Abstract
Whether HbA1c levels are associated with mortality in subjects without known diabetes remains controversial. Moreover, the shape of the dose–response relationship on this topic is unclear. Therefore, a dose–response meta-analysis was conducted. PubMed and EMBASE were searched. Summary hazard ratios (HRs) were calculated using a random-effects model. Twelve studies were included. The summary HR per 1% increase in HbA1c level was 1.03 [95% confidence interval (CI) = 1.01–1.04] for all-cause mortality, 1.05 [95% CI = 1.02–1.07) for cardiovascular disease (CVD) mortality, and 1.02 (95% CI = 0.99–1.07) for cancer mortality. After excluding subjects with undiagnosed diabetes, the aforementioned associations remained significant for CVD mortality only. After further excluding subjects with prediabetes, all aforementioned associations presented non-significance. Evidence of a non-linear association between HbA1c and mortality from all causes, CVD and cancer was found (all Pnon-linearity < 0.05). The dose–response curves were relatively flat for HbA1c less than around 5.7%, and rose steeply thereafter. In conclusion, higher HbA1c level is associated with increased mortality from all causes and CVD among subjects without known diabetes. However, this association is driven by those with undiagnosed diabetes or prediabetes. The results regarding cancer mortality should be treated with caution due to limited studies.
HbA1c, a major component of hemoglobin-glucose adducts1, reflects the average blood glucose level within the prior 2 to 3 months2, and is a well accepted biomarker for glycemic management in diabetic patients over the past several decades. HbA1c has been consistently recommended as a diagnostic biomarker for diabetes by several societies3,4,5. At present, in clinical practice, a variety of diagnostic assays, including ion-exchange chromatography, electrophoresis, immunoassay, are available to measure HbA1c levels6,7. Nonetheless, mounting evidence shows that electrochemical HbA1c sensors (e.g., amperometric nonenzymatic sensors)8 and optical HbA1c sensors (e.g., vibrational spectroscopy)6,9 possibly provide more convenient and cheaper assays for determining HbA1c levels.
Previous studies in diabetic patients demonstrated a significant association between HbA1c levels and all-cause mortality10, which presented a J-shaped pattern11. However, whether these findings can extend into subjects without known diabetes is largely unknown. To fill this gap, some epidemiological studies were conducted but presented inconsistent results. Several studies observed that high HbA1c levels (≥6.5%, 48 mmol/mol) were significantly associated with increased all-cause mortality2,12,13, whereas others failed to find any significant associations with all-cause mortality across the whole HbA1c range14,15. In addition, for the shape of the dose–response relationship between HbA1c levels and mortality, previous studies have suggested a J-shaped pattern12,15, a U-shaped pattern2, and a linear pattern13. Although a 2011 meta-analysis16, including 5 studies, investigated the association of HbA1c levels with mortality from cardiovascular disease (CVD) in non-diabetic population, substantial heterogeneity (I2 = 98.5%) and combination data from cross-sectional study17 with those from prospective studies18,19,20,21 raised concerns for the reliability of its findings.
With more attention to HbA1c currently, investigation of the dose–response relationship between HbA1c and mortality is critical for a better understanding of this biomarker, and can extend its’ application into a broader field. However, to the best of our knowledge, a comprehensive dose–response meta-analysis on the HbA1c-mortality association is not available to date. Therefore, the objectives of our study were to clarify the associations between HbA1c levels and risks of death from all causes, CVD, or cancer in subjects without known diabetes, and to further investigate the exact shape of these associations.
Methods
Search strategy
We conducted this study and reported corresponding results in adherence to the PRISMA statement22. A comprehensive electronic search of PubMed and EMBASE was conducted up to January 2015. Detailed search strategy is presented in the Supplementary List S1. For including additional citations, we performed a manual search of the reference lists of included articles and pertinent reviews. We contacted the original authors to obtain extra information if necessary.
Study selection
Studies were eligible for inclusion if they met the following criteria: (1) participants: subjects without known diabetes; (2) exposure: HbA1c levels measured by methods standardized by the National Glycohemoglobin Standardization Program or International Federation of Clinical Chemistry23; (3) outcome: adjusted risk estimates for at least three quantitative HbA1c categories on the associations of HbA1c levels with risks of death from all causes, CVD, or cancer; (4) study design: prospective cohort study. We excluded studies in which subjects were free of diabetes but suffered from other conditions (e.g., acute coronary syndrome).
