Abstract
The α1β1 collagen receptor is only present in a few epithelial cell types. In the intestine, it is specifically expressed in proliferating crypt cells. This integrin has been reported to be involved in various cancers where it mediates the downstream activation of the Ras/ERK proliferative pathway. We have recently shown that integrin α1β1 is present in two-thirds of colon adenocarcinomas, but the mechanism by which ITGA1 expression is regulated is not known. DNA methylation, involved in ITGA1 repression during megakaryocyte differentiation, is not the mechanism of ITGA1 regulation in colorectal cancer cells. Our in silico analysis of the ITGA1 promoter revealed two response elements for MYC, an oncogenic factor known to regulate cancer cell proliferation, invasion and migration. In situ, the expressions of both MYC and ITGA1 are localized in the lower crypt of the normal colon and correlate in 72% of the 65 analyzed colorectal cancers. MYC pharmacological inhibition or downregulation of expression with short hairpin RNA in HT29, T84 and SW480 cells resulted in reduced ITGA1 expression at both the transcript and protein levels. Chromatin immunoprecipitation assays revealed that MYC was bound to the chromatin region of the ITGA1 proximal promoter, whereas MYC overexpression enhanced ITGA1 promoter activity that was reduced with MAD co-transfection or by the disruption of the response elements. We concluded that MYC is a key regulating factor for the control of ITGA1 expression.
Introduction
Integrins provide dynamic cell to cell linkage and cell attachment to extracellular matrix components. These noncovalently associated αβ heterodimers form a large superfamily of 18 α and 8 β subunits that can form 24 αβ units.1, 2 Three decades after their characterization at the molecular level, integrin functions are now well known and better understood. In contrast to most other cell receptors, they can drive inside-out as well as outside-in signaling.3 Despite the absence of intrinsic enzymatic capability and depending upon the integrin heterodimer, they can recruit different scaffolding and signaling molecules and drive activation of different pathways involved in cancer progression including cell shape, invasion, migration, survival and proliferation.4, 5, 6 The integrin α subunit is crucial for ligand specificity and selective molecule recruitment. ITGA1 (integrin, α1) contains an ‘I‘ domain involved in α1β1 binding to collagen and is crucial for recruiting caveolin-1 and Shc to activate the Ras/MEK/ERK pathway regulating cell proliferation.4, 7, 8 It has been demonstrated that specific amino acids in the ITGA1 cytoplasmic tail are necessary to drive various functions and pathways.9 Pro1142 and Leu1145 are required for extracellular signal-regulated kinase (ERK) activation and cell proliferation, whereas Lys1146 is involved in cell adhesion and migration (activation of p38 mitogen-activated protein kinase); interestingly, substitution of the Lys1151 positively charged amino acid at the COOH-terminus of the α1 tail results in a complete loss of the above functions.9
Integrin α1β1 has been reported to be involved in various cancers. For instance, blocking ITGA1 function, but not ITGA2, resulted in reduced cell adhesion to collagens and invasion in a mouse breast cancer model.10 Similarly, ITGA1 and ITGA2 have been shown to enhance cell invasion through a fibrotic microenvironment comparable to the fibrotic matrix of hepatocellular carcinoma.11 Knockout mice for Itga1 display longer survival with smaller tumors and reduced proliferation and angiogenesis as well as enhanced cell death in lungs bearing a Kras mutation.12 Another indication supporting a critical role for integrin α1β1 in tumorigenicity was obtained with the demonstration in colon cancer cells that α1β1 but not α2β1 can associate with talin and paxillin to activate focal adhesion kinase/Src, resulting in its accumulation in focal aggregates and activation of the p130Cas/c-Jun N-terminal kinase cascade to promote cancer cell invasion.13
We recently reported that ITGA1 was expressed in 65% of colorectal cancers,14 but how its expression is regulated remains unknown. The first description of transcriptional regulation of ITGA1 was in smooth muscle cells where the proximal promoter containing the CArG box for the serum response factor was found within 400 bp upstream from the translation initiation site.15 On the other hand, Cheli et al.16 reported that ITGA1 expression is predominantly downregulated by DNA methylation but not histone modification during differentiation of mononuclear cells toward the megakaryocyte lineage. Whether ITGA1 expression is regulated by a DNA methylation-dependant mechanism in cancers has not yet been investigated but our data showing widespread ITGA1 expression in colorectal cancers14 suggest that other mechanisms could prevail. Indeed, an in silico analysis of the ITGA1 proximal promoter region revealed two CANNTG responsive elements for the MYC transcription factor.
Interestingly, MYC expression is known to be upregulated in up to 70% of colorectal cancers.17, 18 Considering that MYC is involved in various aspects of cancer cell proliferation and invasion,19, 20, 21 functions where the integrin α1β1 also appears to play a role as summarized above, in this study we have investigated the possibility that ITGA1 expression is regulated by MYC in colorectal cancer.
Results
Methylation is not the mechanism of regulation of ITGA1 expression in colorectal cancer cells
Different colon cancer cell lines were screened for ITGA1 expression at the transcript and protein levels. As summarized in Table 1, ITGA1 was found to be highly expressed in HT29, SW480 and Caco-2/15 cells, moderately in T84 and SW620 cells and weakly in DLD1 and HCT116 cells. As downregulation of ITGA1 has been reported to be DNA methylation dependent in megakaryocytic cells,16 we treated HCT116 and DLD1 cells with 5-aza-2′-deoxycytidine for 7 days. This treatment did not trigger ITGA1 expression as compared with dimethyl sulfoxide alone, whereas IGFBP7, known to be epigenetically inactivated in various colon cancer cell lines,22 was induced. Furthermore, treatment of HCT116 cells with a bisulfite agent did not reveal methylated CpGs in the proximal region of the ITGA1 promoter. Together, these results strongly suggest that ITGA1 gene expression is not regulated by DNA methylation in human colon cancer cells.
