Abstract
OBJECTIVES:
Observational studies have shown that colonoscopy reduces colorectal cancer (CRC) incidence and mortality in the general population. We aimed to conduct a meta-analysis quantifying the magnitude of protection by colonoscopy, with screening and diagnostic indications, against CRC in patients with non-malignant findings and demonstrating the potentially more marked effect of screening over diagnostic colonoscopy.
METHODS:
PubMed, EMBASE, and conference abstracts were searched through 30 April 2015. The primary outcomes were overall CRC incidence and mortality. Pooled relative risks (RRs) and 95% confidence intervals (CIs) were calculated using random-effect models.
RESULTS:
Eleven observational studies with a total of 1,499,521 individuals were included. Pooled analysis showed that colonoscopy was associated with a 61% RR reduction in CRC incidence (RR: 0.39; 95% CI: 0.26–0.60; I2=93.6%) and a 61% reduction in CRC mortality (RR: 0.39; 95% CI: 0.35–0.43; I2=12.0%) in patients with non-malignant findings, although there was high heterogeneity for the outcome of CRC incidence. After excluding one outlier study, there was low heterogeneity for the outcome of incidence (I2=44.7%). Subgroup analysis showed that the effect of screening colonoscopy was more prominent, corresponding to an 89% reduction in CRC incidence (RR: 0.11; 95% CI: 0.08–0.15), in comparison with settings involving diagnostic colonoscopy (RR: 0.51; 95% CI: 0.43–0.59; P<0.001).
CONCLUSIONS:
On the basis of this meta-analysis of observational studies, CRC incidence and mortality in patients with non-malignant findings are significantly reduced after colonoscopy. The effect of screening colonoscopy on CRC incidence is more marked than diagnostic colonoscopy.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer-related death throughout the world (1). By means of detection and subsequent resection of precancerous lesions and early-stage CRCs, screening is effective in reducing CRC incidence and mortality, which has already been demonstrated in trials with fecal occult blood test (2, 3, 4, 5, 6) and flexible sigmoidoscopy (7, 8, 9, 10, 11). Evidence for the effectiveness of colonoscopy screening in average-risk general population, however, is still limited as related large-scale randomized trials are still ongoing (12, 13, 14, 15).
Since 2009, mounting evidence from observational studies has shown that colonoscopy screening is associated with reductions in both CRC incidence and mortality (16, 17, 18, 19). However, colonoscopy screening programs have not been implemented in many European countries (20, 21) and most of the Asia-Pacific region (22); even the colonoscopy screening rates in the United States and Germany, where screening programs were introduced early this century, were only 54% by 2013 (23) and ~20–30% by 2012 (24), respectively. A great number of studies from the real-world settings in which indications for colonoscopy included both screening and diagnostic also supported the protective effect of colonoscopy in the general population (25, 26, 27, 28, 29).
Two previous meta-analyses found significant reductions in CRC mortality (and incidence) after (screening) colonoscopy (30, 31), but the generalizability of the findings in the general population is less than ideal due to the heterogeneity of the baseline population, as subjects with malignant findings were enrolled in some included studies but not in others. Ranging from negative findings, hyperplastic polyps, adenomas to serrated lesions, non-malignant findings at the index colonoscopy, which constitutes over 90% of the yield of colonoscopy in clinical practice (32, 33), differ with malignant findings in the following aspects: non-malignant nature, mostly non-surgical treatment, longer surveillance interval, and better prognosis (34, 35). We therefore aim to evaluate the magnitude of protection against CRC by colonoscopy, with screening and diagnostic indications, in patients with non-malignant findings and further determine the potentially more marked effect of screening over diagnostic colonoscopy in the magnitude of reductions in CRC incidence and mortality.
METHODS
Search strategy
The meta-analysis was performed according to MOOSE statement (MOOSE Checklist is available in Supplementary Appendix A online) (36). A comprehensive, computerized literature search was conducted in PubMed and EMBASE from the beginning of indexing for each database to 30 April 2015 by two reviewers (J.P. and L.X.) independently, with no restrictions in language. The search for relevant studies was performed using the following text words and corresponding Medical Subject Heading/Emtree terms: “colonoscopy or endoscopy” AND “colorectal, colon, rectum, or large bowel” AND “cancer, carcinoma, neoplasm, tumo(u)r, or adenocarcinoma” AND “relative risk(s), odds ratio(s), rate ratio(s), risk ratio(s), or hazard ratio(s)” AND “cohort, or case–control” (detailed search strategy is available in Supplementary Appendix B). Abstracts from Digestive Disease Week (DDW) and United European Gastroenterology Week (UEGW) were searched manually. In addition, we searched for additional studies in reference lists of identified articles.
Eligibility criteria
Three reviewers (J.P., L.X., and Y.-F.M.) independently evaluated all of the studies retrieved according to the eligibility criteria. Disagreements were resolved by consensus. Studies were included if they met all of the following criteria: (i) studies from which effect estimates assessing the effect of colonoscopy on CRC incidence and/or mortality in patients with non-malignant findings vs. no colonoscopy were extractable (patients with non-malignant findings were defined as a consecutive collection of both cases detected with non-malignant polyps and those with negative findings at the index colonoscopy; the index colonoscopy was defined as the initial colonoscopy performed during the study period for either screening or diagnostic purpose); (ii) all of the participants with and without the exposure to colonoscopy are from the same population source; (iii) all of the participants had no history of CRC; (iv) all (or the vast majority) of the participants had no history of inflammatory bowel disease and no family history of hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, or sporadic CRC; (v) effect estimates and the corresponding 95% confidence intervals (CIs) were adjusted for age at least; and (vi) studies with an observational design (prospective cohort, retrospective cohort, or case–control studies). For studies with multiple publications from the same population source, only data from the most recent publication was included.
