Abstract
Depression is the major psychiatric ailment of our times, afflicting ~20% of the population. Despite its prevalence, the pathophysiology of this complex disorder is not well understood. In addition, although antidepressants have been in existence for the past several decades, the mechanisms that underlie their therapeutic effects remain elusive. Building evidence implicates a role for the plasticity of specific neuro-circuitry in both the pathophysiology and treatment of depression. Damage to limbic regions is thought to contribute to the etiology of depression and antidepressants have been reported to reverse such damage and promote adaptive plasticity. The molecular pathways that contribute to the damage associated with depression and antidepressant-mediated plasticity are a major focus of scientific enquiry. The transcription factor cyclic AMP response element binding protein (CREB) and the neurotrophin brain-derived neurotrophic factor (BDNF) are targets of diverse classes of antidepressants and are known to be regulated in animal models and in patients suffering from depression. Given their role in neuronal plasticity, CREB and BDNF have emerged as molecules that may play an important role in modulating mood. The purpose of this review is to discuss the role of CREB and BDNF in depression and as targets/mediators of antidepressant action.
Keywords: Antidepressant, depression, hippocampus, neurotrophin, stress
1. Introduction
Depression is a widespread, incapacitating psychiatric ailment, with 10-30% of women and 7-15% of men in a population being afflicted with this disorder at any given time (Briley 2000). Despite its prevalence and social impact, its prognosis and management are often poor, due not only to the heterogeneity of this ailment, but also our lack of knowledge of the pathophysiology underlying this disorder. It is this lack of understanding of the underpinnings of depression that has resulted in no substantial improvement to antidepressant treatments, which were discovered serendipitously more than 50 years ago. Although second generation antidepressant drugs have eliminated some of the side effects associated with their predecessors, there have been few novel therapeutic targets identified that could significantly improve our management of mood disorders. The purpose of this review is to discuss emerging evidence that implicates the transcription factor, cyclic AMP response element binding protein (CREB) and the neurotrophin, brain-derived neurotrophic factor (BDNF) as potential key players in both the etiology and the treatment of depression.
While the neurobiological correlates of this disorder are still poorly understood, building evidence suggests that it is a dysfunction of specific neuro-anatomical foci, notably the hippocampus and the prefrontal cortex (PFC), which may underlie the pathogenesis of depression (Bremner 1995; Sheline et al 1996; Drevets et al 1997). There is considerable evidence demonstrating alterations in brain structure in patients suffering from depression, including volumetric loss, neuronal atrophy and cell death. Hippocampal volumetric loss has been reported in patients suffering from recurrent major depression (Sheline et al 1997), and alterations in PFC volume have also been documented in cases of filial depression (Drevets et al 1997). Additionally, reduced numbers of neurons and glia have been observed in the temporal cortex of patients suffering from unipolar depression (Rajkowska et al 2000). Dramatic changes in mammalian brain structure have also been documented following sustained stress exposure, which serves as an experimental animal model simulating the psychobiology of depression. Among the pathological effects that have been documented following prolonged stress exposure, is damage to limbic structures like the hippocampus and cortex. Several types of adult-onset stressors, including physical and psychosocial stressors, affect hippocampal structure: causing dendritic atrophy in the CA subfields (Watanabe et al 1992a; Vyas et al 2002) and suppressing granule cell neurogenesis within the dentate gyrus (Gould and Tanapat 1999; Pham et al 2003). Lending further credence to the hypothesis that it is alterations in brain structure that underlie depressive disorders, studies clearly demonstrate the reversal of the stress-induced atrophy with antidepressant therapy in animal models (Watanabe et al 1992b; Czeh et al 2001), as well as the reversal of hippocampal volumetric loss in depressed patients administered antidepressant treatment (Sheline et al 2003). Interestingly, antidepressant treatment can also prevent the stress-mediated reduction in hippocampal neurogenesis (Czeh et al 2001), while inhibiting neurogenesis, using irradiation, can hinder antidepressant action in behavioural animal models (Santarelli et al 2003). Taken together, these studies raise the possibility that the pathogenesis and treatment of depression may involve structural plasticity. Depression could, thus, be a consequence of the failure of specific neuronal circuits to show adaptive plasticity when exposed to external stimuli such as stress. The beneficial effects of antidepressant treatments could then arise from the reversal or amelioration of this dysfunction, or through the direct stimulation of adaptive neuronal plasticity. Although current antidepressant treatments exert their acute actions rapidly, the therapeutic benefits exhibit a lag of about 2-3 weeks. An emerging hypothesis suggests that this lag may be a consequence of the requirement to exhibit structural plasticity, which would develop gradually as a cumulative response to sustained treatment with antidepressants (Vaidya and Duman 2001).