Table 1. Evaluation of MYC protein and ITGA1 mRNA and protein expression levels in colorectal cancer cell lines by real-time quantitative PCR (qPCR) and western blot.
| Cell line | MYC protein | ITGA1 transcript | ITGA1 protein |
|---|---|---|---|
| Caco-2/15 | +/− | + | ++ |
| HCT116 | ++ | +/− | +/− |
| DLD1 | + | +/− | +/− |
| HT29 | ++ | ++ | ++ |
| SW480 | ++ | ++ | ++ |
| SW620 | + | + | + |
| T84 | + | + | + |
MYC regulates ITGA1 expression in colorectal cancer cells
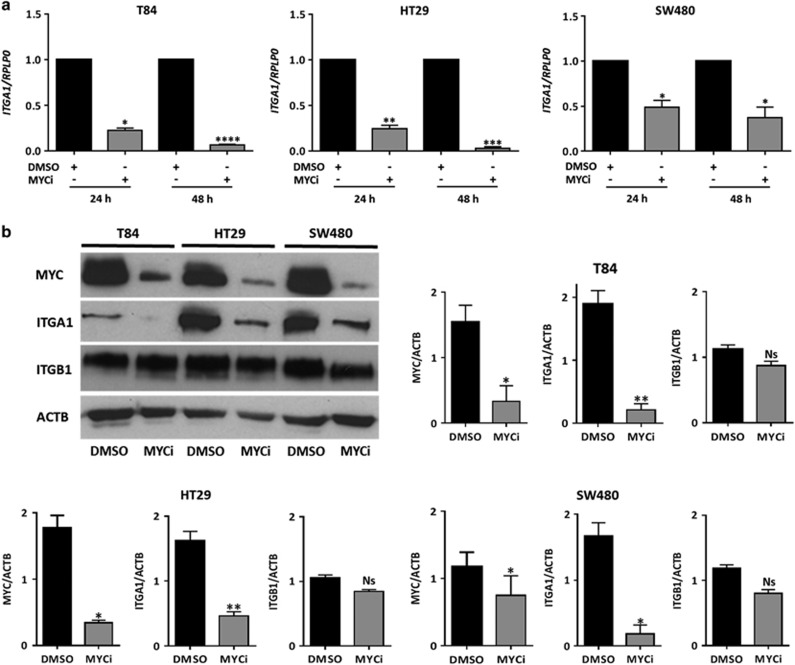
ITGA1 regulation at the transcriptional level in colorectal cancer cells has not been studied. The in silico analysis of its proximal promoter revealed two putative response elements where binding of the oncogenic transcription factor MYC could occur. In light of this finding, we first investigated whether endogenous MYC regulates ITGA1 expression in colorectal cancer cells. As summarized in Table 1, ITGA1 expression at the protein and transcript levels was present in five of the seven tested cell lines (Caco-2/15, HT29, T84, SW480 and SW620), whereas MYC protein was detected at significant levels in four of them. We therefore selected three of the latter to further investigate the implication of MYC on ITGA1 expression. Treatment of the HT29, T84 and SW480 cell lines with the specific MYC inhibitor 10058-F4 used at 50 μM resulted in a significant reduction of MYC and ITGA1 at both transcript and protein levels (Figures 1a and b), whereas the expression of the ITGA1 partner, ITGB1, was not statistically altered (Figure 1b).
Figure 1.
MYC inhibition downregulates ITGA1 expression at the mRNA and protein levels in colorectal cancer cells. (a) T84, HT29 and SW480 cells were treated with the MYC inhibitor 10058-F4 used at 50 μM in dimethyl sulfoxide (DMSO; MYCi) or with DMSO alone for the indicated times. Real-time quantitative PCR (qPCR) levels are indicated as fold changes of ITGA1 relative to the control and normalized to RPLP0 used as housekeeping gene. (b) Representative western blot and densitometric analyses of MYC and ITGA1 expression after treatment of T84, HT29 and SW480 cells with MYCi as in (a). β-Actin (ACTB) was used as loading control. All experiments were performed in triplicate and repeated three times. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
The use of interfering short hairpin RNAs targeting MYC resulted in a significant MYC and ITGA1 downregulation at both the transcript (Figure 2a) and protein levels (Figure 2b) with no effect on ITGB1 in HT29 cells. Similar results were obtained with T84 and SW480 cells. These findings suggest that MYC could regulate ITGA1 expression at the transcript level.
Figure 2.
MYC downregulation inhibits ITGA1 in colorectal cancer cells. Cells were infected with lentivirus encoding a nontargeting short hairpin RNA (sh Ctrl) or shRNA targeting MYC (sh MYC). Cells were selected with puromycin (10 μg/ml) for 10 days before protein or RNA extraction. (a) MYC and ITGA1 mRNA expression was quantified by real-time quantitative reverse-transcriptase–PCR (RT–qPCR) relative to RPLP0 expression. (b) Representative western blot and densitometric analyses showing expression of MYC, ITGA1 and ITGB1 as well as ACTB used as loading control. Experiments were performed in triplicate and repeated three times. *P<0.05, **P<0.01, ***P<0.001.
MYC binds to the ITGA1 promoter in colorectal cancer cells
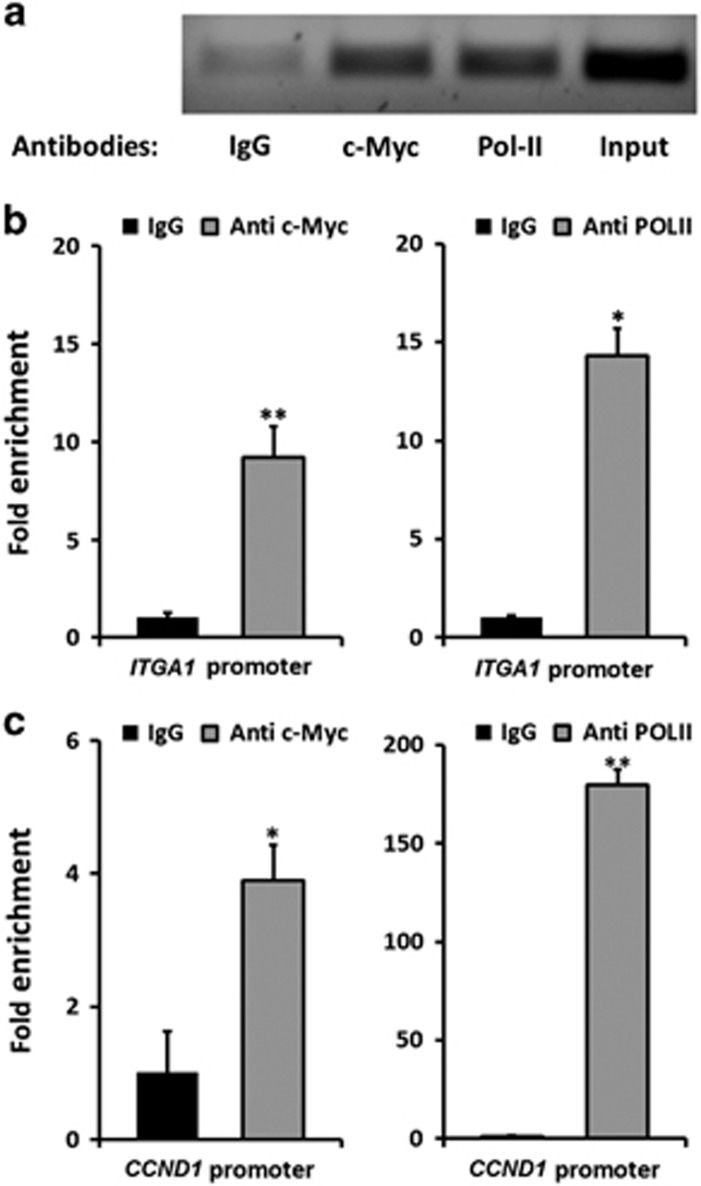
To determine whether MYC associates directly to the region of the ITGA1 promoter in native chromatin, we performed chromatin immunoprecipitation (ChIP) assays in HT29 cells. When using anti-c-Myc antibodies, a significant enrichment of the chromatin region containing the two presumed response elements was observed compared with the IgG control (Figures 3a and b). Occupancy of the promoter by transcription factors does not indicate whether the promoter is repressed or activated. Performing the ChIP assay using anti-RNA-polymerase II (Pol-II) antibodies resulted in a significant enrichment of the same chromatin region compared with the control (Figures 3a and b). As CCND1 is a known target of MYC,23, 24 we used its promoter containing the E-box CACGTG as a positive control. As shown in Figure 3c, there was higher chromatin enrichment when using anti-c-Myc or anti-RNA-Pol-II antibodies compared with the control IgG, indicating that MYC occupies the promoter of the CCND1 gene and activates its expression. These results strongly indicated that the ITGA1 promoter was active, as was the CCND1 promoter, at the time of its association with the MYC transcription factor.