Data extraction and quality assessment
Two reviewers (J.P. and L.X.) extracted the data independently, and disagreements were resolved by consensus. The following data were extracted from each study: first author, publication year, indications for index colonoscopy, study design, setting, study period, number of participants, age at baseline, sex, duration of follow-up, effect estimates with 95% CIs, and adjustments. For studies with several multivariable-adjusted estimates, we extracted those reflecting the greatest degree of control for potential confounders. The primary outcomes were overall CRC incidence and mortality; the secondary outcomes were CRC incidence and mortality according to indications for colonoscopy, site of cancer, sex, and study design. The study quality was assessed using the Newcastle–Ottawa Scale (37), and the studies awarded seven or more stars were considered of high quality.
Statistical analysis
The measure of effect of interest was the relative risk (RR). Odds ratio, rate ratio, risk ratio, or hazard ratio yielded similar estimates of RR (38). Study-specific RR estimates were combined using a random-effects model, which considers both within- and between-study variation (39). Statistical heterogeneity among studies was evaluated by I2 and Q statistics (40). Studies with an I2 of <25%, 25–50%, 50–75%, and >75% were considered to have no, low, moderate, and high heterogeneity, respectively. An I2 of >50% indicated significant heterogeneity (41). Sensitivity analysis was performed to evaluate the robustness of results, in which pooled estimates were computed omitting one study in each turn (42). Subgroup analysis was performed by indications for colonoscopy, site of cancer, sex, and study design. We compared the pooled RR estimates from different subgroups with a test of interaction (43). Publication bias was evaluated by Begg's test and Egger's test (44, 45). All statistical analyses were performed with Stata software, version 12.0 (Stata Corp, College Station, TX). P<0.05 was considered statistically significant.
RESULTS
Literature search
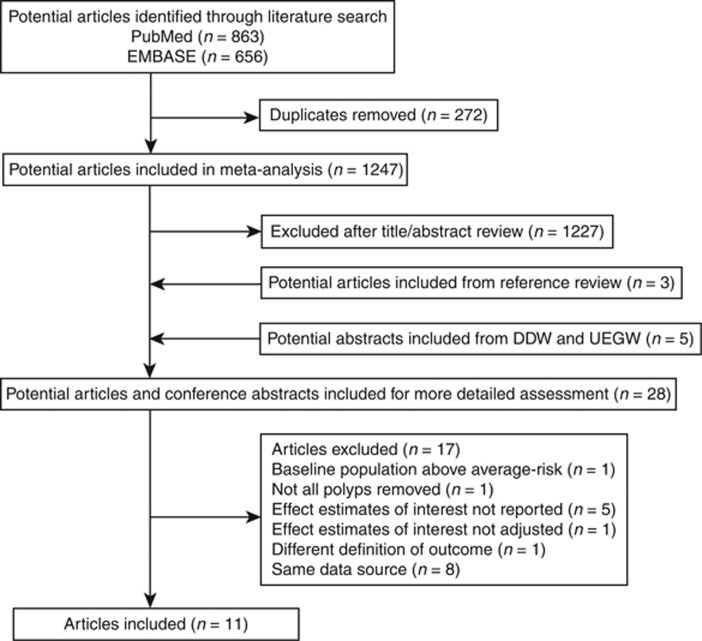
PubMed and EMBASE were searched for relevant studies. As shown in Figure 1, a total of 1,247 studies met our search strategy. After title/abstract review, we excluded 1,227 studies; after including 3 studies from reference review and 5 abstracts from DDW and UEGW, 28 studies remained. Another 17 studies were further excluded for reasons listed as follows: baseline population above average risk (n=1) (46), not all polyps removed (n=1) (47), effect estimates of interest not reported (n=5) (17, 48, 49, 50, 51), effect estimates of interest not adjusted (n=1) (52), different definition of outcome (n=1) (53), and same data source (n=8) (25, 27, 54, 55, 56, 57, 58, 59). Finally, 11 studies were included in the meta-analysis (18, 19, 28, 29, 60, 61, 62, 63, 64, 65, 66).
Figure 1.
Flow diagram of literature search and study selection.
Study characteristics and quality assessment
Details of the 11 included studies are listed in Table 1. Of the 11 observational studies, 5 were cohort studies (18, 60, 61, 62, 63) (3 prospective (18, 60, 61) and 2 retrospective (62, 63)) and 6 were case–control studies (19, 28, 29, 64, 65, 66). A total of 1,499,521 individuals were included, in which 1 study enrolled over 1,000,000 individuals (62), 7 studies enrolled 10,000–100,000 individuals each (18, 28, 60, 61, 63, 64, 65), and the other 3 enrolled <10,000 individuals each (19, 29, 66). Duration of follow-up for cohort studies (or corresponding duration from exposure of colonoscopy to CRC occurrence/death for case–control studies) varied, with three studies of over 10 years (18, 60, 61), seven studies of 5–10 years (19, 28, 29, 62, 63, 64, 65), and one study of <5 years (66). Six studies reported CRC incidence only (19, 29, 61, 63, 64, 66), four reported CRC mortality only (18, 28, 60, 65), and one reported both CRC incidence and mortality (62). Indication(s) for index colonoscopy varied among studies, with screening in three studies (18, 19, 60), screening/diagnostic in five (28, 29, 61, 62, 63), and diagnostic in three (64, 65, 66). Eight studies were conducted in North America (18, 28, 29, 60, 62, 63, 64, 65), and three in Europe (19, 61, 66). In each of the 11 studies, colonoscopy at baseline (combination of polypectomy with removal of all detected lesions and negative colonoscopy) was compared with no colonoscopy.
