Abstract
Purpose
To evaluate retinal nerve fiber layer (RNFL) thickness in migraine patients with unilateral headache.
Methods
A total of 58 patients diagnosed with migraine headache consistently occurring on the same side and 58 age- and sex-matched healthy subjects were evaluated in this cross-sectional study. RNFL thickness was measured using spectral-domain optical coherence tomography, and the side with the headache was com-pared with the contralateral side as well as with the results of healthy subjects.
Results
The mean patient age was 33.05 ± 8.83 years, and that of the healthy subjects was 31.44 ± 8.64 years (p = 0.32). The mean duration of disease was 10.29 ± 9.03 years. The average and nasal RNFL thicknesses were significantly thinner on the side of headache and on the contralateral side compared to control eyes (p < 0.05, for all). Thinning was higher on the side of the headache compared to the contralateral side; however, this difference was not statistically significant.
Conclusions
The RNFL thicknesses were thinner on the side of the headache compared to the contralateral side in the migraine patients with unilateral headache, but this difference was not statistically significant.
Keywords: Migraine, Retinal nerve fiber layer thickness, Unilateral headache
Migraine is a common neurological disorder with episodic headaches and is most common in females between the ages of 20 and 45 years [1,2]. One of the typical criteria for migraine is a unilateral character of the headache, which is more common than the bilateral type [3,4,5].
The pathophysiology of migraine is not clearly understood [6]. The complex pathophysiology of the disease entails proposed vascular and neuronal mechanisms [7]. Cortical spreading depression, first described by Leao [8], is a key event characterized by dramatic changes in cortical potentials, significant transient increases in extracellular ions and neurotransmitters, and transient increases in cortical blood flow followed by sustained flow decreases [9]. This focal regional cerebral blood flow decrease, reported especially in migraine with aura, begins in the posterior circulation [10]. Reduced blood flow and vasospasm are usually limited to one hemisphere [11]. Rarely, hypoperfusion originates from other regions of the brain or even the retina and might also result in retinal infarction due to retinal artery occlusion [12,13]. Although vasospasm of cerebral and retinal blood vessels is transient, recurrent migraine attacks can cause permanent structural changes in the retina.
Optical cohorence tomography (OCT) is a non-invasive and objective cross-sectional imaging technique that provides quantitative in vivo information about the retinal nerve fiber layer (RNFL) [14]. This measurement sensitively evaluates ganglion cell and retinal nerve fiber damage. Several studies have evaluated RNFL thickness in migraine patients using OCT [15,16,17,18,19,20,21]. However, there has been no study evaluating the relationship between RNFL thickness and headache lateralization in migraine patients.
Therefore, the aim of the present study was to evaluate RNFL thickness on the side of the headache and the contralateral side of migraine patients who experience headaches that are almost always on the same side and to compare migraine patients with healthy subjects.
Materials and Methods
The study was an observational, cross-sectional case series that included 58 consecutive patients diagnosed with migraine headaches consistently located on the same side and 58 age- and sex-matched healthy subjects. All participants were Caucasian. The study was approved by the Süleyman Demirel University Department of Medical Sciences ethics committee and was performed according to the tenets of the Declaration of Helsinki.
All participants were aged between 18 years and 45 years. Patients with glaucoma, intraocular pressure higher than 21 mmHg, refractive error greater than ±3 spherical diopters, optic neuropathy, optic disc anomaly, cataract, vitreo-retinal diseases, history of ocular trauma, ocular surgery or ocular laser, diabetes mellitus, hypertension, hyperlipidemia, cardiovascular or renal disease, history of central nervous system disorders including epilepsy, encephalitis, brain tumors, infarction, head trauma, any type of headache except for migraine, and users of drugs, alcohol, and cigarettes were excluded from the study. All measurements were performed during attack-free periods in migraine patients.