Figure 3.
MYC occupies the ITGA1 promoter. (a) ChIP assay with an anti-human c-Myc-specific antibody or with a Pol-II antibody was performed in HT29 cells and real-time quantitative PCR (qPCR) amplification products were migrated on gel. Mock immunoprecipitation with mouse anti-IgG was performed as negative control. (b) Enrichment of the ITGA1 promoter region containing the MYC response elements was calculated by PCR quantification relative to the amount of control chromatin without response element for MYC. (c) Enrichment for the CCND1 promoter containing one complete EBOX response element was used as a positive control. Results represent the mean of three independent experiments, with qPCR performed in triplicate. *P<0.05, **P<0.01.
ITGA1 promoter activity is modulated by the MYC transcription factor
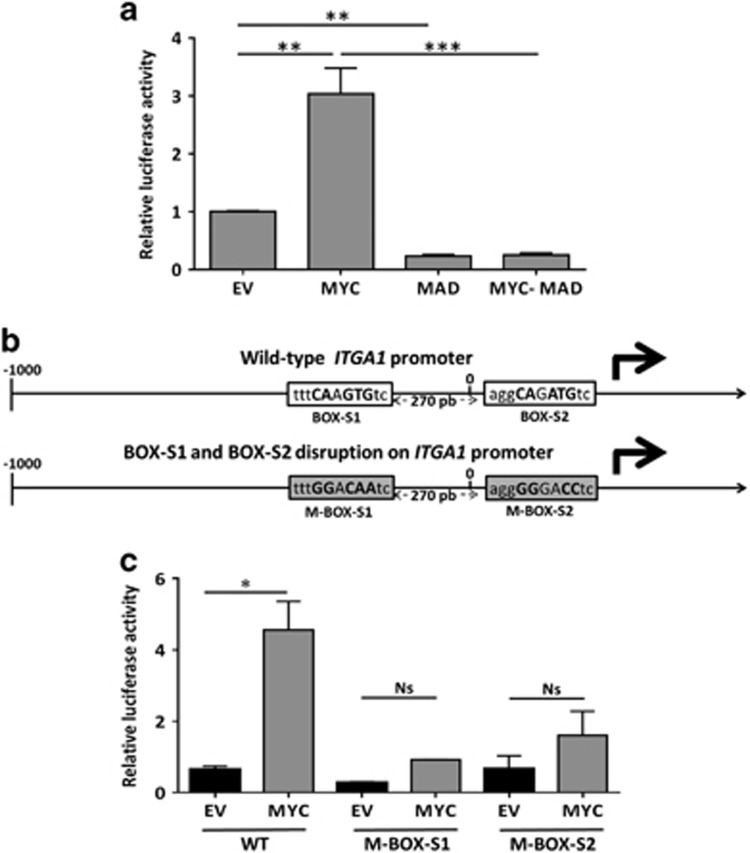
As a first step, the responsiveness of the ITGA1 promoter to MYC was studied in HEK293T cells that have a significant transfection rate. As shown in Figure 4a, MYC overexpression led to a higher activity of the ITGA1 promoter compared with the empty vector. This activity was reduced when equal amounts of MAD, a MYC competitor to binding with MAX,25, 26 was introduced alone or co-transfected with MYC (Figure 4a). As a result, ITGA1 promoter activity was lower than the basal activity, suggesting that MAD inhibited exogeneous as well as endogeneous MYC activities. This result confirmed that MYC regulates ITGA1 expression by inducing the activity of the ITGA1 promoter.
Figure 4.
MYC controls ITGA1 promoter activity at two response elements. (a) The ITGA1 promoter coupled to a Renilla luminescent reporter gene inserted into the pLightSwitch_Prom vector was transfected into HEK293T cells, together with empty vector (EV), a MYC expressing vector (MYC), the dominant negative MAD (MAD) or both (MYC–MAD). Promoter-dependent Renilla activities were determined as fold activation over Renilla activity with vector alone. (b) Schematic representation of the possible response elements that may bind the transcription factor MYC. These incomplete elements have been named BOX-S1 and BOX-S2. Bases in bold are common with the consensus sequence bases in the wild-type promoter. The gray boxes represent site disruption (M-BOX-S1 and M-BOX-S2) after base substitution (bold). (c) A promoter-gene reporter test was performed after transient transfection of HEK293T cells with the wild-type ITGA1 (WT) construct, or the ITGA1 promoter with a disrupted M-BOX-S1 or M-BOX-S2. For each experiment, transfected activators were EV or the vector encoding MYC. Results are represented as the mean±s.e.m. from three independent experiments, each performed in triplicate. *P<0.05, **P<0.01, ***P<0.001.