Table 1. Characteristics of included studies.
| Authors (reference) | Indications for the index colonoscopy | Study design | Setting | Study period | Number of participantsa | Age at enrollment (years)b | Men (%) | Duration of follow-upc | Adjustments | Study qualityd |
| Eldridge et al. (60) | Screening | Prospective cohort | NIH-AARP Study, USA | 1995–2008 | 68,531 (22,780/45,751) | 50–71e | 62 | 11 yearsf | Age, sex, hormone replacement therapy, education, race, diabetes, family history of CRC, and healthy lifestyle score | 6 |
| Nishihara et al. (18) | Screening | Prospective cohort | Nurses' Health Study and Health Professionals Follow-up Study, USA | 1988–2012 | 88,902 (NA/NA) | Men: 42–77e Women: 32–57e | 35.7 | 1,841,586 person-years | Age, sex, calendar year of the questionnaire cycle, body mass index, smoking status, family history of CRC, status with respect to regular use of aspirin, physical activity level, red-meat intake, total caloric intake, alcohol intake, folate intake, calcium intake, multivitamin use, nonsteroidal antiinflammatory drug use, and cholesterol-lowering drug use | 7 |
| Brenner et al. (19)g | Screening | Case–control | Rhine-Neckar, Germany | 1993–2010 | 4,800 (2,516/2,284) | 70h | 59 | 1–10 yearse | Age, sex, county of residence, education, family history of CRC, smoking, body mass index, ever regular use of NSAIDs, ever use of hormone replacement therapy, and ever participation in a general health screening examination | 7 |
| Morois et al. (61) | Screening/diagnostic | Prospective cohort | E3N Study, France | 1990–2008 | 92,048 (37,459/54,589) | Colonoscopy group: 49.9±6.6 Control group: 48.8±6.6 | 0 | 15.4 yearsh | Age, physical activity, smoking status, family history of CRC, educational level, and body mass index | 7 |
| Jacob et al. (62) | Screening/diagnostic | Retrospective cohort | Ontario, Canada | 1996–2007 | 1,089,998 (86,837/1,003,161) | 62f | 45.1 | Incidence: 7 years Mortality: 5 years | Age, sex, comorbidity as measured by the Adjusted Diagnostic Groups case-mix system, neighborhood income quintile, rural residence, and PCP characteristics (age, sex, and country of medical education) | 9 |
| Wang et al. (63) | Screening/diagnostic | Retrospective cohort | SEER-Medicare, USA | 1998–2005 | 53,676 (12,266/41,410) | Colonoscopy group: 73.1±3.8 Control group: 73.3±4.0 | 39.3 | Colonoscopy group: 5 yearsf Control group: 5.3 yearsf | Age, sex, race, zip code, income and educational level, metropolitan county residence, endoscopist subspecialty, and SEER registry stratification | 7 |
| Baxter et al. (28) | Screening/diagnostic | Case–control | SEER-Medicare, USA | 1991–2007 | 37,099 (9,458/27,641) | Cases: 79.9 (70.0–89.9)i Controls: 79.8 (69.1–90.8)i | 42.6 | 9.4 yearsh | Age, sex, race, SEER registry, individual comorbid conditions, socioeconomic status, and urban/rural status | 6 |
| Kahi et al. (29) | Screening/diagnostic | Case–control | Veterans Affairs, USA | 1997–2007 | 2,492 (623/1,869) | 81.22±3.89 | 98.7 | 5.19 yearsf | Age, sex, race, NSAID use, and Charlson comorbidity index. | 5 |
| Mìller and Sonnenberg (64)j | Diagnostic | Case–control | Veterans Affairs, USA | 1981–1993 | 32,702 (16,351/16,351) | Cases (CC): 67.2±9.3 Cases (RC): 66.2±9.4 Controls: 57.0f | 97.8 | Cases (CC): 6.8 yearsf Cases (RC): 6.1 yearsf Controls: 7.1 yearsf | Age, sex, and race. | 4 |
| Mìller and Sonnenberg (65) | Diagnostic | Case–control | Veterans Affairs, USA | 1978–1992 | 20,889 (4,358/16,531) | Cases (CC): 69.1 (68.7–69.5)k Cases (RC): 68.3 (67.8–68.8)k Controls: 57.0 (57.0–57.1)k | 97.7 | Cases: 6.4 yearsf Controls: 8.3 yearsf | Age, sex, race, number of other colorectal procedures, procedures other than colorectal, length of coverage by the Department of Veterans Affairs, and presence of arthritis-related diseases. | 4 |
| Mulder et al. (66) | Diagnostic | Case–control | The Netherlands | 1996–2005 | 8,384 (594/7,790) | Cases: 69.5±11.9 Controls: 69.3±11.9 | 51.7 | 2.8 yearsh | Age, sex, calendar time, duration of follow-up before the date of diagnosis (index date), and IBD. | 7 |
CC, colon cancer; CRC, colorectal cancer; IBD, inflammatory bowel disease; NA, not available; NIH-AARP, National Institutes of Health American Association of Retired Persons; NSAID, nonsteroidal anti-inflammatory drug; RC, rectal cancer.