Data extraction
Two investigators (G.C.Z. and J.H.C.) independently performed study screening through a two-stage method. At the first stage, we scrutinized titles and abstracts to exclude apparently irrelevant studies. At the second stage, we read carefully the full text to further exclude unrelated studies. Two investigators discussed together until consensus was reached when any discrepancy regarding the eligibility of studies occurred.
One investigator (G.C.Z.) performed data extraction, and then another investigator (J.H.C.) checked the results for accuracy. We re-checked data and discussed together to deal with any inconsistent results. Following information was extracted: first author, publication year, study location, mean age at baseline, sample size, mean follow-up duration, assessments of non-diabetic statuses and outcomes, methods of measuring HbA1c, deaths and person-years for each HbA1c level, categories of HbA1c, maximally adjusted risk estimates and corresponding 95% confidence intervals (CIs), and adjustment factors. We extracted information from the publication with the longest follow-up duration when multiple publications derived from the same cohort.
Data synthesis and analysis
In this meta-analysis, hazard ratio (HR) for every 1% increase in HbA1c level was used to assess the HbA1c-mortality association. To support the International Federation of Clinical Chemistry HbA1c system23, we also calculated HR for a 10 mmol/mol increase in HbA1c level. For one study24, relative risk was treated as equivalent to HR. For another study25 whose authors reported risk estimates for men and women separately, we pooled these data to yield an overall estimate using a random-effects model. The Hedges Q statistic was employed to qualitatively assess heterogeneity across studies, with P < 0.10 indicating statistically significant heterogeneity. The I2 statistic was employed to quantify heterogeneity26, with I2 > 50% representing substantial heterogeneity, 30% ≤ I2 ≤ 50% representing moderate heterogeneity, and I2 < 30% representing low heterogeneity27.
We performed a two-stage dose–response meta-analysis to identify whether higher HbA1c level was significantly associated with increased mortality. First, for the purpose of pooling risk estimates from included studies using different HbA1c categorization, we employed the generalized least square regression described by Orsini and colleagues28 to calculate study-specific slopes (linear trends) and 95% CIs for every 1% increase in HbA1c level within each study from the natural logs of maximally adjusted HRs and CIs across categories of HbA1c. Then, we pooled them using a random-effects model to obtain the summary risk estimates. This method is based on specific HbA1c level, distribution of deaths and person-years, and adjusted risk estimates and 95% CIs. Since all included studies reported HbA1c level as range, we designated the midpoint of lower and upper limits as the assigned level. When the highest range was open-ended, we calculated the assigned level by adding the width of the adjacent range to the highest value specified. When the lowest range was open-ended, we calculated the assigned level by subtracting half of the width of the adjacent range from the lowest value specified29. The method described by Hamling and colleagues30 was employed to convert risk estimates if the reference group reported in the original study was not the lowest group. For 4 studies15,19,31,32 whose authors did not report person-years for each HbA1c category, we approximately estimated these data from mean follow-up duration and number of subjects. For studies that provided risk estimates with more than one level of adjustment, we calculated study-specific slopes and 95% CIs with minimally adjusted data to examine whether the observed associations were largely explained by confounding. To determine whether increased mortality was due to inclusion of subjects with undiagnosed diabetes (HbA1c ≥ 6.5%, 48 mmol/mol), we repeated our meta-analyses by excluding these subjects. To investigate whether HbA1c was still associated with mortality in subjects with normal HbA1c level (HbA1c < 5.7%, 39 mmol/mol), we repeated our meta-analyses by further excluding subjects with prediabetes (5.7% ≤ HbA1c ≤ 6.4%, 39 mmol/mol ≤ HbA1c ≤ 46 mmol/mol).
We explored a potential non-linear dose–response relationship between HbA1c levels and mortality using restricted cubic spline function with 3 knots at the 10th, 50th, and 90th percentiles33,34. A Pnon-linearity was obtained by testing the null hypothesis that the estimated value of the second spline equals zero34.
To determine the stability of summary results, we performed sensitivity analyses for all-cause and CVD mortality through three techniques, namely ignoring a single study in turn, repeating meta-analyses by a fixed-effects model, and applying various eligibility criteria. To identify whether the association of HbA1c levels with mortality was modified by age, follow-up duration, sample size, and study location, we conducted subgroup analyses stratified by these study-level characteristics. A Pinteraction between subgroups was calculated by meta-regression. Considering limited studies for CVD and cancer mortality, subgroup analyses were performed only for all-cause mortality.