The transcription factor MYC controls different genes with direct binding to the cognate hexanucleotide CACGTG named the E-box.23, 27 The promoter of ITGA1 contains two response elements within the −400/+315 regulatory segment relative to the transcription start site (Figure 4b).16 The first was CAAGTG (BOX-S1) between −249 and −244 bp, having only one nucleotide (A in the place of C) different from the consensus E-box CACGTG. The second was CAGATG (BOX-S2) from +26 to +31 bp, with a high similarity to CACATG (at +28: G to C).23 To identify whether MYC could bind to the two response elements BOX-S1 and BOX-S2, we changed nucleotides to create two new sites M-BOX-S1 and M-BOX-S2 that could not be recognized by MYC (Figure 4b, bold letters in gray filled boxes). Mutation of the first or second site resulted in a significant decrease of luciferase activity induced by the ITGA1 promoter when activated by MYC compared with the nonmutated promoter (Figure 4c). Taken together, these results strongly suggest that MYC enhances the expression of ITGA1 by direct control from these two sites on the proximal promoter of the ITGA1 gene.
MYC and ITGA1 expression correlates in colorectal cancers
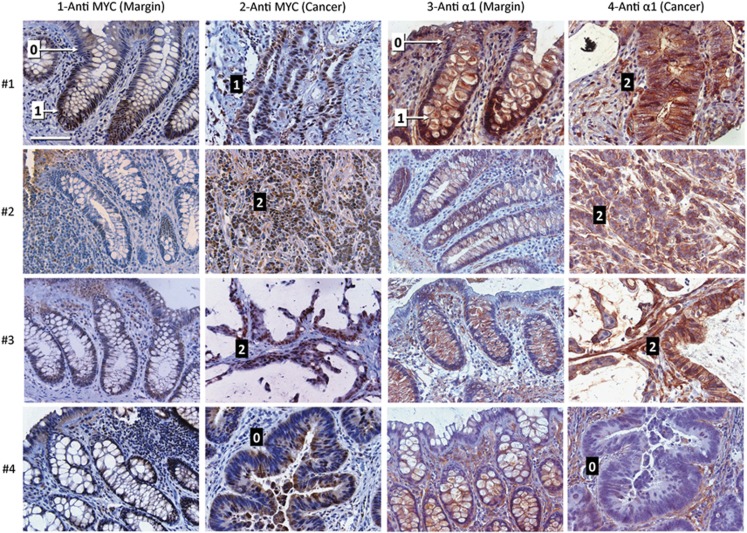
Having demonstrated that MYC controls ITGA1 expression in colorectal tumor cell lines, we investigated whether MYC and ITGA1 expression correlates in colorectal cancers. As ITGA1 is upregulated in both tumor and peritumoral stroma,14 observation of a possible correlation with MYC expression had to be performed by immunohistochemistry. ITGA1 and MYC expression were thus analyzed on tissue microarrays prepared previously from 65 patients including tumors of all 4 stages (see Material and methods for details) and their corresponding resection margins. A semiquantitative system for evaluating the staining intensity was based on the fact that both antigens are expressed in the lower crypts of the normal colon. Thus, in cancers, a score of 1 was given for a staining intensity equivalent to that in the lower half of the normal crypt, whereas a staining intensity equivalent to that of the upper crypt/surface epithelium was scored as 0. A score of 2 was given to a staining intensity stronger than that observed in the normal crypt. Representative images from four patients showing MYC and ITGA1 expression and scoring are shown in Figure 5. Only nuclear staining for MYC and basolateral staining for ITGA1 were considered for the scoring. Overall, as summarized in Table 2, MYC was found to be expressed in 34 of the 65 tumors (52%), whereas ITGA1 was present in 42 tumors (65%) as reported previously.14 MYC and ITGA1 expressions were found to be significantly correlated in 47 patients (72.30%, weighted κ=0.70, P<0.0001; gray boxes in Table 2). These immunohistochemical analyses support, as observed with the cell lines, that MYC is involved in the regulation of ITGA1 expression in colorectal cancer cells in situ.
Figure 5.
Representative immunohistochemical staining for MYC and ITGA1 expression in colon adenocarcinomas and their corresponding resection margins. Immunostaining for MYC and ITGA1 was performed on tissue microarrays containing cancer and corresponding resection margins for 65 patients as described in the Materials and methods. Representative staining from 4 patients (1–4) are illustrated. Row 1 illustrates patient 1 where MYC staining in the cancer was evaluated at ‘1' (black box), as it displays a similar staining intensity to that of the lower crypt in the resection margin (1, white box), whereas the ITGA1 staining, evaluated to be stronger than in the crypt of the resection margin (1, white box), was scored as a ‘2' (black box) in the cancer. In patients 2 and 3, staining intensity for both MYC and ITGA1 were scored at 2. For patient 4, staining intensity for MYC and ITGA1 in the tumor were both comparable to the weak labeling observed on the surface epithelium in the resection margin and were thus scored as ‘0'. For MYC and ITGA1, reference scores corresponding to the weak/negative staining of the upper gland/surface epithelium (0 in white boxes) and staining in the lower gland (1 in white boxes) are indicated in the margins of the images of patient 1. Black boxes display the scores that were given to each tumor for both MYC and ITGA1. Bar in upper left panel is 100 μm.
Table 2. MYC and ITGA1 protein expression correlates in colorectal cancers.
| MYC score | Total | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| ITGA1 score | ||||
| 0 | 21 | 2 | 0 | 23 |
| 1 | 8 | 15 | 1 | 24 |
| 2 | 2 | 5 | 11 | 18 |
| Total | 31 | 22 | 12 | 65 |
Immunohistochemical expression of MYC and ITGA1 were compared with their respective expression in the normal colonic epithelium as shown in Figure 5 where scores of 0, 1 or 2 were attributed in the tumor relative to the staining intensity observed in the corresponding resection margin for the upper gland/surface epithelium (=0), the lower half of the gland (=1) or stronger than the lower half of the gland (=2). MYC and ITGA1 expressions are significantly correlated in 72.3% of the studied tumors (47 cases, weighted κ=0.70, P<0.0001, bold values).
