Abstract
Background:
Evidence linking inflammation and depression is marred by several methodological inconsistencies. Further, varying information is present on the role of gender as a potential confounder in this association.
Aims:
To assess systemic inflammation in drug naοve depression by measuring selected pro-inflammatory (tumor necrosis factor-alpha [TNF-α], interleukin-6 [IL-6]) and anti-inflammatory cytokines (transforming growth factor-beta [TGF-β]) and comparing them with a matched control group. We also aimed at exploring the differences in these markers between genders.
Setting and Design:
The study was a cross-sectional one carried out a teaching cum Tertiary Care Hospital.
Materials and Methods:
We recruited 55 drug naοve cases diagnosed with major depression and compared them for inflammatory markers with a matched apparently healthy control group (n = 42) at baseline. The inflammatory markers were also compared between the genders. Baseline depression and stress levels were assessed using standard measures.
Statistical Analysis Used:
Mann-Whitney U-test.
Results:
In comparison with healthy controls, drug naοve depressed individuals demonstrated significantly raised baseline levels of TNF-α and IL-6 (P < 0.001 for both) but no difference in levels of TGF-β (P = 0.433). Neither the baseline depression nor the stress scores correlated with any of the inflammatory markers (P = 0.955 and 0.816 for TNF-α respectively). Males and females were comparable on the levels of markers studied (P = 0.986, 0.415, and 0.430 for TNF-α, IL-6 and TGF-β respectively).
Conclusion:
There is evidence for higher baseline inflammation in depression prior to starting anti-depressant therapy. Gender does not mediate this observed link between inflammation and depression.
Keywords: Anti-depressant, depression, drug naοve, gender, inflammation
INTRODUCTION
Major depressive disorder (MDD) is now the second leading cause of years lived with disability and a leading cause of disability-adjusted life years according to the Global Burden of Disease Study 2010.[1] The last couple of decades have seen significant research into the biological underpinnings of depression. One such area that has been the focus of researchers in the last decade is the postulated link between inflammation and depression. A growing body of evidence indicates that inflammation is involved in the pathophysiology of depression, albeit in a subtle way. This proposition has mainly come about from three empiric lines of evidence - the presence of raised inflammatory and hypothalamo-pituitary-adrenal axis markers in MDDs, increased rates of major depression in medical conditions with prominent inflammation such as multiple sclerosis and rheumatoid arthritis and finally, the “depressogenic” effects of immunotherapy with cytokines such as interferon alpha in those who undergo these treatments.[2,3,4] However, in a recent cumulative meta-analysis on the same topic, the authors urge caution in interpreting these associations due to many inconsistencies in methodology such as the use of markedly different subgroups and variable study estimates. The same authors concluded that more work needs to be done in clearly establishing the relationship between inflammation and depression.[5]
Previous studies examining the influence of gender in mediating the relationship between systemic inflammatory markers and depression have thrown up conflicting reports. Results of two population-based studies from different nations have shown that the link between clinical depression and elevated levels of inflammatory markers such as C-reactive protein (CRP) is stronger in men than women.[6,7] Similarly, in a large community-based sample of elderly people, the investigators found that the serum level of interleukin-6 (IL-6) was higher among men, but not women, with depression.[8] These findings seem to suggest that certain biological and/or hormonal differences between men and women may mediate the relationship between inflammation and depression. In contrast to these findings; however, several other reports have failed to document any differences in inflammatory markers between men and women both within[9] and outside[10] the context of clinical depression as well one population-based study that showed this relationship to be more robust in women.[11] Clearly, more research is needed to establish the role of gender in the relationship between inflammation and depression because of its potential clinical utility in assisting the development of gender-specific treatments for depression. Another limitation of prior research in this area is that anti-inflammatory markers have not been studied adequately. Indeed, if depression is an inflammatory disease, it should, logically, reflect in corresponding changes in the levels of these markers too.
Against this background, we carried out the present research with two objectives - firstly to compare and contrast the levels of pro-inflammatory markers (tumour necrosis factor-alpha [TNF-α] and IL-6) and anti-inflammatory markers (transforming growth factor-beta [TGF-β]) in drug naïve depressed outpatients versus healthy controls. These markers were selected due to their widely suspected, but unil date unconfirmed role in the pathophysiology of depression.[12,13,14] Second, we aimed to assess gender differences in inflammatory markers among clinical cases of depression. We hypothesized that the baseline levels of inflammatory markers would be higher among cases with depression compared to healthy controls and further, that there would not be any significant differences in levels between the genders at baseline.
