sympathetic nervous system activity [13]. Because discrete obstructive airway events cause arousal from sleep and sympathetic activation, these events are associated with attenuation of the PAT signal [14]. On the following day, patients returned the WP100 device, and the overnight polysomnographic data were uploaded and automatically analyzed using commercial software (zzzPAT ver. 44, Itamar Medical). Despite the gold standard for an OSA diagnosis being the in-laboratory approach using level I polysomnography, the use of the WP100 has been validated to have reasonable reliability and specificity [13,14,15].
In addition to analyzing the PAT, heart rate and pulse oximetry data, the WP100 calculates three indices according to the number of events per hour of sleep: the apnea-hypopnea index (AHI), respiratory disturbance index (RDI) and oxygen desaturation index (ODI). The AHI is calculated as the average number of apnea and hypopnea events per hour of sleep; the RDI is determined based on the total number of apneas, hypopneas and respiratory effort-related arousals per hour of sleep, while the ODI is calculated as the number of oxygen desaturations of at least 4% from baseline per hour of sleep. An AHI of five or more events confirmed a positive OSA diagnosis. Cut-off points of 5, 15 and 30 were used to indicate mild, moderate and severe levels of OSA, respectively [16]. An RDI of five or more events was considered abnormally elevated. Records were accepted when patients had spent at least 6 hours in bed and when good to excellent recordings of arterial oxygen saturation and respiration were achieved.
We also evaluated the participants' ophthalmic results, including the initial best-corrected visual acuity and the presence of a non-perfusion area larger than five disc diameters on FA performed at least 6 months after the diagnosis of the BRVO. Moreover, we assessed patients' snoring behavior and incidence of daytime sleepiness or fatigue using the Berlin questionnaire.
Statistical analyses were performed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). We compared patients both with and without OSA using the Fisher's exact test for categorical data and the Mann-Whitney U-test for numerical data.
Results
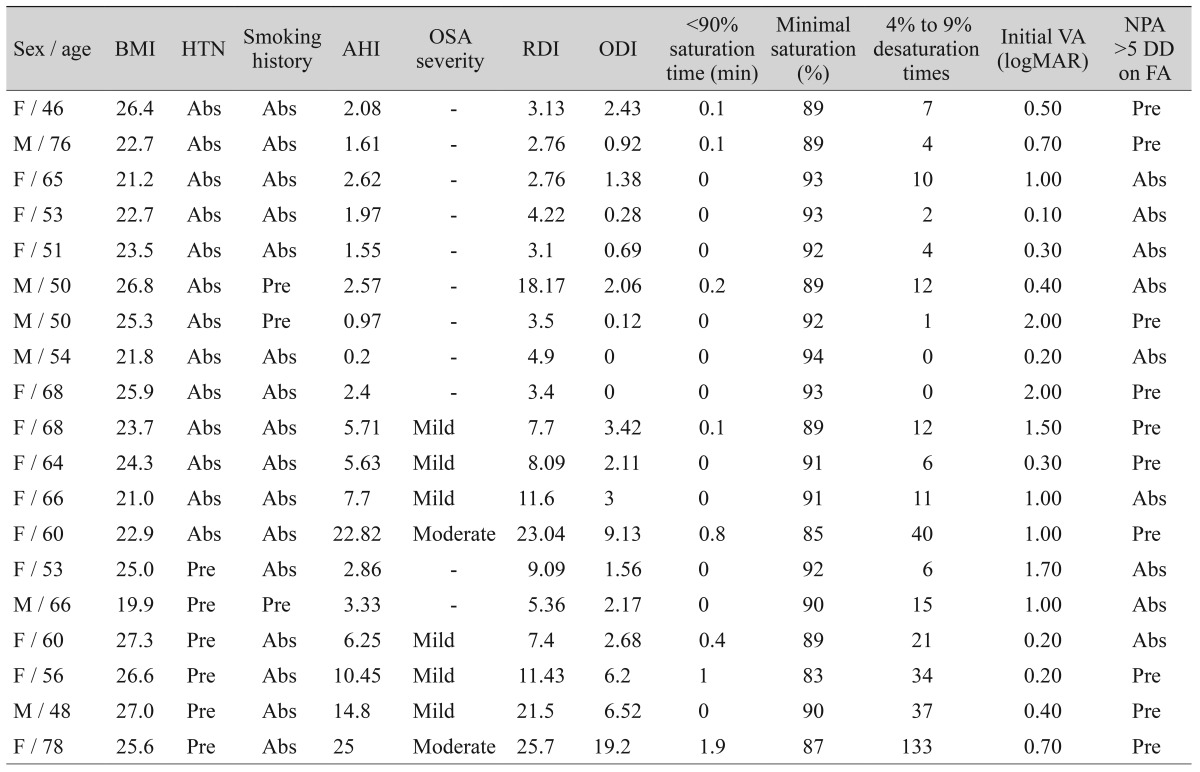