Discussion
In previous works, we have demonstrated that in the normal human intestine, integrin α1β1 expression is restricted to the lower crypts, the site of proliferating cells, and absent in most other epithelial cells.28 In colorectal cancers, we found ITGA1 in the tumor epithelium of 65% of cases.14 This proportion is consistent with our current observation with adenocarcinoma cell lines where ITGA1 was found to be expressed at significant levels in five of the seven tested cell lines. However, not much is known about the regulation of ITGA1 expression in colorectal cancer cells. In differentiating megakaryocytes, ITGA1 expression has been reported to be repressed by selective methylation of the proximal promoter.16 Notwithstanding that promoter methylation exists in these cells as illustrated with IGFBP7,22 the lack of methylated CpGs in the proximal region of the ITGA1 promoter and the fact that 5-aza-2′-deoxycytidine did not trigger ITGA1 expression in DLD1 and HCT116 cells suggests that ITGA1 downregulation in colorectal cancer cells is not DNA methylation dependent.
We thus looked for other mechanisms that could explain ITGA1 upregulation in colorectal cancer cells. Regulation of ITGA1 expression at the transcriptional level was first studied in smooth muscle cells where the ITGA1 promoter appears to be controlled by GATA6, Nkx3.2 and serum response factor transcription factors. However, although GATA6 has been reported to be implicated in colon cancer progression,29 neither serum response factor, which modulates the expression of ITGA1 at the CArG box present in the ITGA1 promoter,15, 30 nor Nkx3.2 expression have been characterized in colorectal cancer cells. N-Myc (MYCN), a member of the MYC family, has been reported to downregulate ITGA1 expression in neuroblastomas but the mechanism has not been elucidated.31 Interestingly, in the intestine MYCN is expressed in the nonproliferating cells of the villus whereas ITGA1, as we observed,28 and MYC32 are both expressed in the crypt, suggesting that MYC could be involved in the regulation of ITGA1. Incidentally, immunohistochemistry analysis of tissue microarrays prepared from 65 patients also revealed a clear correlation between MYC and ITGA1 expression in 72% of the colorectal cancers, strengthening the potential functional link between MYC and the expression of ITGA1 in situ.
The identification of two MYC response elements in the ITGA1 promoter and the functional studies presented herein suggest that MYC is a key regulator of ITGA1 expression in colorectal cancers. Indeed, we first observed that pharmacological inhibition of MYC led to a significant inhibition of ITGA1 expression in three distinct colorectal cancer cell lines. The 10058-F4 inhibitor was used at 50 μM, a concentration that has previously been reported to be sufficient to inhibit Myc transactivation and avoid cytotoxicity.33, 34, 35, 36 Despite a reduction in ITGA1 expression in the three treated cell lines, the inhibitor induced significant MYC downregulation only in HT29 and T84 cells. This effect could be dose dependent and/or due to cell-specific degradation of inactive MYC as previous studies have shown that 10058-F4 induces a dose-dependent reduction in MYC expression in some cell types.33, 34
ITGB1, the only partner of ITGA1 to form the integrin α1β1, contains the cognate CACGTG E-box in its proximal promoter.37 MYCN reduces ITGB1 expression in neuroblastoma cells,38 but MYC regulation of ITGB1 in intestinal epithelial cells has not been reported. The fact that, overall, ITGB1 expression was not significantly altered by pharmacological inhibition or short hairpin RNA silencing of MYC suggests a minor role for this factor on the ITGB1 promoter. It is noteworthy that a relatively weak MYC activity has also been reported for the ITGB4 promoter in colorectal cancer cells.39
In this context, it was important to determine whether ITGA1 is a direct target of MYC. First, ChIP assay confirmed that endogenous MYC binds directly to the chromatin region of the ITGA1 promoter and the positive control CCND1. MYC is a transcription factor that regulates a myriad of genes, ∼15% of all genes,20 either indirectly by inducing factors implicated in the DNA or RNA amplification process, thus acting as a general amplifier, or directly to regulate genes involved in cell proliferation, differentiation, apoptosis migration and invasion.21 MYC/MAX binding to promoters induces MYC interaction with various factors important for RNA-Pol-II recruitment and the elongation process.19 The presence of RNA-Pol-II on the promoter indicates that the gene, if not active, is ready for rapid activation.40, 41 In agreement with this report, our results showed enhanced chromatin enrichment of regions of the ITGA1 and CCND1 promoters when RNA-Pol-II was targeted, suggesting that both of these promoters were in an active state when associated with MYC. The reduction of ITGA1 promoter activity by MAD as observed in the luciferase assay is consistent with the fact that the MAD/MAX complex binds to the same MYC response elements.25, 42 Disruption of either one of the two response elements that we identified in the ITGA1 promoter induced a reduction in promoter activity, suggesting that both are necessary for MYC activation on this promoter. Numerous E-boxes other than the cognate CACGTG have been reported to be responsive43 to MYC/MAX transactivation activity as previously reported.23, 26, 43, 44 A reliable finding was observed in breast cancer for the BRCA1 promoter that contains two non-CACGTG response elements for MYC.45 In addition, high-throughput studies usually focus on the latter cognate element and thus underestimate other MYC-regulated genes such as BRCA1 or ITGA1 in the present study. MYC is known to be upregulated in colorectal cancers17, 18 and is associated with the deregulation of various signaling pathways that contribute to promoting proliferation and invasion of colorectal tumor cells.19, 20, 21 The fact that integrin α1β1 activates the Ras/MEK/ERK pathway,7, 9 which in turn enhances MYC expression,46 suggests that a positive regulatory loop between MYC and the integrin α1β1 could be a mechanism to maintain cell proliferation. This interplay between MYC and integrins has also been reported in colorectal cancer cells, where the antiproliferative ITGA6B splice variant inhibits MYC activity.47 MYC contribution to cell invasion could be, in part, driven by upregulation of the α1β1 integrin, as the latter is reported to activate FAK-Src and p130Cas/c-Jun N-terminal kinase to enhance colon cancer cell invasion.13 MYC regulates other integrin subunits, and regarding the various functions and implications of integrins in colon cancer cell proliferation, migration and survival,39, 47, 48, 49, 50 it is not surprising that the proto-oncogene MYC modulates integrin subunits such as ITGA6 and ITGB4 that are known to influence cancer progression.39, 51, 52 We identified herein a new member of this group by providing for the first time evidence that ITGA1 is a direct target for MYC in cancer.
