Abstract
Electroconvulsive seizure (ECS) induces structural remodelling in the adult mammalian brain, including an increase in adult hippocampal neurogenesis. The molecular mechanisms that underlie this increase in the proliferation of adult hippocampal progenitors are at present not well understood. We hypothesized that ECS may recruit the Sonic hedgehog (Shh) pathway to mediate its effects on adult hippocampal neurogenesis, as Shh is known to enhance the proliferation of neuronal progenitors and is expressed in the adult basal forebrain, a region that sends robust projections to the hippocampus. Here we demonstrate that the ECS-induced increase in proliferation of adult hippocampal progenitors was completely blocked in animals treated with cyclopamine, a pharmacological inhibitor of Shh signalling. Our results suggest that both acute and chronic ECS enhance Shh signalling in the adult hippocampus, as we observed a robust upregulation of Patched (Ptc) mRNA, a component of the Shh receptor complex and a downstream transcriptional target of Shh signalling. This increase was rapid and restricted to the dentate gyrus, where the adult hippocampal progenitors reside. In addition, both acute and chronic ECS decreased Smoothened (Smo) mRNA, the other component of the Shh receptor complex, selectively within the dentate gyrus. However, ECS did not appear to influence Shh expression within the basal forebrain, the site from which it has been suggested to be anterogradely transported to the hippocampus. Together, our findings demonstrate that ECS regulates the Shh signalling cascade and indicate that the Shh pathway may be an important mechanism through which ECS enhances adult hippocampal neurogenesis.
Keywords: adult neuronal progenitors, cyclopamine, dentate gyrus, Patched, Smoothened
Introduction
Electroconvulsive seizure (ECS) serves as a model for activity-dependent neural plasticity in the adult brain. Amongst the structural plasticity that results from ECS is enhanced adult hippocampal neurogenesis, mossy fibre sprouting and synaptic reorganization (Vaidya et al., 1999; Madsen et al., 2000; Malberg et al., 2000). This remodelling is thought to represent adaptive changes, and not injury-induced reorganization, as it occurs in the absence of neuronal death (Dam & Dam, 1986). However, the mechanisms that underlie activity-dependent structural plasticity in the adult brain are poorly elucidated. Neurotrophic and angiogenic molecules have been suggested as putative downstream candidates that contribute to the ECS-induced increase in hippocampal neurogenesis (Newton et al., 2003; Altar et al., 2004), although at present there is a lack of direct evidence to support their role. We hypothesized that a key developmental signalling molecule, Sonic hedgehog (Shh), may be a mediator of the ECS-induced enhancement of adult hippocampal neurogenesis.
During development Shh, a secreted protein, has multiple effects on both neuronal and glial precursors, these include regulation of their proliferation, survival and fate determination (Ingham & McMahon, 2001). Shh has a mitogenic effect on embryonic neuronal precursors (Rowitch et al., 1999; Palma & Ruiz-i-Altaba, 2004), and is involved in the maintenance of telencephalic stem cell niches (Machold et al., 2003). Shh continues to be expressed within the vertical limb of the diagonal band (VDB) in the basal forebrain region of the adult rat (Traiffort et al., 1999), from where it has been suggested to be anterogradely transported to the hippocampus. Intriguingly, the Shh receptor components, Patched (Ptc) and Smoothened (Smo), co-localize within the dentate gyrus, a target of the VDB (Traiffort et al., 1998). The presence of Ptc and Smo within adult neurogenic niches, namely the subgranular zone (SGZ) in the hippocampal dentate gyrus subfield and the subventricular zone (SVZ) lining the lateral ventricles, support a role for Shh in adult neurogenesis (Traiffort et al., 1998). However, few studies have addressed the role of Shh in the mature nervous system. Recent reports indicate that adult hippocampal progenitors express both Ptc and Smo, and Shh enhances their proliferation (Lai et al., 2003; Palma et al., 2005). In addition, injury-induced regeneration of the adult rat facial nerve has also been shown to involve the Shh pathway (Akazawa et al., 2004). Taken together, these studies suggest that Shh continues to have an important role beyond development, and may be reutilized to regulate structural plasticity in the adult brain.
In the present study we addressed whether Shh is involved in the ECS-mediated upregulation of adult hippocampal neurogenesis. We demonstrate that Shh signalling is required for the increase in adult hippocampal progenitor proliferation following ECS, and that ECS differentially regulates the expression of both Ptc and Smo mRNA, selectively in the dentate gyrus subfield of the hippocampus. Taken together, our studies indicate that Shh signalling is regulated by ECS, and suggest that it may play an important role in the ECS-mediated regulation of structural plasticity in the adult brain.
Materials and methods
Animal treatment paradigms
Male Sprague-Dawley rats (200–250 g) bred in our animal-breeding colony were used in all experiments. Animals were group-housed (three to four per cage) and maintained on a controlled 12 h light : 12 h dark cycle with access to food and water ad libitum. All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the TIFR Institutional Animal Ethics Committee. Animals received bilateral ECS treatment via spring-loaded ear clip electrodes (ECT unit, UGO Basile, Comerio, Italy) (frequency: 100 pulses/s; pulse width: 0.9 ms; pulse duration: 0.5 s; current: 70 mA) or sham treatment (application of ear clip electrodes without electrical stimulation). The ECS treatment resulted in grand mal seizures with tonic and clonic convulsive components. For the acute ECS study, animals (n = 9 per group) received a single sham or ECS treatment, and were killed 2 h later. In the time-course experiment, animals (n = 3 per group) were killed 4 h, 6 h and 24 h after acute ECS treatment, and each time point had a separate sham group. For the chronic ECS paradigm, animals (n = 8 per group) received sham or ECS treatment once daily for 10 consecutive days and were killed 2 h after the final ECS treatment. One group of animals was killed 21 h following the final ECS treatment (n = 4 per group). After decapitation, brains were rapidly removed and frozen on dry ice and stored at −70 °C prior to cryostat sectioning and in situ hybridization analysis. In addition, some animals were subjected to acute or chronic ECS/sham treatment and were killed 2 h following treatment, and further processed for either immunohistochemistry (n = 4 per group) or Western blotting (n = 4 per group). For the immunohistochemistry experiments, animals received an overdose of sodium pentobarbital (100 mg/kg, Sigma, MO, USA) and were killed through transcardial perfusion with 4% paraformaldehyde. Coronal sections (50 μm) were generated using a vibratome (The Vibratome Company, MO, USA) and were processed for Shh immunohistochemistry. For the Western blotting experiments, animals were rapidly decapitated, brains were removed and the hippocampi and the VDB were microdissected in sterile physiological saline prior to being frozen in liquid nitrogen and stored at −70 °C.