Begg rank correlation test35 and Egger linear regression test36 were employed to test publication bias, with P < 0.1 indicating publication bias. We conducted all data analyses through STATA software (version12.0, StataCorp, College Station, TX). Statistical significance level was set at P < 0.05 under two-sided test unless otherwise specified.
Results
Study identification and selection
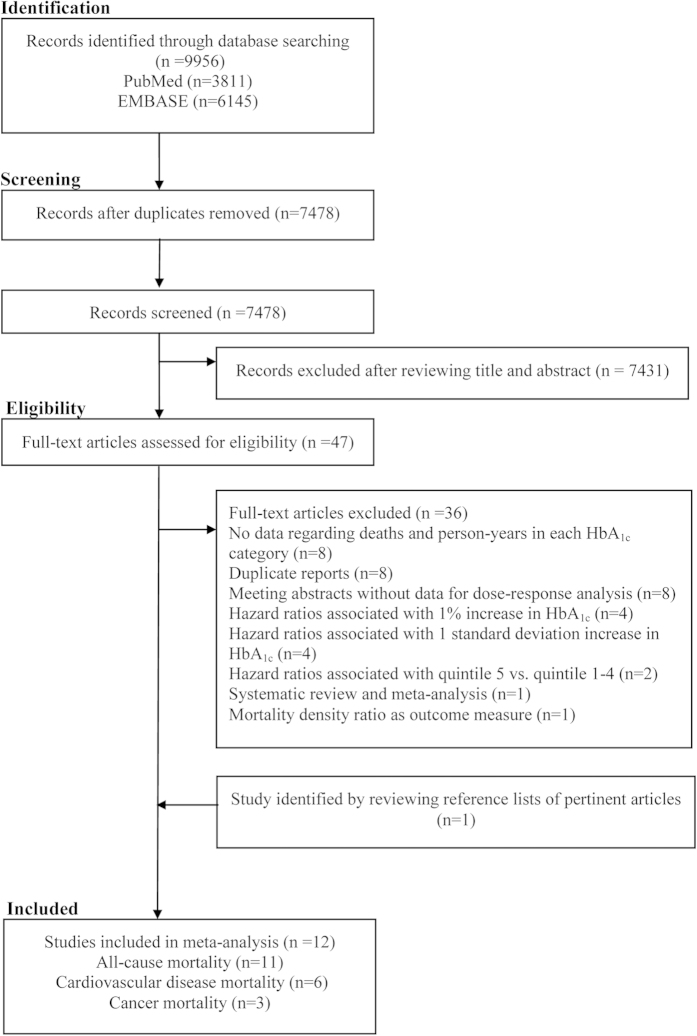
Our comprehensive retrieval identified 3,811 and 6,145 records from PubMed and EMBASE, respectively. After removing duplicates and excluding obviously unrelated records, there were remaining 47 records that were potentially relevant. After checking the full text, 36 were further excluded (detailed reasons for exclusion are shown in Fig. 1). We included two studies25,37 derived from the same cohort, because they reported different outcomes (one reported all-cause mortality37, another reported cancer mortality)25. In addition, we added one study15 through the handsearch. Thus, 12 studies (11 cohorts) were eligible for inclusion (Fig. 1).
Figure 1. The flowchart of identifying relevant studies.
Study characteristics
The characteristics of included studies are summarized in Supplementary Table S1. The HbA1c level ranged from 3.5% (15 mmol/mol)24 to 10.4% (90 mmol/mol)24 across studies. Of included studies, ten provided data necessary to calculate proportions of subjects in three clinical HbA1c ranges (i.e., normal, prediabetes, and undiagnosed). Specifically, proportions of subjects with normal HbA1c level, prediabetes, and undiagnosed diabetes were 85.90%, 11.33%, and 2.77%, respectively. The included studies were published between 200532 and 20152. Four studies were conducted in Europe2,19,31,38, 5 in the USA14,15,24,25,37, and remaining 3 in Asia12,13,32. The mean age of participants at baseline varied from 44.7 years2 to 78.7 years19 across studies. The sample size of included study ranged from 81014 to 26,54924. Our study included a total of 114,102 subjects, consisting of 41,616 men (36.5%) and 72,486 women (63.5%). The mean follow-up duration changed from 5.0 years19 to 14.2 years14, and during 1,161,714 person-years of follow-up, there were a total of 11,301 deaths. Most studies relied on self-reports of participants for the ascertainment of non-diabetic status, and only 2 studies14,38 confirmed it through diagnostic test or use of anti-diabetic medications. Of included studies, the information regarding vital status and causes of death was obtained from diverse sources, including death certificate19,25,31,32,37 and death registry2,12,38. Ten studies used high performance liquid chromatography method to measure HbA1c level, and remaining 2 studies adopted affinity column method14 or turbidimetric immunoinhibition assay method24.