Materials and methods
Cell culture, MYC inhibition and lentivirus-mediated RNA interference
Colorectal cancer cell lines T84, HT29, SW480, SW620, DLD1, HCT116, Caco-2/15 and transformed human embryonic kidney cells (HEK293T) were initially obtained from the American Type Culture Collection (Manassas, VA, USA) or the original investigator (Caco-2/15) and used from original stock kept frozen. All cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies, Burlington, ON, Canada) supplemented with 10% fetal bovine serum, 2 mM GlutaMAX and 10 mM Hepes, except T84 cells that were cultured in Dulbecco's modified Eagle's medium/F12 (Life Technologies) with 5% fetal bovine serum, 2.5 mM GlutaMAX, 15 mM Hepes and 0.5 mM pyruvate.47, 49, 50 All cells were maintained in a 5% CO2-humidified atmosphere at 37 °C and routinely tested for absence of mycoplasm contamination. HCT116 cells were treated with 10 μm of 5-aza-2′-deoxycytidine (Sigma-Aldrich, Oakville, ON, Canada) for 7 days, and the medium was changed every 24 h. T84, HT29 and SW480 cells were treated with the c-Myc inhibitor 10058-F4 (Sigma-Aldrich) (MYCi) at the indicated times and concentrations. Lentivirus sequences, containing short hairpin RNAs targeting MYC obtained from Sigma-Aldrich, were: sh1; 5′-CCGGATCATCATCCAGGACTGTATGCTCGAGCATACAGTCCTGGATGATGATTTTTTG-3′ and sh2; 5′-CCGGCCTGAGACAGATCAGCAACAACTCGAGTTGTTGCTGATCTGTCTCAGGTTTTTG-3′. Viruses were prepared in HEK293T cells as previously described.53 HT29, SW480 and T84 cells, at 60% confluence, were infected for 48 h and selected with puromycin (2.5–10 μg/ml) for 10 days.
RNA extraction and real-time quantitative PCR
Total RNA was extracted with RiboZol reagent according to the manufacturer's instructions (Amresco, OH, USA), quality tested (RNA integrity number >9.0) and reverse transcribed to complementary DNA using Omniscript (Qiagen, Toronto, ON, Canada) as described previously.54 Complementary DNA was amplified using primers specific for ITGA1: forward 5′-CATCAGGTGGGGATGGTAAG-3′ and reverse 5′-TGGCTCAAAATTCATGGTCA-3′, MYC forward 5′-CCTACCCTCTCAACGACAGC-3′ and reverse 5′-CTCTGACCTTTTGCCAGGAG-3′ and RPLP0 forward 5′-GCAATGTTGCCAGTGTCTG-3′ and reverse 5′-GCCTTGACCTTTTCAGCAA-3′. Quantitative PCR monitored with Brilliant II SYBR QPCR Low ROX Master Mix (Agilent, Mississauga, ON, Canada) was performed on a MX3000P Real-Time System (Stratagene, Mississauga, ON, Canada). Relative expression of each gene, normalized to RPLP0 expression,54 was evaluated according to the Pfaffl method55 following MIQE guidelines.56 All experiments were performed in triplicate and replicated three times.
Protein extraction and western blot analysis
Cells were scraped and lysed in Laemmli 1 × solution. After sonication, equal amounts of protein extracts were loaded and separated on 10% SDS–polyacrylamide gel electrophoresis gels under denaturing conditions (4% β-mercaptoethanol). Proteins were transferred onto a nitrocellulose membrane (GE Healthcare, Mississauga, ON, Canada), followed by blocking of nonspecific binding sites with a 10% blotto/0.1% Tween solution. Membranes were then incubated overnight at 4 °C with the following primary antibodies: anti-human integrin α1/CD49a (1:2000, AF5676, R&D Systems, Minneapolis, MN, USA), anti-c-Myc (1:5000, Y69, ab32072, Abcam, Toronto, ON, Canada), anti-β1 (1:1000, BD Biosciences, Mississauga, ON, Canada) and anti-β-actin (1:80 000, C4, Millipore, Etobicoke, ON, Canada) as endogenous loading control. After washing three times with phosphate-buffered saline/Tween 0.1%, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (anti-sheep, 12-342, Millipore; anti-mouse NA931V, anti-rat NA935V and anti-rabbit NA934V, Amersham, Mississauga, ON, Canada). Chemiluminescence was developed using the Immobilon Western Kit (Millipore, WBKLS0100). All experiments were performed in triplicate and repeated three times.
ITGA1 promoter in silico analysis and site-directed mutagenesis
The sequence between −800 and 489 relative to the transcription start site of the human ITGA1 promoter was analyzed for the MYC response element E-box (CACGTG)43, 57 using the MatInspector web-based search algorithm from Genomatix Software (Munich, Germany). The promoter sequence inserted in the pLightSwitch_prom plasmid vector was purchased from Switch Gear Genomics (Menlo Park, CA, USA). The two sites found on the promoter, which we named EBOX-S1 and EBOX-S2, were disrupted using the GeneArt Site-Directed Mutagenesis System following the manufacturer's instructions (Life Technologies). Pairs of oligonucleotide primers used were: EBOX-S1: forward 5′-CGACTTCACGGTGAATTTGGACAATCCGCAGGGGATGGAAGG-3′ and reverse 5′-CCTTCCATCCCCTGCGGATTGTCCAAATTCACCGTGAAGTCG-3′ and EBOX-S2: forward 5′-CACCCTCTCAATGAAAGGGGGACCTCCCTTTAAGGTTTGCTT-3′ and reverse 5'-AAGCAAACCTTAAAGGGAGGTCCCCCTTTCATTGAGAGGGTG-3′. Mutations of these promoter regions were confirmed by DNA sequencing.
Transient transfection and promoter-gene reporter assay
Plasmids were transfected using Effectine reagent following the manufacturer's instructions (Qiagen). Promoter activation reported by Renilla and luciferase activities was measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) and quantified using a luminometer according to the manufacturer's instructions (Berthold, Wildbad, Germany). The HEK293T cell line was used for all luciferase assays. Briefly, 24 h before transfection, HEK293T cells were plated in 12-well plates at 5 × 104 cells/well. Cells were transfected, according to each condition, with vectors expressing the Renilla-coupled ITGA1 promoter (100 ng/well), MYC (100 ng), MAD (100 ng) and firefly luciferase (2 ng) as the transfection control. Plates were incubated at 37 °C for 48 h. Experiments were performed in three separate experiments, each in triplicate.