To study the effect of the Shh antagonist, cyclopamine (Toronto Research Chemicals, Ontario, Canada), on the ECS-mediated increase in hippocampal neurogenesis, animals were divided into four experimental groups: (i) Vehicle + Sham; (ii) Vehicle + ECS; (iii) Cyclopamine + Sham; and (iv) Cyclopamine + ECS (n = 5 per group). Cyclopamine is a naturally occurring plant alkaloid that has been demonstrated to specifically block Shh signalling by binding to the Shh receptor Smo (Cooper et al., 1998; Incardona et al., 1998; Taipale et al., 2000; Chen et al., 2002). This dose of cyclopamine was selected to be within the range used in previous studies (Lai et al., 2003; Palma et al., 2005). Rats were anaesthetized using ketamine (50 mg/kg, Sigma) and xylazine (20 mg/kg, Sigma), and were placed in a Stoelting stereotaxic Instrument (Stoelting, Illinois, USA). All animals received a unilateral intracerebroventricular (i.c.v.) implant with a 28-gauge osmotic minipump cannula at stereotaxic coordinates −0.8 mm posterior from Bregma, −1.4 mm medio-lateral and −4.0 mm ventral to skull surface (Paxinos & Watson, 1998). Prior to implantation, the osmotic minipumps (Alzet Model, 2001, 7 day pump, 1 μL/h; CA, USA) were filled with either vehicle [45% 2-hydroxypropyl-β-cyclodextrin (HBC) in sterile phosphate-buffered saline, Sigma] or cyclopamine [1 mg/mL (w/v) in 45% HBC], and were then connected to polyethylene tubing (Brain infusion Kit, Alzet) via the flow moderator and sealed with Loctite glue. The minipumps were primed by placing them overnight in sterile saline at 37 °C. Following cannula implantation, the osmotic minipumps were attached to the cannula using the polyethylene tubing and sealed with Loctite. The minipumps and tubing were placed subcutaneously between the scapulae and the animals were allowed to recover from the surgery for 2 days. On the third day following surgery, animals from Groups 2 (Vehicle + ECS) and 4 (Cyclopamine + ECS) received ECS treatment once daily for two consecutive days, whereas Groups 1 (Vehicle + Sham) and 3 (Cyclopamine + Sham) received sham treatment. All animals were administered the mitotic marker bromodeoxyuridine (BrdU, 100 mg/kg; Sigma) 45 min after the final ECS/sham treatment, and were killed 2 h later. Animals received an overdose of sodium pentobarbital (100 mg/kg; Sigma) and were killed through transcardial perfusion with 4% paraformaldehyde. Serial coronal sections (50 μm) through the rostro-caudal extent of the hippocampus were generated using a vibratome and were further processed for immunohistochemistry. Some sections from each animal were processed for Cresyl violet staining to determine the integrity of brain parenchyma around the infusion site and to confirm correct cannula placement.
For the receptor antagonist experiments, the drugs or vehicle were administered i.p. 30 min prior to sham or acute ECS treatment. The N-methyl-d-aspartate (NMDA) receptor antagonist 5-methyl-10,11-dihydro-5H-dibenzo (a,d) cyclohepten-5,10-imine maleate (MK-801; 3 mg/kg; Sigma) and the alpha-amino-3-hydroxy-5-methyl-isoxozole propionic acid/kainate (AMPA/KA) receptor antagonist 6,7-dinitro-quinoxaline-2,3-dione (DNQX; 12.5 mg/kg; Sigma) were administered in physiological saline. The treatment groups were as follows: Experiment 1: Vehicle + Sham, Vehicle + Acute ECS, MK-801 + Sham, MK-801 + Acute ECS (n = 4 per group); Experiment 2: Vehicle + Sham, Vehicle + Acute ECS, DNQX + Sham, DNQX + Acute ECS (n = 3 per group). All animals were killed 2 h after the sham/ECS treatment and brains were rapidly removed, frozen on dry ice and stored at −70 °C prior to cryostat sectioning and in situ hybridization analysis.
In situ hybridization
In situ hybridization was carried out as previously described (Nibuya et al., 1995; Dias et al., 2003). In brief, 14-μm-thick coronal sections were cut on the cryostat and thaw mounted onto ribonuclease-free Probe-on plus slides (Electron Microscopy Services, USA). Slides were fixed in 4% paraformaldehyde, acetylated and dehydrated prior to storage at −70 °C. Rat Sonic hedgehog (Shh), Smoothened (Smo) and Patched (Ptc) cRNA probes were generated from transcription-competent plasmids (pGEM-4Z) provided by Dr Martial Ruat (Centre National de la Recherche Scientifique, France) containing Shh (678–1356 bp), Ptc (1765–3587 bp) or Smo (411–2016 bp). All cRNA probes were transcribed using 35S-labelled UTP (Amersham). Slides were incubated for 20-24 h at 60 °C with hybridization buffer [50% formamide, 1 × standard sodium citrate (SSC), 25 × Denhardt’s solution, 40 mm dithiothreitol, 150 μg/mL yeast tRNA, 10% dextran sulphate, 400 μg/mL salmon sperm DNA] containing 35S-UTP-labelled riboprobes at a concentration of 1 × 106 c.p.m./150 μL per slide. After hybridization, the slides were washed in 2 × SSC for 10 min, treated with RNase A (20 μg/mL) at 45 °C for 30 min, followed by stringent washes in decreasing concentrations of SSC, with a final wash in 0.5 × SSC at 60 °C. Slides were air-dried and exposed to Hyperfilm β-max (Amersham) for 2–3 weeks. In addition, some slides were dipped in emulsion (LM-1, RPN40, Amersham) and developed 8 weeks later. Sense riboprobes for Shh, Ptc or Smo, or a ribonuclease (40 μg/mL at 37 °C for 30 min) prehybridization wash did not yield significant hybridization (data not shown), confirming the specificity of the signal observed with these antisense riboprobes.
Immunohistochemistry and immunofluorescence
For BrdU immunohistochemistry, coronal free-floating sections (50 μm), generated using a vibratome, were processed for BrdU immunohistochemistry as described previously (Kulkarni et al., 2002). In brief, following DNA denaturation and acid hydrolysis, sections were incubated overnight with mouse anti-BrdU antibody (1 : 500, Boehringer Mannheim, USA) and then exposed to secondary antibody (biotinylated antimouse IgG, 1 : 500, Vector Laboratories, CA, USA). Signal amplification was carried out with an avidin–biotin complex (Vector Laboratories) and was detected with diaminobenzidine (Sigma). For Shh immunohistochemistry, 50-μm-thick coronal sections containing the VDB region were further processed. Sections were washed in blocking buffer (0.1 m phosphate buffer containing 10% horse serum) and incubated with mouse anti-Shh antibody (5E1, 1 : 250; Developmental Studies Hybridoma Bank, California, USA) for 8 h at 37 °C. Sections were then exposed to biotinylated anti-mouse IgG (1 : 500; Vector Laboratories) followed by streptavidin fluorescein (Amersham). Sections were then mounted onto slides with Fluoromount (Sigma) and imaged using an epifluorescent Zeiss (Axioskop 2 plus) microscope. Experiments with the appropriate isotype control, and in the absence of primary antibody, did not yield any signal indicating the specificity of Shh and BrdU immunohistochemistry.
Double immunohistochemistry and in situ hybridization
Free-floating sections were subjected to fixation and acetylation, prior to being placed in hybridization buffer containing digoxigenin-labelled riboprobes for Smo or Ptc. Sections were then washed with increasing stringency before exposure to an alkaline phosphatase-tagged anti-digoxigenin antibody (Roche). NBT/BCIP (4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate; Roche) was used as the alkaline phosphatase substrate, and the colour reaction was fixed prior to commencement of the immunohistochemistry protocol. Sections were blocked in 10% horse serum in 0.1 m phosphate buffer containing 3% Triton X-100 prior to incubation with an anti-doublecortin antibody (1 : 250; Santa Cruz) overnight. Sections were washed and then exposed to secondary antibody (biotinylated anti-goat IgG, 1 : 250, Vector Laboratories). Signal amplification was carried out with an avidin–biotin complex (Vector Laboratories) and was detected with diaminobenzidine (Sigma).
Western blot analysis
Frozen brain tissue from the hippocampus and VDB was Dounce homogenized in 0.1 m phosphate buffer containing the protease inhibitors phenyl methyl sulphonyl fluoride (725 μg/mL; Amersham), pepstatin A (12.5 μg/mL; Amersham) and leupeptin (31.25 μg/mL; USB, OH, USA). Brain homogenates were incubated on ice and then centrifuged at 13,000 g for 10 min at 4 °C in a Heraeus Biofuge Stratos centrifuge, and supernatants were processed further for Western blotting. Aliquots of brain extracts containing 25 μg total protein were subjected to electrophoresis on a 14% sodium dodecyl sulphate–polyacrylamide gel.
