Abstract
Sex-determining region Y box 6 (SOX6) has been described as a tumor-suppressor gene in several cancers. Our previous work has suggested that SOX6 upregulated p21Waf1/Cip1(p21) expression in a p53-dependent manner; however, the underlying mechanism has remained elusive. In this study, we confirmed that SOX6 can suppress cell proliferation in vitro and in vivo by stabilizing p53 protein and subsequently upregulating p21. Co-immunoprecipitation and immunocytofluorescence assays demonstrated that SOX6 can promote formation of the p14ARF-HDM2-p53 ternary complex by promoting translocation of p14ARF (p14 alternate reading frame tumor suppressor) to the nucleoplasm, thereby inhibiting HDM2-mediated p53 nuclear export and degradation. Chromatin immunoprecipitation combined with PCR assay proved that SOX6 can bind to a potential binding site in the regulatory region of the c-Myc gene. Furthermore, we confirmed that SOX6 can downregulate the expression of c-Myc, as well as its direct target gene nucleophosmin 1 (NPM1), and that the SOX6-induced downregulation of NPM1 is linked to translocation of p14ARF to the nucleoplasm. Finally, we showed that the highly conserved high-mobility group (HMG) domain of SOX6 is required for SOX6-mediated p53 stabilization and tumor inhibitory activity. Collectively, these results reveal a new mechanism of SOX6-mediated tumor suppression involving p21 upregulation via the p14ARF-HDM2-p53 axis in an HMG domain-dependent manner.
Introduction
SOX (Sry-type HMG box) family proteins are a conserved group of transcriptional regulators defined by the presence of a highly conserved high-mobility group (HMG) domain that mediates their capacity for DNA binding.1, 2, 3 SOX proteins are expressed in various cell lineages, where they have critical roles in cell fate determination and in differentiation of developing tissues. Depending on their interacting partners and target sequences, SOX proteins can act as either transcriptional activators or repressors to modulate expression of different genes.3
SOX proteins are classified into eight groups (A–H), based on the amino-acid sequences in the HMG domain.1, 2 SOX6 (sex-determining region Y box 6) belongs to the D group, and its HMG domain recognizes the (A/T)(A/T)CAA(A/T) highly conserved sequence. However, binding of SOX6 to DNA alone does not elicit transcriptional regulation, and binding of other transcriptional factors to adjacent DNA sites is required.1, 4, 5 Studies by our lab and others have confirmed the tumor-suppressive function of SOX6 in various human malignancies, including chronic myeloid leukemia,6 esophageal squamous cell carcinoma7 and hepatocellular carcinoma.8, 9 SOX6 can act as either a positive or negative modulator of its various target genes. For example, SOX6 suppresses cell proliferation by promoting SOCS3 expression in K562 and primary erythroid cells, and this process is mediated by binding of the HMG domain to its double-binding site in the regulatory region of the SOCS3 gene.6 Whereas in pancreatic β-cells, SOX6 suppresses cyclin D1 expression by binding with β-catenin and HDAC1 at the CCND1 gene promoter, which leads to suppression of cell proliferation.10 However, the underlying mechanisms involved in the tumor suppressor roles of SOX6 have not been fully addressed.
We have previously reported that SOX6 can inhibit HCC cell proliferation via upregulation of the p21 protein in a p53-dependent manner.8 However, little is known about the mechanism that mediates the role of SOX6 in p53 activation. In the current study, we identified a new mechanism by which the SOX6-mediated decrease in expression of c-Myc and nucleophosmin 1 (NPM1) may lead to the stabilization and activation of p53 protein through the p14ARF-HDM2-p53 axis; ultimately, these molecular events contribute to the HMG domain-dependent tumor inhibitory activity of SOX6.
Results
SOX6-mediated suppression of cell proliferation is HMG domain-dependent
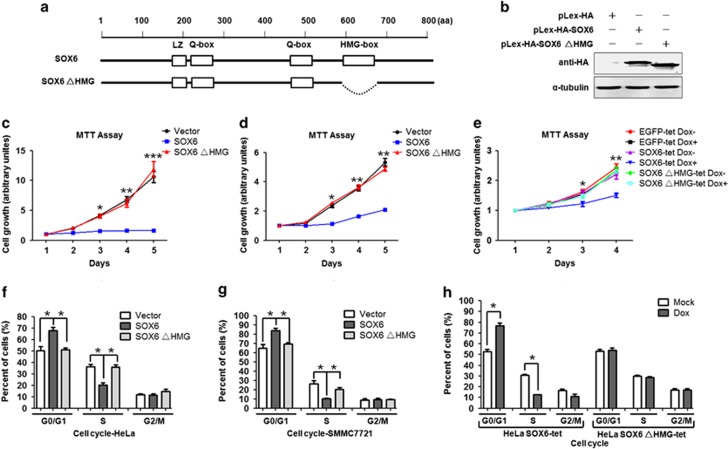
The HMG domain is crucial for the function of SOX6.6, 10 To explore whether the HMG domain is required for suppression of cell proliferation induced by SOX6, both wild-type and HMG domain-deleted (ΔHMG) SOX6 expression plasmids were generated (Figures 1a and b) and transiently transfected into HeLa and SMMC7721 cells. MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay showed that overexpression of SOX6 significantly suppressed proliferation of the HeLa and SMMC7721 cells, as compared with control cells transfected with empty vector. In contrast, transfection with the SOX6ΔHMG mutant had no effect on cell proliferation (Figures 1c and d). In line with these findings, doxycycline (Dox)-mediated conditional expression of SOX6 in HeLa SOX6-tet cells also led to significantly suppressed cell proliferation, and this effect was absent in HeLa EGFP-tet cells as well as in HeLa SOX6ΔHMG-tet cells (Figure 1e). Flow cytometry analysis showed that both SOX6-transfected HeLa and SMMC7721 cells had significantly increased percentage of the G0/G1-phase cells and decreased percentage of the S-phase cells, which was absent in the SOX6ΔHMG-transfected cells (Figures 1f and g and Supplementary Figure S1A). Meanwhile, Dox-induced SOX6 expression in the HeLa SOX6-tet cells also led to cell cycle arrest in the G0/G1 phase, but not in the HeLa SOX6ΔHMG-tet cells (Figure 1h and Supplementary Figure S1B). Moreover, no effect of SOX6 on cell apoptosis was observed (Supplementary Figure S1C). Taken together, these results suggest that SOX6 might act as an important suppressor of cell proliferation and that such function is critically dependent on the presence of the HMG domain.
Figure 1.
HMG domain mediates SOX6-induced suppression of cell proliferation via cell cycle arrest in the G0/G1 phase. (a) Schematic of SOX6 and SOX6ΔHMG (HMG domain deletion) depicting the functional domain. (b) Ectopic expression of SOX6 or SOX6ΔHMG was measured in HeLa cells transfected with pLex-HA-SOX6 or pLex-HA-SOX6ΔHMG vectors. Proliferation of HeLa (c) or SMMC7721 (d) cells transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector, or of HeLa-tet cells (e) was measured using MTT assay. For the HeLa-tet cells, each cell type was cultured in medium with Dox (2 μg/ml) or control medium for 96 h. Data (c–e) shown are the mean±s.d. of 5 independent experiments (*P<0.05, **P<0.01 and ***P<0.001, Student's t-test, two-sided). The column diagram of the cell cycle of HeLa (f) or SMMC7721 (g) cells transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector, or of HeLa-tet cells (h), was measured using flow cytometry. Data (f–h) shown are the mean±s.d. of 5 independent experiments (*P<0.05, χ2 test, two-sided). The statistical tests (c–h) are justified as appropriate and meet the assumptions of the tests. The variance between the groups is similar. aa, Amino acids.