The molecular and cellular mechanisms that (i) contribute to the damage which is implicated in the pathogenesis of depression and (ii) the repair that has been hypothesized to contribute to the therapeutic effects of antidepressant treatment remain unclear. While previous research efforts studied alterations in monoamine neurotransmitter levels, receptors or receptor-coupled second messenger systems, more recent efforts have focused on intracellular cascades and the regulation of gene expression. Exploring the role of signalling cascades and transcription factors dysregulated in depression and recruited by antidepressant therapies will most likely yield valuable data, since these molecules can affect the expression of diverse downstream target molecules. One such candidate transcriptional regulator is CREB, a transcription activator that is implicated in both stress- (Hatalski and Baram 1997) and antidepressant-induced transcriptional regulation (Conti et al 2002). The cAMP-CREB signalling cascade is critical to the generation of new neurons in the rodent hippocampus (Nakagawa et al 2002), and also facilitates their subsequent morphological maturation (Fujioka et al 2004). Thus, given its neuro-protective and survival-enhancing properties, it is an attractive candidate molecule that may be targeted by antidepressant therapy.
2. Cyclic AMP response element binding preotein
CREB is a nuclear protein, belonging to the family of leucine zipper transcription factors that are expressed in a variety of tissues and serve diverse functions. This family consists not only of transcriptional activators, including CREB and activating transcription factor (ATF), but also repressor molecules like cAMP response element modulator (CREM) and inducible cAMP early repressor (ICER). CREB contains a basic leucine zipper motif with which it can homodimerize or heterodimerize (to CREM or ATF) to form the functional dimer, and a DNA-binding domain with which it recognizes and binds to promoter cAMP response element (CRE) sequences. Phosphorylation of a serine residue (S133) in its kinase-inducible domain is critical to mediate its effects, as this permits recruitment of co-activator proteins and allows initiation of transcription. Phosphorylation, and hence activation, of CREB can be accomplished by a number of upstream signalling cascades. The canonical pathway that leads to CREB phosphorylation is the cAMP-protein kinase A (PKA) pathway (see reviews Montminy et al 1997; Casare et al 1999). The PKA cascade is known to be perturbed in animal models of depression and also serves as a target for antidepressant treatments and has been reviewed elsewhere (Shelton et al 2000; Tardito et al 2006). Besides the cAMP-PKA pathway, several other signalling cascades are also known to affect CREB activity through phosphorylation of the S133 and other serine residues, notably the MAP kinase signalling pathway (MEK/ERK), the calcium-CaMKII/IV cascade, as well as phospholipase C (PLC) signalling (Johannessen et al 2004). Studies indicate that these pathways are perturbed in patients suffering from depression (Dwivedi et al 2006) and are also known to be influenced by antidepressant treatment (Tiraboschi et al 2004; Fumagalli et al 2005).
2.1 CREB, animal models of depression and antidepressants
While the hippocampal expression of CREB is reduced in response to stress exposure (Alfonso et al 2006), CREB expression in the brain is increased following chronic, though not acute, administration of a variety of antidepressants (Nibuya et al 1996). A diverse body of antidepressants, including serotonin-selective reuptake inhibitors (SSRIs), norepinephrine-selective reuptake inhibitors (NRIs), monoamine oxidase inhibitors (MAOIs) and electroconvulsive seizure therapy (ECS), all upregulate CREB expression in distinct brain regions that include the hippocampus and cerebral cortex. While these antidepressants have distinct acute targets, their chronic administration results in CREB upregulation. It will be interesting to know whether antidepressants can influence the translation of CREB, given recent reports that antidepressant treatment can influence translational machinery (Dagestad et al 2006). CREB may then serve as a common post-receptor target and thus be a central integrator for diverse upstream signalling cascades recruited by antidepressant treatment. CREB thus emerges as an exciting common target for distinct classes of antidepressants (figure 1).
Figure 1.
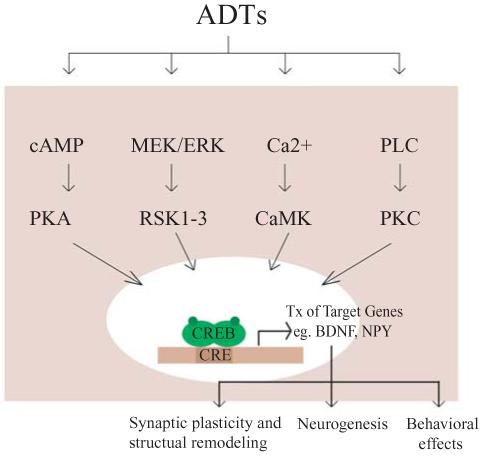
The CREB cascade. CREB is regulated by diverse signalling pathways, and can thus serve as a central integrator for the actions of numerous external stimuli, including antidepressants (ADT). Antidepressants which have distinct acute targets are known to recruit several signal transduction pathways, including cyclic AMP – protein kinase A (cAMP-PKA) cascade, the MAP kinase signalling pathway (MEK/ERK) as well as calcium and phospholipase C (PLC) signalling. A downstream consequence of enhanced CREB function is thought to be the increased expression of target genes like the neurotrophin, brain-derived neurotrophic factor (BDNF), and neuropeptide Y (NPY), which may contribute to the ADT-mediated changes in structural plasticity and behaviour.