Purified mouse N-terminal Shh protein was used in the Western blotting experiments as a positive control (R & D Systems, USA). Proteins in gel were then electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane (Amersham) and subjected to immunoblot analysis. Following electrophoretic transfer, the PVDF membrane was blocked with 5% non-fat dry milk in buffer and then probed with a rat anti-Shh IgG antibody (MAB 464; 1 : 10,000; R & D Systems) overnight at 4 °C. This antibody is raised against the N-terminal peptide (amino acid 25–198) of the mouse Shh protein. The membranes were then incubated with peroxidase-conjugated antirat antibody (1 : 10,000; Amersham) and developed with an enhanced chemiluminescence system (Amersham) followed by exposure to Hyperfilm-ECL (Amersham) for 2 min. Immunoblots were stained with Ponceau (Sigma) to confirm equal loading and transfer of proteins.
Cell counting analysis
Quantification of the BrdU-positive cells was carried out on coded sections by an experimenter blind to the study code. BrdU-positive cells within the SGZ and granule cell layer (GCL) of the dentate gyrus were counted. Sections were counterstained with cresyl violet (0.1%, Sigma) to aid visualization of the GCL of the dentate gyrus. The average number of BrdU-positive cells within the SGZ and GCL of the dentate gyrus per section was determined in each animal (12 sections/animal) from all treatment conditions. For quantification of the SVZ progenitors, every third striatal section was processed for BrdU immunohistochemistry. Using a stereology-based protocol (Sailor et al., 2003), BrdU-positive cells present on the lateral face of the lateral ventricle were counted (six sections/animal; Bregma 1.6–0.2: n = 3–4 per group). The area in which the cells were counted was traced using a NIH image 1.62 software (Scion image, USA) to yield the number of BrdU-positive cells/mm2.
Autoradiogram quantification
Levels of Shh, Ptc and Smo transcripts were analysed using the Macintosh-based Scion Image software (Scion, USA). To correct for non-linearity, 14C standards were used for calibration. For Shh mRNA, we analysed the expression in both the horizontal and vertical limbs of the diagonal band (VDB) as well as the neocortex. We have shown the data for only the VDB as it does not differ from data we obtained in the horizontal limb of the diagonal band. For Ptc and Smo mRNA, we quantified expression in the dentate gyrus GCL and in the SVZ. An equivalent area was outlined for each sample, and optical density measurements from both sides of four individual sections from each animal were analysed, from which the mean value was calculated. Levels of Shh immunoreactivity were quantified by outlining the Shh band in the autoradiogram and by determining the optical density using densitometric analysis (Scion image 1.62).
Statistical analysis
Results were subjected to statistical analysis using the program Prism (Graphpad, USA). Experiments with two groups were analysed for differences using the unpaired Student’s t-test, with significance determined at P < 0.05. Experiments with four groups were subjected to statistical analyses using two-way ANOVA followed by the Bonferroni post-hoc test, with significance determined at P-values < 0.05. Data are expressed as means ± SEM, with percentages indicating percentage of Sham results.
Results
The Shh signalling inhibitor cyclopamine prevents the ECS-mediated increase in the proliferation of dentate granule cell progenitors
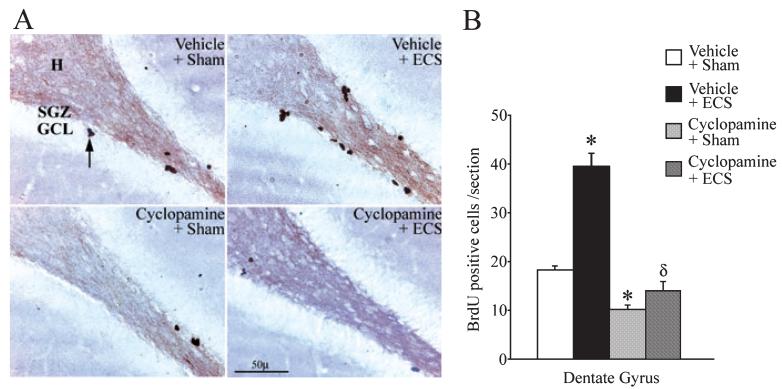
To examine whether the inhibition of Shh signalling would alter the ability of ECS to enhance the proliferation of dentate granule cell progenitors in the adult rat hippocampus, animals received cyclopamine (1 μg/μL per hour) delivered i.c.v. via osmotic minipumps prior to ECS administration. Cyclopamine is a selective pharmacological inhibitor of Shh signalling (Berman et al., 2002), which is thought to function through direct binding to Smo, the signalling component of the Shh receptor complex (Chen et al., 2002). The effects of ECS on the proliferation of adult dentate granule cell progenitors were assessed using the mitotic marker BrdU. Cyclopamine completely prevented the ECS-mediated increase in the number of BrdU-positive cells per section within the dentate gyrus (Fig. 1). BrdU-positive cells were found to be localized to the SGZ at the border of the hilus and the GCL (Fig. 1A), and showed a significant induction (twofold) following ECS treatment, which was absent in animals that received cyclopamine. In agreement with a previous study (Lai et al., 2003), we also observed a significant decline (~ 45%) in the number of BrdU-positive cells per section in animals that received only cyclopamine as compared with the vehicle-treated controls (Fig. 1B). ECS administration to cyclopamine-treated animals failed to induce an increase in the number of BrdU-positive cells in the dentate gyrus, indicating that inhibition of Shh signalling blocks the effects of ECS on dentate granule cell progenitor proliferation.
Fig. 1.
Cyclopamine prevents the electroconvulsive seizure-mediated increase in the proliferation of dentate granule cell progenitors. Rats received i.c.v. administration of either vehicle or cyclopamine through osmotic minipumps prior to the administration of sham or electroconvulsive seizure (ECS) treatment, as described in Materials and methods. The number of proliferating progenitors within the subgranular zone (SGZ)/granule cell layer (GCL) of the dentate gyrus was determined using the mitotic marker BrdU. ECS administration resulted in a significant increase in the number of BrdU-positive cells per section within the dentate gyrus of the vehicle + ECS group (B; *P < 0.001 as compared with the Vehicle + Sham treatment). Cyclopamine pretreatment significantly reduced the number of BrdU-positive cells per section in the Cyclopamine + Sham group (B; *P < 0.001 as compared with the Vehicle + Sham treatment group). ECS administration to animals pretreated with cyclopamine (Cyclopamine + ECS group; B) did not significantly increase the number of BrdU-positive cells within the dentate gyrus as compared with the Cyclopamine + Sham treatment, indicating that cyclopamine prevents the ability of ECS to enhance the proliferation of dentate granule cell progenitors. The Cyclopamine + ECS group had significantly decreased numbers of BrdU-positive cells per section as compared with the Vehicle + ECS group (δP < 0.001; B). Shown are representative images of the dentate gyrus region from Vehicle + Sham, Vehicle + ECS, Cyclopamine + Sham and Cyclopamine + ECS groups (A). BrdU-positive cells (arrow A) were observed in the SGZ, at the border of the hilus (H) and the GCL. The results are expressed as the mean ± SEM (n = 5 per group) number of BrdU-positive cells per section in the dentate gyrus. *P < 0.001 indicates significantly different from Vehicle + Sham; δP < 0.001 indicates significantly different from Vehicle + ECS (ANOVA and Bonferroni post-hoc test).
We also examined the number of BrdU-positive cells/mm2 observed within the SVZ, lining the lateral ventricles, the other major neurogenic region in the adult rat brain. We did not observe any effect of either ECS, cyclopamine or an interaction between these treatments on the number of BrdU-positive cells/mm2 in the SVZ (Vehicle + Sham, 5546 ± 269.1 cells/mm2; Vehicle + ECS, 5195 ± 287.54 cells/mm2; Cyclopamine + Sham, 6276 ± 449.92 cells/mm2; Cyclopamine + ECS, 6193 ± 615.72 cells/mm2, n = 3-4 per group, P > 0.05). Treatment with cyclopamine did not have any effect on the duration or the pattern of seizures induced following ECS treatment, as all animals in the ECS treatment groups exhibited grand mal seizures with tonic-clonic convulsions. Animals were checked for cannula placement to confirm correct localization and subjected to Cresyl violet staining to ensure that there was no tissue damage (data not shown).