Numbers in parentheses represented number of participants in colonoscopy/control group (cohort studies), or number of cases/controls (case–control studies).
Values for age were presented as median (interquartile range) or mean±s.d. unless indicated otherwise.
Duration of follow-up for cohort studies, and duration from the exposure of colonoscopy to CRC occurrence/death for case–control studies.
Study quality was assessed based on the Newcastle–Ottawa Scale (range, 0–9 stars), details see Supplementary Appendices C and D online.
Range.
Mean.
Effect estimate was extracted from authors' reply letter by Brenner et al. (68).
Median.
Median (range).
Effect estimate was calculated by pooling the two separate estimates for colon and rectal cancer.
Mean (95% CI).
Effect estimate of the study by Brenner et al. (19) was extracted from authors' reply letter in which a widely accepted definition of screening exposure was adopted (67, 68). One study by Mìller and Sonnenberg (64) separately reported effect estimates for colon and rectal cancer, and we included the combined RR by pooling the two estimates using a random-effect model.
Strategies for excluding CRC cases to form the group of patients with non-malignant findings at the index colonoscopy varied among studies: two studies excluded CRC cases diagnosed at the index colonoscopy (64, 65), four studies excluded CRC cases diagnosed at or within 6 months (exclusion window) of the index colonoscopy (28, 29, 63, 66), one study used a longer exclusion window of 12 months (19), three studies used variable exclusion windows ranging from 0 to 24 or 36 months (18, 60, 61), and one study used a variable exclusion window ranging from 0 to 60 months (62).
Results for study quality assessment are also shown in Table 1 (for details see Supplementary Appendices C and D). Six out of the 11 studies were awarded seven or more stars, indicating high study quality.
Primary outcomes
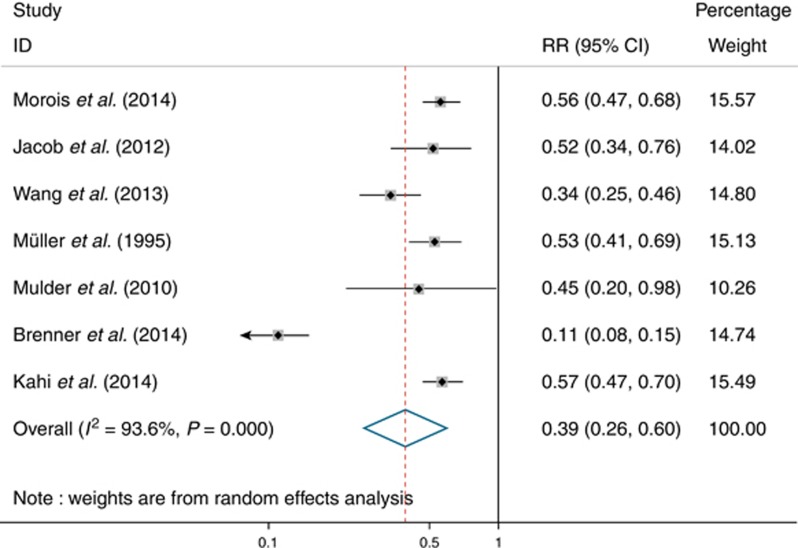
Seven studies were included for outcome of overall CRC incidence. Pooling by a random-effect model (Figure 2) yielded a pooled RR of 0.39 (95% CI: 0.26–0.60), corresponding to a 61% RR reduction in CRC incidence after colonoscopy in patients with non-malignant findings. There was evidence of high heterogeneity among studies (I2=93.6%, P<0.001). Sensitivity analysis revealed that the study by Brenner et al. (19) substantially influenced pooled RR. After excluding this study, there was evidence of low heterogeneity (I2=44.7%, P=0.11), and pooled RR was 0.51 (95% CI: 0.43–0.59). Funnel plot asymmetry test for publication bias was negative using both Begg's test (P=0.07) and Egger's test (P=0.43).
Figure 2.
Forest plot of reduction in colorectal cancer incidence after colonoscopy in patients with non-malignant findings.
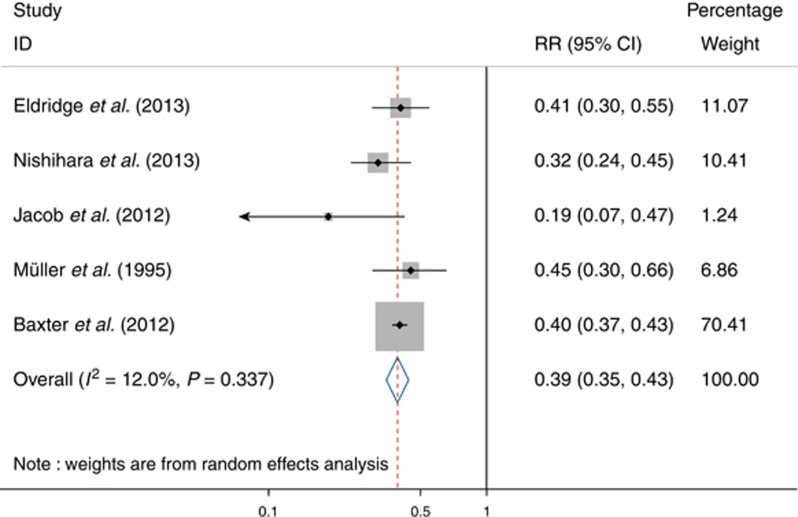
Five studies were included for outcome of overall CRC mortality. Pooling by a random-effect model (Figure 3) yielded a pooled RR of 0.39 (95% CI: 0.35–0.43), corresponding to a 61% RR reduction in CRC mortality after colonoscopy in patients with non-malignant findings. There was no evidence of heterogeneity among studies (I2=12.0%, P=0.34). Sensitivity analysis further confirmed the robustness of our findings. Funnel plot asymmetry test for publication bias was negative using both Begg's test (P=0.22) and Egger's test (P=0.35).