A detailed neurological examination was performed for each patient. Migraine was diagnosed according to the International Classification of Headache Disorder second edition [3]. The migraine patients with headaches that consistently occur on the same side were included in the study. Clinical and demographic characteristics of the patients were noted and included gender, age, migraine variables such as severity of headache, duration of headache, duration of attack, side of headache, and frequency of attacks. The severity of headache was assessed by the Migraine Disability Assessment questionnaire that evaluates headache-related disability [22].
Detailed ophthalmological examinations were performed containing spherical equivalent, best-corrected visual acuity, slit lamp biomicroscopy, intraocular pressure with Goldmann applanation tonometry, central corneal thickness, anterior chamber depth, and axial length (PacScan 300AP, Biometric/Pachymeter; Sonomed, New York, NY, USA). RNFL thickness measurements were performed using spectral-domain OCT (Spectral OCT SLO; OPKO/OTI Instrumentation, Miami, FL, USA). Average, temporal, nasal, inferior, and superior quadrant peripapillary RNFL thicknesses were recorded. Both eyes of migraine patients and the right eyes of the control group were included in this study. The findings in the migraine group were compared with the findings in healthy subjects. Additionally, in migraine patients, the mean RNFL thicknesses were compared between the side of headache and the contralateral side.
SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used to analyze data. The normality of distribution for continuous variables was tested with the Kolmogorov-Smirnov test. Categorical variables were shown as frequency and percentages. For the comparisons of parametric data of two groups, Student's t-test was used for parametric variables and Mann-Whitney U-test was used for non-parametric variables. Categorical variables were compared between the groups by the chi-square test. Pearson's correlation was used to determine the strength of the relationship between the variables. Comparisons of parametric data of the three groups were performed with one-way ANOVA testing. Levene's test was used to determine homogeneity of variances and in case of homogeneity of variance, post hoc Tukey's test was used. Data were presented as mean ± standard deviation. A p-value less than 0.05 was considered to be significant.
Results
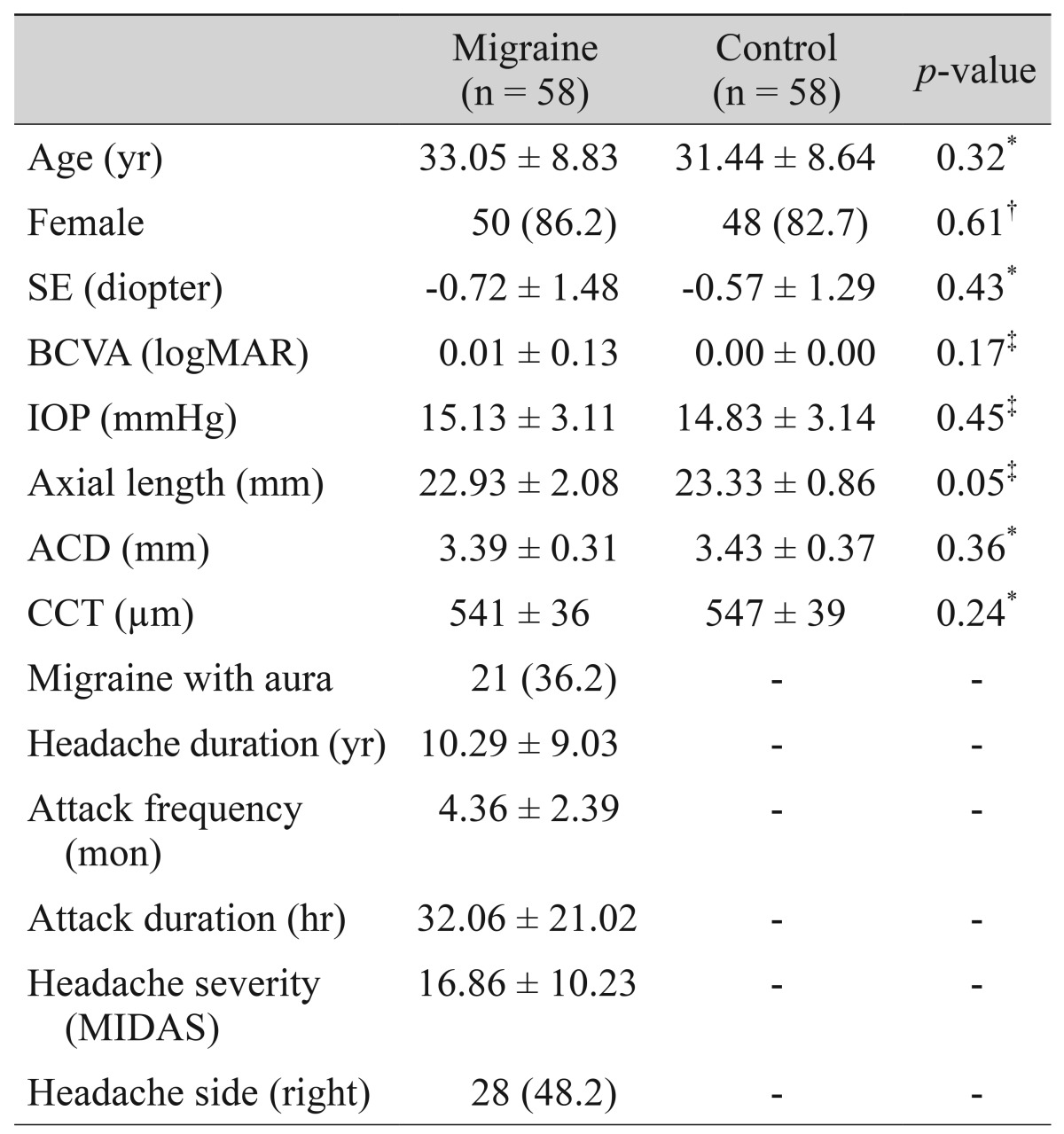
The demographic and clinical characteristics of all subjects are summarized in Table 1. There were no statistically significant differences between the migraine patients and healthy subjects in terms of age, sex, visual acuity, spherical equivalent, intraocular pressure, axial length, anterior chamber depth, and central corneal thickness. The mean age of the patients and controls were 33.05 ± 8.83 years and 31.44 ± 8.64 years, respectively (p = 0.32).
Table 1. Demographic and clinical characteristics in migraine patients and healthy controls.
Values are presented as mean ± SD or number (%).
