Abstract
Background:
Lithium, which is frequently used in the treatment of mood disorder, can lead to various types of thyroid dysfunctions. Although clinical examination and biochemical assessment are fundamental to any thyroid work-up of lithium-treated patients, assessment findings vary widely depending on the investigator. Ultrasonographic measurement of thyroid volume has, therefore, been performed in lithium treatment populations and found to be a sensitive tool.
Aim:
We aimed to determine and compare thyroid gland volume using Ultrasonography and laboratory parameters, (thyroid-stimulating hormone [TSH], T3, and T4) in long-term lithium and other mood stabilizers treated patients with mood disorder.
Materials and Methods:
In this cross-sectional study, we performed ultrasonography examinations and thyroid function test of 30 patients on lithium treatment and 30 patients on other mood stabilizers.
Results:
The ultrasonographically measured thyroid volume was significantly increased in patients receiving lithium therapy as compared to the patients receiving other mood stabilizers. The total triiodothyronine (T3) was significantly increased with trends toward increased total thyroxine (T4) and decreased TSH in patients receiving lithium therapy as compared to the patients receiving other mood stabilizers.
Conclusion:
These results highlight the need of including ultrasonographic measurement of thyroid volume as a part of standard thyroid work-up before initiating lithium prophylaxis and during follow-up. Additional studies on the incidence and mechanism of lithium associated hyperthyroidism are needed.
Keywords: Lithium, mood disorder mood stabilizer, thyroid function test, thyroid ultrasonography
INTRODUCTION
Lithium has been used for the treatment and prophylaxis of mood disorders since 1950s. Patients taking lithium usually require to take the drug on a long-term basis, which makes the issue of long-term toxicity an important one. Among the various side effects recognized, one of the major side effects is the development of various types of thyroid abnormalities.[1] High rates of hypothyroidism, goiter 30-55%[2,3,4,5,6,7] and thyrotoxicosis has been reported.[8,9] Female gender and starting lithium at a later age are the main risk factors.[3,10,11,12] Both the mechanism of its therapeutic action and the mechanisms of its various adverse effects are still incompletely understood. Lithium is concentrated by the thyroid at levels 3-4 times that in plasma and might exert a direct toxic effect on the thyroid gland.[13]
The most important clinically relevant action purposed is the inhibition of thyroid hormone release.[14] This may result in the development of goiter and hypothyroidism. Independent effects on the hypothalamic-pituitary-thyroid axis and the receptor mediated mechanism of thyroid hormone action may contribute. The immunological influence of lithium on thyroid antibody concentrations leading to a more rapid onset of thyroid autoimmunity resulting in goiter and hypothyroidism and possibly also a state of hyperthyroidism in some cases has been hypothesized.[14] Lithium-induced direct damage of thyroid cells with consequent release of thyroglobulin and thyroid hormones into the circulation has been purposed as possible mechanism of hyperthyroidism associated with long-term lithium use.[15] Additional risk factors such as iodine deficiency, smoking, or goitrogens may leads to thyroid dysfunction.[7]
Although clinical inspection and palpation of the thyroid gland are fundamental to any physical examination of lithium-treated patients, the assessment of findings varies widely depending on the investigator.[16] Biochemical assessment includes measurement of serum concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), and thyroid peroxidase antibody and ultrasonographic scanning of thyroid has therefore been performed in lithium-treated populations and found to be a sensitive tool.[14,17] Unlike other imaging techniques, ultrasonography does not involve radiation exposure and is low in cost, fast, and relatively accurate.[18,19] Only few studies up to now have used both ultrasonography measurement and biochemical assessment along with clinical palpation in age and gender matched lithium-treated patients and controls.[20] Decades of clinical use of lithium and availability of new diagnostic tools for thyroid abnormalities have extended the interest to other aspects, including autoimmunity, hyperthyroidism, and morphological changes.[21]
Most of the earlier study have used clinical inspection, palpitation and measurement of thyroid hormone to assess the change in thyroid status in lithium-treated patients of mood disorders.[22,23,24,25] Only few studies up to now have used both ultrasonography measurement and biochemical assessment along with clinical palpation in age and gender matched lithium-treated patients and controls.[7,14,20] Unlike most of the previous studies where normal was taken as control, we include patients with mood disorder as a control because impairment of the hypothalamic pituitary thyroid axis stemming from the mood disorder may leads to changes observed in the thyroid state. Although cross-sectional in design, the main advantage of our study is that this is the first study using ultrasonography to determine thyroid volume along with clinical assessment and thyroid hormone measurement in age and gender matched Indian population.