Figure 3.
Forest plot of reduction in colorectal cancer mortality after colonoscopy in patients with non-malignant findings.
Secondary outcomes
Subgroup analyses were conducted for the following secondary outcomes of CRC incidence (Table 2). As for indications, screening colonoscopy was associated with greater protection (RR: 0.11; 95% CI: 0.08–0.15) than screening/diagnostic and diagnostic colonoscopies (RR: 0.51; 95% CI: 0.43–0.59; Pinteraction<0.001). As for site of cancer, colonoscopy was associated with a 28% non-statistically significant reduction in proximal CRC incidence (RR: 0.72; 95% CI: 0.50–1.03), whereas protection against distal CRC (RR: 0.32; 95% CI: 0.20–0.50) was much stronger (Pinteraction=0.01). As for sex, results were similar for studies in men (RR: 0.55; 95% CI: 0.47–0.64) and women (RR: 0.56; 95% CI: 0.47–0.66; Pinteraction=0.88). As for study design, results were also similar for cohort (RR: 0.47; 95% CI: 0.34–0.65) and case–control studies (RR: 0.35; 95% CI: 0.16–0.77; Pinteraction=0.50).
Table 2. Subgroup analyses for reduction in colorectal cancer incidence after colonoscopy in patients with non-malignant findings.
| Subgroups | Number of studies | Pooled RR (95% CI) | I 2 (%) | Pheterogeneity | Pinteraction |
|---|---|---|---|---|---|
| Indications for colonoscopy | |||||
| Screening (19) | 1 | 0.11 (0.08–0.15) | NA | NA | |
| Screening/diagnostic and diagnostic (29, 61, 62, 63, 64, 66) | 6 | 0.51 (0.43–0.59) | 44.7 | 0.11 | <0.001 |
| Site of cancer | |||||
| Proximal CRC (29, 61, 62, 63) | 4 | 0.72 (0.50–1.03) | 69.9 | 0.02 | |
| Distal CRC (29, 61, 62, 63) | 4 | 0.32 (0.20–0.50) | 75.7 | 0.01 | 0.01 |
| Sex | |||||
| Men (29, 62, 64) | 3 | 0.55 (0.47–0.64) | 0.0 | 0.89 | |
| Women (61, 62) | 2 | 0.56 (0.47–0.66) | 0.0 | 0.82 | 0.88 |
| Study design | |||||
| Cohort (61, 62, 63) | 3 | 0.47 (0.34–0.65) | 73.7 | 0.02 | |
| Case–control (19, 29, 64, 66) | 4 | 0.35 (0.16–0.77) | 96.3 | <0.001 | 0.50 |
CI, confidence interval; CRC, colorectal cancer; NA, not available; RR, relative risk.
Subgroup analyses were conducted for the following outcomes of CRC mortality (Table 3). As for indications, screening colonoscopy was associated with somewhat greater protection (RR: 0.36; 95% CI: 0.29–0.46) than screening/diagnostic and diagnostic colonoscopies (RR: 0.40; 95% CI: 0.32–0.49), but the difference between subgroups was not statistically significant (Pinteraction=0.51). As for the site of cancer, colonoscopy was associated with less protection against proximal CRC mortality (RR: 0.57; 95% CI: 0.52–0.63) than distal CRC (RR: 0.18; 95% CI: 0.11–0.31; Pinteraction<0.001). As for sex, colonoscopy provided a similar magnitude of protection for men (RR: 0.36; 95% CI: 0.32–0.40) and women (RR: 0.23; 95% CI: 0.10–0.54; Pinteraction=0.30). As for study design, results were similar in the cohort (RR: 0.34; 95% CI: 0.26–0.45) and case–control studies (RR: 0.40; 95% CI: 0.37–0.43; Pinteraction=0.26).
Table 3. Subgroup analyses for reduction in colorectal cancer mortality after colonoscopy in patients with non-malignant findings.
| Subgroups | Number of studies | Pooled RR (95% CI) | I 2 (%) | Pheterogeneity | Pinteraction |
|---|---|---|---|---|---|
| Indications for colonoscopy | |||||
| Screening (18, 60) | 2 | 0.36 (0.29–0.46) | 10.4 | 0.29 | |
| Screening/diagnostic and diagnostic (28, 62, 65) | 3 | 0.40 (0.32–0.49) | 25.7 | 0.26 | 0.51 |
| Site of cancer | |||||
| Proximal CRC (18, 28, 62) | 3 | 0.57 (0.52–0.63) | 0.0 | 0.66 | |
| Distal CRC (18, 28, 62) | 3 | 0.18 (0.11–0.31) | 63.9 | 0.06 | <0.001 |
| Sex | |||||
| Men (18, 28, 62, 65) | 4 | 0.36 (0.32–0.40) | 0.0 | 0.69 | |
| Women (18, 28, 62) | 3 | 0.23 (0.10–0.54) | 93.5 | <0.001 | 0.30 |
| Study design | |||||
| Cohort (18, 60, 62) | 3 | 0.34 (0.26–0.45) | 28.1 | 0.25 | |
| Case–control (28, 65) | 2 | 0.40 (0.37–0.43) | 0.0 | 0.57 | 0.26 |
CI, confidence interval; CRC, colorectal cancer; RR, relative risk.