Chromatin immunoprecipitation
ChIP assay was performed as previously described.53 Briefly, HT29, SW480 and T84 cells fixed with 1% paraformaldehyde (Fisher Scientific, Ottawa, ON, Canada) were scraped and lysed in cell lysis buffer. After nuclear extraction, DNA was sonicated (Branson Sonifier 250, Crystal Electronics, Newmarket, ON, Canada) to ∼1000 bp DNA fragments. DNA, precleared for 1 h with protein A agarose/salmon sperm DNA (Millipore), was incubated overnight at 4 oC with anti-c-Myc (9E10 ChIP grade, Abcam), anti-RNA-Pol-II (Millipore) or anti-IgG (Millipore) antibodies. DNA–protein–antibody complexes were then collected with agarose beads and washed. Immunoprecipitates were then eluted and DNA–protein crosslinks were reversed at 65 oC for 16 h. Samples were then treated with RNase A (Roche Diagnostics, Laval, QC, Canada) and proteinase K (Roche Diagnostics). Finally, DNA fragments were purified using a QIAquick spin kit (Qiagen). Differential chromatin enrichment was quantified using real-time quantitative PCR. The ITGA1 promoter region including the two sites identified for MYC was targeted for amplification using the forward 5′-CAGTGAGATTTCAGAGACCAAG-3′ and reverse 5′- CTGGCTGGGCCACTTATC-3′ primers. As the CCND1 gene (coding for CyclinD1) has been reported to be a target of c-Myc,23 we used its promoter as a positive control and the primers were: forward 5′-GAAACTTGCACAGGGGTTGT-3′ and reverse 5′-GCCAAAGAATCTCAGCGACT-3′. For a negative control segment without a MYC response element, we used a sequence located 5- kb upstream from the ITGA1 initiation start site; the primers were: forward 5′-GGAGGGAGAAACACCTATTTTA-3′ and reverse 5′-GGAACTTAAACTTCACCATGAG-3′. Results represent the mean of three independent experiments, performed in triplicate. For illustration purposes, real-time quantitative PCR amplification products were migrated on a 1% agarose gel (Roche Diagnostics) and images of bands were taken using a gel documentation system (MBI, Dorval, QC, Canada).
Tissue microarray and immunohistochemical analysis
MYC and ITGA1 expression were analyzed in adenocarcinoma tissues and paired margins, obtained from 65 patients with their written informed consent according to a protocol approved by the Institutional Human Subject Review Board of the Centre Hospitalier Universitaire de Sherbrooke. Tissue microarray preparation and characterization have been described previously.53 Tumor staging was according to TNM (tumor, node, metastasis) classification and the array included 8 stage 1, 23 stage 2, 26 stage 3 and 8 stage 4 tumor samples and corresponding resection margins. The immunohistochemical procedure for ITGA1 expression was as previously reported.14 The same protocol was used for MYC detection except for the use of a Tris-EDTA pH 8.0/Tween-20 0.05% buffer for antigen retrieval and incubation with anti-c-Myc rabbit primary antibody (clone Y69, ab32072, Abcam) at 4 °C overnight followed by incubation with anti-rabbit biotinylated secondary antibody (GE Healthcare) for 1 h at room temperature. The staining intensity for each paired tumor was scored as 0 or 1 for staining intensity corresponding to the surface epithelium or the lower half of the normal crypt, respectively, or 2 if stronger that the normal crypt as illustrated in Figure 5.
Statistical analysis and presentation of results
Prism 6.04 software (GraphPad Software, San Diego, CA, USA) was used for data presentation and statistical calculation using the Student's paired t-test (two sided). Results are presented as mean±s.e.m. and those with P≤0.05 were considered to be statistically significant. For correlation analysis, SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA) and StatXact software version 6 (Cytel Software Corporation, Cambridge, MA, USA) were used and the weighted κ (quadratic) was calculated for correlation significance between MYC and ITGA1.
Acknowledgments
We thank Elizabeth Herring for technical support and reviewing the manuscript, Eric Tremblay for virus production, Dr Marie-Pierre Garand of the Biostatistics Facility of the Centre de Recherche du CHUS for assistance in statistical analyses and Dr Andrea Quaroni (Cornell University, Ithaca) for the gift of Caco-2/15 cells. This work was supported by the Canadian Institute of Health Research Grants MOP-97836 and MOP-123415 (to J-FB). J-FB is the recipient of the Canadian Research Chair in Intestinal Physiopathology. JCC is a scholar of the Fonds de la Recherche du Québec–Santé (FRQS). J-FB and JCC are members of the Fonds de la Recherche en Santé du Québec-funded Centre de Recherche of the Centre Hospitalier Universitaire de Sherbrooke.
The authors declare no conflict of interest.
References
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 2010; 339: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol 2007; 8: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 2010; 11: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 1997; 9: 691–700. [DOI] [PubMed] [Google Scholar]
- Beausejour M, Noel D, Thibodeau S, Bouchard V, Harnois C, Beaulieu JF et al. Integrin/Fak/Src-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: selective engagement and roles of PI3-K isoform complexes. Apoptosis 2012; 17: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Signal Transduct 2011; 2011: 738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol 2000; 2: E13–E14. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 1998; 94: 625–634. [DOI] [PubMed] [Google Scholar]
- Abair TD, Bulus N, Borza C, Sundaramoorthy M, Zent R, Pozzi A. Functional analysis of the cytoplasmic domain of the integrin {alpha}1 subunit in endothelial cells. Blood 2008; 112: 3242–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Navre M, Werb Z, Bissell MJ. alpha1 and alpha2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol Biol Cell 1999; 10: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zeisberg M, Lively JC, Nyberg P, Afdhal N, Kalluri R. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res 2003; 63: 8312–8317. [PubMed] [Google Scholar]
- Macias-Perez I, Borza C, Chen X, Yan X, Ibanez R, Mernaugh G et al. Loss of integrin alpha1beta1 ameliorates Kras-induced lung cancer. Cancer Res 2008; 68: 6127–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Slambrouck S, Grijelmo C, De Wever O, Bruyneel E, Emami S, Gespach C et al. Activation of the FAK-src molecular scaffolds and p130Cas-JNK signaling cascades by alpha1-integrins during colon cancer cell invasion. Int J Oncol 2007; 31: 1501–1508. [PubMed] [Google Scholar]
- Boudjadi S, Carrier JC, Beaulieu JF. Integrin alpha1 subunit is up-regulated in colorectal cancer. Biomark Res 2013; 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Hayashi K, Nishida W, Momiyama T, Uchida A, Ochi T et al. Smooth muscle cell phenotype-dependent transcriptional regulation of the alpha1 integrin gene. J Biol Chem 1997; 272: 26643–26651. [DOI] [PubMed] [Google Scholar]
- Cheli Y, Kanaji S, Jacquelin B, Chang M, Nugent DJ, Kunicki TJ. Transcriptional and epigenetic regulation of the integrin collagen receptor locus ITGA1-PELO-ITGA2. Biochim Biophys Acta 2007; 1769: 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora K, Chan S, Evan G, Gabra H, Markham N, Stewart J et al. c-myc oncogene expression in colorectal cancer. Cancer 1987; 59: 1289–1295. [DOI] [PubMed] [Google Scholar]