The primary aim of this study was to see the ultrasonographically measured changes in thyroid gland volume, biochemical parameters (TSH, T3 and T4) and to compare the above findings with gender and age-matched Lithium naοve patients of mood disorder receiving other mood stabilizers such as sodium valproate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
MATERIALS AND METHODS
Study design and population
This was a cross-sectional study of lithium-treated patients with mood disorders and of gender-and age matched lithium naive patients on other mood stabilizers. Subjects attending outpatient or in-patient Departments of Central Institute of Psychiatry (CIP) were included in the study. All subjects provided written informed consent prior to participating in the study. CIP is an academic psychiatry center with tertiary care facility, has a total bed capacity for 673 patients with more than 55,500 patients attending the outpatient clinic every year, which includes males, females and children. It kept detailed documentation of patient history, course of illness, and pharmacological treatment. Study was passed by the Ethics Committee of the Institute. Lithium-treated subjects of both sexes who fulfilled the following criteria were eligible for the study: Age 18-60 years, a diagnosis of mood disorder according to diagnostic criteria of International Classification of Diseases-10-Diagnostic Criteria for Research (ICD-10-DCR) (WHO, 1992),[26] continuous and documented maintenance treatment with lithium at adequate blood levels (≥0.6-1.2 mmol/L) for one or more years. Control subjects were gender-and age-matched, who fulfilled the following criteria: Age 18-60 years, age and gender matched patients with mood disorder according to ICD-10 DCR, (WHO, 1992),[26] who have been on mood stabilizer other than lithium including sodium valproate, carbamazepine, divalproex sodium, oxcarbazepine, and lamotrigine for 1 year or more, no prior use of lithium. The study's exclusion criteria for case group: Were use of mood stabilizers other than lithium, the study's exclusion criteria for control group, a history of use of lithium and the study's exclusion criteria applied to both groups, Any past or family history of thyroid disorder, a history of complete/subtotal thyroidectomy, a history of exposure to thyroid supplements or antithyroid drugs, Iodide, sulfonamide, amiodarone interferons interleukins-2, β-Blockers, or any special diets that might interfere with iodine metabolism. All subjects also underwent a clinical examination and palpation of the neck and thyroid gland.
Measures
A semi-structured pro-forma was used for recording demographic details like age, sex, marital status, religion, education, occupation, socioeconomic status, habitat, and family history of thyroid disorder, as well as clinical data such as duration of illness total duration of treatment, continuous duration of treatment, age of onset of mood disorder, history of medical or psychiatric illness, family history of medical or psychiatric illness and premorbid personality. It also included details of physical examination of all organ systems and mental status examination. Finally, diagnosis of the patient according to ICD-10 DCR, (WHO, 1992)[26] and the drugs taken were also recorded.
Acquisition of ultrasound images
The ultrasound images were acquired with a conventional ultrasound scanner, Envisor HD (Phillips) ultrasound scanner. Ultrasonographic examination of the thyroid was performed using a linear 3-12 MHz transducer with the patient in a supine position and his or her neck hyper extended. The volume of the thyroid lobes was determined using the ellipsoid model by measuring the maximum transversal (T), horizontal (H), and longitudinal (L) diameters. The volume (ml) was calculated with the formula for ellipsoids as described by Brunn et al.:[27] T (cm) Χ H (cm) Χ L (cm) Χ 0.479. Sonographic examinations were performed by the trained radiologist.