Chronic administration of antidepressants not only affects CREB expression, but also CREB activity and CREB-mediated transcription. Chronic antidepressant therapy upregulates cellular PKA activity and increases the translocation of PKA to the nucleus (Nestler et al 1989). Enhanced CRE binding (Itoh et al 2004) and, studies using CRE-LacZ reporter mice (Thome et al 2000), indicate a region-specific increased rate of CRE-mediated transcription observed following antidepressant treatment. It has been suggested that antidepressants recruit CREB to upregulate the transcription of target genes that then lead to the trophic effects, including neurogenesis and neuronal plasticity, which underlie antidepressant action (figure 1).
2.2 Pro- and anti-depressant activity of CREB
CREB can, itself, act in a manner analogous to that of antidepressants. Viral-mediated over-expression of CREB in the hippocampus has an antidepressant-like effect in behavioural models of depression (Chen et al 2001a), notably the learned helplessness and forced swim test. Furthermore, chronic administration of rolipram, an inhibitor of phosphodiesterases that downregulate cAMP-CREB signalling, has antidepressant actions in several behavioural tests (Itoh et al 2004). However, it is interesting to note that the role of CREB is highly dependent on its temporal and spatial regulation. CREB over-expression in the basolateral amygdala has pro- or anti-depressive responses depending on whether the over-expression of CREB precedes or follows training in the learned helplessness model of depression (Wallace et al 2004). Expression of CREB before training increases both escape failures and immobility in the forced swim test, which are pro-depressive effects. However, induction of CREB in the basolateral amygdala after training decreases the number of escape failures, an antidepressant-like response. This suggests that the timing of CREB regulation within the basolateral amygdala may be key for the various associative changes that culminate in behavioural responses. CREB expression before training may cause a strong association thus promoting a fear-conditioned state and enhancing the learned helplessness phenotype. In striking contrast, the expression of CREB in this region after the learned helplessness paradigm results in an antidepressant-like response. This temporal consequence to CREB expression in the amygdala could be a result of different target genes being regulated by CREB. Given that CREB works in conjunction with other key transcription factors (CREM, ICER, ATF) (Masquilier and Sassone-Corsi 1992), future studies are required to address whether the composition of the transcription factors that bind to the CRE sites prior and post-training for learned helplessness are different, thus resulting in distinct functional outcomes. Interestingly, while CREB expression in the hippocampus elicits antidepressant-like responses (Chen et al 2001a), it has pro-depressive effects when expressed in the nucleus accumbens (Pliakas et al 2001). Viral mediated over-expression of CREB in the accumbens causes pro-depressive reactions in animal models of depression (Pliakas et al 2001), while over-expression of the dominant negative form of CREB (mCREB) results in antidepressant-like action (Pliakas et al 2001; Newton et al 2002). Thus, CREB exhibits a region-specificity in its actions, and this is a theme that will be further discussed later.
2.3 CREB and downstream target genes
While it seems clear that CREB has a role to play in pathogenesis of depression and in antidepressant action, the mechanistic details of the same remain obscure, in part due to the complexity of the CREB gene itself. The CREB gene can generate multiple transcripts and protein products that can serve as both transcriptional activators and as repressors. However, identifying downstream target genes, those with the necessary CRE binding sites, is crucial in revealing the action of CREB. Of the several molecules that are regulated by CREB, studying brain-derived neurotrophic factor (BDNF) seems a promising avenue given that this neurotrophin could stimulate structural plasticity and repair stress- or depression-induced damage. The BDNF gene, which consists of five individual exons in the rat, has a promoter that contains a cAMP response element and a calcium response element, both of which can bind the transcriptional-activator CREB (Tabuchi et al 2002). Additionally, the antidepressant-induced upregulation of BDNF expression is lost in CREB deficient mice (Conti et al 2002). Besides BDNF, several other molecules that are strongly implicated in depression are also known to be regulated by CREB, such as tyrosine hydroxylase (TH) (Gueorguiev et al 2006), corticotrophin releasing factor (CRF) (Itoi et al 1996), dynorphin (Carlezon et al 1998) and neuropeptide Y (NPY) (Pandey 2003). The effects of CREB on mood will not only depend upon the specific target gene that is altered, but also the region of the brain in which this regulation takes place and the consequent changes on structure and function of the specific neuro-circuitry. For example, the increased expression of CREB in the hippocampus is likely to contribute to trophic changes through the recruitment of BDNF and NPY, resulting in enhanced neurogenesis (Hansel et al 2001; Pencea et al 2001) and reversal of damage. A possible consequence of these changes may be to enhance the ability of the hippocampus to exert an adaptive influence on stress reactivity by terminating the stress responses mediated via the hypothalamo-pituitary-adrenocortical (HPA) axis, incidentally, known to be dysfunctional in depression (Carroll et al 1976). In contrast, enhanced CREB in the hypothalamus would increase CRF thus leading to the maladaptive hyper-activation of the HPA axis. This means that a simplistic upregulation of CREB in the entire brain may not have any therapeutic relevance, and that attempts to use this molecule as a target for drug development requires identifying mechanisms that allow for a region-specific recruitment of CREB.
