Abstract
Purpose of the review
This article reviews our current knowledge regarding the role of sex and sex hormones in regulating innate immune responses to viral infections, which may account for the described sex differences in immunity to HIV-1.
Recent findings
Prominent sex differences exist in various infectious and autoimmune diseases. Biological mechanisms underlying these differences include the modulation of immunological pathways by sex hormones and gene dosage effects of immunomodulatory genes encoded by the X chromosome. During HIV-1 infections, females have been shown to present with lower viral load levels in primary infection, though their progression to AIDS is faster in comparison to males when accounting for viral load levels in chronic infection. HIV-1-infected females furthermore tend to have higher levels of immune activation and interferon-stimulated gene expression in comparison to males for the same viral load, which has been associated to innate sensing of HIV-1 by toll-like receptor 7 and the consequent Interferon α-production by plasmacytoid dendritic cells.
Summary
Improvement in understanding the mechanisms associated with sex-differences in HIV-1-mediated immunopathology will be critical in order to take sex-differences into consideration when designing experimental and clinical studies in HIV-1-infected populations.
Keywords: HIV, sex, TLR7, Interferon alpha, immune activation
Introduction
The incidence, outcome and severity of many infectious and autoimmune diseases differ between women and men(1). A strong sex bias is observed for many autoimmune diseases(2), which are much more common in females, including autoimmune thyroid diseases, systemic sclerosis, systemic lupus erythematosus. Furthermore, the incidence and severity of several microbial infections, including malaria and tuberculosis(3), influenza(4), hepatitis(5) and HIV-1(6) are different between the sexes(7). In HIV-1 infection, clinical studies have shown faster disease progression and stronger immune activation in females compared to males for the same level of viral replication, as well as better control of initial viremia in women during primary infection. This review will summarize recent findings on the immune mechanisms that underlie sex-specific differences in the manifestations of HIV-1 disease, and in particular the role of immune activation.
Direct and indirect effects of sex chromosomes on antiviral immunity
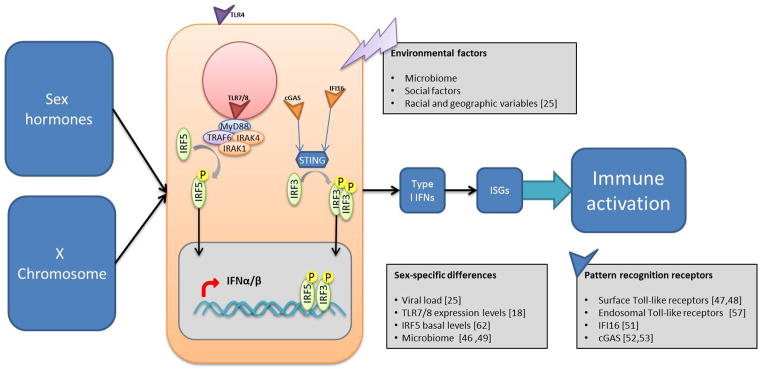
In general, women have been reported to display an overall reduced susceptibility to viral infections, potentially due to stronger innate, cellular and humoral immune responses compared to men(1, 8–11). This enhanced level of immune responses and activation, however, can have detrimental consequences for disease outcomes resulting from immune pathology, in particular for chronic persistent infections. The biological mechanisms that lead to these sex-specific differences in the manifestations of viral infections are incompletely understood, but could result from sex-specific environmental risk factors, sex differences in the microbiome(12), steroid hormones secreted by gonads(9, 13) and direct effects of X and Y chromosome-linked factors(14–16) (figure 1).
Figure 1. Direct and indirect sex-specific determinants modulate viral recognition and immune activation.
In additional to environmental factors, X chromosome gene dosage and sex hormones can influence immune cell function, including innate sensing of viral components, type I IFN production and immune activation.