MATERIALS AND METHODS
Design and setting
This was a cross-sectional study conducted in a teaching cum Tertiary Care Hospital of Puducherry, South India between the months of February 2014 and December 2014. This public sector multi-specialty hospital caters mainly to rural patients coming from low socioeconomic status. Most of the patients can directly walk-in and seek services without a prior appointment. Apart from this, the hospital also caters to referral patients from nearby hospitals. All patients attending the psychiatry outpatient clinic during the period of study are first screened by a qualified psychiatrist (senior resident) for psychiatric morbidity. Those who are deemed to have an underlying diagnosis are allotted an appointment for detailed assessment. On this day, they are asked to come with a reliable informant to give history and are worked up by a junior resident following which they are allotted a diagnosis, and a management plan is formulated after discussion with a consultant psychiatrist.
Patient population
For the present study, 55 consecutive drug naïve patients who received a diagnosis of major depression as per Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, Text Revision[15] in the age group of 18-65 years were recruited. In all of them, the diagnosis was additionally confirmed using the mini-international neuropsychiatric interview (M.I.N.I.).[16] They constituted the cases. We excluded patients with a suspected organic or substance-induced mood disorder, with fever or clinical evidence of active infection or wounds or with co-morbid medical conditions such as diabetes, immunological disorders such as systemic lupus erythematosus or endocrinopathies such as hypothyroidism. Patients who were already receiving steroids and other immuno-modulatory therapies and those with a history of mania or hypomania were also excluded. To select drug naïve patients, relevant information was collected from both the patient and informant and cross-checked with available records. All patients who had received prior treatment with anti-depressants and even anti-psychotics were excluded as were those with any prior contact with a psychiatrist or a neurologist. The latter was done to ensure that only drug naïve patients were selected into the study. Age and gender matched individuals (n = 42) were selected from consenting hospital staff after applying the same exclusion criteria used for cases. Their physical health status was deemed to be normal after assessing their electronic or physical health records, available for all institute staff. All of them returned negative screening tests for psychiatric morbidity using M.I.N.I. screen.[16] They constituted the controls for the study.
Assessments done
Basic and relevant sociodemographic details were collected by using a semi-structured proforma. The cases were rated on the following instruments at baseline:
Hamilton Depression Rating Scale (HDRS): This is the most widely used clinician-administered rating scale for depression. We used the original 17-item version of the scale which enquires about symptoms of depression over the past week. Semi-structured interview guidelines are available to use and score the items on the scale.[17]
Presumptive Stressful Life Events Scale (PSLES): This is a 51-item scale developed and validated specifically for the Indian population.[18] The items, which include both desirable and undesirable stressful events, are quantified with weighted scores. For analysis, we used the total of the weighted scores corresponding to the stressful life events that were reported in the last 1 year preceding the hospital contact.
All assessments were conducted by a single trained rater (AM). In addition, all information was collected and cross-verified separately with the key informant to minimize recall bias. Subsequently, 5 ml of blood was drawn from the participants for assessing the baseline levels of TNF-α, IL-6 and TGF-β, the procedures for which are detailed in Kim et al.[19]
The study protocol had prior approval from the Institute Human Ethics Committee. Written informed consent was obtained from all volunteers after explaining the salient features of the research in the local language (Tamil).
Data analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) - PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA). Normality of data was assessed using the Shapiro-Wilk test. Continuous co-variates were expressed as mean with standard deviation (SD) or median with interquartile range and compared between groups using the Student's t-test or Mann-Whitney U-test depending on their distribution. Discrete co-variates were expressed as frequencies and percentages and compared using Chi-square (χ2) or Fisher's exact test. Due to non-normal distributions of TNF-α, IL-6 and TGF-β, nonparametric test (Mann-Whitney U-test) was used to compare their levels at baseline between cases and controls and between genders. All statistical analysis was done at 95% confidence interval and P < 0.05 was considered significant.
RESULTS
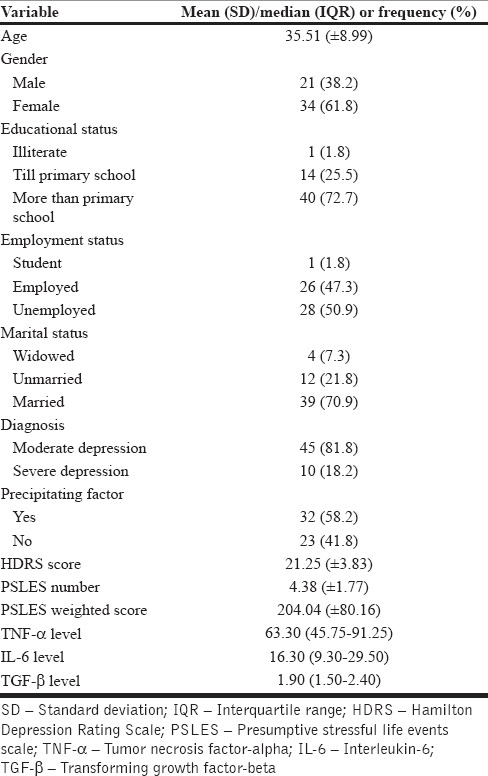
The mean (±SD) age of the cases was 35.51 (±8.99) while that of the controls was 35.07 (±8.87) and were not significantly different (t = 0.239, P = 0.812). The gender distribution of the two groups (34 females and 21 males among the cases and 29 females and 13 males among controls) were also similar (χ2 = 0.736, df = 1, P = 0.391). The distribution of other baseline sociodemographics among cases is shown in Table 1.