SE = spherical equivalent; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimal angle of resolution; IOP = intraocular pressure; ACD = anterior chamber depth; CCT = central corneal thickness; MIDAS = Migraine Disability Assessment.
*Independent t-test; †Chi-square test; ‡Mann-Whitney U-test.
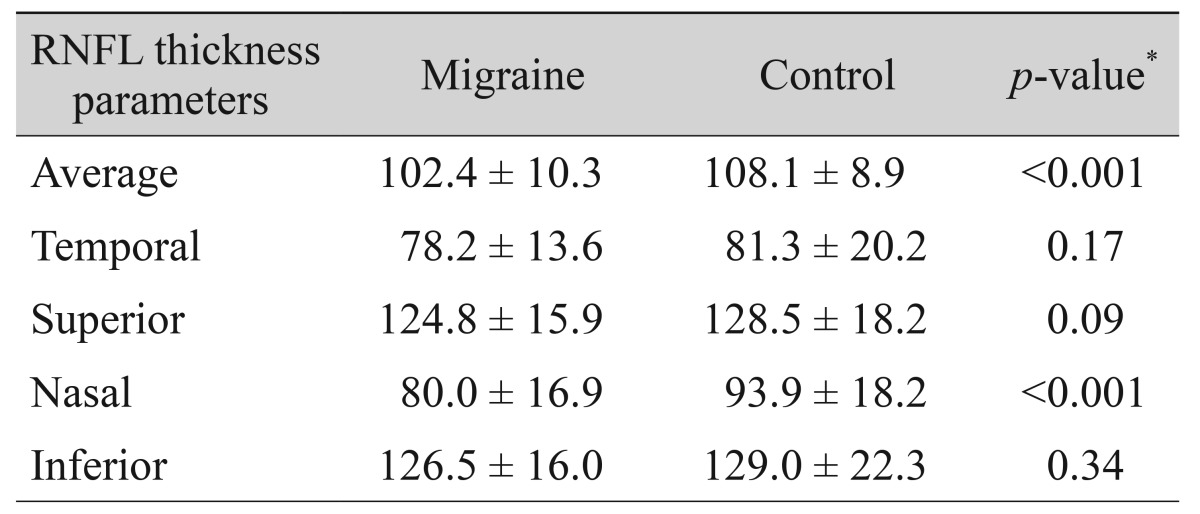
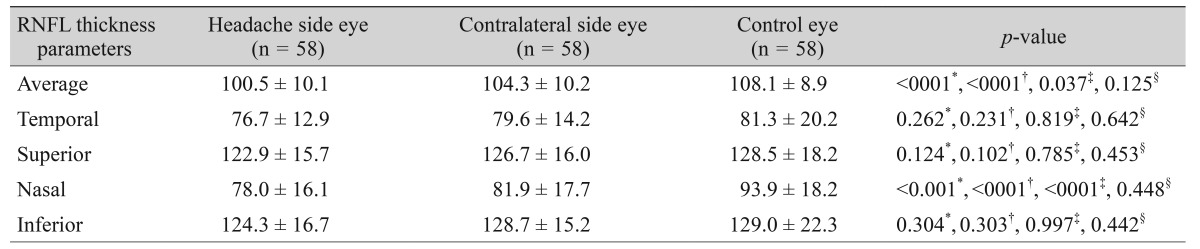
The mean RNFL thicknesses in each quadrant of the migraine and control groups are given in Table 2. In migraine patients, the average and nasal RNFL thicknesses were significantly thinner compared to healthy subjects (102.4 and 80.0 µm vs. 108.1 and 93.9 µm, respectively). The mean RNFL thicknesses on the two sides of migraine patients and healthy subjects are given in Table 3. The average and nasal RNFL thicknesses in both sides of the migraine patients were significantly thinner compared to the controls (p < 0.05, for all). In migraine patients, RNFL thicknesses were thinner on the side of headache than the contralateral side, but this difference was not statistically significant (Table 3).
Table 2. Comparison of the RNFL thickness in migraine patients and controls.
Values are presented as mean ± SD.
RNFL = retinal nerve fiber layer.
*Independent t-test.
Table 3. RNFL thickness in eyes of patients with migraine and control eyes.
Values are presented as mean ± SD.
RNFL = retinal nerve fiber layer.
*One-way analysis of variance; †Comparison between control eyes and ipsilateral eyes by post hoc test; ‡Comparison between control eyes and contralateral eyes by post hoc test; §Comparison between ipsilateral eyes and contralateral eyes by post hoc test.