Several genes on the X chromosome can potentially influence immunocompetence(15), in particular those loci on the X-chromosome encoding for genes involved in immune regulation. These include for example foxp3, the lineage-defining transcription factor of regulatory T cells, IL-2Rγ, a common cytokine receptor, and the pattern recognition receptors (PRR) Tlr7 and Tlr8, which are known to sense HIV-1 ssRNA. Importantly, X-chromosome inactivation is considered to be a random process and an estimated 10% of the X-chromosome escapes inactivation(17), which may lead to an over-expression of certain gene products. For example, Tlr7 mRNA was shown to be higher expressed in cells from females compared to males(18).
Besides the direct role of X-linked factors, the indirect effects of sex chromosomes, namely the immune modulation by steroid hormones, play a prominent role in sex-specific outcomes. Estrogens mediate their immunomodulatory function through binding to the intracellular estrogen receptor (ER) α or ERβ. ERα is ubiquitously expressed by immune cells, and signals via ERα or ERβ complexes that translocate to the nucleus, thereby regulating transcriptional activity of many genes involved at different stages of immune cell maturation, as well as regulation and maturation of immune responses. However, the precise molecular mechanisms and pathways leading to sex-based differences are largely unknown and require further investigation(19). In this review, we will discuss the consequences of these sex-specific differences in immunity for HIV-1 disease.
Sex Differences in HIV-1-associated immune activation and immunopathology
Sex differences have been described for diverse aspects of HIV-1 infection and disease, including transmission, pathogenesis, morbidity, mortality, and responses to antiretroviral treatment(8, 20). In addition to gender- and sex-specific social and political factors, including gender inequalities and limited access to health care, the influence of biological factors also importantly contributes to the differential outcome of HIV-1 infection between women and men. Sex differences in HIV-1 immunopathogenesis will be the topic of this review article, with particular focus on the role of immune activation. Understanding the biological factors underlying these sex differences is important, as women are over-proportionally affected by the HIV-1 epidemic, and in particular young women in Sub-Saharan Africa. HIV-1 infection represents now a leading cause of death in women in their reproductive age(21) and according to the WHO HIV/AIDS has become the main cause of death in adolescent women(22).
Marked sex differences in the manifestations of HIV-1 infections have been described in several larger cohort studies(23, 24). In primary HIV-1 infection, females tend to have lower plasma viral load levels compared to men(25). However, in chronic infections, women with the same viral load as men have a 1.6-fold higher risk of developing AIDS or, equivalently, women with half the viral load of men have a similar time to AIDS progression as men(23). Interestingly, sex differences in viral load are more pronounced in individuals with higher CD4 T cell counts, suggesting that differences in viral load might be lost at later disease stages(26). Furthermore, sex-specific differences in CD4+ T cell counts have been reported in several studies, with higher CD4+ T cell counts in HIV-1-infected women compared to men(27–32). The precise mechanisms responsible for these reported sex differences in viral load and CD4+ T cell counts remain unknown. However, a role of sex hormones has been postulated(23), based on the observation of fluctuating viral loads during the menstrual cycle(33) and lack of differences in CD4+ T cell counts between women and men above the age of 50 years(34).
Untreated chronic HIV-1 infection is characterized by strong systemic immune activation that persists at elevated levels compared to non-HIV-1-infected individuals, even following effective antiretroviral therapy. This immune activation is characterized by B and T cell activation(35), high T cell turnover(36) and increased levels of pro-inflammatory mediators(37). It is now well established that the level of immune activation is a strong predictor for HIV-1 disease progression(38), with some studies demonstrating that the level of immune activation serves as a better correlate than plasma viral load levels(39–41). Even HIV-1 controllers have been shown to have abnormally high T cell activation levels compared to HIV-1-negative individuals(42). Interestingly, plasma type I Interferon (IFN) levels inversely correlated with CD4+ T cell counts and positively with plasma viral load levels and activation status of CD8+ T cells(43). Chronic immune activating and inflammation have also been associated with accelerated immune aging and non-AIDS related morbidities and mortalities, including cardiovascular diseases(44, 45) and non-AIDS-defining malignancies. Taken together, the level of immune activation clearly contributes to HIV-1 disease progression and can differ between women and men(9).