SOX6-mediated suppression of xenograft tumorigenesis is HMG domain-dependent
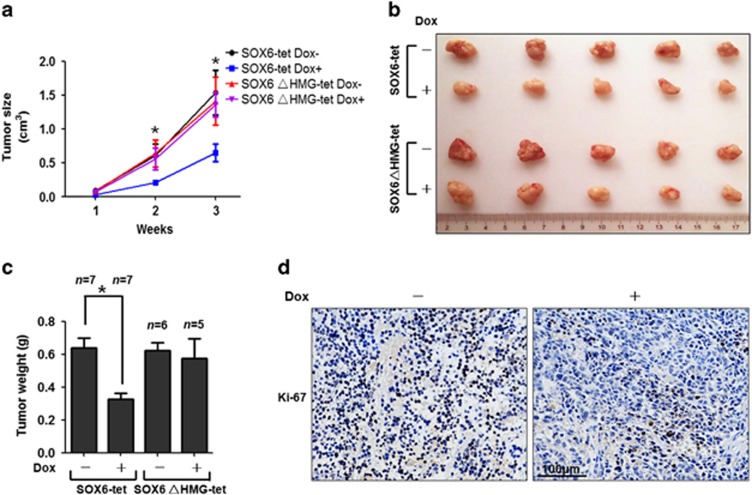
We further investigated whether the HMG domain was involved in the tumor inhibitory activity of SOX6 in vivo. HeLa SOX6-tet or SOX6ΔHMG-tet cells were subcutaneously injected into the flanks of nude mice. In parallel, one group of mice was fed with Dox dissolved in 10% sucrose solution, and a second group of mice was fed with 10% sucrose solution to serve as the control. As expected, the Dox feeding induced suppression of xenograft tumorigenesis in the mice injected with the HeLa SOX6-tet cells, but not in the mice injected with the HeLa SOX6ΔHMG-tet cells. As shown in Figures 2a and b, the between-group difference in tumor size reached statistical significance on day 14 postinjection and the difference became much more significant at day 21, when the mice were killed under anesthesia. Similarly, weights of the tumor blocks in mice injected with the HeLa SOX6-tet cells were significantly smaller in the Dox-treated group compared with that in non-Dox-treated mice (Figure 2c). In contrast, the Dox treatment produced no obvious effects on the tumor size and weight in mice injected with the HeLa SOX6ΔHMG-tet cells (Figures 2a–c). Moreover, immunohistochemical assay revealed that the percentage of Ki-67-positive cells in tumors formed by the HeLa SOX6-tet cells was lower in the presence of Dox compared with that in the absence of Dox (Figure 2d). These results suggest that SOX6 can suppress xenograft tumorigenesis in vivo, and that this effect is HMG domain-dependent.
Figure 2.
HMG domain mediates SOX6-induced suppression of tumorigenesis. (a) Growth curve of tumors formed by subcutaneous injection of HeLa SOX6-tet or SOX6ΔHMG-tet cells into the flank of nude mice, after which the mice were fed with or without Dox (1 mg/ml) in a 10% sucrose solution. Data shown are the mean±s.d. (*P<0.05, Student's t-test, two-sided). (b) Representative tumor blocks from each group taken at killing. (c) Average tumor weights (g) of tumor blocks. Data shown are the mean±s.d. (n, number of mice in each group, *P<0.05, Student's t-test, two-sided). (d) Representative micrographs showing Ki-67 staining in tumor tissues from the mice injected with HeLa SOX6-tet cells and fed without (left panel) or with (right panel) Dox. The statistical tests (a and c) are justified as appropriate and meet the assumptions of the tests. The variance between the groups is similar.
HMG domain and p53 are required for SOX6-induced p21 upregulation
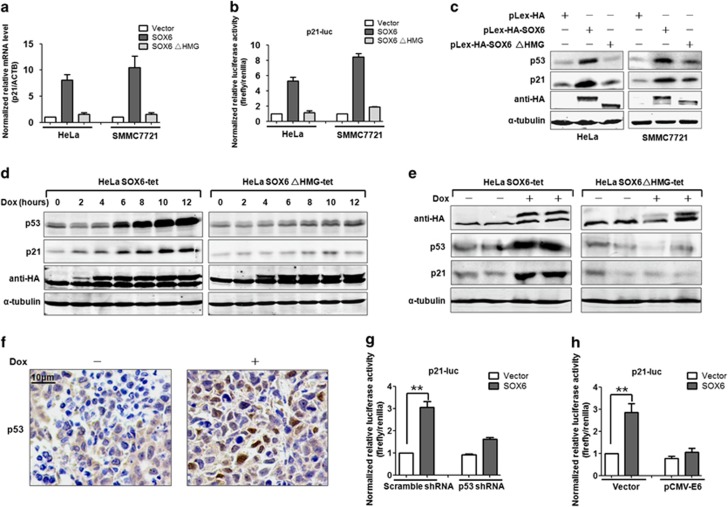
Previous studies have demonstrated that SOX6 can upregulate the protein level of p21.7, 8 To test whether SOX6-induced p21 expression was dependent upon the presence of its HMG domain, the mRNA level and promoter activity of the p21 gene was detected in HeLa and SMMC7721 cells that had been transiently transfected with SOX6 or SOX6ΔHMG expression plasmids. Quantitative reverse transcription–PCR (qPCR) indicated that ectopic-SOX6 expression in both HeLa and SMMC7721 cells led to obvious increase in p21 mRNA level (Figure 3a). In line with the observed mRNA changes, the dual-luciferase assay showed that ectopic-SOX6 expression could increase the transcriptional activity of the p21 gene promoter (Figure 3b). In contrast, ectopic expression of SOX6ΔHMG failed to induce p21 expression. These data demonstrate the necessity of the HMG domain for SOX6-induced p21 expression.
Figure 3.
HMG domain mediates p21 activation in a p53-dependent manner. (a) The mRNA levels of p21 were measured by qPCR (SYBR Green) in HeLa and SMMC7721 cells that had been transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. β-Actin (ACTB) was used as the internal control. Data shown are the mean±s.d. of six independent experiments performed in triplicate. (b) A dual-luciferase assay was performed to analyze the SOX6-mediated regulation of the p21 promoter in HeLa and SMMC7721 cells that had been co-transfected with pGL3-p21-luciferase, PRL-TK and pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. Data shown are the mean±s.d. of five independent experiments performed in triplicate. (c) Western blot analysis of p53 and p21 protein levels in HeLa and SMMC7721 cells that had been transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. α-Tubulin was used as the internal control. (d) Western blot analysis of p53 and p21 protein levels in HeLa SOX6-tet or SOX6△HMG-tet cells along with the time after addition of Dox (2 μg/ml). (e) Western blot analysis of p53 and p21 protein levels in tumor lysates of nude mice. (f) Representative micrographs showing p53 staining of tumor tissues from the mice that had been injected with HeLa SOX6-tet cells and fed without (left panel) or with (right panel) Dox. (g) A dual-luciferase assay was conducted in HeLa cells co-transfected with pGL3-p21-luciferase, PRL-TK, pLex-HA-SOX6 and p53 shRNA vector or scrambled shRNA (control) vector. (h) A dual-luciferase assay was conducted in HeLa cells co-transfected with pGL3-p21-luciferase, PRL-TK, SOX6 and pCMV-E6 or control vector. Data shown (g and h) are the mean±s.d. of five independent experiments (**P<0.01, Student's t-test, two-sided). The statistical tests are justified as appropriate and meet the assumptions of the tests. The variance between the groups is similar.