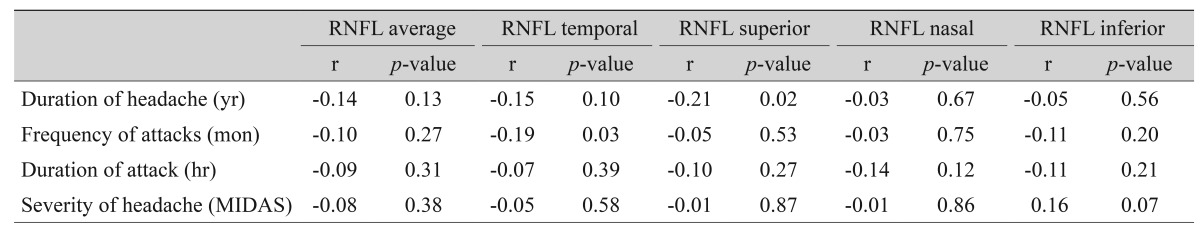
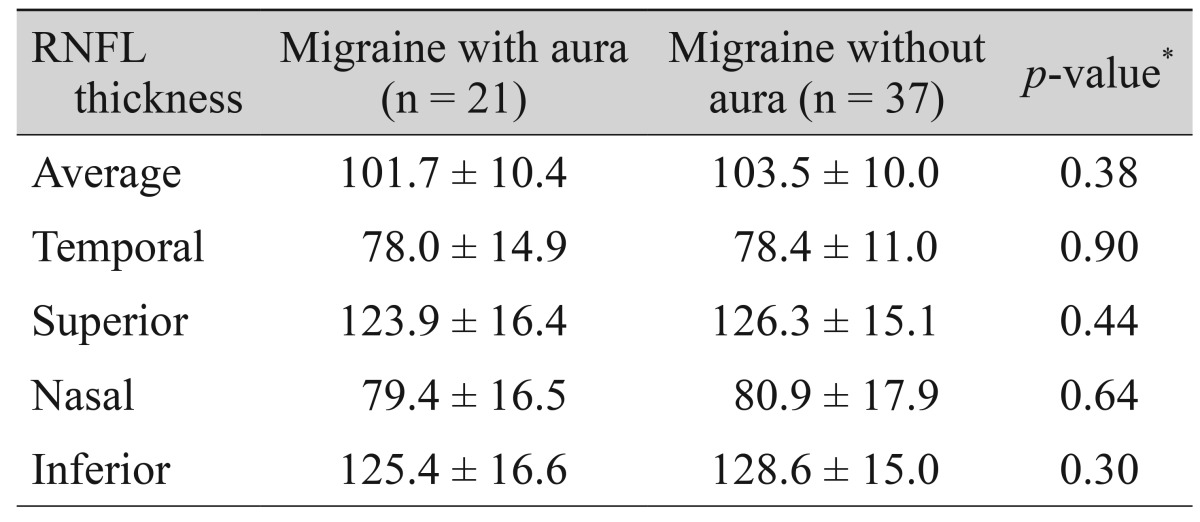
The correlations between RNFL thicknesses and migraine headache variables are shown in Table 4. Two significant correlations were found between the duration of headache and superior RNFL thickness (r = -0.21, p = 0.02) and frequency of attacks and temporal-RNFL thickness (r = -0.19, p = 0.03). We also observed no significant differences in RNFL thicknesses between patients with aura and without aura (Table 5).
Table 4. The correlations between the retinal nerve fiber layer thickness and migraine headache variables.
RNFL = retinal nerve fiber layer; MIDAS = Migraine Disability Assessment.
Table 5. The RNFL thickness in migraine subgroups.
Values are presented as mean ± SD.
RNFL = retinal nerve fiber layer.
*Independent t-test.
Discussion
In the present study, we evaluated the RNFL thickness on the headache side and contralateral side of headache in migraine patients with unilateral headache. We observed that RNFL thicknesses were significantly thinner bilaterally in migraine patients compared to healthy subjects. Although, this thinning was higher on the side of the headache compared to the contralateral side, this difference was not statistically significant.
RNFL thinning was previously shown in migraine patients using OCT [16,17,18,19,20,21]. However, to our knowledge, this is the first study to investigate the relationship between migraine headache lateralization and RNFL thickness in migraine patients with unilateral headache.
Migraine is a common, chronic neurological disorder characterized by episodic headaches of unknown pathophysiology [1,6]. There are several theories with regards to the etiology of optic nerve damage in migraine patients including vascular abnormalities such as vasospasm or focal ischemia [15]. Supporting this hypothesis, Kara et al. [23] reported that arterial vessel resistances in central retinal artery and posterior ciliary artery were higher in migraine patients in periods with no headaches using color Doppler sonography.