This study included a total of 19 patients (6 males and 13 females). Among these, 13 had no concurrent illness, including hypertension, while six participants had well-controlled hypertension. None of these patients had taken medication except for hypertension medication. The mean age of patients was 59.6 ± 9.4 years (range, 46 to 78 years). None of the patients were obese, and they all had a body mass index (BMI) of <30. Ten patients disclosed on the questionnaire that they snored during sleep, and four patients complained of excessive daytime sleepiness. Overall, 42.1% of our patients (8 of 19) were found to have AHI values reflective of OSA, and 57.9% (11 of 19) were diagnosed with an abnormally elevated RDI (Table 1). Among the 13 patients who had no concurrent illness (including hypertension), the average AHI was 4.4 ± 5.9, with 30.8% of the patients (4 of 13) testing positive for OSA. Three of these patients were ranked as mild OSA, and one had moderate OSA. For the patients with hypertension, the average AHI was found to be 10.4 ± 8.4, with 66.7% of the patients (4 of 6) testing positive for OSA: three with mild OSA and one with moderate OSA.
Table 1. Demographic characteristics and polysomnographic results of enrolled patients.
BMI = body mass index; HTN = hypertension; AHI = apnea-hypopnea index; OSA = obstructive sleep apnea; RDI = respiratory disturbance index; ODI = oxygen desaturation index; VA = visual acuity; logMAR = logarithm of the minimum angle of resolution; NPA = non-perfusion area; DD = disc diameter; FA = fluorescein angiography; Abs = absence; Pre = presence.
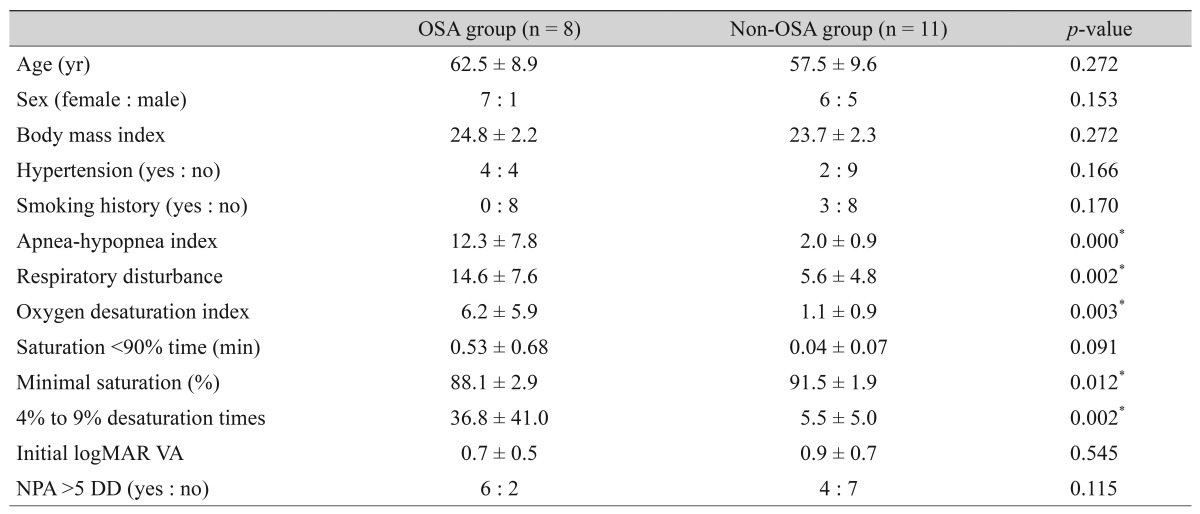
Table 2 shows the results of a comparison within the groups both with and without OSA. There were no differences in age, sex, BMI, hypertension history, smoking history, and time (minutes) spent at less than 90% oxygen saturation in the OSA and non-OSA groups. The OSA group had an average AHI of 12.3 ± 7.8, of which seven patients were ranked as mild OSA and two as moderate OSA. In the non-OSA group, the average AHI was found to be 2.0 ± 0.9. For all patients, AHI showed a positive correlation with RDI (γ = 0.587, p = 0.000), ODI (γ = 0.700, p = 0.000), minimal oxygen saturation (γ = -0.488, p = 0.005), and 4% to 9% desaturation times (γ = 0.752, p = 0.000). However, AHI had no significant correlation with patient age, weight, height, BMI, and time (minutes) spent at less than 90% oxygen saturation.
Table 2. Demographic data for patients with OSA and for those without OSA.
OSA = obstructive sleep apnea; logMAR = logarithm of the minimum angle of resolution; VA = visual acuity; NPA = non-perfusion area; DD = disc diameter.
*p < 0.05.
We also analyzed the differences in ophthalmic examinations within the OSA group and the non-OSA group. The initial visual acuity was not statistically different between the two groups. Although it was not statistically different, the OSA group contained relatively more patients with non-perfusion areas larger than five disc diameters on FA compared to the non-OSA group (75.0% vs. 36.4%).