Influence of acute and chronic ECS administration on Shh mRNA and protein expression in the adult rat brain
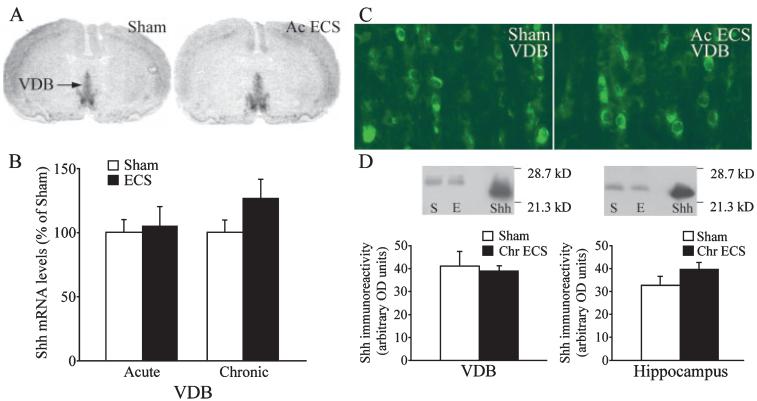
The effects of acute and chronic ECS treatment on the expression of Shh mRNA were determined using in situ hybridization analysis. Shh mRNA has been reported to be expressed in the basal forebrain region of the adult rat brain in the VDB and also within layer V of neocortex (Traiffort et al., 1999, 2001). Our results confirm this basal expression, and indicate that neither acute nor chronic ECS appear to significantly alter the mRNA levels of Shh in either the VDB (Fig. 2A and B) or the neocortex (Acute ECS experiment: Sham, 100 ± 6.76%; Acute ECS, 95.07 ± 18.9%. Chronic ECS experiment: Sham, 100 ± 3.71%; Chronic ECS, 113.13 ± 15.3%; n = 3-4 per group, P > 0.05). We did not detect any Shh mRNA expression within the hippocampus of either sham- or ECS-treated animals (data not shown). To address the influence of acute ECS treatment on the expression of Shh protein we used Shh immunofluorescence and Western blot analysis. Using Shh immunofluorescence, we did not detect any change in the number of Shh-immunopositive cells observed within the VDB (Fig. 2C) following ECS treatment. The Shh immunoreactivity in layer V of neocortex was also unaltered following ECS treatment, and no Shh immunopositive cells were observed in the hippocampus (data not shown). However, it has been suggested that Shh may be transported to the hippocampus from the VDB via the fornix pathway (Traiffort et al., 2001; Lai et al., 2003). Therefore, we quantitatively analysed the expression of Shh protein in the VDB and hippocampus following acute and chronic ECS. Our Western blotting experiments did not reveal any change in the levels of Shh immunoreactivity in either the VDB or the hippocampus (Fig. 2D).
Fig. 2.
Influence of acute and chronic electroconvulsive seizure (ECS) administration on the expression of Sonic hedgehog (Shh) mRNA within the vertical limb of the diagonal band (VDB), and Shh protein within the VDB and hippocampus. Rats received either acute or chronic ECS/sham treatment as described in Materials and methods, and the levels of Shh mRNA in the VDB were determined using in situ hybridization analysis and quantitative densitometry. Representative autoradiograms from a sham and acute ECS-treated animal are shown with an arrow indicating the VDB (A). Levels of Shh mRNA in the VDB were not altered by either acute or chronic ECS treatment (B). The results are expressed as the mean ± SEM percentage of sham (Acute ECS experiment n = 9 per group; Chronic ECS experiment, n = 8 per group; Student’s t-test). Shh immunoreactivity was observed in cell bodies within the VDB, and neither the number of immunopositive cells nor the intensity of staining appeared to be altered following either acute or chronic ECS treatment (C; n = 4 per group). Shown are representative fluorescence photomicrographs of Shh immunoreactivity in the VDB from a sham and acute ECS-treated animal (C). Representative immunoblots (D) show Shh levels in the VDB and hippocampus after sham (S) or chronic ECS (E) treatment, Shh represents the positive control of purified N-terminal Shh protein. Neither acute nor chronic ECS was found to alter the expression of Shh protein levels in the VDB or the hippocampus. Levels of Shh immunoreactivity following sham or chronic ECS treatment are represented as arbitrary OD units and results are expressed as mean + SEM (n = 4 per group, P < 0.05, Student’s t-test).
Acute and chronic ECS upregulate the expression of Ptc mRNA and downregulate the expression of Smo mRNA in the dentate gyrus
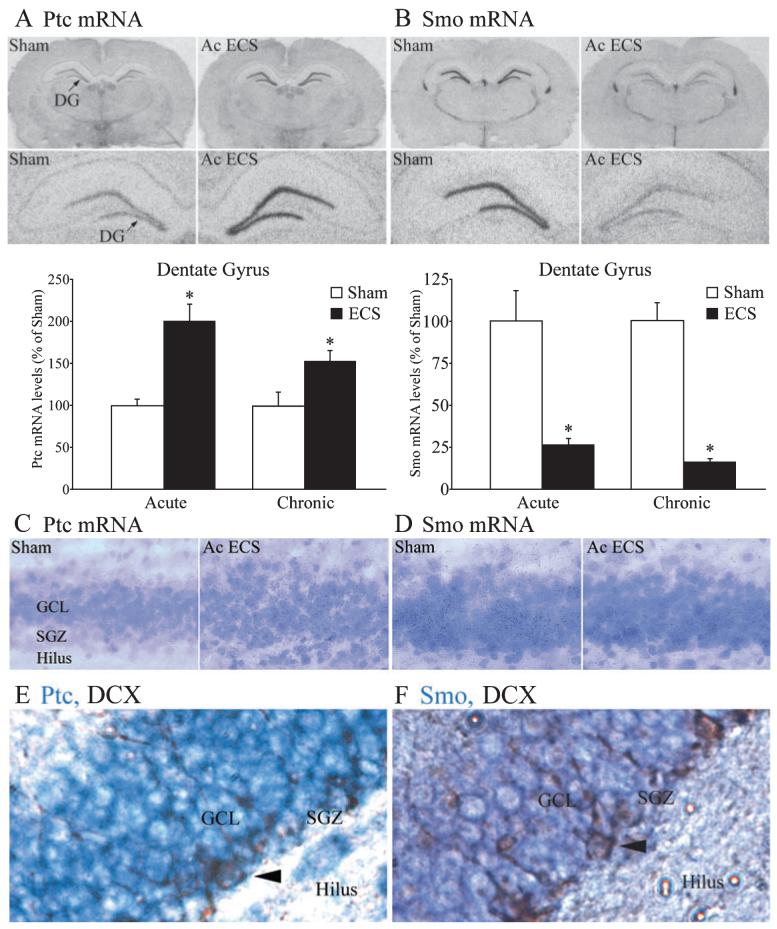
We next examined the influence of both acute and chronic ECS on mRNA expression of the Shh receptor complex, which consists of Ptc and Smo. The expression of Ptc mRNA was significantly increased within the dentate gyrus region of the hippocampus 2 h following acute or chronic ECS treatment (Fig. 3A). Emulsion autoradiography revealed that the cellular localization of upregulated Ptc mRNA was within the GCL of the dentate gyrus. The silver grains were observed in both the GCL as well as the SGZ, and following ECS treatment were upregulated through the GCL including the SGZ (Fig. 3C). This upregulation of Ptc mRNA was restricted to the dentate gyrus and not observed in any other hippocampal subfield. The other neurogenic brain region that is also known to express high levels of Ptc mRNA is the SVZ; however, neither acute nor chronic ECS influence Ptc mRNA expression in the SVZ (Acute ECS experiment: Sham, 100 ± 7.11%; Acute ECS, 115.07 ± 17.24%. Chronic ECS experiment: Sham, 100 ± 7.06%; Chronic ECS, 114.43 ± 8.21%; n = 6 per group, P > 0.05). We also examined the time course of the acute ECS-mediated regulation of Ptc mRNA (Table 1). The regulation of Ptc mRNA was time-dependent, and was observed to be maximal at 2 h following acute ECS administration. The upregulation of Ptc mRNA was transient and returned to baseline 4 h following acute ECS treatment. We then addressed whether chronic ECS treatment (once daily for 10 days) results in sustained elevation of Ptc mRNA levels in the dentate gyrus, but found that 21 h after the final ECS treatment Ptc mRNA levels did not significantly differ between the ECS- and sham-treated groups (Sham, 100 ± 1.95%; Chronic ECS + 21 h, 101.39 ± 5.27%; n = 4 per group, P > 0.05).