Our previous work identified the involvement of p53 in SOX6-induced p21 upregulation.8 Consistent with this, the overexpression of SOX6 in both HeLa and SMMC7721 cells led to the simultaneous upregulation of p53 and p21 protein levels (Figure 3c). On the other hand, stable knockdown of SOX6 expression in HeLa cells led to a decrease in the levels of both p53 and p21 protein (Supplementary Figure S2A). To further demonstrate the above observation, we repeated the experiment in HeLa SOX6-tet cells. Consistent with the results obtained from the transient transfection system, protein levels of both p53 and the direct downstream molecule p21 were simultaneously increased in a time-dependent manner (Figure 3d). This finding was also supported by the observed upregulation of p53 and p21 proteins in the xenograft tumor tissues from the Dox-treated mice that had been injected with the HeLa SOX6-tet cells (Figures 3e and f). The above results indicated that SOX6 can upregulate p21 expression by increasing the protein levels of p53.
It is known that p53 is inactivated by the human papillomavirus (HPV) E6 protein in the HPV-positive HeLa cell line, and that this process is mediated by ubiquitination-dependent degradation. However, we were still able to detect the endogenous p53 protein in the HeLa cell line, perhaps owing to the incapability of endogenous E6 protein to completely degrade all p53 proteins (Figures 3c–e). Thus, SOX6 may upregulate p21 in HeLa cells by reversing the inactivation of p53.
To further confirm if the SOX6-induced transcriptional activation of p21 may be dependent on p53, we performed dual-luciferase assays in HeLa cells with ectopically expressed short hairpin RNA (shRNA) against the p53 or E6 protein. The SOX6-mediated p21 activation was significantly reduced when p53 was further inactivated (Figures 3g and h). Moreover, Huh7 cells that harbored a p53 loss-of-function mutant showed neither the SOX6-induced upregulation of p21 expression nor inhibition of cell proliferation (Supplementary Figures S2B–S2D). Collectively, these data provide additional evidence that p53 activation is required for the SOX6-induced upregulation of p21 expression.
SOX6 stabilizes p53 protein by inhibiting nuclear export and ubiquitination of the p53 protein
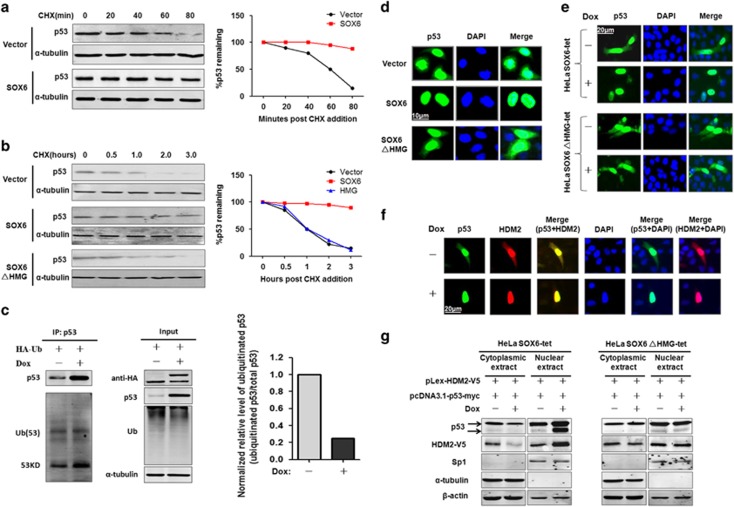
To better understand the mechanism by which SOX6 activates p53, we first used qPCR to detect the p53 mRNA level in both HeLa and SMMC7721 cells that overexpressed SOX6. However, the p53 mRNA level remained unchanged in both cell types (Supplementary Figure S3A). We next used a turnover assay to test whether SOX6 could affect the ubiquitination-dependent degradation of p53. As shown in Figures 4a and b, overexpression of SOX6, and not SOX6ΔHMG, significantly extended the half-life of endogenous p53 protein in both HeLa and SMMC7721 cells. Consistent with this result, the ubiquitination of p53 protein was markedly decreased (Figure 4c). The above results suggest that SOX6 upregulates p53 by inhibiting its ubiquitination-dependent degradation, rather than by promoting its transcription.
Figure 4.
SOX6 increases the stability of p53 protein by inhibiting the nuclear export and ubiquitination of p53. Turnover assay of p53 protein in HeLa cells (a) and SMMC7721 cells (b) that had been transfected with SOX6, SOX6ΔHMG or control expression vector with cycloheximide (CHX) (100 μg/ml) treatment at 48 h post transfection for the indicated times. (c) Immunoprecipitation and western blot assays were conducted to determine the effect of SOX6 on p53-ubiquitination/HDM2 activity in HeLa SOX6-tet cells transfected with a ubiquitination expression vector and treated with or without Dox (2 μg/ml) for 48 h. (d) Immunocytofluorescence staining of p53 in HeLa cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. Nuclei (blue) are stained with 4'-6-diamidino-2-phenylindole (DAPI). (e) Immunocytofluorescence staining of p53 in HeLa SOX6-tet cells that had been transfected with pcDNA3.1-p53-myc and treated with or without Dox (2 μg/ml) for 48 h. (f) Immunocytofluorescence staining of p53 and HDM2 in the same field of HeLa SOX6-tet cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HDM2-V5 plasmids. Colocalization of p53 and HDM2 was analyzed by merging the images of p53 and HDM2 in the same field. (g) Western blot was performed to detect the cytoplasmic or nuclear protein levels of p53 and HDM2 in HeLa SOX6-tet and SOX6ΔHMG-tet cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HDM2-V5 plasmids. Cytoplasmic and nuclear proteins were extracted at 48 h of transfection, and purity of the nuclear and cytoplasmic extracts was indicated by SP1 and α-tubulin, respectively.
It is well known that HDM2, a RING-finger ubiquitin E3 ligase, can initiate the ubiquitination-dependent degradation of p53 protein in the cytoplasm upon binding to and exporting p53 to the cytoplasm, and that phosphorylation of the p53 protein sites Ser15, Ser20 and Ser37 can block this HDM2-mediated activity.11 To elaborate the mechanism of SOX6-mediated p53 stabilization, we assessed the phosphorylated levels of p53 in SOX6-overexpressing cells. However, we observed no effect of SOX6 on the phosphorylation status of the p53 protein (Supplementary Figure S3B). Nor did we observe any effect of SOX6 on the interaction of HDM2 and p53, suggesting that the decreased ubiquitination of the p53 protein might involve inhibition of the p53-ubiquitination activity of HDM2 (Supplementary Figure S3C). Because the p53 protein is known to be primarily ubiquitinated and degraded in the cytoplasm,11 we considered the possibility that SOX6 is able to reduce the export of p53 protein from the nucleus to the cytoplasm, consequently stabilizing the p53 protein. As expected, SOX6 significantly induced the nuclear accumulation of the p53 protein (Figures 4d and e). As blocking of the HDM2-mediated nuclear export is known to abrogate HDM2-mediated p53 degradation,11, 12 we next detected the subcellular localization of HDM2 in the HeLa SOX6-tet cells and found that HDM2 and p53 were simultaneously accumulated in the nucleus following induction of SOX6 expression (Figure 4f). To confirm this observation, the cytoplasmic and nuclear protein fractions of HeLa SOX6-tet cells were separated and subjected to western blot assay. The nuclear levels of HDM2 and p53 proteins were significantly increased after Dox treatment, whereas the cytoplasmic levels of HDM2 and p53 proteins were decreased (Figure 4g). Likewise, the SOX6ΔHMG mutant had no effect on the nuclear accumulation of p53 and HDM2. Taken together, these results suggest that overexpression of SOX6 can effectively inhibit the nuclear export and ubiquitination of p53, subsequently stabilizing p53 in an HMG-dependent manner.