Very recently, Ren et al. reported the development and characterization of a nonhuman primate model that reflected the sex differences observed in HIV-1 infection in humans(46)*. Rhesus macaques that were infected intrarectally with SHIV showed sex differences in gut innate immune responses, with females mounting a faster and more robust local mucosal pro-inflammatory immune response in comparison to males. Moreover, analysis of the bacterial community structures in the rectal mucosa revealed a significantly higher expansion of specific bacterial subsets in the rectal mucosa of female than male macaques during acute infection. This study suggests that the local mucosal innate immune activation that is augmented early in infection and changes in the microbiome could contribute to faster disease progression in females(46)*. A key role for translocation of microbial products from the intestinal lumen to the systemic circulation, due to damages in intestinal barriers, has been suggested as a driver of persistent immune activation in HIV-1 infections(47). Among those translocated commensal microbial products are potent activators of several PRRs(48), leading to the production of pro-inflammatory cytokines, including TNFα. The composition of the gut microbiome might therefore have an impact on immune activation. Recent findings suggest a bidirectional interaction between the microbiome and sex hormones(16). In one study(49), the microbiota of opposite-sex twin pairs was assessed. Interestingly, before puberty, the microbiome between opposite sexes was as similar as the microbiome of same-sex twin pairs. However after puberty, the microbiome became more disparate, suggesting that hormonal levels can shape the microbiome composition(49). Taken altogether, there is emerging evidence that sex hormones can modulate the microbiome, and thereby influence the expression of sex-related phenotypes(16).
Innate sensing of HIV-1
The innate immune system plays a central role in the sensing of microbial infections and in initiating antiviral immune responses(50). HIV-1 encodes for multiple pathogen-associated molecular patterns (PAMPs) that can be recognized by nucleic acid sensors of the toll-like receptor (TLR) family, and the recently discovered intracellular PRRs interferon-inducible protein 16 (IFI16) and cyclic GMP-AMP synthase (cGAS) that sense reverse transcription products early in the viral replication cycle (figure 1). IFI16, an interferon-stimulated gene (ISG) binds DNA products of HIV-1 reverse transcription, including truncated DNA and a DNA segment of the HIV-1 long terminal repeat region(51). Knockdown of IFI16 results in increased permissiveness towards HIV-1 infection and enhanced virus replication, suggesting that IFI16 functions as a viral sensor and restriction factor. The recently identified enzyme cGAS, a cytosolic DNA sensor, triggers the production of type I IFN and other cytokines after sensing of HIV-1 and other retroviruses(52, 53). Both intracellular sensors signal via STING, thereby activating downstream TBK1 and the transcription factors interferon-regulatory factor (IRF) 3 and IRF7 to drive cell intrinsic innate immune responses(50).
Among the TLR family, TLR3, 7, 8 and 9 that are confined to the endosomes(54), have been described to sense several nucleic acid intermediates generated during the viral life cycle (55, 56). TLR3 is activated by dsRNA, TLR7 by ssRNA and short dsRNA and TLR8 detects short ssRNA and ssRNA breakdown products(57). Intracellularly, HIV-1 ssRNA can be recognized by TLR7 on plasmacytoid dendritic cells (pDCs) and TLR8 on monocytes and XCR1− DCs. However during the viral life cycle a dimeric state of HIV-1 viral RNA is present(58), which potentially allows for the recognition by TLR3 on monocytes and XCR1+ DCs(59). Whereas there is solid evidence for TLR7-mediated activation of pDCs by HIV-1 ssRNA(55), resulting in type I IFN production(60), other cell types appear to be less sensitive to HIV-1-encoded PAMPs. Ligand engagement of TLRs also leads to the activation of pro-inflammatory and antimicrobial responses via pathways involving JAK/Stat signaling, NF-κB and IRF3, IRF5 and IRF7(61). Recent data by our group suggest a critical role of IRF5 in mediating the observed sex differences in IFNa production by pDCs in response to TLR7 stimulation. Basal levels of IRF5 in pDCs from females were significantly higher compared to males and correlated with the percentage of IFNα-secreting pDCs(62)*. Interestingly, knockout of the Esr1 gene in the hematopoietic compartment or DC lineage of B6 mice reduced IRF5 mRNA expression in pDCs and IFNα production compared to wt mice, indicating that ERα can modulate IRF5 levels and thus the IFNα pathway. Taken together, sensing of HIV-1-derived oligonucleotides by intercellular PRRs, and in particular endosomal TLRs, results in the production of pro-inflammatory cytokines and antiviral type I IFN, and these proinflammatory responses are differentially regulated in women and men(62).