Table 1.
Baseline sociodemographic characteristics of the cases
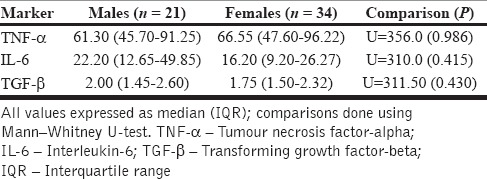
The comparison of baseline inflammatory markers between cases and controls is depicted in Table 2. Notably, the levels of pro-inflammatory markers were significantly higher at baseline among the cases group indicating higher systemic levels of inflammation in depression. A gender-based stratified analysis of the cases did not reveal significant differences for any of the markers studied [Table 3]. Neither the HAM-D scores (for TNF-α, rs = 0.008, P = 0.955, for IL-6, rs = −0.122, P = 0.386, for TGF-β, rs = 0.049, P = 0.730) nor the PSLES scores (for TNF-α, rs = 0.033, P = 0.816, for IL-6, rs = −0.219, P = 0.114, for TGF-β, rs = 0.081, P = 0.616) correlated significantly with any of the assayed parameters.
Table 2.
Comparison of baseline inflammatory markers between cases and controls
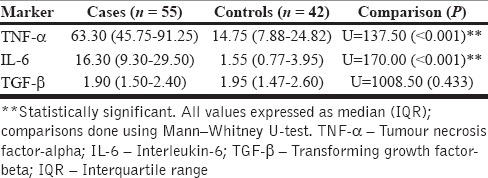
Table 3.
Comparison of baseline inflammatory markers between genders
DISCUSSION
This study shows that drug naïve depressed subjects at baseline have elevated levels of systemic inflammation when compared with the general population and that no differences were present between depressed men and women with respect to the inflammatory markers. The first part of our findings concurs with the conclusions from two recent meta-analysis measuring cytokine concentrations in patients with MDD[20,21] and other reports, including cerebrospinal fluid estimates, which have shown evidence for increased levels of inflammatory markers in MDD.[22,23] It is necessary to replicate this association in different cultural settings because, as pointed out by Slavich and Irwin,[24] the baseline levels of inflammation may differ as function of social and other kinds of stress and threats which may presumably vary across settings and consequently, influence vulnerability to depression - the so-called social signal transduction theory of depression. Already, we know from many classic papers that the presentation of depression varies across cultures, and its sufferers from developing countries are more likely to present with somatic symptoms of depression.[25,26] These somatic symptoms (including malaise, lassitude, muscle and joint aches and loss of appetite) are important components of the “sickness behavior” that are, at a molecular level, thought to be due to the effects of pro-inflammatory cytokines on the brain.[27] Taking these into consideration, our findings assume significance as it reinforces the association between inflammation and depression in a different social and cultural setting. Furthermore, to the best of our knowledge, very few studies have demonstrated elevated levels of baseline inflammation in drug naïve depression as we have done and therefore, these findings are of additional interest.
The second finding from our study was that baseline inflammatory marker levels in depression were comparable between the genders. Our findings differ from the three large population-based studies that were carried out in the West and showed the relationship between inflammatory markers such as CRP and IL-6 to be stronger in males than females.[6,7,8] Contrastingly, Ma et al. found that self-rated depression was associated with elevated high-sensitivity CRP (hs-CRP) only among women.[11] However, in this study, the authors did not control for the potential confounding effect of oral contraceptive pill usage and hormonal therapy that may have contributed to this relationship by impacting adiposity and inflammation directly. In an illuminating study that specifically controlled for the confounding effects of obesity and medications such as oral contraceptive pills, the investigators showed that major depressive symptoms continued to be significantly associated with hs-CRP concentrations in men but not women.[28] It has been postulated that the CRP levels may vary as a function of the hormonal levels and that many other, as yet unidentified, factors may play a role in mediating these observed gender differences in studies.[6] Genetic and ethnic variations in circulating levels of several adipokines and inflammatory markers have also been shown and further, that they may possibly interact with depression resulting in sex-specific variations in inflammatory markers across regions and cultures.[29,30] We explain our findings, partially, based on these documented variations in baseline inflammatory markers across cultures and recommend further large population-based studies to confirm or refute our preliminary results.