3. Brain-derived neurotrophic factor
BDNF is the most widely expressed member of the nerve growth factor family of growth regulators, collectively termed the neurotrophins. The neurotrophins play a critical role in the development of the brain and continue to have a seminal role in shaping plasticity in the mature nervous system (Thoenen 1995). They mediate their effects through the stimulation of a family of the tyrosine kinase-coupled receptors, known as trks (tropomyosin receptor kinase), which signal through the MAP kinase signalling cascade. The specific trk that mediates BDNF signalling is known as the trkB receptor. The BDNF gene has a complex structure, with 4 distinct non-coding 5′ exons with their unique promoters, and one common 3′ coding exon that generates the mature BDNF protein (Timmusk et al 1993). BDNF has a well-established role in the development, survival and differentiation of select populations of neurons (Hoefer and Barde 1988; Alderson et al 1990) and is capable of augmenting ongoing neurogenesis in the adult brain (Pencea et al 2001). BDNF has also been shown to elicit rapid action potentials thus influencing neuronal excitability (Kafitz et al 1999) and it has a demonstrable role in activity-dependent synaptic plasticity events like long-term potentiation, learning tasks and memory (Bramham and Messaoudi 2005). Thus, BDNF is involved in structural remodeling, neuronal plasticity and synaptic restructuring, and is promising as a candidate molecule underlying the structural changes associated with depression and animal models of depression, and as a potential target for antidepressants.
3.1 BDNF in animal models of depression
A large body of literature already documents the role of BDNF in the developing brain, and there is growing support for the continued role of BDNF in shaping adult brain structure and function. Existing data from animal models supports a role for BDNF in the pathophysiology of depression. Prolonged exposure to several stressors, including immobilization stress, forced swim stress as well as conditioned footshock, results in a down-regulation of BDNF expression in the hippocampus (Smith et al 1995; Rasmusson et al 2002). Animal models like learned helplessness also result in a decline in hippocampal BDNF expression (Song et al 2006). A recent study indicates that BDNF regulation by social stress is mediated through epigenetic mechanisms of methylation, and hence transcriptional repression, of the BDNF III and IV promoters (Tsankova et al 2006). Our unpublished studies reveal that the same promoters (III and IV) may also be targets for another stress model, immobilization, raising the possibility that it is these specific promoters that are recruited by stress to alter BDNF expression. It is likely that environmental perturbations result in sustained chromatin remodeling and epigenetic programming thus influencing the transcriptional expression of the BDNF gene. Perturbed BDNF expression could have strong functional correlates on both the structure and activity of stress neuro-circuitry. Indeed, it is hypothesized that the down-regulation of BDNF levels may contribute to the stress-induced damage observed in the hippocampus.
3.2 BDNF as a target of antidepressant action
Antidepressant therapies, including physical activity – a natural antidepressant, electroconvulsive seizures, and pharmacological antidepressants robustly increase the expression of this neurotrophin in the hippocampus and cortex (Nibuya et al 1995; Dias et al 2003; Farmer et al 2004). Additionally, the stress-mediated reduction in BDNF expression can be prevented with pre-administration of antidepressant therapy (Nibuya et al 1995). Distinct classes of antidepressants appear to regulate the BDNF gene through differential recruitment of individual BDNF promoters (Dias et al 2003). In addition, they appear to be capable of reversing the epigenetic shut down of the BDNF III and IV promoters caused by stress (Tsankova et al 2006). Indeed, overexpression of histone deacetylases, which would de-repress the epigenetic control of the BDNF promoters, is shown to upregulate hippocampal BDNF expression and exert antidepressant-like effects in animal models (Tsankova et al 2006). Furthermore, truncated trkB over-expressing mice (Saarelainen et al 2003), which show reduced BDNF signalling, are resistant to the effects of antidepressants in the forced swim model of depression. In addition, some of the structural changes in the hippocampus that ensue from sustained antidepressant treatment are mediated, at least in part, by BDNF. The mossy fiber sprouting seen in the hippocampus following chronic ECS is partially lost in BDNF heterozygote mice (Vaidya et al 1999). BDNF also appears to have a role in the increased survival, but not the enhancement of proliferation, of adult hippocampal progenitors observed following chronic antidepressant treatment (Sairanen et al 2005). Finally, the effects of the antidepressant desipramine in the forced swim test are lost in adult-onset forebrain knockouts of BDNF, thus indicating that BDNF plays a role in the behavioural actions of antidepressants (Monteggia et al 2004). Taken together, these reports clearly implicate BDNF in both the pathogenesis of depression and as a target/mediator of antidepressant action.