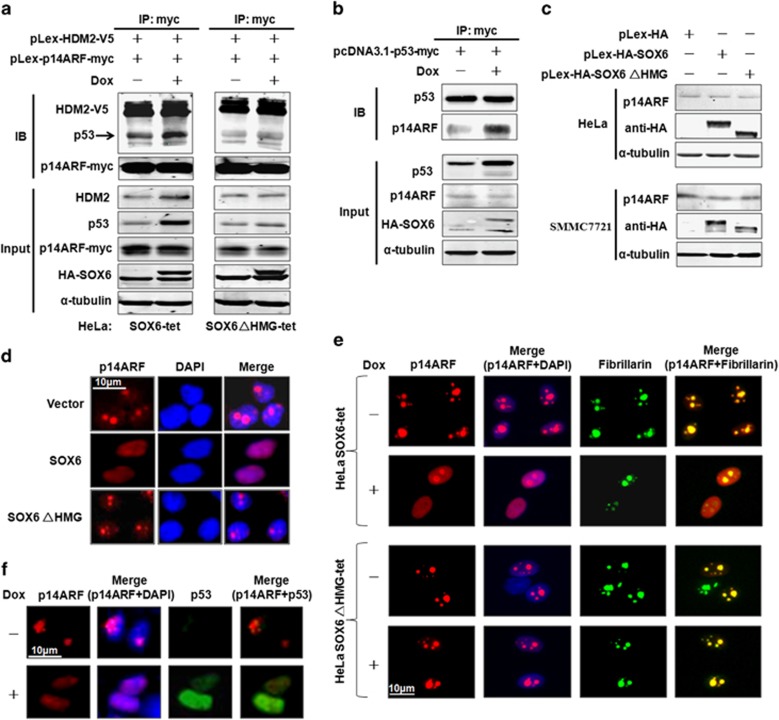
SOX6 promotes formation of the p14ARF-HDM2-p53 ternary complex by inducing translocation of p14ARF to the nucleoplasm
Previous studies have revealed that the p14 alternate reading frame tumor suppressor (p14ARF) is capable of inhibiting the nuclear export and p53-ubiquitination activity of HDM2, as well as increasing the stability of the p53 protein by forming the p14ARF-HDM2-p53 ternary complex.13, 14, 15 To test whether SOX6 can promote formation of the p14ARF-HDM2-p53 complex, HeLa SOX6-tet cells were co-transfected with pLex-p14ARF-myc and pLex-HDM2-V5 plasmids, and the cell lysates were subjected to co-immunoprecipitation assay. More p53 protein was pulled down in the anti-myc-tagged p14ARF immunoprecipitates of the Dox-treated HeLa SOX6-tet cells compared with that of the HeLa SOX6ΔHMG-tet cells (Figure 5a). Conversely, more p14ARF protein was pulled down in the anti-myc-tagged p53 immunoprecipitates of the Dox-treated HeLa SOX6-tet cells (Figure 5b). It has been reported that p53 cannot bind to p14ARF directly and that the p14ARF-p53 association is bridged by HDM2.13 In line with this, p14ARF also efficiently pulled down HDM2 (Figure 5a). Thus, our data strongly suggest that SOX6 may promote formation of the p14ARF-HDM2-p53 ternary complex.
Figure 5.
SOX6 promotes formation of the p14ARF-HDM2-p53 ternary complex and translocation of p14ARF to the nucleoplasm. (a) HeLa SOX6-tet and SOX6ΔHMG-tet cells were co-transfected with pLex-p14ARF-myc or pLex-HDM2-V5 plasmids with or without Dox (2 μg/ml) treatment, and the cell lysates were subjected to co-immunoprecipitation by anti-myc-tag antibody. One-tenth of the total cell lysate volume was subjected to direct western blot analysis. (b) HeLa SOX6-tet cells were transfected with the pCDNA3.1-p53-myc plasmid and the cell lysates were subjected to co-immunoprecipitation (co-IP) by anti-myc-tag antibody. (c) Western blot was performed to analyze the p14ARF protein levels in HeLa and SMMC7721 cells that had been transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. (d) Immunocytofluorescence staining of p14ARF in HeLa cells that had been co-transfected with pLex-p14AF and pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. (e) Immunocytofluorescence staining of p14ARF in HeLa SOX6-tet and SOX6ΔHMG-tet cells that had been transfected with pLex-p14ARF and treated with or without Dox (2 μg/ml) for 48 h. Fibrillarin was used as a nucleolar marker. (f) Immunocytofluorescence staining of p14ARF and endogenous p53 in HeLa SOX6-tet cells that had been transfected with pLex-p14ARF and treated with or without Dox (2 μg/ml) for 48 h. IP, immunoprecipitate; IB, immunoblotting.
We next investigated the mechanism by which SOX6 promotes formation of the p14ARF-HDM2-p53 ternary complex. Testing of the transcriptional regulation of SOX6 on the p14ARF gene by qPCR assay showed no change of p14ARF mRNA level in either HeLa or SMMC7721 cells overexpressing SOX6 (data not shown). Similarly, SOX6 overexpression produced no effects on the protein level of p14ARF, suggesting that the SOX6-induced p14ARF-HDM2-p53 ternary complex formation is unrelated to p14ARF expression (Figure 5c). Testing of the effect of SOX6 on the subcellular distribution of p14ARF protein by immunocytofluorescence assay indicated that SOX6 overexpression in HeLa cells can promote translocation of p14ARF from the nucleolus to the nucleoplasm (Figures 5d and e). Furthermore, the observed SOX6-mediated p53 induction was accompanied by the translocation of p14ARF to the nucleoplasm, indicating that p53 is induced by translocation of p14ARF to the nucleoplasm (Figure 5f).