Tan et al. [15] evaluated RNFL thickness in patients with migraine using a scanning laser polarimetry. The researchers reported that there was no differences in RNFL thickness in migraine patients compared to healthy subjects. Several studies have also evaluated RNFL in migraine patients by OCT [16,17,18,19,20,21], which is a more advanced application of RNFL evaluation and produces higher resolution images [14]. Martinez et al. [16] used OCT to measure the RNFL and reported reduced RNFL thickness in the temporal quadrant in migraine patients. Also, different results have been reported in other studies [17,18,19,20,21]. Sorkhabi et al. [17] detected a significant thinning in the nasal quadrant-RNFL in migraine patients. Gipponi et al. [18] and Kirbas et al. [20] found a significant thinning in the superior quadrant RNFL in migraine patients. Another study reported that only the average RNFL thickness was significantly thinner in migraine patients [19]. These distinct results may be explained with the use of different methods and sample sizes, racial differences, and the lack of standardized patients in terms of migraine variables such as severity of headache, duration of headache, duration of attack, and frequency of attacks. Ekinci et al. [21] evaluated subgroups of migraine with aura and without aura. The researchers reported a significant thinning of RNFL in all quadrants in migraine patients with aura, but no significant differences in migraine patients without aura. Cerebral hypoperfusion commonly occurs in the posterior region of one hemisphere during the aura period [16]. Therefore, RNFL thicknesses may be more affected in migraine patients with aura compared to migraine patients without aura. In the present study, we found that the average and nasal RNFL thicknesses were significantly thinner in patients with and without aura compared to healthy subjects. Although RNFL thicknesses were thinner in patients with aura compared to patients without aura, these differences were not statistically significant. This result may be due to small sample size and a lack of standardized patients. Selective nasal RNFL involvement might be explained pathophysiologically by a differential vulnerability of retinal axons to ischemia. Moreover, RNFL thicknesses were thinner on the headache side compared to the contralateral side in all quadrants, but these differences were not statistically significant. During a migraine, reduced blood flow and vasospasm was reported to usually occur in one hemisphere, but other areas of the brain and even the retina may also be influenced by hypoperfusion [11,12]. Therefore, the bilateral RNFL involvement we observed might be a reflection of diffuse involvement of the brain and neuronal structures in migraine.
Additionally, Martinez et al. [16] reported a significant correlation between RNFL thickness and Migraine Disability Assessment scores and duration of migraine disease. On the contrary, Gipponi et al. [18] relate the RFNL thickness just to the presence of migraine and put forth that it does not depend on illness duration and frequency. In our study, a significant correlation was found between headache duration and superior RNFL thickness, and attack frequency and temporal RNFL thickness. However, relationships between the RNFL thickness and migraine headache variables should be studied further.
In addition, there are some studies of unilateral involvement in migraine patients [24,25]. Friberg et al. [24] found reduced blood flow velocities in the middle cerebral artery on the affected side during migraine episodes in seven patients. Hougaard et al. [25] studied 20 patients with unilateral headache and investigated the relationship between migraine with aura and structural gray matter abnormalities. Although the researchers found no differences in gray matter structure with regards to aura symptoms in migraine patients they identified thinning of the ipsilateral hemisphere cortical thickness in the inferior frontal gyrus. The finding of more prominent RNFL thinning in headache side might be related with lateralized structural abnormalities in the cortex, indicating that the hemisphere on the side of pain is affected more.
As noted above, a handful of literature reported controversial findings of RNFL thickness in migraine while some reports show no change and some reported thinning in different quadrants. In line with findings by Sorkhabi et al. [17] and Yulek et al. [19], we found average and nasal thinning of RNFL in migraine patients.
This study has some limitations. It is a single-center study and has a relatively small sample size. Furthermore, these findings need to be confirmed in a larger patient group.