Fig. 3.
Acute and chronic electroconvulsive seizures (ECS) regulate the expression of the Shh receptor components Patched (Ptc) and Smoothened (Smo) in the dentate gyrus. Rats received either acute or chronic sham/ECS treatment as described in Materials and methods, and mRNA levels of the Shh receptors, Ptc and Smo in the hippocampal dentate gyrus subfield were determined using in situ hybridization analysis and quantitative densitometry. Representative autoradiographs of Ptc (A) and Smo (B) mRNA expression from a sham and acute ECS-treated animal are shown with an arrow indicating the dentate gyrus (DG). Levels of Ptc mRNA and Smo mRNA in the dentate gyrus hippocampal subfield are shown in the bar graphs below the representative autoradiograms. Acute and chronic ECS significantly enhanced the expression of Ptc mRNA within the dentate gyrus, whereas the expression of Smo mRNA was significantly decreased following both acute and chronic ECS treatment. The results are expressed as the mean ± SEM percentage of sham (Acute ECS experiment n = 9 per group; Chronic ECS experiment, n = 8 per group). *P < 0.05 indicates significantly different from sham (Student’s t-test). Cellular localization of the ECS induction of Ptc mRNA and the downregulation of Smo mRNA was visualized with emulsion autoradiography and then counterstained with Cresyl violet. Shown are representative emulsion sections through the granule cell layer (GCL) for Ptc (C) and Smo (D) mRNA from a sham and acute ECS-treated animal. Note the presence of black silver grains, in both the Ptc and Smo emulsion, through the entire GCL including the subgranular zone (SGZ) at the border of the GCL and the hilus. Shown are representative images indicating the expression of Ptc mRNA (purple, E) and Smo mRNA (purple, F) in doublecortin-immunopositive adult hippocampal progenitors (arrowhead) within the SGZ.
Table 1.
Time course of acute ECS-mediated regulation of Ptc and Smo mRNA in the dentate gyrus
| Ptc mRNA (%) |
Smo mRNA (%) |
|||
|---|---|---|---|---|
| Treatment | Sham | Acute ECS | Sham | Acute ECS |
| ECS + 2 h | 100 ± 11.44 | 199.91 ± 27.76 | 100 ± 17.92 | 26.54 ± 3.38 |
| ECS + 4 h | 100 ± 7.85 | 109.83 ± 4.62 | 100 ± 21.04 | 7.78 ± 1.98 |
| ECS + 6 h | 100 ± 3.94 | 106.22 ± 5.92 | 100 ± 4.37 | 45.91 ± 10.98 |
| ECS + 24 h | 100 ± 9.36 | 88.21 ± 3.41 | 100 ± 17.18 | 64.96 ± 13.69 |
Rats received acute ECS treatment as described in Materials and methods, and were killed 2, 4, 6 and 24 h following the sham/ECS treatment. Levels of Ptc and Smo mRNA were determined by in situ hybridization and densitometric analysis. The results are expressed as mean ± SEM percentage of sham; n = 3–4 per group.
In striking contrast to the ECS-mediated increase in Ptc mRNA, a robust and significant downregulation of Smo mRNA within the GCL of the hippocampal dentate gyrus region was observed 2 h following both acute and chronic ECS administration (Fig. 3B). Emulsion autoradiography revealed that Smo mRNA is expressed in the GCL as well as in the SGZ, and after ECS treatment appears to be reduced in both these areas of the dentate gyrus subfield (Fig. 3D). Smo mRNA expression, in contrast to the relatively wider expression of Ptc mRNA, is restricted predominantly to the neurogenic regions, namely the dentate gyrus and SVZ in the adult rat brain (Traiffort et al., 1999). The downregulation of Smo mRNA observed following both acute and chronic ECS treatment was restricted to the dentate gyrus (Fig. 3B) and not observed in the SVZ (Acute ECS experiment: Sham, 100 ± 6.09%; Acute ECS, 119.12 ± 10.89%. Chronic ECS experiment: Sham, 100 ± 7.92%; Chronic ECS, 97.22 ± 4.72%; n = 6 per group, P > 0.05). The time course of the acute ECS-mediated regulation of Smo mRNA revealed a maximal downregulation of Smo mRNA (~ 90%) 4 h following acute ECS administration (Table 1). At 6 h following ECS Smo mRNA levels in the dentate gyrus were still ~55% reduced as compared with sham-treated animals. Twenty-four hours after ECS treatment no significant differences were observed in Smo mRNA expression as compared with the sham group, indicating a return to baseline (Table 1). When we examined the levels of Smo mRNA 21 h after chronic ECS treatment (once daily for 10 days) to address whether repeated ECS treatment results in a sustained decrease of Smo mRNA levels in the dentate gyrus, we found that Smo mRNA did not significantly differ between the ECS- and sham-treated groups (Sham, 100 ± 17.78%; Chronic ECS + 21 h, 77.45 ± 2.30%; n = 4 per group, P > 0.05). Taken together, these results indicate that both acute and chronic ECS treatment induce a substantial regulation of the Shh receptor complex resulting in enhanced mRNA levels for Ptc, whilst concomitantly downregulating Smo mRNA expression.
To examine whether adult hippocampal progenitors express Ptc and Smo mRNA, we performed combined immunohistochemistry and in situ hybridization with an anti-doublecortin antibody to label hippocampal progenitors and digoxigenin-labelled riboprobes for Ptc or Smo. Doublecortin-positive cells, which reside in the SGZ, were found to be positively labelled with both Ptc (Fig. 3E) and Smo (Fig. 3F) mRNA.
Influence of the NMDA receptor antagonist MK-801 on the acute ECS-mediated regulation of Ptc and Smo mRNA
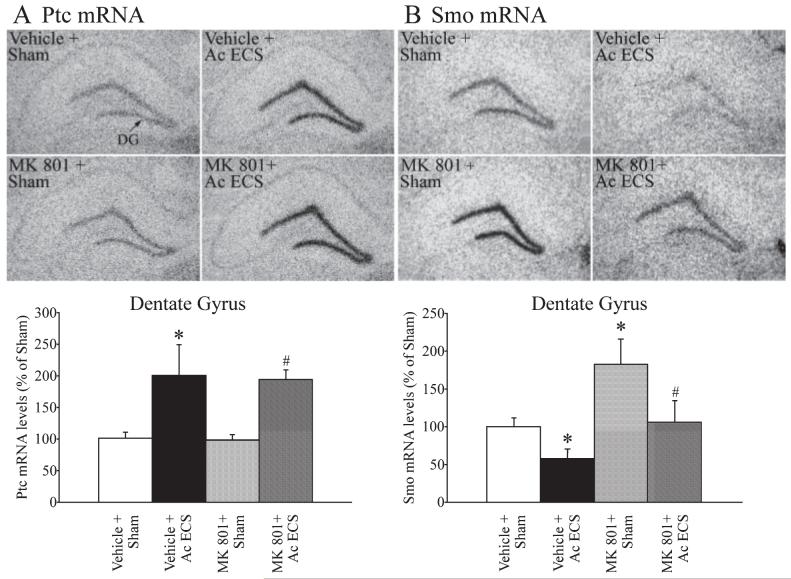
To examine the possibility that NMDA receptors contribute to the ECS-mediated regulation of Ptc and Smo mRNA levels in the dentate gyrus, the influence of the non-competitive NMDA receptor antagonist MK-801 on this ECS-mediated effect was addressed. Pretreatment with MK-801 prior to ECS administration did not alter the ECS-mediated upregulation of Ptc mRNA in the GCL of the dentate gyrus (Fig. 4A). In addition, administration of MK-801 alone did not influence basal expression of Ptc mRNA. In contrast, MK-801 treatment resulted in a substantial increase (~ 80%) in the basal expression of Smo mRNA within the dentate gyrus (Fig. 4B). This finding suggests that the NMDA receptor contributes to maintaining the basal expression levels of Smo mRNA in the hippocampus. Although an influence of MK-801 on basal Smo mRNA expression was observed, no significant interaction was seen in the effects of MK-801 and ECS on Smo mRNA levels (Fig. 4B). Indeed, the ECS-mediated decline in Smo mRNA was similar (~ 40%) in both the vehicle- and MK-801-pretreated groups (Fig. 4B). These results suggest that the NMDA receptor does not contribute to the ECS-mediated regulation of Ptc and Smo mRNA levels in the hippocampus, but indicates that the basal expression of at least one component of the Shh receptor complex, namely Smo, is regulated by the NMDA receptor. The MK-801-pretreated animals did exhibit ataxia, and the number of generalized clonic seizures observed in the MK-801 + ECS group was lower than the Sham + ECS group. However, this effect of MK-801 on the ECS-induced seizure pattern does not appear to alter the ability of ECS to regulate either Ptc or Smo mRNA expression.