- Erisman MD, Rothberg PG, Diehl RE, Morse CC, Spandorfer JM, Astrin SM. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol 1985; 5: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. MYC on the path to cancer. Cell 2012; 149: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol 2006; 16: 253–264. [DOI] [PubMed] [Google Scholar]
- Sabo A, Kress TR, Pelizzola M, de Pretis S, Gorski MM, Tesi A et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature 2014; 511: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Igarashi S, Nojima M, Maruyama R, Yamamoto E, Kai M et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis 2010; 31: 342–349. [DOI] [PubMed] [Google Scholar]
- Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J et al. Genomic targets of the human c-Myc protein. Genes Dev 2003; 17: 1115–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Li TW, Ko KS, Xia M, Lu SC. Switch from Mnt-Max to Myc-Max induces p53 and cyclin D1 expression and apoptosis during cholestasis in mouse and human hepatocytes. Hepatology 2009; 49: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer DE, Kretzner L, Eisenman RN. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell 1993; 72: 211–222. [DOI] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol 2000; 16: 653–699. [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 1991; 251: 1211–1217. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 1992; 102: 427–436. [DOI] [PubMed] [Google Scholar]
- Belaguli NS, Aftab M, Rigi M, Zhang M, Albo D, Berger DH. GATA6 promotes colon cancer cell invasion by regulating urokinase plasminogen activator gene expression. Neoplasia 2010; 12: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida W, Nakamura M, Mori S, Takahashi M, Ohkawa Y, Tadokoro S et al. A triad of serum response factor and the GATA and NK families governs the transcription of smooth and cardiac muscle genes. J Biol Chem 2002; 277: 7308–7317. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Fukuzawa M. MYCN downregulates integrin alpha1 to promote invasion of human neuroblastoma cells. Int J Oncol 2008; 33: 815–821. [PubMed] [Google Scholar]
- Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S et al. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol 2005; 25: 7868–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MJ, Cheng YC, Liu CR, Lin S, Liu HE. A small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle arrest, apoptosis, and myeloid differentiation of human acute myeloid leukemia. Exp Hematol 2006; 34: 1480–1489. [DOI] [PubMed] [Google Scholar]
- Lin CP, Liu JD, Chow JM, Liu CR, Liu HE. Small-molecule c-Myc inhibitor, 10058-F4, inhibits proliferation, downregulates human telomerase reverse transcriptase and enhances chemosensitivity in human hepatocellular carcinoma cells. Anticancer Drugs 2007; 18: 161–170. [DOI] [PubMed] [Google Scholar]
- Mo H, Henriksson M. Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation. Proc Natl Acad Sci USA 2006; 103: 6344–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Giap C, Lazo JS, Prochownik EV. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene 2003; 22: 6151–6159. [DOI] [PubMed] [Google Scholar]
- Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B. A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells. Proc Natl Acad Sci USA 2003; 100: 8164–8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golen CM, Soules ME, Grauman AR, Feldman EL. N-Myc overexpression leads to decreased beta1 integrin expression and increased apoptosis in human neuroblastoma cells. Oncogene 2003; 22: 2664–2673. [DOI] [PubMed] [Google Scholar]
- Ni H, Dydensborg AB, Herring FE, Basora N, Gagne D, Vachon PH et al. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005; 24: 6820–6829. [DOI] [PubMed] [Google Scholar]
- Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, Richmond TA et al. A high-resolution map of active promoters in the human genome. Nature 2005; 436: 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 2007; 130: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Queva C, Eisenman RN. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev 1997; 11: 44–58. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Kretzner L, Blackwood EM, Eisenman RN, Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science 1990; 250: 1149–1151. [DOI] [PubMed] [Google Scholar]
- Oster SK, Ho CS, Soucie EL, Penn LZ. The myc oncogene: MarvelouslY Complex. Adv Cancer Res 2002; 84: 81–154. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu J, Borowicz S, Collins C, Huo D, Olopade OI. c-Myc activates BRCA1 gene expression through distal promoter elements in breast cancer cells. BMC Cancer 2011; 11: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff E, Houben R, Loffler S, Troppmair J, Lee JE, Rapp UR. Regulation of c-myc expression by Ras/Raf signalling. Oncogene 1998; 16: 211–216. [DOI] [PubMed] [Google Scholar]
- Dydensborg AB, Teller IC, Groulx JF, Basora N, Pare F, Herring E et al. Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer 2009; 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit YD, Larrivee JF, Groulx JF, Stankova J, Vachon PH, Beaulieu JF. Integrin alpha8beta1 confers anoikis susceptibility to human intestinal epithelial crypt cells. Biochem Biophys Res Commun 2010; 399: 434–439. [DOI] [PubMed] [Google Scholar]
- Basora N, Desloges N, Chang Q, Bouatrouss Y, Gosselin J, Poisson J et al. Expression of the alpha9beta1 integrin in human colonic epithelial cells: resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int J Cancer 1998; 75: 738–743. [DOI] [PubMed] [Google Scholar]
- Groulx JF, Giroux V, Beausejour M, Boudjadi S, Basora N, Carrier JC et al. Integrin alpha6A splice variant regulates proliferation and the Wnt/beta-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014; 35: 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Tanaka Y, Liu ZJ, Yagita H, Okumura K, Kosugi A et al. Down-regulation of alpha6 integrin, an anti-oncogene product, by functional cooperation of H-Ras and c-Myc. Genes Cells 2001; 6: 337–343. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF. Integrin alpha6beta4 in colorectal cancer. World J Gastrointest Pathophysiol 2010; 1: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudjadi S, Bernatchez G, Beaulieu JF, Carrier JC. Control of the human osteopontin promoter by ERRalpha in colorectal cancer. Am J Pathol 2013; 183: 266–276. [DOI] [PubMed] [Google Scholar]
- Dydensborg AB, Herring E, Auclair J, Tremblay E, Beaulieu JF. Normalizing genes for quantitative RT-PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am J Physiol Gastrointest Liver Physiol 2006; 290: G1067–G1074. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA et al. MIQE precis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 2010; 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Chung S, Kim KM, Jung KC, Park C, Hahm ER et al. Determination of binding constant of transcription factor myc-max/max-max and E-box DNA: the effect of inhibitors on the binding. Biochim Biophys Acta 2004; 1670: 217–228. [DOI] [PubMed] [Google Scholar]