c-Myc is a direct mediator of SOX6-induced p53 activation
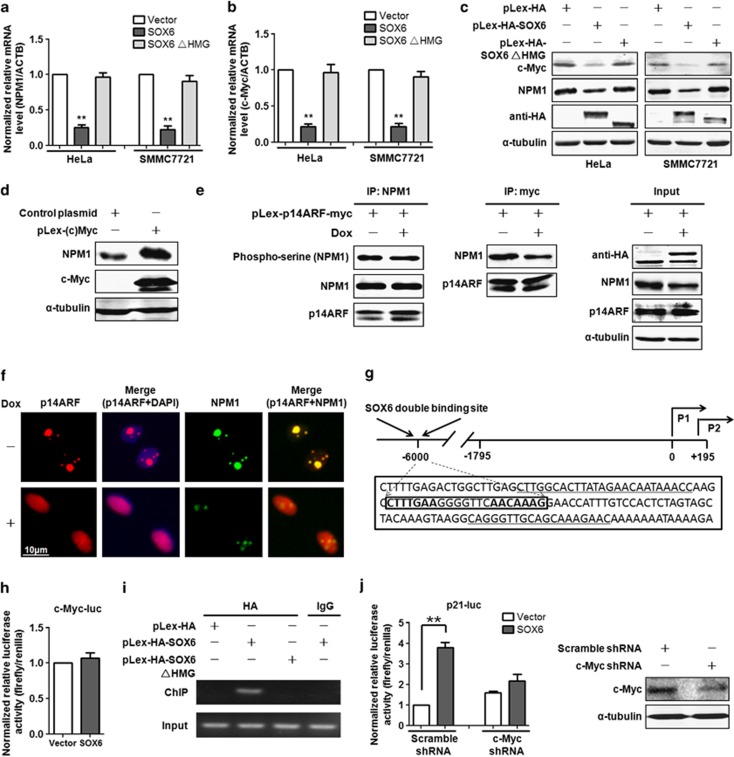
We next investigated the mechanism responsible for the SOX6-induced nucleoplasmic translocation of p14ARF. NPM1 was considered first, as it has been reported to target p14ARF to the nucleolus and to block p14ARF-mediated p53 activation.16, 17 Interestingly, we found that SOX6 was able to downregulate the mRNA and protein levels of NPM1 and its direct inducer c-Myc in HeLa and SMMC7721 cells (Figures 6a–c), and that these effects were HMG domain-dependent. As NPM1 is a direct target gene of c-Myc,17 the SOX6-mediated downregulation of NPM1 may be owing to the downregulation of c-Myc. To test this notion, we transfected c-Myc expression plasmids into HeLa cells, and detected the NPM1 protein level. Indeed, overexpression of c-Myc upregulated the NPM1 protein level (Figure 6d). In addition, it has been reported that phosphorylation of NPM1 can inhibit its ability to bind with p14ARF, thereby preventing nucleolar sequestration of p14ARF;18 thus, we assessed whether SOX6 affects the phosphorylation status of NPM1 and the interaction of NPM1 and p14ARF. The results showed no change in the level of phosphorylated NPM1 upon induction of SOX6 expression (Figure 6e). Further, SOX6 had no influence on the interaction of NPM1 and p14ARF, as observed in anti-NPM1 immunoprecipitates. Although, a reduced level of NPM1 protein was noted in anti-myc-tagged p14ARF immunoprecipitates, which might be due to the SOX6-induced downregulation of NPM1 expression (Figure 6e). To further confirm that the SOX6-mediated translocation of p14ARF to the nucleoplasm is associated with the downregulation of NPM1, we assessed the effect of SOX6 on the expression of NPM1 and the subcellular distribution of p14ARF using an immunocytofluorescence assay. As expected, we observed the SOX6-mediated translocation of p14ARF to the nucleoplasm accompanied by downregulation of NPM1 (Figure 6f).
Figure 6.
SOX6-mediated p53 activation occurs via the direct downregulation of c-Myc. mRNA levels of NPM1 (a) and c-Myc (b) were measured by qPCR in HeLa and SMMC7721 cells that had been transfected with pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. (c) Western blot was performed to analyze the protein levels of NPM1 and c-Myc in HeLa and SMMC7721 cells as above. (d) Western blot was performed to analyze the protein levels of NPM1 in HeLa cells overexpressing c-Myc. (e) Co-immunoprecipitation (co-IP) was performed to analyze the effect of SOX6 on the phosphorylation status of NPM1 and the interaction of NPM1 and p14ARF in HeLa SOX6-tet cells that had been transfected with pLex-p14ARF-myc plasmid and treated with or without Dox (2 μg/ml) for 48 h. To facilitate the analysis, the amount of the pulled-down NPM1 protein was aligned. (f) Immunocytofluorescence staining of p14ARF and endogenous NPM1 in HeLa SOX6-tet cells that had been transfected with pLex-p14ARF-myc plasmid and treated with or without Dox (2 μg/ml) for 48 h. (g) A schematic of the c-Myc gene P1 and its upstream region. The predicted SOX6 double-binding site is at ~6000 bp upstream of P1 transcription start site (TSS). For experimentation, the detailed sequence of the SOX6 double-binding site in bold face was boxed and the underlined sequences served as the primer sequences for ChIP combined with PCR assay (ChIP-PCR). P1, c-Myc gene promoter 1; P2, c-Myc gene promoter 2. (h) A dual-luciferase assay was performed to analyze the effect of SOX6 on c-Myc gene promoter transactivity. (i) Binding of SOX6 to its potential double-binding site in the regulatory region of c-Myc gene was analyzed by ChIP-PCR. (j) A dual-luciferase assay was performed in HeLa cells to analyze the effect of c-Myc on SOX6-mediated p21 gene promoter activation. The knockdown efficiency of c-Myc shRNA in HeLa cells was analyzed by western blot. Data shown (a, b and j) are the mean±s.d. of five independent experiments (**P<0.01, Student's t-test, two-sided). The statistical tests are justified as appropriate and meet the assumptions of the tests. The variance between the groups is similar.
Considering our evidence showing the HMG domain as a mediator of the SOX6 effects on the expression of c-Myc and its direct target gene NPM1, we investigated whether c-Myc is a direct mediator of SOX6-induced p53 activation. However, analysis of the c-Myc gene promoter region identified no SOX6 double-binding site (Figure 6g). Consistent with this finding, a dual-luciferase assay with a luciferase reporter plasmid containing the c-Myc gene promoter region (−1795 to 195 bp) also detected no downregulation of the c-Myc gene promoter activity in response to SOX6 overexpression (Figure 6h). As it has been previously reported that the upstream sequence region far away from the c-Myc gene promoters may harbor regulatory capacity for c-Myc expression,19 we searched the region from 10 000 bp upstream to 1000 bp downstream of the c-Myc gene promoter 1 transcription start site for a SOX6 binding site. A putative double-binding site for SOX6 (CTTTGAA and AACAAAG) was found at ~6000 bp upstream of the promoter 1 transcription start site (Figure 6g). Chromatin immunoprecipitation (ChIP) combined with PCR assay was performed in HeLa cells to demonstrate the interaction of SOX6 with the predicted double-binding site. As shown in Figure 6i, SOX6 was able to specifically bind to the sequence containing the double-binding site, and the SOX6ΔHMG mutant was not able to bind. Further, a dual-luciferase assay demonstrated that the enhancement of SOX6-induced luciferase activity under the control of the p21 gene promoter was significantly lower in HeLa cells transfected with the c-Myc shRNA, as compared with that in control cells (1.36±0.19 vs 3.80±0.24, P=0.029) (Figure 6j).
Discussion
Previous studies have reported that SOX6 acts as a tumor-suppressor gene and is frequently downregulated in different human malignancies.7, 8, 9 We and others have previously shown that SOX6 is able to upregulate the protein level of p21,7, 8 a key negative regulator of cell cycle progression.20, 21 However, the underlying mechanism had not been precisely defined. Here, we reported the observation for the first time that SOX6, in an HMG domain-dependent manner, mediates tumor suppression and p21 upregulation via the p14ARF-HDM2-p53 axis.