Laboratory measurements
Venous blood was drawn on the day of Sonographic evaluation and analyzed for TSH, total thyroxine (T4), and total triiodothyronine (T3) at the clinical laboratory of the CIP, Ranchi, India, with the commercially available Syntron® Elisa test kit, Bioresearch, Inc., India. Normal reference value was taken as T3 = 72-184 μg/dl, T4 = 5-13 μg/dl, TSH = 0.5-4 μ-IU/l.
Venous blood was drawn after 24 h of last lithium dose and serum lithium estimation was done at the clinical laboratory of the CIP, Ranchi, India. Ion selective electrode method was used,[28] with the commercially available Esylyte® Tranasia biomedicals, Mumbai, India.
Statistical analysis
All analyses were performed with the computer software program, Statistical Package for Social Sciences, version 16.0 (SPSS-16.0) for Windows. Demographic and clinical differences between the groups were tested with McNemar's Chi-square test to compare categorical variables. The paired t-test was employed for continuous variables such as age, thyroid volume, and thyroid hormone levels. Descriptive statistics was used to calculate mean, percentage, and standard deviation of the sample. The level of significance was taken as P < 0.05 (two-tailed).
RESULTS
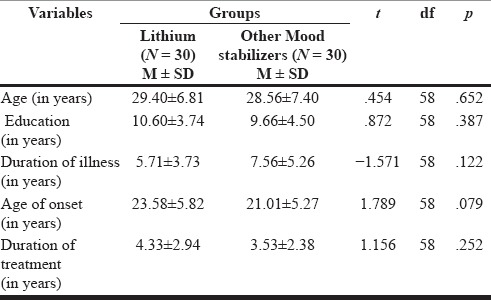
In our study, 60 patients (30 cases, 30 controls) with the diagnosis of mood disorder fulfilling the inclusion and exclusion criteria were selected. The patients who were receiving lithium treatment were similar to the control group patients who were receiving other mood stabilizers with respect to age, education, marital status, religion, occupation, and habitat. The mean age of patients was 29.40 ± 6.81 years in the lithium group and 28.56 ± 7.40 years in the other mood stabilizers group. Both the groups had 25 (83.3%) males and 5 (16.7%) females [Table 1]. More than 50% of the patients were from lower socioeconomic status in both the group because the catchment area of our institute is economically underdeveloped. Thyroid was palpable only in 5 (16.6%) patients in lithium group and not palpable in any of the patient of other mood stabilizer group. Both the groups had similar duration of illness, age of onset of mood disorder, continuous duration of treatment, and education in years. The mean age of onset was 23.58 ± 5.82 years for the lithium group and 21.01 ± 5.27 years for the other mood stabilizer group. The total duration of treatment was 4.33 ± 2.94 years for the lithium group and 3.53 ± 2.38 years for the other mood stabilizers group [Table 2]. No statistically significant difference could be observed in any of the sociodemographic or clinical parameters between two groups. There was no significant group difference in terms of past and family history of any psychiatric or any major medical illness, treatment history, and premorbid personality.
Table 1.
Comparison of Sociodemographic profiles between patients on lithium and on other mood stabilizers
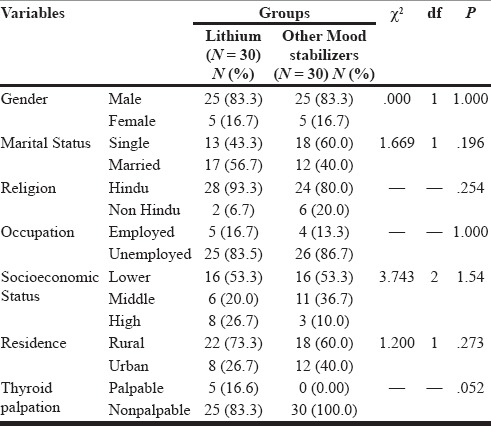
Table 2.