Sex differences in TLR7-mediated IFNα response are modulated by estrogens
Initial in vitro studies have shown significant sex-dependent differences in TLR7-induced IFNα production by pDCs, with higher levels of IFNα production by pDCs from females compared to males(63). Based on this data, Meier et al reported sex differences in the IFNα production by pDCs stimulated with HIV-1 and HIV-1-derived TLR7 ligands(9). This observation of sex-specific differences was restricted to pDCs, and neither myeloid dendritic cells (mDCs) nor monocytes presented sex differences in HIV-1-induced cytokine production. Interestingly, the percentage of IFNα-producing pDCs significantly correlated with plasma progesterone concentrations(9), indicating a possible role of sex hormones in modulating the TLR7/IFNα pathway in pDCs. In line with this, an important role of estrogen in regulating TLR responsiveness of pDCs has been recently elucidated(13). In a study by Seillet et al, the transplantation of human female CD34+ progenitor cells into either female or male humanized NOD/SCID/β2m−/− mice showed that pDCs developing in female mice exhibited an increased frequency of IFNα-producing cells compared to pDCs developing in male mice, indicating that the response of human pDCs is shaped by female sex hormones(13). To evaluate whether estrogens were responsible for the sex differences in IFNα production in humans, the effect of estrogen treatment in postmenopausal women on TLR7-mediated IFNα production by pDCs was analyzed in a longitudinal clinical trial study. 17β-Estradiol treatment markedly enhanced TLR7-dependent production of IFNα by pDCs from postmenopausal women(13). In a follow-up study, Laffont et al showed that blockage of estrogen receptor signaling during pDC development in vitro inhibited TLR7-mediated IFNα production by pDCs(64). Interestingly, X chromosome dosage contributed to the observed sex bias, as transplanted human pDCs derived from females had an increased TLR7-mediated IFNα production compared to pDCs from males, independent of the sex of the recipient mice(64). Taken together, these data clearly established an important role for estrogens in regulating innate immune functions and in particular the IFNα response of pDCs to viral infections.
The persistent production of type I IFN by pDCs has been linked to chronic immune activation in HIV-1 infection(65, 66) and correlated with markers of disease progression(43). The important role of type I IFN in viral pathogenesis was demonstrated in a recent studies assessing the role of type I IFN in acute versus chronic LCMV infection(67). Whereas blocking of IFNα during acute infection had detrimental consequences on the outcome of the LCMV infection, blocking of IFNα during chronic LCMV infection allowed for enhanced immune control(68, 69). In a humanized mice model, depletion of pDCs in acute HIV-1 infection using a monoclonal antibody abolished the induction of type I IFN and ISGs(70)*. During chronic infection, depletion of pDCs reduced HIV-1-induced depletion of T cells in lymphoid organs, despite increased levels of viral replication(70)*. These studies reflect the importance of the antiviral effects that IFNα exerts during acute infection, however also show that during persistent infection, the continuous production of IFNα results in an increase in immunopathology. Very recently, the timing of type I IFN-induced immune signatures was analyzed in detail during acute SIV-1 infection of rhesus macaques(71)**. Blockade of the IFNα/β receptor in acute infection reduced the levels of antiviral gene expression, increased the SIV reservoir size and accelerated the progression to AIDS, similar to the detrimental effects observed during acute LCMV infection. In contrast, administration of IFNα during primary infection initially prevented systemic infection, though persistent administration resulted in an increase in SIV reservoir size and accelerated disease markers. Altogether, these studies show the importance of IFNα-induced innate responses for the overall disease course of persistent viral infections(71)**.