DISCUSSION
Overview
This meta-analysis shows that CRC incidence and mortality in patients with non-malignant findings were both 61% lower after colonoscopy. The protective effect was more prominent after screening colonoscopy, corresponding to an 89% reduction in CRC incidence.
Interpretations of study findings
Our study is the first meta-analysis to quantify the magnitude of protection against CRC that patients with non-malignant findings benefit from colonoscopy. When interpreting the study results, both the overall effect of colonoscopy and the individual effect of screening colonoscopy derived from subgroup analysis are informative. As regular colonoscopy screening has not been implemented even in many developed countries (20, 21), the primary outcome, which estimated the benefit derived from both screening and diagnostic colonoscopies, reflected the effect of regular colonoscopy in routine clinical practice. Subgroup analysis of screening colonoscopy provides data on the maximum cases of CRCs and CRC-related deaths that may be prevented in patients with non-malignant findings by population-based screening programs in standardized conditions, which is more important from a public health perspective.
There are several explanations for our findings. First, removal of all detected polyps (i.e., clearing colonoscopy) is the main modality responsible for the decreased CRC risk (69, 70), while individuals with negative findings are inherently associated with lower risks of developing CRC even compared with postpolypectomy individuals (71). Second, interval CRCs could hardly be avoided because of factors such as missed lesions at the index colonoscopy, rapid growth of specific type of neoplasms, and incomplete resection of polyps (72). Therefore, both the aspects should be considered when interpreting the study findings.
In subgroup analysis, our study showed more prominent protection against CRC incidence by screening colonoscopy than colonoscopy with indications of screening/diagnostic and diagnostic (Pinteraction<0.001), and, similar tendency was observed for CRC mortality (RR: 0.36 (0.29–0.46) vs. 0.40 (0.32–0.49); Pinteraction=0.51), as screening detects a different spectrum of findings (e.g., fewer polyps) compared with that diagnosed in the symptomatic population (35, 73, 74). Our results showed that colonoscopy was less effective in preventing proximal CRC incidence and mortality (both Pinteraction<0.05) than distal CRC in patients with non-malignant findings, which might be explained by several factors concerning endoscopists, patients, and tumor biology: proximal serrated polyps could be easily missed by endoscopists because of flat or sessile appearance; patients' poor bowel preparation usually results in incomplete colonoscopy examination; differences in tumor biology exist between proximal and distal lesions of the colorectum (75, 76).
Novelty of the study
Two previous meta-analyses are important studies on the effect of colonoscopy (30, 31). Brenner et al. (30) found that screening colonoscopy is associated with 69 and 68% reductions in CRC incidence and mortality, respectively, and Elmunzer et al. (31) concluded that colonoscopy reduces CRC mortality by 57%. Novelty of our meta-analysis are threefold. First, in the two meta-analyses, patients with malignant findings were enrolled in some included studies but not in others. The significant heterogeneity of baseline population may strongly affect generalizability of their results in the general population. Therefore, we enrolled in our meta-analysis patients with non-malignant findings, a more homogeneous group constituting over 90% of the yield of colonoscopy in clinical practice (32, 33) and featured with non-malignant nature, mostly non-surgical treatment, longer surveillance interval, and better prognosis compared with malignant findings (34, 35). Second, the effect of screening colonoscopy and the effect of colonoscopy regardless of indication were separately reported in the two meta-analyses, without comparison, whereas our subgroup analysis found a more prominent effect of screening colonoscopy over screening/diagnostic and diagnostic colonoscopies on reducing CRC incidence. Third, with expanded colonoscopy indications (including both screening and diagnostic), study outcomes (including both incidence and mortality), and an updated inclusion of recent studies (18, 29), our study (n=1,499,521) is responsible for a more robust conclusion with a larger sample size.
Study limitations
Our study has several limitations. First, in addition to excluding detected CRCs (CRCs diagnosed at or within 6 months of the index colonoscopy) to arrive at non-malignant findings at the index colonoscopy, five of the eleven included studies also excluded interval CRCs (CRCs diagnosed within 6 to 36 (or even 60) months of the index colonoscopy) (18, 19, 60, 61, 62). As interval CRCs certainly argue against the protective effect of colonoscopy (77, 78), results of our study might be biased, causing overestimation of the magnitude of protection by colonoscopy. Therefore, study results should be interpreted with caution. Second, it should be noted that indications for colonoscopy according to original publications of some studies may not reflect real circumstances, e.g., studies by Nishihara et al. (18) and Eldridge et al. (60) initiated earlier than the nationwide introduction of screening colonoscopy. This may offer one of the explanations for the non-significant difference between the effect of screening vs. screening/diagnostic and diagnostic colonoscopy on CRC mortality. Third, statistical heterogeneity was significant for outcome of incidence. This might be explained by the differences in population enrolled, intervention strategy, and study designs. After excluding the study by Brenner et al. (19) (screening was the only indication for colonoscopy), statistical heterogeneity became non-significant.