Comparison of clinical profiles between patients on lithium and on other mood stabilizers
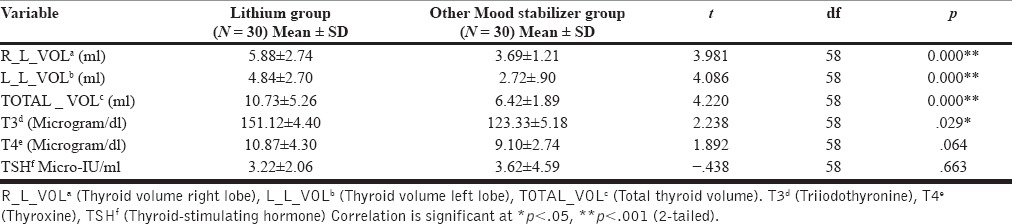
Significantly higher right, left, and total thyroid volume were observed in lithium group subjects as compared to subjects taking other mood stabilizers (all P < 0.001) [Table 3]. Significantly increased mean T3 (microgram/dl) value was observed in the lithium group (151.12 ± 4.40) as compared to the other mood stabilizers group (123.33 ± 5.18) (P = 0.029) [Table 3]. Similar trend toward higher mean value of T4 (microgram/dl) (10.87 ± 4.30) was noted in the lithium group as compared to the other mood stabilizers group (9.10 ± 2.74); although, it was not statistically significant. A slight lower mean value of TSH (micro-IU/ml) was observed in the lithium group (3.22 ± 2.06) as compared to other mood stabilizers group [Table 3]. However, the difference between groups was not statistically significant. Our finding suggests trends toward hyperthyroidism in lithium-treated patients as compared to lithium naοve patients receiving other mood stabilizers.
Table 3.
Comparison of thyroid profile between mood disorder subjects on lithium and on other mood stabilizers
In the lithium group, the age of patients in years was found to positively correlate with left lobe volume (r = 0.391) and total volume (r = 0.376). Similarly, the age of onset of mood disorder was found to correlate positively with right lobe volume (r = 0.392), left lobe volume (r = 0.394), and total thyroid volume (r = 0.407) [Table 4].
Table 4.
Correlation of Sociodemographic profile with ultrasonographically measured thyroid volumes and biochemical thyroid parameters (T3, T4, TSH) in lithium group patients (N = 30)
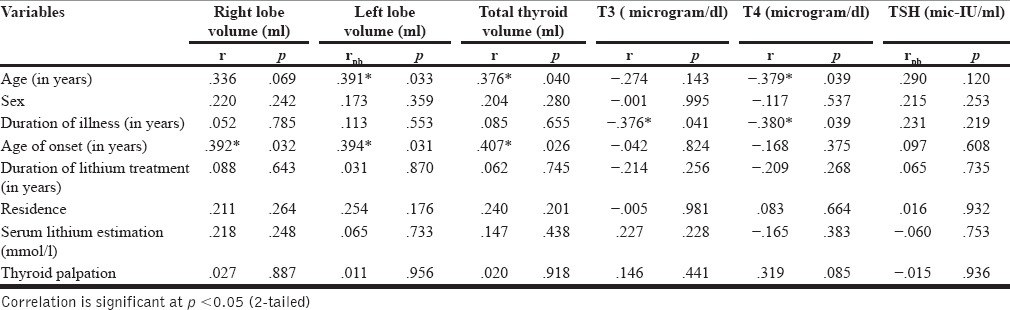
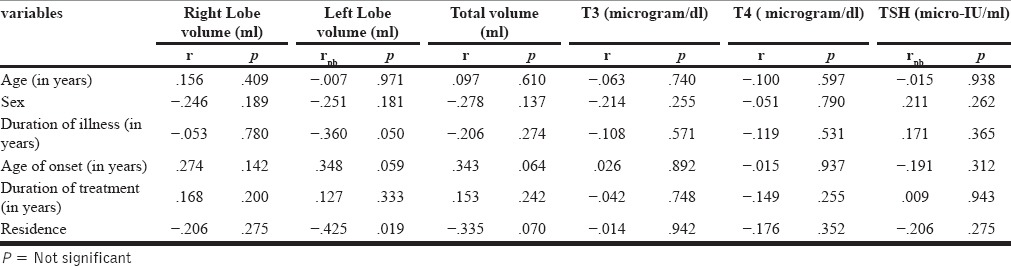
Significantly negative correlation was found between age of patients in years and T4 (r = −0.379) duration of illness and T3 (r = −0.376), T4 (r = −0.380) [Table 4]. There was no significant correlation between gender, duration of lithium treatment, age of onset of mood disorder, serum lithium level, and serum level of T3, T4, and TSH [Table 4]. No significant correlation was found between ages, gender, duration of illness, age of onset, duration of treatment, and with any of the thyroid parameters in control group [Table 5].