Fig. 4.
Influence of pretreatment with the NMDA receptor antagonist 5-methyl-10,11-dihydro-5H-dibenzo (a and d) cyclohepten-5,10-imine maleate (MK-801) on the acute ECS-mediated regulation of Patched (Ptc) and Smoothened (Smo) mRNA. MK-801 (3 mg/kg) was administered 30 min prior to sham/acute ECS treatment as described in Materials and methods. The levels of Ptc and Smo mRNA within the dentate gyrus (DG) were determined using in situ hybridization analysis and quantitative densitometry. Representative autoradiograms for Ptc (A) and Smo (B) mRNA expression in the dentate gyrus from the Vehicle + Sham, Vehicle + Acute ECS, MK-801 + Sham and MK-801 + Acute ECS groups are shown. Levels of Ptc mRNA and Smo mRNA in the dentate gyrus hippocampal subfield are shown in the bar graphs below the representative autoradiograms. Acute ECS significantly increased the expression of Ptc mRNA and decreased Smo mRNA within the dentate gyrus (*P < 0.05 as compared with Vehicle + Sham). Pretreatment with MK-801 did not significantly influence either the basal expression or the ECS-mediated increase in Ptc mRNA levels (A). MK-801 treatment resulted in a significant increase in the basal expression of Smo mRNA levels, but did not influence the ECS-mediated decrease in Smo mRNA expression. The results are expressed as the mean ± SEM percentage of Vehicle + Sham (n = 4 per group). *P < 0.05 indicates significantly different from Vehicle + Sham; #P < 0.05 indicates significantly different from MK-801 + Sham (ANOVA and Bonferroni post-hoc test).
Influence of the AMPA/KA receptor antagonist DNQX on the acute ECS-mediated regulation of Ptc and Smo mRNA
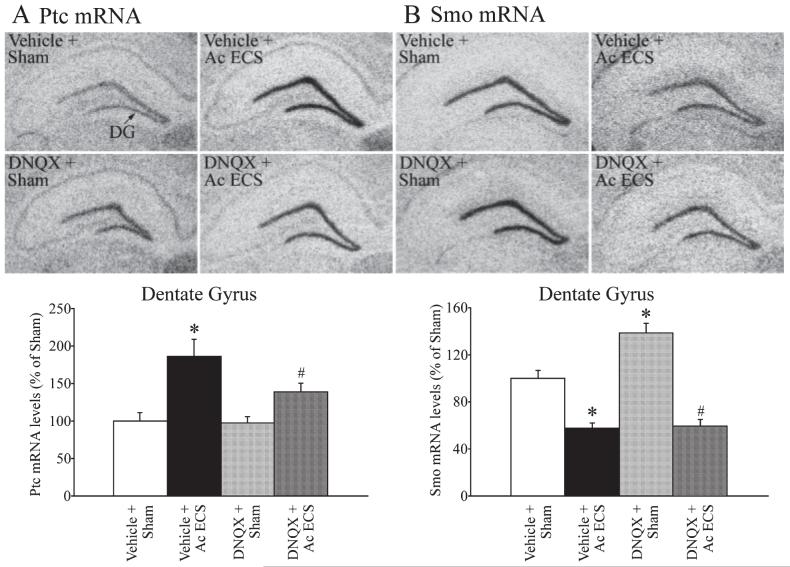
To address the role of the other ionotropic glutamate receptors, namely AMPA/KA, in the ECS-mediated regulation of Ptc and Smo mRNA levels in the dentate gyrus, we used the AMPA/KA receptor antagonist DNQX. Treatment with DNQX before acute ECS administration did not significantly influence the ECS-induced regulation of either Ptc or Smo mRNA (Fig. 5A and B). The DNQX pretreatment did not alter in any way the pattern or intensity of ECS-induced seizures. The upregulation of Ptc mRNA was observed in both vehicle- and DNQX-pretreated animals following ECS administration (Fig. 5A). The ECS-induced increase in Ptc mRNA levels in vehicle treated animals was ~ 85% and the induction in DNQX-treated animals was ~40%; however, this did not result in a statistically significant interaction effect between DNQX and ECS treatments (Fig. 5B, two-way ANOVA, P > 0.05). Administration of DNQX alone had no effect on the basal expression of Ptc mRNA in the dentate gyrus (Fig. 5A). DNQX treatment significantly increased (~ 38%) the basal expression of Smo mRNA (Fig. 5B), suggesting that the AMPA/KA receptors contribute to the regulation of basal Smo mRNA levels. However, the ECS-mediated decrease in Smo levels does not appear to involve the AMPA/KA receptors as acute ECS treatment resulted in a significant decline in Smo mRNA expression in animals that were pretreated with either vehicle or DNQX (Fig. 5B). Collectively the findings indicate that the AMPA/KA ionotropic glutamate receptors contribute to the basal expression of Smo mRNA, but not the ECS-mediated regulation of either Ptc or Smo mRNA expression in the hippocampus.
Fig. 5.
Influence of pretreatment with the AMPA/KA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) on the acute electroconvulsive seizures (ECS)-mediated regulation of Patched (Ptc) and Smoothened (Smo) mRNA. DNQX (12.5 mg/kg) was administered 30 min before sham/acute ECS treatment, as described in Materials and methods. The mRNA levels of Ptc and Smo within the dentate gyrus were determined using in situ hybridization analysis and quantitative densitometry. Representative autoradiogram images of Ptc (A) and Smo (B) mRNA expression in the dentate gyrus from the following groups: Vehicle + Sham, Vehicle + Acute ECS, DNQX + Sham and DNQX + Acute ECS are shown. Levels of Ptc and Smo mRNA in the dentate gyrus hippocampal subfield are shown in bar graphs below the representative autoradiograms. Acute ECS significantly upregulated the expression of Ptc mRNA, and downregulated Smo mRNA within the dentate gyrus (*P < 0.05 as compared with Vehicle + Sham). Pretreatment with DNQX did not significantly influence either the basal expression or the ECS-mediated increase in Ptc mRNA levels (A). DNQX treatment resulted in a significant upregulation in the basal expression of Smo mRNA levels but did not influence the ECS-mediated downregulation of Smo mRNA expression. The results are expressed as the mean ± SEM percentage of Vehicle + Sham (n = 3 per group). *P < 0.05 indicates significantly different from Vehicle + Sham; #P < 0.05 indicates significantly different from MK-801 + Sham (ANOVA and Bonferroni post-hoc test).