3.3 Pro- and anti-depressant activity of BDNF
BDNF is not only a potential target for antidepressant action, but it can, itself, act as an antidepressant. Infusion of exogenous BDNF into the ventricles, midbrain or the hippocampus of rodents has an antidepressant-like effect in several models of depression (Siuciak et al 1997; Shirayama et al 2002; Eisch et al 2003). In addition, BDNF receptor ‘trkB’ overexpressing mice exhibit antidepressant-like responses in the forced swim test (Koponen et al 2005). However, in a manner reminiscent of CREB, the spatial expression of BDNF is critical in its antidepressant-like activity. Whereas increased BDNF expression in the hippocampus appears to be antidepressant in nature (Shirayama et al 2004), increased BDNF activity in the ventral tegmental area (VTA) or nucleus accumbens (NAc) results in a pro-depressive state (Eisch et al 2003). Further confirming a region-specificity in the effects of BDNF on depression-associated behaviours, the blunting of BDNF action in the nucleus accumbens by over-expression of truncated BDNF TrkB receptors has an antidepressant-like activity (Eisch et al 2003). Thus, similar to the actions of CREB, BDNF shows a distinct region-specificity in its activity as a pro- or anti- depressant.
4. Region specificity in the action of CREB and BDNF
Depressive disorders and sustained stress exposure do involve a dysregulation of the CREB-BDNF cascade, while antidepressant therapies potentially recruit these molecules to mediate their effects. Interestingly, both CREB and BDNF have the capacity to exert both pro- or antidepressant-like effects, depending on their spatio-temporal regulation. For the purposes of this review we have focused upon two key neuro-anatomical foci that are strongly implicated in depression, namely the hippocampus and the ventral tegmental area – nucleus accumbens (VTA-NAc) reward circuitry (figure 2).
Figure 2.

Sagittal schematic of the adult rat brain. Shown are the two major circuits, regulated by the CREB-BDNF cascade that, have a relevance to depression. The hippocampus (Hip) projects to the hypothalamus (Hypo) via a multi-synaptic pathway. This circuit is thought have a critical inhibitory control over the hypothalamo-pituitary-adrenocortical stress response pathway. The other circuit where CREB and BDNF have been shown to perturb depression-related behaviours is the meso-limbic dopaminergic pathway. This pathway consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc) and modulate reward perception.
4.1 CREB and BDNF in the hippocampus
The hippocampus, as previously described, is a key limbic region whose structure and function is compromised in mood disorders. The hippocampus is also of particular relevance given it plays a critical role in inhibiting the HPA axis, which is the major mediator of systemic stress responses (Jacobson and Sapolsky 1991) (figure 2). In the hippocampus, increased activity of the CREB-BDNF cascade results in anti- depressive responses. Hippocampal overexpression of BDNF and CREB is capable of mimicking both the structural consequences of sustained antidepressant treatment as well as exerting antidepressant-like behavioural effects (Chen et al 2001a; Vaidya and Duman 2001; Shirayama et al 2004; Malberg and Blendy 2005). Indeed, activation of the cAMP-CREB cascade results in increased neurogenesis of dentate granule cell progenitors (Nakagawa et al 2002), and increased dendritic length and branching (Fujioka et al 2004). Additionally, transgenic mice that over-express the dominant negative form of CREB show reduced levels of hippocampal neurogenesis (Giachino et al 2005), mimicking the effects observed in animal models of depression. It is possible that CREB, a transcriptional activator of BDNF, recruits this neurotrophin to mediate its effects on structural plasticity (figure 3). BDNF, in addition to being a target of CREB, can itself recruit this particular transcription factor by activating the MAP kinase cascade (Arthur et al 2004) (figure 1), thus setting up a potential positive feed-back loop. Taken together, elevated CREB-BDNF, through their protective influences on vulnerable hippocampal neurons and their ability to directly promote structural reorganization, could result in repair of this region known to be damaged in depression. In addition, BDNF can alter neurotransmitter release (Tyler et al 2002) and itself elicit an activation of postsynaptic neurons (Kafitz et al 1999), and may thus have potential protective functional consequences on hippocampal circuitry known to be dysfunctional in depression. A direct consequence of enhanced hippocampal function would be a restoration of the inhibitory control exerted on the stress response pathway of the HPA axis. In addition, the well-established role of BDNF and CREB in hippocampal-dependent learning and memory (Tyler et al 2002; Mizuno and Giese 2005) may play a critical role in ameliorating the cognitive symptoms associated with depression.
Figure 3.
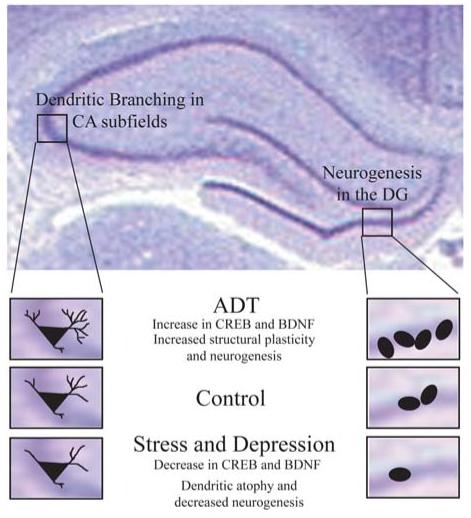
Role of CREB and BDNF in hippocampal structural plasticity in response to antidepressants and in animal models of depression. Shown are the hippocampal subfields, where dentate gyrus (DG) neurogenesis and CA subfield dendritic branching is regulated by both antidepressants and in depression models. While antidepressants enhance CREB-BDNF and promote neurogenesis and dendritic sprouting, animal models of depression are associated with a decline in CREB-BDNF, dendritic atrophy and decreased neurogenesis.