Table 5.
Correlation of Sociodemographic profile with ultrasonographically measured thyroid volumes and biochemical thyroid parameters (T3, T4, TSH) in other mood stabilizers group patients (N = 30)
DISCUSSION
Although cross-sectional in design, this study has the advantages as it included gender and age matched control belonging to same geographical area, so to eliminate the chances of normal variation in the prevalence of thyroid disorder primarily due to regional differences in iodine availability. To best of our knowledge, in most of the previous studies healthy volunteer has been used as a control, this study includes patients with mood disorder as a control, to eliminate the chances of variation in thyroid status due to impairment of the hypothalamic pituitary thyroid axis stemming from the mood disorder itself.[29]
A single ratter using same laboratory and same laboratory technique, ensured uniformity in biochemical assessment of thyroid status. For assessment of thyroid status both ultrasonography and laboratory measurement along with clinical inspection and palpation of thyroid gland was done. To best of our knowledge, only four studies up to now have used ultrasonography both in lithium patients and controls. Although clinical inspection and palpation of the thyroid gland are fundamental to any physical examination of lithium-treated patients, the assessment of findings varies widely depending on the investigator.[16] Ultrasonographic measurement of thyroid size has therefore been performed in lithium-treated populations and found to be a sensitive tool.[14,17] Unlike other imaging techniques, ultrasonography does not involve radiation exposure and is low in cost, fast, and relatively accurate.[18,19]
Ultrasonographically measured thyroid volume was found significantly increased in lithium-treated patients, as reported in previous studies, increased volume was found more common in patients treated with lithium for 1-5 years (44%) or more than 10 years (50%) than in patients who never received lithium (16%).[20,30] The volumes of the right and the left thyroid lobes were significantly higher in the lithium-treated than among normal controls.[7] Several mechanisms have been hypothesized to explain the development of increase thyroid volume, like Lithium is concentrated by the thyroid and inhibits thyroidal iodine uptake.[31]
Various studies[16,22,23,24,25] have used the laboratory assessment of serum T3, T4, and TSH to study the effects of lithium on thyroid gland. In our study, significantly increased mean T3 value was observed in the lithium group as compared to the other mood stabilizers group. Similar trend toward higher mean value of T4 was noted in the lithium group as compared to the other mood stabilizers group; although, it was not statistically significant. A slight lower mean value of TSH was also observed in the lithium group as compared to other mood stabilizers group. However, the difference between groups was not statistically significant. Our finding suggests trends toward hyperthyroidism in lithium-treated patients as compared to lithium naοve patients receiving other mood stabilizers. Whereas, hypothyroidism is described as commonly known effect of lithium use[1,7,14,32] hyperthyroidism associated with lithium use has also been described.[1,9,23,33,34,35] A large retrospective review demonstrated that lithium-associated silent thyroiditis and lithium-associated hyperthyroidism had a much higher incidence (1.3 and 2.7 cases per 1000 person-years, respectively) than that seen in the general population (0.03-0.28 and 0.8-1.2, respectively).