Discussion
The concept that a key developmental signalling pathway, the Shh cascade, may be recruited by paradigms like ECS that enhance adult hippocampal neurogenesis is particularly interesting, as it implies that Shh may be a putative mediator through which a dynamic control can be exerted on adult neurogenesis. The major finding of our study revealed that blockade of Shh signalling abolished the ECS-mediated induction of adult hippocampal neurogenesis by preventing the enhanced proliferation of hippocampal progenitors. Our results also demonstrated that key components of the Shh cascade are regulated by ECS. Ptc and Smo mRNA, which encode proteins that together constitute the Shh receptor complex, were differentially regulated by both acute and chronic ECS. This regulation was selectively observed in the dentate gyrus region of the hippocampus, a major neurogenic site in the adult rat brain. Collectively, these findings provide evidence that the Shh pathway may be an important mechanism through which ECS regulates hippocampal neurogenesis in the adult brain.
During development, Shh influences the proliferation of various neuronal precursors including cerebellar granule cell (Dahmane & Ruiz-i-Altaba, 1999; Wechlser-Reya & Scott, 1999), neocortical (Palma & Ruiz-i-Altaba, 2004) and spinal cord progenitors (Rowitch et al., 1999). Recent reports (Lai et al., 2003; Palma et al., 2005) indicate that Shh also regulates the basal proliferation of adult hippocampal progenitors. The present study provides the first evidence that Shh signalling may be required to upregulate adult hippocampal neurogenesis above basal levels. Our experiments using the Smo antagonist cyclopamine demonstrated that the ability of ECS to increase the proliferation of adult hippocampal progenitors requires intact Shh signalling. The mechanisms through which Shh exerts a mitogenic effect on adult hippocampal progenitors are unknown, although several possibilities can be suggested. Shh could directly regulate G1 phase cyclins (Kenney & Rowitch, 2000), transcription factors such as N-myc (Kenney et al., 2003), regulators of chromatin structure-like helicases (Oliver et al., 2003), and repress cell-cycle inhibitors like cyclin G2 (Bennin et al., 2002). The effects of Shh could also involve the regulation of growth factors such as insulin-like growth factor-2, which is upregulated in response to Shh signalling (Hahn et al., 2000) and is also a transcriptional target of ECS (Newton et al., 2003).
In contrast to adult hippocampal progenitors, the proliferation of SVZ progenitors was not altered by cyclopamine, ECS or their combination. The lack of a proliferative effect on SVZ progenitors following ECS is consistent with a previous study that ECS selectively influences adult hippocampal progenitors (Malberg et al., 2000). Previous reports (Lai et al., 2003; Machold et al., 2003) indicate that Shh is sufficient to induce proliferation of hippocampal progenitors, but are less clear about the proliferative effects on SVZ progenitors (Charytoniuk et al., 2002a; Machold et al., 2003; Palma et al., 2005). It has been suggested that the predominant effect of Shh within the SVZ is the maintenance of the neurogenic niche through effects on the stem cell pool (Machold et al., 2003; Palma et al., 2005). Our data indicate that cyclopamine does not alter the proliferation of SVZ progenitors, in contrast to the clear reduction in the proliferation of SGZ progenitors, further supporting the possibility that SGZ and SVZ progenitors respond differentially to Shh.
Given that ECS requires Shh signalling to enhance adult hippocampal neurogenesis, we examined whether ECS influences Shh expression within the adult rat brain. Our results confirmed the basal expression of Shh mRNA within the VDB and in the neocortex of the adult rat, and the absence of Shh transcripts in the hippocampus (Traiffort et al., 1999). It has been suggested that basal forebrain structures, which project to the hippocampus (Nyakas et al., 1987), may anterogradely transport Shh to this neurogenic site (Traiffort et al., 2001; Lai et al., 2003). Indeed, our study supports this contention as we detected Shh protein in hippocampal extracts, despite the absence of Shh mRNA. ECS did not alter Shh mRNA expression in the VDB, or the levels of Shh protein within the VDB or the hippocampus. However, our results indicating that ECS upregulates a Shh-transcriptional target gene suggest that enhanced Shh signal may be perceived in the hippocampus. Shh signals by binding Ptc, relieving the Ptc-mediated inhibition of the Shh signalling receptor Smo, which results in recruitment of Gli transcription factors that influence Shh transcriptional target genes, including Ptc itself (Chen & Struhl, 1998; Murone et al., 1999). Previous reports indicate that the absence of Shh downregulates Ptc, while enhanced Shh signalling increases Ptc transcription, setting up a feedback control (Goodrich et al., 1996; Charytoniuk et al., 2002b). A striking effect we observed following ECS was a dramatic and selective upregulation of Ptc mRNA within the hippocampal dentate gyrus region, suggesting that ECS results in enhanced Shh signalling. An intriguing possibility is that ECS may induce an activity-dependent increase in hippocampal Shh secretion or may modify anterograde transport from the basal forebrain to the hippocampus. The rapid upregulation of Ptc could act to temporally and spatially alter the Shh morphogen gradient via receptor-dependent internalization (Casall & Struhl, 2004; Gallet & Therond, 2005), and may be a reason why we do not pick up an observable increase in Shh protein levels within the hippocampus following ECS. The upregulation of Ptc mRNA in the dentate gyrus was observed in both the GCL as well as the SGZ, where adult hippocampal progenitors reside. In addition, our results as well as a previous report (Lai et al., 2003) indicate that adult hippocampal progenitors express the Shh receptor components Ptc and Smo. Taken together, this raises the possibility that adult hippocampal progenitors may perceive an enhanced Shh signal following ECS.
In addition to the ECS-mediated increase in Ptc mRNA, we observed a rapid and robust reduction in Smo mRNA expression restricted to the dentate gyrus hippocampal subfield following both acute and chronic ECS. Although several studies have examined the post-transcriptional regulation of Smo (Alcedo et al., 2000; Denef et al., 2000; Ingham et al., 2000), few have addressed its transcriptional regulation (Akazawa et al., 2004). It has been suggested that unlike Ptc, Smo does not appear to be transcriptionally regulated by the Shh signal (Zhu et al., 2003). However, the heptahelical Shh signalling receptor Smo bears homology to G-protein-coupled receptors (DeCamp et al., 2000), which exhibit transcriptional downregulation in response to enhanced ligand. At present we do not understand the mechanisms that underlie the ECS-induced decrease in hippocampal Smo mRNA. Further studies are required to address whether the effects of ECS on Smo mRNA involve enhanced Shh signalling, a role for Ptc, a direct transcriptional repression or an altered stability in Smo mRNA. Nevertheless, our studies show that ECS does dramatically regulate the expression of Shh receptor components, Ptc and Smo. Enhanced Ptc could act as a feedback control restricting the Shh signal (Incardona et al., 2000) and as an opponent to Smo function (Chen & Struhl, 1996; Bergstein et al., 2002). In addition, reduced Smo mRNA could further regulate the response to Shh signalling following ECS. This raises the possibility that ECS, through regulation of the Shh receptor components, could act to control the Shh signal as a potent but transient mitogen.
It is also possible that ECS may directly regulate Ptc and Smo mRNA independent of an effect on the ligand, Shh (Borjigin et al., 1999). Given that ECS robustly enhances glutamate release in the hippocampus (Stewart & Reid, 2002), we examined the contribution of the NMDA and AMPA/KA glutamatergic receptors to the ECS-mediated regulation of Ptc and Smo. Our studies reveal that ECS does not regulate either Ptc or Smo expression in the hippocampus via the NMDA or AMPA/KA receptors. Further studies are required to explore the mechanisms of ECS regulation of these Shh receptor components. Interestingly, our studies revealed a dramatic and selective induction in the hippocampal expression of Smo, but not Ptc, mRNA following treatment with the NMDA or AMPA/KA receptor antagonists. This provides the first evidence for a role of glutamatergic ionotropic receptors in regulating Smo mRNA levels, and suggests an intriguing possibility that activity could influence Shh signalling by regulating the basal expression of the Shh signalling receptor, Smo.