The findings of this study must be discerned in the context of its limitations. First, purposive nonprobability sampling was done and the sample size was limited. Hence, our results need to be viewed as preliminary and require validation in larger samples. Second, the possibility that some of the participants were carrying minor infections that may have influenced the markers studied cannot be ruled out entirely despite our best efforts to exclude those with obvious ailments. Third, ours was a clinical sample drawn from a single Tertiary Care Centre and hence, the findings need replication in other settings for confirmation. Fourth, we did not study any markers of obesity or adipokines that could have impacted the inflammatory indices measured. The strengths of the study include a sampling of drug naïve depressed individuals to eliminate confounding effects of prior psychotropic treatment. The inclusion of a matched control group adds to the methodological strengths of the work. Certainly, ours is one of the very few studies to have simultaneously studied pro- and anti-inflammatory markers in depression as well as the influence of gender on inflammatory marker status. Further questions that arise specifically from our findings is whether and to what extent inflammation plays a causal role in depression or, in other words, is depression an inflammatory disorder? A suggested design for future research is to examine the levels of inflammatory markers in relatives of probands with major depression and study them prospectively to establish these causal links. Future work with longer term prospective designs from various ethnic and cultural settings should be carried out to confirm our preliminary findings.
CONCLUSION
There is evidence for elevated levels of inflammatory markers in drug naïve depression when compared with general population. Gender, seemingly, does not exert influence on baseline inflammatory status. Though our findings need replication in larger samples for confirmation, they seem to suggest a clear association between inflammation and depression among Indian subjects. In addition, it appears that the status of gender as a potential confounder in the link between inflammation and depression may not be relevant in clinical samples. Whether inflammation is causally linked to depression continues to be unclear and needs further evaluation. Research into the links between inflammation and depression is still at a very nascent stage but has the potential to improve our understanding and enhance the therapeutic armamentarium necessary to tackle depressive disorders that continue to challenge clinicians and contribute significantly to the global burden of disease.
Financial support and sponsorship
The work was funded by an intramural research grant from Jawaharlal Institute of Postgraduate Medical Education and Research (Sanction No. JIP/Res/Intra-MD, MS/sec/04/2014 dated 07/11/2014).
Conflicts of interest
There are no conflicts of interest
Acknowledgments
The authors gratefully acknowledge the funding support received from the institute and sincerely thank Ms. Aparna Sundaresh, Ph.D scholar as well as the staff of the Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research for their timely help at various stages of this research.
REFERENCES
- 1.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capuron L, Miller AH. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 4.Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- 5.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: Data from the third national health and nutrition examination survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 7.Elovainio M, Aalto AM, Kivimäki M, Pirkola S, Sundvall J, Lönnqvist J, et al. Depression and C-reactive protein: Population-based health 2000 study. Psychosom Med. 2000;71:423–30. doi: 10.1097/PSY.0b013e31819e333a. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: Results from the health, aging and body composition study. Biol Psychiatry. 2003;54:566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 9.Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2008;106:249–55. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–8. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Chiriboga DE, Pagoto SL, Rosal MC, Li W, Merriam PA, et al. Association between depression and C-reactive protein. Cardiol Res Pract. 2010;2011:286509. doi: 10.4061/2011/286509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015;229:27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Kim YK. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6:1298–304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [DSM-IV] [Google Scholar]
- 16.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh G, Kaur D, Kaur H. Presumptive stressful life events scale (PSLES) - A new stressful life events scale for use in India. Indian J Psychiatry. 1984;26:107–14. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–53. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29:32–8. doi: 10.1002/da.20876. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:722–6. doi: 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teja JS, Narang RL, Aggarwal AK. Depression across cultures. Br J Psychiatry. 1971;119:253–60. doi: 10.1192/bjp.119.550.253. [DOI] [PubMed] [Google Scholar]
- 26.Weiss MG, Raguram R, Channabasavanna SM. Cultural dimensions of psychiatric diagnosis. A comparison of DSM-III-R and illness explanatory models in South India. Br J Psychiatry. 1995;166:353–9. doi: 10.1192/bjp.166.3.353. [DOI] [PubMed] [Google Scholar]
- 27.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–64. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vetter ML, Wadden TA, Vinnard C, Moore RH, Khan Z, Volger S, et al. Gender differences in the relationship between symptoms of depression and high-sensitivity CRP. Int J Obes (Lond) 2013;37(Suppl 1):S38–43. doi: 10.1038/ijo.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV, et al. Ethnic differences in serum adipokine and C-reactive protein levels: The multiethnic cohort. Int J Obes (Lond) 2014;38:1416–22. doi: 10.1038/ijo.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder I, Marsland AL, Cheong J, Muldoon MF, Ferrell RE, Manuck SB. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C-reactive protein. Brain Behav Immun. 2010;24:160–7. doi: 10.1016/j.bbi.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