4.2 CREB and BDNF in the VTA-NAc pathway
The VTA-NAc dopaminergic pathway is the primary reward circuitry of the mammalian brain. Lack of pleasure seeking, anhedonia, is a characteristic symptom observed in all depressed patients, thus strongly implicating this circuitry in the pathophysiology of this disease (Nestler and Carlezon 2006). While dysfunction of the VTA-NAc circuit is thought to underlie the anhedonia associated with depression, antidepressants have been postulated to reverse this dysfunction. In stark contrast to the effects of CREB-BDNF in the hippocampus, activation of the CREB-BDNF cascade in the VTA-NAc pathway results in pro-depressive like behaviour (Malberg and Blendy 2005). Increased CREB activity in the NAc results in a phenotype characteristic of depression, including reduced reward experience or ‘anhedonia’ and increased immobility in the forced swim test, symbolizing behavioural ‘despair’ (Pliakas et al 2001). Additionally, increased BDNF activity in this pathway also results in a similar pro-depressive state (Eisch et al 2003). The effects of BDNF are thought to be mediated via up-regulating CREB expression, through activation of the MAPK/ERK pathway (Arthur et al 2004). Thus BDNF, upstream of CREB, would recruit specific target genes – with promoter CRE elements – that then affect the functioning of the VTA-NAc circuitry, resulting in a depression-like phenotype. CREB is known to positively modulate levels of dynorphin within the NAc (Carlezon et al 1998), and this upregulation of the endogenous opioid dynorphin could mediate the pro-depressive effects of CREB. Dynorphin is a natural ligand at the κ opioid receptors, these being present on the terminals of the meso-limbic dopaminergic neurons that originate from the VTA and innervate the NAc. Increased dynorphin, through the κ opioid receptor, negatively modulates dopaminergic transmission. Although at present it is unknown if CREB induction in the accumbens actually results in enhanced dynorphin release within the VTA, it has been hypothesized that such a change in the context of the VTA-nucleus accumbens pathway could result in dysphoria and lack of pleasure seeking (figure 4) (Nestler and Carlezon 2006). This raises the possibility that enhanced BDNF-CREB in the accumbens may, through a regulation of opioid signalling, result in an anhedonic state thus contributing to the pro-depressive effects, whereas an abrogation of BDNF-CREB signalling in this region could have beneficial consequences on behaviour and exert an antidepressant-like effect.
Figure 4.

The role of the BDNF-CREB cascade in the ventral tegmental area (VTA) – nucleus accumbens (NAc) pathway. Enhanced BDNF-CREB signalling in the NAc in animal models of stress and depression is associated with increased dynorphin expression. Dynorphin, through a feedback inhibitory loop, activates κ opioid receptors on VTA neurons and results in an inhibitory effect on the dopaminergic neurons projecting from the VTA to the NAc. Decreased activation of the VTA-NAc pathway is associated with the pro-depressive effects induced by overexpression of CREB-BDNF in this circuit.
5. CREB and BDNF mutant mice: Animal models for depression?
Although converging lines of evidence clearly point to a role for CREB and BDNF in depression-related behaviour, CREB/BDNF loss of function mutants do not exhibit classical symptoms observed in animal models of depression. The major caveat is that most of the studies done in mutant mice do not allow for either a spatial or temporal control over the silencing of CREB and BDNF expression, hence making it difficult to dissect out both the developmental effects as well as the region-specific consequences of CREB/BDNF loss upon the depression related neuro-circuitry. However, there do appear to be specific behavioural changes in CREB deficient mice and BDNF heterozygote mice, and careful analysis of these animals may provide insights into the mood modulatory effects of these molecules.
5.1 CREB mutant mice
CREB-deficient mice have been reported to exhibit an antidepressant response in the footshock and tail suspension tests (Valverde et al 2004). Given that antidepressants are believed to recruit CREB to mediate their effects, it is contrary to expectations for CREB deficient mice to display an antidepressant-like phenotype. However, this behaviour of CREB-deficient mice mimics that seen with an abrogation of CREB function, using dominant negative CREB (mCREB) overexpression, in the nucleus accumbens of rats (Newton et al 2002). The CREB-deficient mice show reduced CREB throughout the brain, and it appears that lowered CREB in the NAc is able to exert an antidepressant-like phenotype overriding any pro-depressive consequences of lowered CREB expression. Future studies clearly require a controlled spatio-temporal targeted disruption of CREB to reveal the role of this molecule in distinct neuronal circuits relevant to mood modulation.