[15] Increased T3 and T4 along with decreased TSH found in our study could be possibly due to increased concentration of lithium in thyroid gland and direct damage[13,15] of thyroid cells with compensatory hypertrophy leading to increase thyroid volume and release of thyroid hormones into the circulation. Another reason could be autoimmune thyroiditis[36] or granulomatous thyroiditis.[37] As in our study starting lithium at a later age has been reported as the main risk factors for increased thyroid volume in lithium-treated patients.[14] However, we have found no significant correlation between gender, duration of illness, duration of lithium treatment, habitat, serum lithium level, palpable thyroid, and thyroid volume in both groups. Several investigators showed higher incidences of lithium-induced thyroid abnormalities in women.[11,14] However Perrild et al.[20] reported no gender difference with regard to thyroid volume, in our study we could not find any correlation between female gender and total thyroid volume (P = 0.280) [Table 4], this could be due to small sample size of female patients. Unlike our study significant positive correlation was found between duration of lithium treatment,[11,14] serum lithium level[7] and thyroid volume. Duration of lithium treatment and thyroid volume was not found to be significantly correlated also.[7]
In our study, significantly negative correlation was found between age of patients in years and T4 (r = −0.379) duration of illness and T3 (r = −0.376), T4 (r = −0.380) [Table 4]. There was no significant correlation between gender, duration of lithium treatment, age of onset of mood disorder, serum lithium level, and serum level of T3, T4, and TSH [Table 4]. Significantly positive correlation has been found between female gender, duration of treatment, starting lithium at later age, serum lithium level, and hypothyroidism.[3,7,12,14,20,38,39] No correlation was found between serum lithium level and T3, T4, and TSH values. Hyperthyroidism was not found significantly correlated with gender.[40]
The design of our study has few limitations like this was a cross-sectional study conducted at a random point in time, concomitant antipsychotics used in the patients could be a confounding factor, small sample size and measurement of thyroid volume by single radiologist.
CONCLUSION
Our study has produced several significant findings like ultrasonographically measured thyroid volume was significantly increased in patients receiving lithium therapy as compared to the patients receiving other mood stabilizers, measurements of total triiodothyronine (T3), total thyroxine (T4) and TSH, finding suggests trends toward hyperthyroidism in lithium-treated patients as compared to lithium naοve patients receiving other mood stabilizers and enlargement of thyroid can be diagnosed reliably only by ultrasonography and not by clinical inspection or palpation. Starting lithium at a later age was found as the risk factor for increased thyroid volume in lithium-treated patients but duration of lithium treatment and serum lithium level was not associated with any change thyroid status.
Ultrasonographic measurement of thyroid volume should be included as part of the standard thyroid work-up before initiating lithium prophylaxis and during follow-up of long-term lithium-treated patients. Hyperthyroidism can cause or exacerbate mania, so it is important to be aware of this association during lithium treatment. Additional studies on the incidence and mechanism of lithium associated hyperthyroidism are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgments
This study was supported by the Central Institute of Psychiatry, Ranchi, Government of India.