In conclusion, our studies provide the first evidence that ECS induces a rapid regulation of key genes of the Shh cascade, and that Shh signalling is required for ECS to enhance adult hippocampal neurogenesis. This implies that Shh may be reutilized in the adult brain to induce activity-dependent neural plasticity, in particular that involving the regulation of adult neurogenesis. In addition, our data provide evidence that glutamatergic transmission may work to maintain basal expression of the Shh signalling receptor Smo, further supporting a link between neuronal activity and the Shh signalling cascade. Given the role of ECS as a potent anti-depressant treatment (Duman & Vaidya, 1998) and the requirement of adult hippocampal neurogenesis in the behavioural actions of antidepressants (Santarelli et al., 2003), our studies raise the exciting possibility that Shh signalling may also be a potential therapeutic target for anti-depressant action. The developmental role of Shh is far better understood in contrast to its function in the adult brain; however, recent reports (Lai et al., 2003; Akazawa et al., 2004; Palma et al., 2005), including the present study, support an emerging role for Shh in regeneration and repair in the adult mammalian brain.
Acknowledgements
This work was supported by a Wellcome Trust Senior Fellowship to V.V. (04082003114133). We thank Dr Rohit Mittal, TIFR, for input on the Shh immunoblotting experiments. We are grateful to Prof. Martial Ruat (CNRS, France) for giving us the Shh, Smo and Ptc cDNA. We are thankful to Dr Ashok Vaidya for comments on the manuscript.
Abbreviations
- AMPA/KA
alpha-amino-3-hydroxy-5-methyl-isoxozole propionic acid/kainate
- BrdU
bromodeoxyuridine
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- ECS
electroconvulsive seizures
- GCL
granule cell layer
- HBC
2-hydroxypropyl-β-cyclodextrin
- i.c.v.
intracerebroventricular
- MK-801
5-methyl-10,11-dihydro-5H-dibenzo (a,d) cyclohepten-5,10-imine maleate
- NMDA
N-methyl-d-aspartate
- Ptc
Patched
- PVDF
polyvinylidene fluoride
- SGZ
subgranular zone
- Shh
Sonic hedgehog
- Smo
Smoothened
- SSC
standard sodium citrate
- SVZ
subventricular zone
- VDB
vertical limb of the diagonal band.
References
- Akazawa C, Tsuzuki H, Nakamura Y, Sasaki Y, Ohsaki K, Nakamura S, Arakawa Y, Kohsaka S. The upregulated expression of Sonic Hedgehog in motor neurons after rat facial nerve axotomy. J. Neurosci. 2004;24:7923–7930. doi: 10.1523/JNEUROSCI.1784-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Zou Y, Noll N. Posttranscriptional regulation of smoothened is part of a selfcorrecting mechanism in the Hedgehog signaling system. Mol. Cell. 2000;6:457–465. doi: 10.1016/s1097-2765(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J. Neurosci. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennin DA, Don AS, Brake T, McKenzie JL, Rosenbaum H, Ortiz L, DePaoli-Roah AA, Horne MC. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 2002;277:27449–27467. doi: 10.1074/jbc.M111693200. [DOI] [PubMed] [Google Scholar]
- Bergstein I, Leopold PL, Sato N, Panteleyev AA, Christiano AM, Crystal RG. In vivo enhanced expression of Patched dampens the Sonic hedgehog pathway. Mol. Ther. 2002;6:258–264. doi: 10.1006/mthe.2002.0628. [DOI] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Deng J, Wang MM, Lee X, Blackshaw S, Snyder SH. Circadian rhythm of Ptc1 transcription in the pineal regulated by adrenergic stimulation and cAMP. J. Biol. Chem. 1999;274:35012–35015. doi: 10.1074/jbc.274.49.35012. [DOI] [PubMed] [Google Scholar]
- Casall A, Struhl G. Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound patched protein. Nature. 2004;431:76–80. doi: 10.1038/nature02835. [DOI] [PubMed] [Google Scholar]
- Charytoniuk D, Porcel B, Gomez JR, Faure H, Ruat M, Traiffort E. Sonic hedgehog signaling in the developing and adult brain. J. Physiol. Paris. 2002a;96:9–16. doi: 10.1016/s0928-4257(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Intrastriatal Sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur. J. Neurosci. 2002b;16:2351–2357. doi: 10.1046/j.1460-9568.2002.02412.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for Patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development. 1998;125:4943–4948. doi: 10.1242/dev.125.24.4943. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Dam AM, Dam M. Quantitative neuropathology in electrically induced generalized convulsions. Convulsive Ther. 1986;2:77–89. [PubMed] [Google Scholar]
- DeCamp DL, Thompson TM, de Sauvage FJ, Lerner MR. Smoothened activates Galphai-mediated signaling in frog melanophores. J. Biol. Chem. 2000;275:26322–26327. doi: 10.1074/jbc.M004055200. [DOI] [PubMed] [Google Scholar]
- Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover of subcellular localization of Patched and Smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Duman RS, Vaidya VA. Molecular and cellular actions of chronic electroconvulsive seizures. J. ECT. 1998;14:181–193. [PubMed] [Google Scholar]
- Gallet A, Therond PP. Temporal modulation of the Hedgehog morphogen gradient by a patched-dependent targeting to lysosomal compartment. Dev. Biol. 2005;277:51–62. doi: 10.1016/j.ydbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon AP, Scott MP. Conservation of the Hedgehog/Patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, Zimmer A, Muller U, Samson E, Quintanilla-Martinez L, Zimmer A. Patched target IGF-2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J. Biol. Chem. 2000;275:28341–28344. doi: 10.1074/jbc.C000352200. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrumalkaloid cyclopamine inhibits Sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H. Receptor-mediated endocytosis of soluble and membrane tethered Sonic hedgehog by Patched-1. PNAS. 2000;97:12044–12049. doi: 10.1073/pnas.220251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nystedt S, Nakano Y, Brown D, Van den Heuvel M, Taylor AM. Patched represses the Hedgehog signaling pathway by promoting modification of the smoothened protein. Curr. Biol. 2000;10:1315–1318. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. N-myc upregulation by Sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G1 cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 2000;20:9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin GJ, Gritli-Linde A, Delloyade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ. Sonic hedgehog signaling by the Patched-Smoothened receptor complex. Curr. Biol. 1999;9:76–84. doi: 10.1016/s0960-9822(99)80018-9. [DOI] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J. Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakas C, Luiten PG, Spencer DG, Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Res. Bull. 1987;18:533–545. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. PNAS. 2003;100:7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Briolne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz-i-Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Ruiz-i-Altaba A. Hedgehog-Gli signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA, USA: 1998. [Google Scholar]
- Rowitch DH, St-Jacques B, Lee SMK, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailor KT, Dhodda VK, Rao VL, Tempsey RJ. Osteopontin infusion into normal adult rat brain fails to increase cell proliferation in dentate gyrus and subventricular zone. Acta Neurochir. Suppl. 2003;86:181–185. doi: 10.1007/978-3-7091-0651-8_39. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman RS, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Antidepressant mechanisms: functional and molecular correlates of excitatory amino acid neurotransmission. Mol. Psychiatry. 2002;7:S15–S22. doi: 10.1038/sj.mp.4001014. [DOI] [PubMed] [Google Scholar]
- Taipale T, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signaling components in the developing and adult rat nervous system. Eur. J. Neurosci. 1999;11:3199–3214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniyuk DA, Faure H, Ruat M. Regional distribution of Sonic hedgehog, Patched and Smoothened mRNA in the adult rat brain. J. Neurochem. 1998;70:1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Moya KL, Faure H, Hassig R, Ruat M. High expression and axonal transport of aminoterminal Sonic hedgehog in the adult hamster brain. Eur. J. Neurosci. 2001;14:839–850. doi: 10.1046/j.0953-816x.2001.01708.x. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- Wechlser-Reya RJ, Scott MP. Control of neuronal precursor population by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Zheng L, Suyama K, Scott MP. Altered localization of drosophila protein activates Hedgehog signal transduction. Genes Dev. 2003;17:1240–1252. doi: 10.1101/gad.1080803. [DOI] [PMC free article] [PubMed] [Google Scholar]