Studies assessing the consequences of sex differences in IFNα production in treatment naïve, HIV-1-infected individuals showed that higher IFNα production in females was associated with higher levels of T cell activation, defined by CD38+HLA-DR+ T cells, in females in comparison to males after adjusting for viral load(9). Giving that the expression of T cell activation markers has been shown to predict the rate of untreated HIV-1 disease progression(72, 73), these data indicate a critical role of the TLR7/IFNα pathway in the sex-specific manifestations of HIV-1 disease. Chang et al furthermore demonstrated that the higher IFNα production observed in females was also associated with higher expression levels of several ISGs in treatment-naïve, chronically HIV-1- infected individuals(74). Overall, these data demonstrate that stronger HIV-1-dependent ISG induction in women compared to men, for the same viral load levels(74), is associated with the higher levels of immune activation observed in HIV-1 infected females, providing a possible mechanism for the faster disease progression described in infected women.
Conclusion
Recent studies have started to provide first insights into the pathways by which sex chromosomal factors and sex hormones regulate antiviral immunity, and in particular the type I IFN response to infections, leading to advances in our knowledge of how sex can influence HIV-1-mediated immunopathology. However, there remain large gaps in our understanding of the precise mechanisms leading to sex-based differences in immunity, and how these can be therapeutically modulated. A better understanding of these mechanisms will be critical in order to take sex-specific factors into consideration when designing experimental and clinical studies in HIV-1-infected populations.
Key points.
Prominent sex differences exists in the manifestations of primary and chronic HIV-1 infection
Sex-dependent differences in the TLR7/IFNα pathway lead to higher levels of ISG expression in female, resulting in stronger immune activation in HIV-1-infected females
These sex differences in the inflammatory response to HIV-1 might be responsible for the described faster disease progression in females compared to males after controlling for viral load levels
Estrogens can positively regulate the TLR7/IFNα pathway through cell-intrinsic pathways
Acknowledgments
none
Financial support
SZ is supported by a GILEAD grant. MA receives funding from the National Health Institute (NIH), the Deutsche Forschungsgemeinschaft (DFG), the German Center for Infection Research (DZIF), the Ragon Institute of MGH, MIT and Harvard, and the Leibniz Gemeinschaft.
Footnotes
Disclosure information: SZ received a grant from GILEAD Förderprogramm Infektiologie.
Conflicts of interest
None
References
- 1.van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. The Journal of infectious diseases. 2014;209(Suppl 3):S79–80. doi: 10.1093/infdis/jiu159. [DOI] [PubMed] [Google Scholar]
- 2.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Frontiers in Neuroendocrinology. 2014;35(3):347–69. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. The Journal of infectious diseases. 2014;209(Suppl 3):S100–6. doi: 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel G, Arck PC. Sex, immunity and influenza. The Journal of infectious diseases. 2014;209(Suppl 3):S93–9. doi: 10.1093/infdis/jiu020. [DOI] [PubMed] [Google Scholar]
- 5.Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: does sex play a role? The Journal of infectious diseases. 2014;209(Suppl 3):S81–5. doi: 10.1093/infdis/jiu057. [DOI] [PubMed] [Google Scholar]
- 6.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. The Journal of infectious diseases. 2014;209(Suppl 3):S86–92. doi: 10.1093/infdis/jiu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein SL. Sex differences in prophylaxis and therapeutic treatments for viral diseases. Handb Exp Pharmacol. 2012;(214):499–522. doi: 10.1007/978-3-642-30726-3_22. [DOI] [PubMed] [Google Scholar]
- 8.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays : news and reviews in molecular, cellular and developmental biology. 2012;34(12):1050–9. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature medicine. 2009;15(8):955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villacres MC, Longmate J, Auge C, Diamond DJ. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Human immunology. 2004;65(5):476–85. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes and immunity. 2009;10(5):509–16. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–12. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C, Arnal JF, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119(2):454–64. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 14.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nature reviews Immunology. 2010;10(8):594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 15.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews Immunology. 2008;8(9):737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markle JG, Fish EN. SeXX matters in immunity. Trends in immunology. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Lockshin MD. Nonhormonal explanations for sex discrepancy in human illness. Annals of the New York Academy of Sciences. 2010;1193:22–4. doi: 10.1111/j.1749-6632.2009.05293.x. [DOI] [PubMed] [Google Scholar]