In this study, we further confirmed that SOX6 is able to suppress cell proliferation through a mechanism involving upregulation of p21 expression and blockade of the cell cycle at the G0/G1 phase in HeLa and SMMC7721 cells containing wild-type p53, but not in Huh7 cells, which contain a mutant p53. Consistent with this result, SOX6 was also found to upregulate the protein level of p53 and transcriptionally upregulate p21 expression; the suppressed p53 reduced the SOX6-induced p21 upregulation. In vivo analysis further confirmed the ability of SOX6 to suppress tumorigenesis and upregulate p53 as well as its direct target gene p21. Therefore, this study provides convincing evidence of SOX6 upregulating p21 by activating p53.
Based on the extended half-life and decreased ubiquitination level of endogenous p53 in the SOX6-overexpressing cells, we concluded that SOX6 was able to activate p53 by increasing stability of the p53 protein, rather than by promoting the transcription of p53. The ubiquitination-dependent degradation of p53 is known to be initiated mainly by HDM2, an E3 ligase that can degrade p53 via binding to and exporting of the p53 protein to cytoplasm.11, 21, 22, 23, 24 In this study, we found that SOX6 does not affect the binding of p53 with HDM2 but does induce the nuclear accumulation of p53 and HDM2 simultaneously. These findings suggest that SOX6 might stabilize the p53 protein by inhibiting the HDM2-mediated p53 nuclear export and subsequent ubiquitination. Besides HDM2, the E6 protein of HPV can also promote degradation of the p53 protein in a ubiquitination-dependent manner. As the HPV-positive HeLa cells were used as the cell model in this study, it is also possible that SOX6 stabilized the p53 protein by interfering with the E6 protein. At the moment, we cannot rule out this possibility, but our observation of SOX6 stabilizing the p53 protein in HPV-negative SMMC7721 cells suggests that HDM2 might be a key factor in the pathway of SOX6-induced p53 stabilization. Further studies are needed, however, to investigate the potential correlation between SOX6 and E6-mediated p53 degradation in HeLa cells.
Numerous studies have demonstrated that the tumor suppressor protein p14ARF can stabilize and activate p53 by inhibiting the E3 ligase activity and nuclear export of HDM2, which is followed by formation of the p14ARF-HDM2-p53 ternary complex.13, 14, 15, 25, 26, 27 Consistent with those reports, we found that SOX6 can trigger formation of the p14ARF-HDM2-p53 ternary complex via induction of translocation of p14ARF from the nucleolus to the nucleoplasm. The increase in p14ARF-HDM2-p53 ternary complex would be expected to inhibit the nuclear export and p53-ubiquitination activity of HDM2, thereby leading to stabilization of p53 protein. As the binding of p53 with p14ARF was bridged by HDM2, we predicted that increased binding of p14ARF and HDM2 would also be observed when a noticeable increase of the p14ARF-bound p53 was present in HeLa cells overexpressing SOX6. Unexpectedly, although, overexpression of SOX6 did not affect the interaction of p14ARF and HDM2. One possible reason might be that p14ARF could bind to HDM2 in both nucleolus and nucleoplasm,28, 29 so that the SOX6-induced translocation of p14ARF could increase the formation of p14ARF-HDM2-p53 ternary complex but not affect the ability of p14ARF to bind with HDM2. Of course, we cannot rule out the possibility of interference of the E6 protein in HeLa cells, and further study is needed to elucidate this mechanism.
NPM1 (also called nucleolar phosphoprotein B23, NO38 or numatrin) is an abundant and highly conserved protein that is distributed most prominently in the nucleolus. It has been shown that NPM1 can target p14ARF to the nucleolus and block p14ARF-mediated p53 activation, and NPM1 knockdown is able to trigger translocation of p14ARF from the nucleolus to the nucleoplasm.16, 30, 31 As SOX6 was able to downregulate the expression of NPM1, it was reasonable to postulate that SOX6-induced p14ARF translocation might be mediated by NPM1. Consistent with this postulation, we found that SOX6-mediated translocation of p14ARF to the nucleoplasm is accompanied by a decrease in NPM1 protein level. Moreover, SOX6-mediated downregulation of NPM1 decreases the level of p14ARF bound to NPM1. It has been reported that ATM-mediated phosphorylation of NPM1 can inhibit the binding of NPM1 with p14ARF, and thereby promoting the translocation of p14ARF to nucleoplasm.18, 32 Besides, AKT-mediated phosphorylation of NPM-Ser48 can prevent the oligomerization of NPM1, and subsequently results in nucleoplasmic localization of p14ARF, constitutive HDM2 inhibition and stabilization of p53.33 However, we observed no effect of SOX6 on the phosphorylation of NPM1. Collectively, our data indicate that SOX6-mediated NPM1 downregulation is at least one pathway contributing to translocation of p14ARF and to formation of the p14ARF-HDM2-p53 ternary complex.
Interestingly, the proto-oncogene c-Myc, which directly regulates the expression of NPM1,17 was also shown to be suppressed by SOX6 in our study. Of note, a potential double-binding site for SOX6 was identified at ~6000 bp upstream of the c-Myc gene promoter, suggesting that c-Myc may be a potential direct target gene of SOX6. Consistent with this notion, ChIP combined with PCR assay demonstrated the interaction of SOX6 protein with the DNA sequence containing this predicted double-binding site and knockdown of c-Myc expression significantly suppressed the SOX6-induced p21 upregulation. Thus, we predicted that the SOX6-mediated NPM1 downregulation and subsequent p53 activation might occur through its direct effector c-Myc. A previous study showed that c-Myc overexpression not only induced p14ARF transcription but also stabilized p14ARF by inhibiting the ubiquitin ligase activity of ULF in normal fibroblast cells.34 However, although SOX6 inhibited c-Myc expression, the mRNA and protein levels of p14ARF did not change when SOX6 was overexpressed in HeLa and SMMC7721 cancer cells, suggesting that c-Myc upregulated p14ARF activity via inhibition of NPM1-dependent p14ARF nucleolar sequestration in the HeLa and SMMC7721 cells overexpressing SOX6.
The HMG domain is a crucially important functional domain of the SOX proteins, as it mediates the ability of these proteins to bind DNA and interact with other transcriptional factors.1, 2, 3, 6, 10 In the current study, both in vitro and in vivo analyses confirmed that the HMG domain was also a key element of SOX6-mediated tumor suppression and p21 upregulation. Deletion of this domain abolished the ability of SOX6 to bind to its regulatory site in the c-Myc gene and to inhibit c-Myc expression. Importantly, the SOX6ΔHMG mutant showed a loss of ability to induce the nucleoplasmic translocation of p14ARF as well as of the subsequent formation of p14ARF-HDM2-p53 ternary complex, thereby abolishing the effect of SOX6 on p53 activation. Of note, SOX6 is a member of the Sox D group, which is characterized by low DNA-binding affinity when binding without a partner; therefore, SOX6 exerts its transcriptional regulatory functions in a manner dependent on high affinity and specificity of other transcription factors. However, the particular transcription factor that contributes to the SOX6-mediated regulation of c-Myc expression has not been explored in this study and further study should be conducted.