5.2 BDNF mutant mice
Studies using mice with perturbations in BDNF signalling indicate a complex behavioural phenotype. While neither trkB-mutant mice (Saarelainen et al 2000) nor BDNF heterozygotes (+/−) (Lyons et al 1999) display classical depression-like behaviours, trkB overexpressing mice clearly exhibit antidepressant-like behaviour in the forced swim test (Koponen et al 2005). A recent study taking advantage of conditional BDNF knockouts, in which the BDNF loss of function is restricted to the adult forebrain, reveals that there is a gender-specific propensity to develop depressive behaviours, with the female mice showing an enhanced incidence of depression-like behaviours (Monteggia et al 2006). This is particularly interesting given the fact that the occurrence of depression is higher in women (Noble 2005), and use of this model can be made to understand the sexual dimorphism displayed with respect to the consequences of reduced BDNF signalling. What is clear is that a critical future direction for studies employing genetic perturbations of BDNF is the requirement to target the perturbation of BDNF to specific neuronal regions and at particular ages. This line of scientific enquiry is likely to provide important insights into the role of BDNF in depression models.
6. Clinical studies
While a robust body of literature exists suggesting a role for CREB and BDNF in depression as well as in antidepressant action, a majority of the work is restricted to animal models. However, building evidence from clinical studies also supports the idea that CREB and BDNF are involved in the pathogenesis of depression and in the mechanism of antidepressant action.
Postmortem studies show a decrease in CREB expression in the cerebral cortex of patients not undergoing antidepressant therapy, while CREB immunoreactivity increases in response to antidepressant treatment (Dowlatshahi et al 1998). Similarly, BDNF immunoreactivity is considerably decreased in the brains of treatment-naive patients, while antidepressant therapy increases BDNF expression to levels resembling that of healthy control individuals (Chen et al 2001b). Serum BDNF, which is believed to be reflective of BDNF levels in the brain, also shows a similar trend, with decreased levels of serum BDNF seen in patients suffering from depression. This decline in serum BDNF levels is negatively correlated with the MADRS (Montgomery-Ashberg Depression Rating Scale), i.e. with the severity of the depressive symptoms (Karege et al 2002).
Insights from genetic linkage studies provide further support for a role of CREB and BDNF in depressive disorders. The CREB1 locus is thought to have an epistatic influence in defining susceptibility to recurrent, early-onset, major depression (Zubenko et al 2003). The BDNF locus has been implicated in altered vulnerability for bipolar disorder (Neves-Pereira et al 2002) and childhood depression (Kaufman et al 2006). In addition, a single nucleotide polymorphism, val66met, in the BDNF coding region has a strong association with childhood-onset mood disorder (Strauss et al 2005) and geriatric depression (Hwang et al 2005). The val66met polymorphism is correlated with a decreased hippocampal volume and abnormal hippocampal activation, which may underlie an enhanced vulnerability to the development of depression (Bueller et al 2005). Furthermore, cell biological studies have revealed that the met-BDNF variant shows disruption of activity-dependent release (Egan et al 2003), raising the possibility that the met allele may result in deficits in the ability to mount BDNF-induced remodeling of neuronal circuits. In support of such a hypothesis, a recent study indicates that healthy individuals with the met allele exhibit reduced experience-dependent structural plasticity in the cortex (Kleim et al 2006). Taken together, these studies suggest that BDNF may be critical in mediating adaptive-responses to environmental changes such as stress and the inability to exhibit such an adaptive response could substantially enhance the susceptibility to stress-related disorders like depression.
7. Conclusions
The pathways and signalling cascades dysregulated in depression are undoubtedly far more complex than previously suspected. While it would be simplistic to consider the BDNF-CREB cascade as the only intracellular pathway involved, what emerges is that CREB and BDNF are likely to be key molecules involved in mood regulation. The current state of knowledge on the role of the CREB-BDNF cascade in depression provides strong impetus to further understand the regulation and action of CREB and BDNF in depression and following antidepressant therapy. In addition, CREB and BDNF may serve as potential therapeutic targets for the development of new drugs that modulate mood. However, given the region specificity of the effects of the BDNF-CREB cascade on behaviour, a critical requirement would be to identify upstream signalling cascades that recruit these molecules in a region specific fashion. A combination of both preclinical and clinical studies are required to further explore the role of the CREB-BDNF cascade in depression, as this could shed new light on the pathogenesis and effective management of depressive disorders.