In conclusion, the average and nasal RNFL thicknesses were significantly thinner in migraine patients compared to healthy subjects and the RNFL thicknesses were thinner in the side of headache compared to the contralateral side, but this finding was not statistically significant. The RNFL thinning related to migraine headache lateralization may depend on the focal retinal blood flow decrease theory in migraine.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 2.Waters WE, O'Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38:613–616. doi: 10.1136/jnnp.38.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Headache Classification. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 4.Kudrow L. Current aspects of migraine headache. Psychosomatics. 1978;19:48–57. doi: 10.1016/S0033-3182(78)71035-2. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia MS, Gupta R. Migraine: clinical pattern and psychiatric comorbidity. Ind Psychiatry J. 2012;21:18–21. doi: 10.4103/0972-6748.110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl 2):2–7. doi: 10.1111/j.1468-2982.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- 7.Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27(Suppl 2):S86–S90. doi: 10.1007/s10072-006-0577-z. [DOI] [PubMed] [Google Scholar]
- 8.Leao AA. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 9.Friberg L, Olesen J, Lassen NA, et al. Cerebral oxygen extraction, oxygen consumption, and regional cerebral blood flow during the aura phase of migraine. Stroke. 1994;25:974–979. doi: 10.1161/01.str.25.5.974. [DOI] [PubMed] [Google Scholar]
- 10.Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 11.Wang SJ. Epidemiology of migraine and other types of headache in Asia. Curr Neurol Neurosci Rep. 2003;3:104–108. doi: 10.1007/s11910-003-0060-7. [DOI] [PubMed] [Google Scholar]
- 12.Killer HE, Forrer A, Flammer J. Retinal vasospasm during an attack of migraine. Retina. 2003;23:253–254. doi: 10.1097/00006982-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Rahman AM, Gilhotra JS, Selva D. Dynamic focal retinal arteriolar vasospasm in migraine. Indian J Ophthalmol. 2011;59:51–53. doi: 10.4103/0301-4738.73717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan FU, Akarsu C, Gullu R. Retinal nerve fiber layer thickness is unaffected in migraine patients. Acta Neurol Scand. 2005;112:19–23. doi: 10.1111/j.1600-0404.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, Proupim N, Sanchez M. Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br J Ophthalmol. 2008;92:1069–1075. doi: 10.1136/bjo.2008.137471. [DOI] [PubMed] [Google Scholar]
- 17.Sorkhabi R, Mostafaei S, Ahoor M, Talebi M. Evaluation of retinal nerve fiber layer thickness in migraine. Iran J Neurol. 2013;12:51–55. [PMC free article] [PubMed] [Google Scholar]
- 18.Gipponi S, Scaroni N, Venturelli E, et al. Reduction in retinal nerve fiber layer thickness in migraine patients. Neurol Sci. 2013;34:841–845. doi: 10.1007/s10072-012-1103-0. [DOI] [PubMed] [Google Scholar]
- 19.Yulek F, Dirik EB, Eren Y, et al. Macula and retinal nerve fiber layer in migraine patients: analysis by spectral domain optic coherence tomography. Semin Ophthalmol. 2015;30:124–128. doi: 10.3109/08820538.2013.833270. [DOI] [PubMed] [Google Scholar]
- 20.Kirbas S, Tufekci A, Turkyilmaz K, et al. Evaluation of the retinal changes in patients with chronic migraine. Acta Neurol Belg. 2013;113:167–172. doi: 10.1007/s13760-012-0150-x. [DOI] [PubMed] [Google Scholar]
- 21.Ekinci M, Ceylan E, Cagatay HH, et al. Retinal nerve fibre layer, ganglion cell layer and choroid thinning in migraine with aura. BMC Ophthalmol. 2014;14:75. doi: 10.1186/1471-2415-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–S28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 23.Kara SA, Erdemoglu AK, Karadeniz MY, Altınok D. Color Doppler sonography of orbital and vertebral arteries in migraineurs without aura. J Clin Ultrasound. 2003;31:308–314. doi: 10.1002/jcu.10181. [DOI] [PubMed] [Google Scholar]
- 24.Friberg L, Olesen J, Iversen HK, Sperling B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- 25.Hougaard A, Amin FM, Hoffmann MB, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia. 2015;35:3–9. doi: 10.1177/0333102414532378. [DOI] [PubMed] [Google Scholar]