Discussion
Several mechanisms by which OSA evokes RVO have been proposed. First, the change in retinal microcirculation caused by sleep apnea is related to the occurrence of RVO [11]. Respiratory events caused by OSA result in hypoxia, hypercapnia and activation of the sympathetic nervous system. Hypoxia-induced vasodilation of the central retinal artery can compress the adjacent central retinal vein and interfere with retinal blood f low. Hypercapnia-induced cerebral vasodilation can increase the intracranial and cerebral spinal fluid pressure, inducing papilledema and elevated venous pressure in the optic nerve head. This vasodilation also has the effect of reducing the rate of retinal circulation. Moreover, activated sympathetic tone can also stimulate increases in arterial blood pressure. These three factors all impact hemodynamic changes in the retinal artery, which contribute to the occlusion of the retinal vein located in the same adventitial sheath and may help explain why RVO increases the incidence of OSA [11].
Second, the thrombogenicity of sleep apnea patients is another probable mechanism for the promotion of RVO in OSA [12]. Intermittent hypoxia produces reactive oxygen species and inflammatory cytokines (interleukin-6 and tumor necrosis factor-α), which increase the expression and release of tissue factors of vascular endothelial cells and trigger the extrinsic coagulation pathway and platelet aggregation. Reactive oxygen species and inflammatory cytokines also impair the repair capacity of endothelial cells and suppress fibrinolysis. All these factors may contribute to the hypercoagulable state frequently present in OSA and are able to trigger RVO [12].
In this prospective consecutive study, we found that the rate of OSA was high in BRVO patients (42.1% in all BRVO patients and 30.8% in BRVO patients without systemic diseases). It was significantly higher than the overall prevalence of OSA in the normal, middle-aged Korean population (male, 4.5%; female, 3.2%) [17]. The rate of OSA in RVO patients in this study was comparable to that of a previous study by Glacet-Bernard et al. [11], which reported an OSA prevalence of 37% in RVO patients. We found the rate of OSA in BRVO patients without any disease to be high. These results were obtained despite the exclusion of BRVO patients with concomitant diseases besides hypertension; therefore, the prevalence of OSA in all BRVO patients may be much higher.