Abbreviations used
- ATF
Activating transcription factor
- BDNF
brain-derived neurotrophic factor
- CRE
cAMP response element
- CREB
cyclic AMP response element binding protein
- CREM
cAMP response element modulator
- ECS
electroconvulsive seizure therapy
- HPA
hypothalmo-pituitary-adrenocortical
- NPY
neuropeptide Y
- PFC
prefrontal cortex
- PKA
protein kinase A
- VTA
ventral tegmental area
Footnotes
Corresponding editor: Vidyanand Nanjundiah
References
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Frick LR, Silberman DM, Palumbo ML, Genaro AM, Frasch AC. Regulation of Hippocampal Gene Expression is Conserved in Two Species Subjected to Different Stressors and Antidepressant Treatments. Biol. Psych. 2006;59:244–251. doi: 10.1016/j.biopsych.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J. Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM. MRI-based measurement of hippocampal volume in patients with combat related posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley M. Understanding antidepressants. Martin Dunitz; Londona: 2000. [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carroll, Curtis GC, Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch. Gen. Psychiatry. 1976;33:1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- Cesare DD, Fimia GM, Sassone-Corsi P. Signalling routes to CREM and CREB: plasticity in transcriptional activation. TIBS. 1999;24:281–285. doi: 10.1016/s0968-0004(99)01414-0. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol. Psychiatry. 2001a;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry. 2001b;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioural or endocrine responses to antidepressant drugs. J. Neurosci. 2002;22:3236–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA. 2001;98:12796–12780. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagestad G, Kuipers SD, Messaoudi E, Bramham CR. Chronic fluoxetine induces region-specific changes in translation factor eIF4E and eEF2 activity in the rat brain. Eur. J. Neurosci. 2006;23:2814–2818. doi: 10.1111/j.1460-9568.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased temporal cortex CREB concentrations and anti-depressant treatment in major depression. Lancet. 1998;352:1754–755. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature (Lonodon) 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signalling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol. Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signalling facilitates the morphological maturation of newborn neurons in adult hippocampus. J. Neurosci. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J. Neurochem. 2005;93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gueorguiev VD, Cheng SY, Sabban EL. Prolonged activation of cAMP-response element-binding protein and ATF-2 needed for nicotine-triggered elevation of tyrosine hydroxylase gene transcription in PC12 cells. J. Biol. Chem. 2006;281:10188–10195. doi: 10.1074/jbc.M513806200. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature (London) 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′,5′-monophosphate-regulatory element binding activity. Mol. Endocrinol. 1997;11:2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer MM, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature (London) 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol. Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.10.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behaviour, CRE-binding activity and BDNF level in learned helplessness rats. Eur. J. Pharmacol. 2004;498:135–142. doi: 10.1016/j.ejphar.2004.07.084. [DOI] [PubMed] [Google Scholar]
- Itoi K, Horiba N, Tozawa F, Sakai Y, Sakai K, Abe K, Demura H, Suda T. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137:2389–2396. doi: 10.1210/endo.137.6.8641191. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrinol. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cellular Signalling. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol. Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006 doi: 10.1038/nn1699. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Koponen E, Rantamaki T, Voikar V, Saarelainen T, MacDonald E, Castren E. Enhanced BDNF signalling is associated with an antidepressant-like behavioural response and changes in brain monoamines. Cell. Mol. Neurobiol. 2005;25:973–980. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. TIPS. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Masquilier D, Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J. Biol. Chem. 1992;267:22460–22466. [PubMed] [Google Scholar]
- Mizuno K, Giese KP. Hippocampus-dependent memory formation: do memory type-specific mechanisms exist? J. Pharmacol. Sci. 2005;98:191–197. doi: 10.1254/jphs.crj05005x. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA. 2004;20:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-Derived Neurotrophic Factor Conditional Knockouts Show Gender Differences in Depression-Related Behaviours. Biol. Psych. 2006 doi: 10.1016/j.biopsych.2006.03.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annual. Rev. Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim J, Lee R, Malberg JE, Chen J, Steffen C, Zhang Y, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.09.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Duman RS. Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J. Neurochem. 1989;53:1644–1647. doi: 10.1111/j.1471-4159.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J. Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am. J. Hum. Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble RE. Depression in women. Metabolism. 2005;54:49–52. doi: 10.1016/j.metabol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol. Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricles of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwan BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;271:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirviö J, Castrén E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioural effects. J. Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioural effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shelton RC. Cellular mechanisms in the vulnerability to depression and response to antidepressants. Psychiatr. Clin. North. Am. 2000;23:713–729. doi: 10.1016/s0193-953x(05)70193-3. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioural models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Weigand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kventnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol. Biochem. Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Strauss J, Barr CL, George CJ, Devlin B, Vetro A, Kiss E, Baji I, King N, Shaikh S, Lanktree M, Kovacs M, Kennedy JL. Brain-derived neurotrophic factor variants are associated with childhood-onset mood disorder: confirmation in a Hungarian sample. Mol. Psych. 2005;10:861–867. doi: 10.1038/sj.mp.4001685. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J. Biol. Chem. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signalling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol. Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storn D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J. Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct the tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neuro-trophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signalling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Duman RS. Depression – emerging insights from neurobiology. Br. Med. Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S, Gass P, Kretz O, Mitchell JM, Schutz G, Maldonado R. Modulation of anxiety-like behaviour and morphine dependence in CREB-deficient mice. Neuropsychopharmacology. 2004;29:1122–1133. doi: 10.1038/sj.npp.1300416. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioural models of depression and anxiety. Biol. Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwan BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992a;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwan BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur. J. Pharmacol. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Maher B, Hughes HB, 3rd, Zubenko WN, Stiffler JS, Kaplan BB, Marazita ML. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;123:1–18. doi: 10.1002/ajmg.b.20073. [DOI] [PubMed] [Google Scholar]