- 18.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–72. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296(5573):1642–4. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 20.Moore AL, Mocroft A, Madge S, Devereux H, Wilson D, Phillips AN, et al. Gender differences in virologic response to treatment in an HIV-positive population: a cohort study. Journal of acquired immune deficiency syndromes (1999) 2001;26(2):159–63. doi: 10.1097/00042560-200102010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization 2014 - Department of Maternal N, Child and Adolescent Health. Health for the World’s Adolescents - A second chance in the second decade. 2014. WHO/FWC/MCA/14.05. [Google Scholar]
- 23.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352(9139):1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 24.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. The New England journal of medicine. 2001;344(10):720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 25.Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, Little S, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. The Journal of infectious diseases. 2011;203(4):442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinsztejn B, Smeaton L, Barnett R, Klingman K, Hakim J, Flanigan T, et al. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4(+) T-cell count. Antiviral therapy. 2011;16(7):1057–62. doi: 10.3851/IMP1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loupa CV, Rodriguez B, McComsey G, Gripshover B, Salata RA, Valdez H, et al. Gender differences in human immunodeficiency virus (HIV) RNA and CD4 cell counts among new entrants to HIV care. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2006;12(4):389–91. doi: 10.1111/j.1469-0691.2006.01368.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore AL, Kirk O, Johnson AM, Katlama C, Blaxhult A, Dietrich M, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. Journal of acquired immune deficiency syndromes (1999) 2003;32(4):452–61. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 29.Collazos J. Sexual dysfunction in the highly active antiretroviral therapy era. AIDS reviews. 2007;9(4):237–45. [PubMed] [Google Scholar]
- 30.Nicastri E, Angeletti C, Palmisano L, Sarmati L, Chiesi A, Geraci A, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS (London, England) 2005;19(6):577–83. doi: 10.1097/01.aids.0000163934.22273.06. [DOI] [PubMed] [Google Scholar]
- 31.Prins M, Robertson JR, Brettle RP, Aguado IH, Broers B, Boufassa F, et al. Do gender differences in CD4 cell counts matter? AIDS (London, England) 1999;13(17):2361–4. doi: 10.1097/00002030-199912030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mocroft A, Gill MJ, Davidson W, Phillips AN. Are there gender differences in starting protease inhibitors, HAART, and disease progression despite equal access to care? Journal of acquired immune deficiency syndromes (1999) 2000;24(5):475–82. doi: 10.1097/00126334-200008150-00013. [DOI] [PubMed] [Google Scholar]
- 33.Greenblatt RM, Ameli N, Grant RM, Bacchetti P, Taylor RN. Impact of the ovulatory cycle on virologic and immunologic markers in HIV-infected women. The Journal of infectious diseases. 2000;181(1):82–90. doi: 10.1086/315207. [DOI] [PubMed] [Google Scholar]
- 34.Maini MK, Gilson RJ, Chavda N, Gill S, Fakoya A, Ross EJ, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourinary medicine. 1996;72(1):27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95(1):249–55. [PubMed] [Google Scholar]
- 36.Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nature medicine. 1999;5(1):83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 37.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clinical microbiology reviews. 2001;14(4):753–77. doi: 10.1128/CMR.14.4.753-777.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179(4):859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 40.Choudhary SK, Vrisekoop N, Jansen CA, Otto SA, Schuitemaker H, Miedema F, et al. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. Journal of virology. 2007;81(16):8838–42. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS (London, England) 2003;17(13):1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 42.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. The Journal of infectious diseases. 2008;197(1):126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PloS one. 2013;8(2):e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(4):435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein B, Malow R. A critical review of HIV-related interventions for women prisoners in the United States. The Journal of the Association of Nurses in AIDS Care : JANAC. 