REFERENCES
- 1.Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: A current perspective. Thyroid Res. 2013;6:3. doi: 10.1186/1756-6614-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus JH, John R, Bennie EH, Chalmers RJ, Crockett G. Lithium therapy and thyroid function: A long-term study. Psychol Med. 1981;11:85–92. doi: 10.1017/s0033291700053307. [DOI] [PubMed] [Google Scholar]
- 3.Bocchetta A, Mossa P, Velluzzi F, Mariotti S, Zompo MD, Loviselli A. Ten-year follow-up of thyroid function in lithium patients. J Clin Psychopharmacol. 2001;21:594–8. doi: 10.1097/00004714-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Cayköylü A, Capoglu I, Unüvar N, Erdem F, Cetinkaya R. Thyroid abnormalities in lithium-treated patients with bipolar affective disorder. J Int Med Res. 2002;30:80–4. doi: 10.1177/147323000203000112. [DOI] [PubMed] [Google Scholar]
- 5.Ozpoyraz N, Tamam L, Kulan E. Thyroid abnormalities in lithium-treated patients. Adv Ther. 2002;19:176–84. doi: 10.1007/BF02848693. [DOI] [PubMed] [Google Scholar]
- 6.Schiemann U, Hengst K. Thyroid echogenicity in manic-depressive patients receiving lithium therapy. J Affect Disord. 2002;70:85–90. doi: 10.1016/s0165-0327(00)00374-8. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M, Blumentritt H, Finke R, Schlattmann P, Adli M, Baethge C, et al. Using ultrasonography to determine thyroid size and prevalence of goiter in lithium-treated patients with affective disorders. J Affect Disord. 2007;104:45–51. doi: 10.1016/j.jad.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MT, Burman KD, Hofeldt FD, Kidd GS. Lithium-associated thyrotoxicosis. Am J Med. 1986;80:1245–8. doi: 10.1016/0002-9343(86)90697-2. [DOI] [PubMed] [Google Scholar]
- 9.Barclay ML, Brownlie BE, Turner JG, Wells JE. Lithium associated thyrotoxicosis: A report of 14 cases, with statistical analysis of incidence. Clin Endocrinol (Oxf) 1994;40:759–64. doi: 10.1111/j.1365-2265.1994.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 10.Vincent A, Baruch P, Vincent P. Early onset of lithium-associated hypothyroidism. J Psychiatry Neurosci. 1993;18:74–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Kirov G. Thyroid disorders in lithium-treated patients. J Affect Disord. 1998;50:33–40. doi: 10.1016/s0165-0327(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 12.Johnston AM, Eagles JM. Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry. 1999;175:336–9. doi: 10.1192/bjp.175.4.336. [DOI] [PubMed] [Google Scholar]
- 13.Berens SC, Wolff J, Murphy DL. Lithium concentration by the thyroid. Endocrinology. 1970;87:1085–7. doi: 10.1210/endo-87-5-1085. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus JH, Kirov G, Harris BB. Effect of lithium on the thyroid and endocrine glands. In: Bauer M, Grof P, Müller-Oerlinghausen B, editors. Lithium in Neuropsychiatry - The Comprehensive Guide. Oxfordshire: Informa Healthcare; 2006. pp. 259–70. [Google Scholar]
- 15.Miller KK, Daniels GH. Association between lithium use and thyrotoxicosis caused by silent thyroiditis. Clin Endocrinol (Oxf) 2001;55:501–8. doi: 10.1046/j.1365-2265.2001.01381.x. [DOI] [PubMed] [Google Scholar]
- 16.Jarløv AE, Nygaard B, Hegedüs L, Hartling SG, Hansen JM. Observer variation in the clinical and laboratory evaluation of patients with thyroid dysfunction and goiter. Thyroid. 1998;8:393–8. doi: 10.1089/thy.1998.8.393. [DOI] [PubMed] [Google Scholar]
- 17.Loviselli A, Bocchetta A, Mossa P, Velluzzi F, Bernardi F, del Zompo M, et al. Value of thyroid echography in the long-term follow-up of lithium-treated patients. Neuropsychobiology. 1997;36:37–41. doi: 10.1159/000119358. [DOI] [PubMed] [Google Scholar]
- 18.Knudsen N, Bols B, Bülow I, Jørgensen T, Perrild H, Ovesen L, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999;9:1069–74. doi: 10.1089/thy.1999.9.1069. [DOI] [PubMed] [Google Scholar]