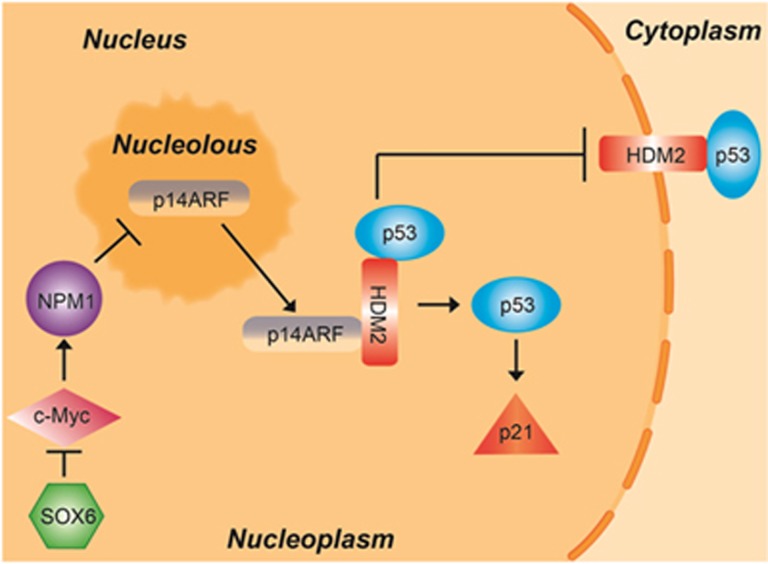
Based on the collective discoveries of our study, as well as those reported from other labs, we hypothesize that binding of SOX6 to its double-binding site in the upstream regulatory region of the c-Myc gene leads to downregulated expression of c-Myc and its direct target gene NPM1, which in turn triggers translocation of p14ARF from the nucleolus to the nucleoplasm. The nucleoplasmic p14ARF promotes formation of the p14ARF-p53-HDM2 ternary complex and inhibits the nuclear export and p53-ubiquitination activity of HDM2, thereby stabilizing and activating p53. Finally, activation of p53 upregulates the expression of p21 and causes cell cycle arrest in the G0/G1 phase (Figure 7).
Figure 7.
The pathway of SOX6-mediated p21 upregulation.
In summary, this study uncovered a new mechanism by which SOX6 suppresses tumor proliferation through the p14ARF-HDM2-p53 axis.
Materials and methods
Cell lines
HeLa cervix cancer cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were recently authenticated by short tandem repeat profiling. Huh7 and SMMC7721 liver cancer cells were maintained as a laboratory stock. HeLa EGFP-tet, SOX6-tet and SOX6ΔHMG-tet polyclonal stable cells were constructed in our laboratory. All cells were free of mycoplasma contamination detected by Hoechst staining and were maintained in either Dulbecco's modified Eagle's medium or RPMI-1640 supplemented with 10% fetal bovine serum (Gibco, Life Technologies, Carlsbad, CA, USA).
Plasmids
The pCMV-E6, pCMV-HA-SOX6, pGL3-p21-luciferase, pGL3-HDM2-luciferase and PRL-TK plasmids were maintained as laboratory stocks.8, 35 The plenti6-TREpitt-EGFP and pELNS-PBX3-IRES-neo plasmids used to construct HeLa EGFP-tet cells were kindly provided by Professor Zhiqian Zhang (Beijing Institute for Cancer Research, Peking University Health Science Center, Beijing, China). The pCMV-HA-SOX6ΔHMG plasmid was generated from pCMV-HA-SOX6 using QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA, USA). The pLex-HA-SOX6, pLex-HA-SOX6ΔHMG, plenti6-TREpitt-SOX6 and plenti6-TREpitt-SOX6ΔHMG plasmids were constructed based on pCMV-HA-SOX6 and pCMV-HA-SOX6ΔHMG plasmids. The pGL3-(c)Myc-luciferase plasmid was constructed by PCR from HeLa cDNA, and the PCR products were subsequently ligated into the pGL3-basic plasmid after digestion with NheI and HindIII. The pcDNA3.1-p53-myc was constructed by PCR from HeLa cDNA, and the PCR products were subsequently ligated into the pcDNA3.1/myc-His(−) B plasmid after digestion with XhoI and EcoRI. The pLex-HDM2-V5, pLex-p14ARF-V5 and pLex-(c)Myc plasmids were constructed by PCR from HeLa cDNA, and the PCR products were subsequently ligated into the pLex plasmid. The primer sequences used for plasmid construction are listed in Supplementary Table S1.
RNA interference
shRNA plasmids were constructed by annealing two oligonucleotides and subsequently ligated into the pRNA-U6.1/Neo vector after digestion with BamHI and HindIII. The RNA interference assay was performed as described previously.8 The oligonucleotide sequences used for shRNA construction are listed in Supplementary Table S2.
Cell viability assays
Cell viability assays were carried out in 96-well plates as described previously.8
Tumorigenicity assays in nude mice
The effect of SOX6 on the tumorigenesis ability of HeLa cells was investigated in vivo as described previously.8 Briefly, 5 × 106 HeLa SOX6-tet or HeLa SOX6ΔHMG-tet cells were suspended in 100 μl phosphate-buffered saline and then injected subcutaneously into the armpit of 6-week-old female C57/BL6 nude mouse. HeLa SOX6-tet cells were injected into one side, and HeLa SOX6ΔHMG-tet cells were injected into the other side of the same mouse. To ensure at least five mice in each group could be used to statistical analysis, we chose eight mice into each group for tumorigenicity assay. If the mouse died or did not form tumor during the study, it would be excluded. Sixteen mice were not randomly but evenly divided into two groups according to body weight. One group of mice were fed with Dox (1 mg/ml) dissolved in 10% sucrose solution, and the other group of mice were fed with 10% sucrose solution as the control. Tumor formation in nude mice was monitored over a 3-week period. The investigator was not blinded during the study. The tumor volume was calculated by formula V=0.5 × L × W2, where L is the the length of the tumor and W is the width of the tumor. All mouse care procedures and experiments were designed and carried out according to the guidelines established by the Institutional Animal Care and Use Committee at the Peking University Health Science Center. All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Peking University Health Science Center.
Cell cycle assay
Cells were seeded in a 6-cm dish and allowed to grow for 16–24 h, and then transiently transfected with the plasmids indicated in the relevant Results section or treated with Dox (2 μg/ml). Two days later, the cells were washed with phosphate-buffered saline two times, resuspended in 2 ml of 75% ethanol, fixed overnight, resuspended in 200 μl phosphate-buffered saline with 20 μl RNaseA (Roche, Basel, Switzerland), and incubated at 37 °C for 30 min, after which the cell suspension was transferred into a new tube, mixed with 50 μl of PI staining solution (51-66211E; BD Biosciences, San Jose, CA, USA), and analyzed in a flow cytometer (FACSCalibur; BD Biosciences).
RNA extraction and qPCR
RNA extraction, reverse transcription and qPCR were carried out as described previously.8 The primer sequences used for qPCR are listed in Supplementary Table S3.
Dual-luciferase reporter assay
Dual-luciferase reporter assays were carried out as described previously.8
Immunoprecipitation and immunoblots
Cellular extracts were prepared in lysis buffer (50 mm HEPES (pH 7.4), 1.5 mm EDTA, 150 mm NaCl, 10% glycerol, 10 mm NaF, 1 mm Na3VO4, 0.5 mM dithiothreitol, 1% Triton X-100 and 1% Protease Inhibitor Cocktail (P8340; Sigma, St Louis, MO, USA) and subjected to immunoprecipitation and/or immunoblot analysis as described previously.8 The antibodies used are listed in Supplementary Table S4.