Fourth, results of our study might be biased due to several other factors. Overestimation of the protective effect of colonoscopy might be caused by selection bias introduced by observational studies, e.g., participants in the colonoscopy (exposed) group tended to be more health-conscious (79), whereas underestimation of the results might be caused by contamination of the control (unexposed) group, e.g., individuals with adenomas in this group may present with symptoms and therefore receive colonoscopy examination with polypectomy (80). Moreover, the initial age for screening in one study (18) is earlier than the guideline-recommended 50 years of age. In this sense, our results should be interpreted with caution, and randomized trials may better resolve this problem. Fifth, our study did not quantify individual CRC risk after either polypectomy or negative colonoscopy, as only one study by Nishihara et al. (18) reported effect estimates in subgroups of patients with polyps and those with negative findings.
CONCLUSIONS
In conclusion, findings from this meta-analysis of observational studies indicate that CRC incidence and mortality in patients with non-malignant findings are significantly reduced after colonoscopy, especially after screening colonoscopy. This provides additional evidence for the effectiveness of colonoscopy in the general population.
Study Highlights
Guarantors of the article: Zhao-Shen Li, MD and Liang-Hao Hu, MD.
Specific author contributions: Jun Pan contributed to the study concept and design, data acquisition and interpretation, and drafting and final approval of the manuscript; Lei Xin and Yi-Fei Ma contributed to the data acquisition, data analysis and interpretation, and revision and final approval of the manuscript; and Zhao-Shen Li and Liang-Hao Hu contributed to the study concept and design, data analysis and interpretation, drafting and revision of the manuscript, and final approval of the manuscript.
Financial support: None.
Potential competing interests: None.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- Ferlay J, Soerjomataram I, Ervik M et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer: Lyon, France. 2013. Available at http://globocan.iarc.fr Accessed on 4 May 2015. [Google Scholar]
- Mandel JS, Church TR, Bond JH et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–1607. [DOI] [PubMed] [Google Scholar]
- Scholefield JH, Moss S, Sufi F et al. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut 2002;50:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronborg O, Jorgensen OD, Fenger C et al. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol 2004;39:846–851. [DOI] [PubMed] [Google Scholar]
- Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008;95:1029–1036. [DOI] [PubMed] [Google Scholar]
- Shaukat A, Mongin SJ, Geisser MS et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–1114. [DOI] [PubMed] [Google Scholar]
- Hoff G, Grotmol T, Skovlund E et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ 2009;338:b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin WS, Edwards R, Kralj-Hans I et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–1633. [DOI] [PubMed] [Google Scholar]
- Segnan N, Armaroli P, Bonelli L et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian randomized controlled trial–SCORE. J Natl Cancer Inst 2011;103:1310–1322. [DOI] [PubMed] [Google Scholar]
- Schoen RE, Pinsky PF, Weissfeld JL et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme O, Loberg M, Kalager M et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MF, Bretthauer M, Zauber AG et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy 2012;44:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero E, Castells A, Bujanda L et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697–706. [DOI] [PubMed] [Google Scholar]
- Clinical Trials.gov Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) 2012. Available at http://clinicaltrials.gov/ct2/show/study/NCT01239082 Accessed on 4 May 2015.
- Sali L, Grazzini G, Carozzi F et al. Screening for colorectal cancer with FOBT, virtual colonoscopy and optical colonoscopy: study protocol for a randomized controlled trial in the Florence district (SAVE study). Trials 2013;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahi CJ, Imperiale TF, Juliar BE et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770–775. [DOI] [PubMed] [Google Scholar]
- Manser CN, Bachmann LM, Brunner J et al. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc 2012;76:110–117. [DOI] [PubMed] [Google Scholar]
- Nishihara R, Wu K, Lochhead P et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Jansen L et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014;146:709–717. [DOI] [PubMed] [Google Scholar]
- Segnan N, Patnick J, von Karsa L. European guidelines for quality assurance in colorectal cancer screening and diagnosis. European Union: Luxembourg. 2010. [DOI] [PubMed] [Google Scholar]
- Altobelli E, Lattanzi A, Paduano R et al. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med 2014;62:132–141. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Ng SC, Chan FK et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut 2015;64:121–132. [DOI] [PubMed] [Google Scholar]
- Meester RG, Doubeni CA, Zauber AG et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer, advance online publication, 12 March 2015; doi:10.1002/cncr.29336. [DOI] [PMC free article] [PubMed]
- Brenner H, Altenhofen L, Stock C et al. Expected long-term impact of the German screening colonoscopy programme on colorectal cancer prevention: Analyses based on 4,407,971 screening colonoscopies. Eur J Cancer 2015;51:1346–1353. [DOI] [PubMed] [Google Scholar]
- Baxter NN, Goldwasser MA, Paszat LF et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- Singh H, Nugent Z, Demers AA et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010;139:1128–1137. [DOI] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Seiler CM et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- Baxter NN, Warren JL, Barrett MJ et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol 2012;30:2664–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahi CJ, Myers LJ, Slaven JE et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology 2014;146:718–725. [DOI] [PubMed] [Google Scholar]
- Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmunzer BJ, Singal AG, Sussman JB et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis. Gastrointest Endosc 2015;81:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol 1995;90:353–365. [PubMed] [Google Scholar]
- Lieberman DA, Weiss DG, Bond JH et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343:162–168. [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Rex DK, Winawer SJ et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Pox C, Kuipers EJ et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Management of lesions detected in colorectal cancer screening. Endoscopy 2012;44 Suppl 3:SE140–SE150. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O'Connell D et alThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed on 4 May 2015.