- 19.Hegedus L, Bennedbaek FN. Nonisotopic techniques of thyroid imaging. In: Braverman LE, Utiger RD, editors. Werner and Ingba's the Thyroid. A fundamental and Clinical Text. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 373–83. [Google Scholar]
- 20.Perrild H, Hegedüs L, Baastrup PC, Kayser L, Kastberg S. Thyroid function and ultrasonically determined thyroid size in patients receiving long-term lithium treatment. Am J Psychiatry. 1990;147:1518–21. doi: 10.1176/ajp.147.11.1518. [DOI] [PubMed] [Google Scholar]
- 21.Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi: 10.1186/1745-0179-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuruvilla K, Karunanidhi A, Kanagasabapathy AS. Effects of long-term lithium carbonate treatment on thyroid function in psychiatric patients. Indian J Psychiatry. 1983;25:98–101. [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava AS, Behere PB, Agrawal JK. Effect of lithium therapy on thyroid function: A retrospective study on Indian patients. Indian J Psychiatry. 1993;35:131–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus JH. The effects of lithium therapy on thyroid and thyrotropin-releasing hormone. Thyroid. 1998;8:909–13. doi: 10.1089/thy.1998.8.909. [DOI] [PubMed] [Google Scholar]
- 25.Kirov G, Tredget J, John R, Owen MJ, Lazarus JH. A cross-sectional and a prospective study of thyroid disorders in lithium-treated patients. J Affect Disord. 2005;87:313–7. doi: 10.1016/j.jad.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 27.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound (author's transl) Dtsch Med Wochenschr. 1981;106:1338–40. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 28.Bertholf RL, Savory MG, Winborne KH, Hundley JC, Plummer GM, Savory J. Lithium determined in serum with an ion-selective electrode. Clin Chem. 1988;34:1500–2. [PubMed] [Google Scholar]
- 29.Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–14. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 30.Ozsoy S, Mavili E, Aydin M, Turan T, Esel E. Ultrasonically determined thyroid volume and thyroid functions in lithium-naïve and lithium-treated patients with bipolar disorder: A cross-sectional and longitudinal study. Hum Psychopharmacol. 2010;25:174–8. doi: 10.1002/hup.1093. [DOI] [PubMed] [Google Scholar]
- 31.Lazarus JH. Effect of lithium on the thyroid gland. In: Weetman AP, Grossman A, editors. Pharmacotherapeutics of the Thyroid Gland. Berlin: Springer-Verlag; 1997. pp. 207–18. [Google Scholar]
- 32.Kleiner J, Altshuler L, Hendrick V, Hershman JM. Lithium-induced subclinical hypothyroidism: Review of the literature and guidelines for treatment. J Clin Psychiatry. 1999;60:249–55. [PubMed] [Google Scholar]
- 33.Yassa R, Saunders A, Nastase C, Camille Y. Lithium-induced thyroid disorders: A prevalence study. J Clin Psychiatry. 1988;49:14–6. [PubMed] [Google Scholar]
- 34.Chow CC, Cockram CS. Thyroid disorders induced by lithium and amiodarone: An overview. Adverse Drug React Acute Poisoning Rev. 1990;9:207–22. [PubMed] [Google Scholar]
- 35.Siyam FF, Deshmukh S, Garcia-Touza M. Lithium-associated hyperthyroidism. Hosp Pract. 2013;41:101–4. doi: 10.3810/hp.2013.08.1073. [DOI] [PubMed] [Google Scholar]
- 36.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 37.Sinnott MJ, McIntyre HD, Pond SM. Granulomatous thyroiditis and lithium therapy. Aust N Z J Med. 1992;22:84. doi: 10.1111/j.1445-5994.1992.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 38.Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: Sex differences. J Psychiatry Neurosci. 2002;27:104–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Gracious BL, Findling RL, Seman C, Youngstrom EA, Demeter CA, Calabrese JR. Elevated thyrotropin in bipolar youths prescribed both lithium and divalproex sodium. J Am Acad Child Adolesc Psychiatry. 2004;43:215–20. doi: 10.1097/00004583-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]