Immunocytofluorescence
Cells were seeded in a 24-well plate at 1 × 105 cells per well, allowed to grow for 16–24 h and then transiently transfected with the plasmids indicated in the relevant Results section or treated with Dox (2 μg/ml). Two days later, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline for 20 min, permeabilized with 0.3% Triton X-100 for 10 min and subsequently incubated with antibodies. Finally, the cells were counterstained with 4′-6-diamidino-2-phenylindole and examined under a Leica inverted fluorescence microscope (DM130CCB; Leica, Solms, Germany). The antibodies use are listed in Supplementary Table S4.
Extraction of cytoplasmic and nuclear proteins
Cytoplasmic and nuclear proteins were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific Pierce, Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer's specifications.
ChIP
The ChIP assay was performed as described previously.36 HeLa cells were transfected with the pLex-HA-SOX6, pLex-HA or pLex-SOX6ΔHMG plasmid. The lysates were incubated with rabbit anti-HA-tag-ChIP grade (ab9110; Abcam) or immunoglobulin G from rabbit serum (I5006; Sigma).
Gray-scale scanning analysis
Gray-scale scanning was used to analyze the protein level on western blots, which was performed using the Gel-Pro Analyzer 4.5 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
For statistical analysis, two-tailed Student's t-test and χ2 test were performed using the Statistical Analysis System software (SAS 9.1 TS level 1M3, SAS Institute, Cary, NC, USA). In all cases, a P-value of <0.05 was considered significant.
Acknowledgments
This work was supported by grants from the 973 Program (2015CB554000), the National S & T Major Project for Infectious Diseases (2012ZX10004-904), the Doctoral Fund and of the Ministry of Education of China (20110001120017), the National Natural Science Foundation of China (81372603) and the 111 Project (B07001).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development 2013; 140: 4129–4144. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 1999; 27: 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol 2000; 227: 239–255. [DOI] [PubMed] [Google Scholar]
- Cantu' C, Grande V, Alborelli I, Cassinelli L, Cantu' I, Colzani MT, et al. A highly conserved SOX6 double binding site mediates SOX6 gene downregulation in erythroid cells. Nucleic Acids Res 2011; 39: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N. Sox6, jack of all trades: a versatile regulatory protein in vertebrate development. Dev Dyn 2011; 240: 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu C, Ierardi R, Alborelli I, Fugazza C, Cassinelli L, Piconese S et al, Sox6 enhances erythroid differentiation in human erythroid progenitors. Blood 2011; 117: 3669–3679. [DOI] [PubMed] [Google Scholar]
- Qin YR, Tang H, Xie F, Liu H, Zhu Y, Ai J, et al. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma. Clin Cancer Res 2011; 17: 46–55. [DOI] [PubMed] [Google Scholar]
- Xie Q, Chen X, Lu F, Zhang T, Hao M, Wang Y et al. Aberrant expression of microRNA 155 may accelerate cell proliferation by targeting sex-determining region Y box 6 in hepatocellular carcinoma. Cancer 2012; 118: 2431–2442. [DOI] [PubMed] [Google Scholar]
- Guo X, Yang M, Gu H, Zhao J, Zou L. Decreased expression of SOX6 confers a poor prognosis in hepatocellular carcinoma. Cancer Epidemiol 2013; 37: 732–736. [DOI] [PubMed] [Google Scholar]
- Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T et al. SOX6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem 2007; 282: 19052–19061. [DOI] [PubMed] [Google Scholar]
- Dai C, Gu W. P53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med 2010; 16: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Nie L, Kawai H, Yuan ZM. Subcellular distribution of p53 and p73 are differentially regulated by MDM2. Cancer Res 2001; 61: 6703–6707. [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 1998; 92: 713–723. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y. Mutations in human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol Cell 1999; 3: 579–591. [DOI] [PubMed] [Google Scholar]
- Midgley CA, Desterro JM, Saville MK, Howard S, Sparks A, Hay RT, et al. An N-terminal p14ARF peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 2000; 19: 2312–2323. [DOI] [PubMed] [Google Scholar]
- Korgaonkar C, Hagen J, Tompkins V, Frazier AA, Allamargot C, Quelle FW et al, Nucleophosmin (B23) targets ARF to nucleoli and inhibits its function. Mol Cell Biol 2005; 25: 1258–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Haggerty TJ, Barrett JF, Guo Q, Wonsey DR, Dang CV. Characterization of nucleophosmin (B23) as a Myc target by scanning chromatin immunoprecipitation. J Biol Chem 2001; 276: 48285–48291. [DOI] [PubMed] [Google Scholar]
- Velimezi G, Liontos M, Vougas K, Roumeliotis T, Bartkova J, Sideridou M et al. Functional interplay between the DNA-damage-response kinase ATM and ARF tumor suppressor protein in human cancer. Nat Cell Biol 2013; 15: 967–977. [DOI] [PubMed] [Google Scholar]
- Wierstra I, Alves J. The c-myc promoter: still MysterY and challenge. Adv Cancer Res 2008; 99: 113–333. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays 1998; 20: 1020–1029. [DOI] [PubMed] [Google Scholar]
- Levine AJ. P53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–331. [DOI] [PubMed] [Google Scholar]
- Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J 1998; 17: 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Levine AJ. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA 1999; 96: 3077–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994; 54: 1169–1174. [PubMed] [Google Scholar]
- Zhang Y, Xiong Y. Control of p53 ubiquitination and nuclear export by MDM2 and ARF. Cell Growth Differ 2001; 12: 175–186. [PubMed] [Google Scholar]
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J 1998; 17: 5001–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tyler T, Saadatmandi N, Lee C, Borgstrom P, Gjerset RA. Enhanced tumor suppression by a p14ARF/p53 bicistronic adenovirus through increased p53 protein translation and stability. Cancer Res 2003; 63: 3646–3653. [PubMed] [Google Scholar]
- Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol 1999; 1: 20–26. [DOI] [PubMed] [Google Scholar]
- Lohrum MA, Ashcroft M, Kubbutat MH, Vousden KH. Identification of a cryptic nucleolar-localization signal in MDM2. Nat Cell Biol 2000; 2: 179–181. [DOI] [PubMed] [Google Scholar]
- Bertwistle D, Sugimoto M, Sherr CJ. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol 2004; 24: 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki S, Peltonen K, Latonen L, Kiviharju TM, Ojala PM, Meek D et al, Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell 2004; 5: 465–475. [DOI] [PubMed] [Google Scholar]
- Lee C, Smith BA, Bandyopadhyay K, Gjerset RA. DNA damage disrupts the p14ARF-B23(nucleophosmin) interaction and triggers a transient subnuclear redistribution of p14ARF. Cancer Res 2005; 65: 9834–9842. [DOI] [PubMed] [Google Scholar]
- Hamilton G, Abraham AG, Morton J, Sampson O, Pefani DE, Khoronenkova S et al, AKT regulates NPM dependent ARF localization and p53mut stability in tumors. Oncotarget 2014; 5: 6142–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kon N, Zhong J, Zhang P, Yu L, Gu W. Differential effects on ARF stability by normal versus oncogenic levels of c-Myc expression. Mol Cell 2013; 51: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang J, Cheng J, Ding S, Li M, Sun S et al, An integrated analysis of SOCS1 down-regulation in HBV infection-related hepatocellular carcinoma. J Viral Hepat 2013; 21: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Jiang W, Chen X, Zhang C, Li H, Hou W, et al. Alpha-fetoprotein acts as a novel signal molecule and mediates transcription of Fn14 in human hepatocellular carcinoma. J Hepatol 2012; 57: 322–329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.