As mentioned above, previous studies have shown an association between RVO and OSA [11,12]. However, they did not use any exclusion criteria, and their investigations included both branch and central RVO patients. Because branch and central RVO have different mechanisms of pathogenesis, it will be worthwhile to evaluate the prevalence of OSA in each subtype. It would also be valuable to investigate patients with no systemic disease because the inclusion of patients with many systemic diseases could confound the impact of OSA in BRVO since other systemic diseases also act as risk factors for BRVO. Our current study is the first to evaluate the prevalence of OSA in a series of BRVO patients with no concomitant, OSA-associated diseases.
Although it was not statistically proven, we found that OSA patients would have experienced a more severe form of BRVO. In addition, 75.0% of the OSA group had a non-perfusion area greater than five disc diameters on FA performed at least 6 months after the diagnosis of the BRVO, in comparison to 36.4% in non-OSA patients. This result may have occurred because OSA patients experience more hypoxia events during sleep. However, further clinical studies using a larger cohort of patients will be needed to assess whether OSA affects the clinical manifestation of BRVO and whether OSA treatment could improve the visual outcome in BRVO.
The major limitations of this study were the small number of enrolled patients and the lack of a control group. The results were based on a single-night measurement with a portable device. We were unable to calculate the exact prevalence of OSA in BRVO patients using this study alone. Therefore, this result must be regarded as preliminary. Further study with a control group will be needed to determine whether the prevalence of OSA is higher in BRVO patients and also to show the differences of the OSA indices between BRVO patients and a normal population.
This was a preliminary study that was conducted to investigate the prevalence of OSA in BRVO patients without certain risk factors. This study included 19 participants and suggested that OSA is common in BRVO patients (42.1%). Especially, we found a higher than expected rate of OSA (30.8%) in 13 BRVO patients without any other systemic risk factors. Our findings suggest that OSA could be an additional risk factor in the pathogenesis of BRVO or at least a frequently associated condition that could function as a triggering factor. However, further study with a control group and a large study population is needed to determine whether the prevalence of OSA is higher in BRVO patients.
As retinal specialists, we encounter many BRVO patients that do not exhibit any obvious risk factors for OSA. However, our findings suggest that OSA could be a risk factor in the pathogenesis of BRVO in patients with no concomitant diseases, such as hypertension and diabetes. Even if it is not an additional independent risk factor per se, OSA may represent a frequently associated condition that functions as a triggering factor for BRVO. Therefore, it is vital to recognize OSA in those with BRVO as a method to ensure the general health of the patient.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A3051984).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yau JW, Lee P, Wong TY, et al. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–910. doi: 10.1111/j.1445-5994.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–441. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 3.Risk factors for branch retinal vein occlusion: the Eye Disease Case-control Study Group. Am J Ophthalmol. 1993;116:286–296. [PubMed] [Google Scholar]
- 4.Zhao J, Sastry SM, Sperduto RD, et al. Arteriovenous crossing patterns in branch retinal vein occlusion: the Eye Disease Case-Control Study Group. Ophthalmology. 1993;100:423–428. doi: 10.1016/s0161-6420(93)31633-7. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173:7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol (1985) 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 11.Glacet-Bernard A, Leroux les Jardins G, Lasry S, et al. Obstructive sleep apnea among patients with retinal vein occlusion. Arch Ophthalmol. 2010;128:1533–1538. doi: 10.1001/archophthalmol.2010.272. [DOI] [PubMed] [Google Scholar]
- 12.Chou KT, Huang CC, Tsai DC, et al. Sleep apnea and risk of retinal vein occlusion: a nationwide population-based study of Taiwanese. Am J Ophthalmol. 2012;154:200–205.e1. doi: 10.1016/j.ajo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Bar A, Pillar G, Dvir I, et al. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 14.Pang KP, Gourin CG, Terris DJ. A comparison of polysomnography and the WatchPAT in the diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137:665–668. doi: 10.1016/j.otohns.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Zou D, Grote L, Peker Y, et al. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 16.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 17.Kim J, In K, You S, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]