2010;21(5):380–94. doi: 10.1016/j.jana.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Ren W, Ma Y, Yang L, Gettie A, Salas J, Russell K, et al. Fast disease progression in simian HIV-infected female macaque is accompanied by a robust local inflammatory innate immune and microbial response. AIDS (London, England) 2015;29(10):F1–8. doi: 10.1097/QAD.0000000000000711. In this study a novel nonhuman primate model for the investigation of sex differences in HIV-1 disease progression was used to study sex differences in local innate immune activation and gut microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nature reviews Microbiology. 2012;10(9):655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. The Journal of infectious diseases. 2009;199(8):1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altfeld M, Gale M., Jr Innate immunity against HIV-1 infection. Nature immunology. 2015;16(6):554–62. doi: 10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- 51.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(48):E4571–80. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–15. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clinical immunology (Orlando, Fla) 2008;126(3):235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nature structural & molecular biology. 2015;22(2):109–15. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 58.Moore MD, Hu WS. HIV-1 RNA dimerization: It takes two to tango. AIDS reviews. 2009;11(2):91–102. [PMC free article] [PubMed] [Google Scholar]
- 59.Junt T, Barchet W. Translating nucleic acid-sensing pathways into therapies. Nature reviews Immunology. 2015;15(9):529–44. doi: 10.1038/nri3875. [DOI] [PubMed] [Google Scholar]
- 60.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. Journal of virology. 2007;81(15):8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical microbiology reviews. 2009;22(2):240–73. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-alpha Production in Women. 2015 doi: 10.4049/jimmunol.1501684. This study showed that basel levels of IRF5 in pDCs differ between females and males and demonstrate that higher IRF5 levels lead to higher IFNα production upon TLR7 stimulation. Furthermore, genetic ablation of the ERα in mice demonstrated that TLR7-mediated IFNα-production in pDCs is partially regulated by ERα signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. Journal of immunology (Baltimore, Md : 1950) 2006;177(4):2088–96. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 64**.Laffont S, Rouquie N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-Chromosome Complement and Estrogen Receptor Signaling Independently Contribute to the Enhanced TLR7-Mediated IFN-alpha Production of Plasmacytoid Dendritic Cells from Women. Journal of immunology (Baltimore, Md : 1950) 2014 doi: 10.4049/jimmunol.1303400. This study investigated the respective contribution of X chromosome dosage and sex hormones on TLR7-mediated IFNα production and showed that that female sex hormones, estrogens, and X chromosome complement independently contribute to the enhanced TLR7-mediated IFNα response of pDCs in women. [DOI] [PubMed] [Google Scholar]
- 65.Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes and infection/Institut Pasteur. 2011;13(1):14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Current opinion in HIV and AIDS. 2011;6(5):411–8. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odorizzi PM, Wherry EJ. Immunology. An interferon paradox. Science. 2013;340(6129):155–6. doi: 10.1126/science.1237568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–11. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–7. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70*.Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, et al. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS pathogens. 2014;10(7):e1004291. doi: 10.1371/journal.ppat.1004291. This study investigated the role of pDC in HIV-1 infection and pathogenesis using a humanized mice model. Depletion of pDC confirmed the important role of pDCs in the control of HIV-1 replication, as well as their contribution to HIV-1-induced immunopathogenesis during chronic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71**.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511(7511):601–5. doi: 10.1038/nature13554. Sandler et al studied the timing of IFN-induced innate immune responses during SIV transmission and acute SIV infection. Their results indicate that the timing of IFN-induced immune responses affects the overall disease outcome and highlights the detrimental consequences of persistent immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 73.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. Journal of acquired immune deficiency syndromes (1999) 1993;6(8):904–12. [PubMed] [Google Scholar]
- 74.Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES, et al. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. The Journal of infectious diseases. 2013;208(5):830–8. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]