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 1999;8:15–17. [Google Scholar]
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterchio M, Manno M, Klar N et al. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control 2005;16:865–875. [DOI] [PubMed] [Google Scholar]
- Murakami R, Tsukuma H, Kanamori S et al. Natural history of colorectal polyps and the effect of polypectomy on occurrence of subsequent cancer. Int J Cancer 1990;46:159–164. [DOI] [PubMed] [Google Scholar]
- Kavanagh AM, Giovannucci EL, Fuchs CS et al. Screening endoscopy and risk of colorectal cancer in United States men. Cancer Causes Control 1998;9:455–462. [DOI] [PubMed] [Google Scholar]
- Brenner H, Arndt V, Sturmer T et al. Long-lasting reduction of risk of colorectal cancer following screening endoscopy. Br J Cancer 2001;85:972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeneck L, Paszat LF, Saskin R et al. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol 2010;105:1627–1632. [DOI] [PubMed] [Google Scholar]
- Steffen A, Weber MF, Roder DM et al. Colorectal cancer screening and subsequent incidence of colorectal cancer: results from the 45 and Up Study. Med J Aust 2014;201:523–527. [DOI] [PubMed] [Google Scholar]
- Barret M, Boustiere C, Canard JM et al. Factors associated with adenoma detection rate and diagnosis of polyps and colorectal cancer during colonoscopy in France: results of a prospective, nationwide survey. PLoS One 2013;8:e68947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubeni CA, Weinmann S, Adams K et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med 2013;158:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister M, Chang-Claude J, Brenner H. Validity of self-reported endoscopies of the large bowel and implications for estimates of colorectal cancer risk. Am J Epidemiol 2007;166:130–136. [DOI] [PubMed] [Google Scholar]
- Baxter NN, Warren J, Barrett MJ et al. The association between colonoscopy and colorectal cancer mortality in a U.S. cohort according to site of cancer and colonoscopist specialty. Gastroenterology 2011;140:S74–S-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob B, Moineddin R, Sutradhar R et al. 658 Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc 2012;75:AB155. [DOI] [PubMed] [Google Scholar]
- Kahi CJ, Myers LJ, Slaven JE et al. 85 endoscopic prevention of colorectal cancer in older individuals. Gastroenterology 2013;144:S19. [Google Scholar]
- Morois S, Cottet V, Racine A et al. Tu1184 colonoscopy, family history of colorectal cancer and colorectal tumor risk: results from a French prospective cohort. Gastroenterology 2012;142:S768–S-769. [Google Scholar]
- Wang YR, Cangemi JR, Loftus EV et al. 766 decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the united states: a population-based analysis of the SEER-Medicare Linked Database, 1998-2005. Gastrointest Endosc 2013;77:AB166. [Google Scholar]
- Eldridge RC, Doubeni CA, Fletcher RH et al. Uncontrolled confounding in studies of screening effectiveness: an example of colonoscopy. J Med Screen 2013;20:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morois S, Cottet V, Racine A et al. Colonoscopy reduced distal colorectal cancer risk and excess cancer risk associated with family history. Cancer Causes Control 2014;25:1329–1336. [DOI] [PubMed] [Google Scholar]
- Jacob BJ, Moineddin R, Sutradhar R et al. Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc 2012;76:355–364. [DOI] [PubMed] [Google Scholar]
- Wang YR, Cangemi JR, Loftus EV Jr et al. Risk of colorectal cancer after colonoscopy compared with flexible sigmoidoscopy or no lower endoscopy among older patients in the United States, 1998-2005. Mayo Clin Proc 2013;88:464–470. [DOI] [PubMed] [Google Scholar]
- Mìller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med 1995;123:904–910. [DOI] [PubMed] [Google Scholar]
- Mìller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med 1995;155:1741–1748. [DOI] [PubMed] [Google Scholar]
- Mulder SA, van Soest EM, Dieleman JP et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol 2010;22:437–443. [DOI] [PubMed] [Google Scholar]
- Pan J, Xin L, Li ZS. Modifying the definition of screening exposure to settle existing differences. Gastroenterology 2014;147:717. [DOI] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Jansen L et al. Reply: To PMID 25075945. Gastroenterology 2014;147:717–718. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Zauber AG, Ho MN et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–1981. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O'Brien MJ et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Turner D, Xue L et al. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA 2006;295:2366–2373. [DOI] [PubMed] [Google Scholar]
- le Clercq CM, Bouwens MW, Rondagh EJ et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut 2014;63:957–963. [DOI] [PubMed] [Google Scholar]
- Keddie N, Hargreaves A. Symptoms of carcinoma of the colon and rectum. Lancet 1968;2:749–750. [DOI] [PubMed] [Google Scholar]
- Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 1999;94:3039–3045. [DOI] [PubMed] [Google Scholar]
- Soetikno RM, Kaltenbach T, Rouse RV et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 2008;299:1027–1035. [DOI] [PubMed] [Google Scholar]
- Rex DK, Ahnen DJ, Baron JA et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Singh PP, Murad MH et al. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:1375–1389. [DOI] [PubMed] [Google Scholar]
- Samadder NJ, Curtin K, Tuohy TM et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology 2014;146:950–960. [DOI] [PubMed] [Google Scholar]
- Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Stock C, Hoffmeister M. In the era of widespread endoscopy use, randomized trials may strongly underestimate the effects of colorectal cancer screening. J Clin Epidemiol 2013;66:1144–1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.