Association of HbA1c levels with all-cause mortality
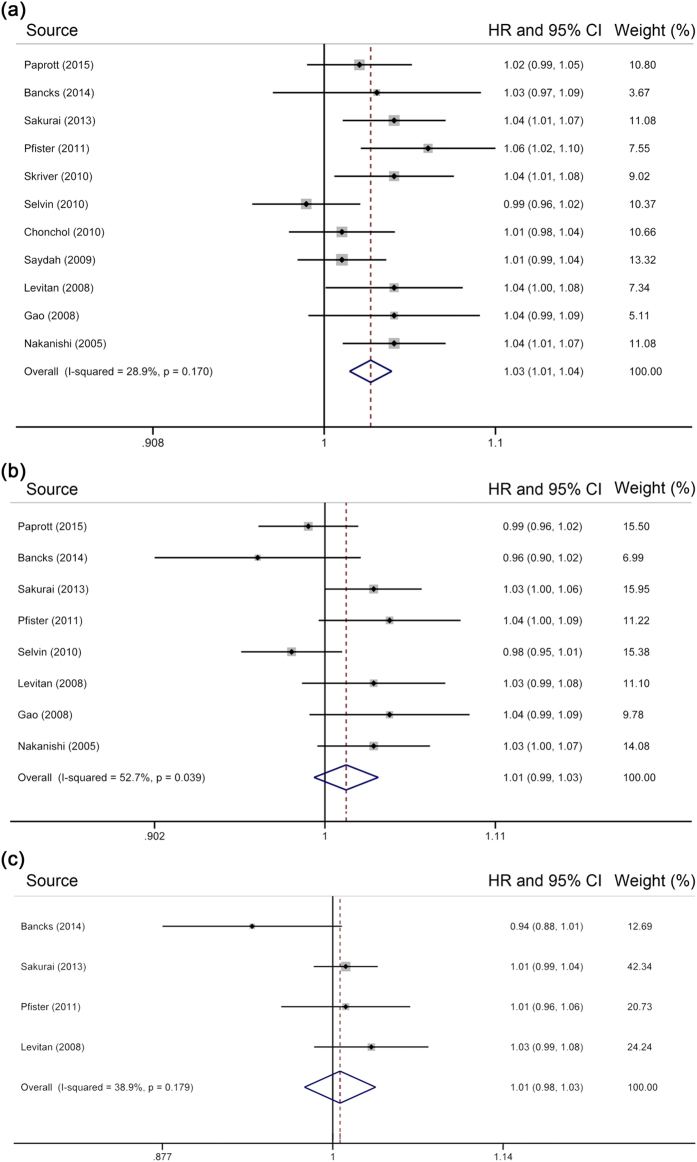
Eleven individual studies were eligible for the two-stage dose–response analysis of HbA1c levels and all-cause mortality, involving a sum of 113,526 subjects and 11,301 deaths. The summary HR per 1% increase in HbA1c level was 1.03 [95% CI = 1.01–1.04] (for every 10 mmol/mol increase, 1.03 [95% CI = 1.01–1.04]), with evidence of low heterogeneity (I2 = 28.9%, P = 0.17) (Fig. 2a). Parallel analysis with minimally adjusted data from 8 individual studies2,13,14,15,24,31,32,37 produced an HR of 1.03 [95% CI = 1.02 –1.05]. However, the initial pooled result presented non-significance after excluding participants with undiagnosed diabetes [HR = 1.01, 95% CI = 0.99–1.03] (Fig. 2b)2,12,13,19,24,31,32,37. After further excluding subjects with prediabetes, a non-significant risk estimate was observed [HR = 1.01, 95% CI = 0.98–1.03] (Fig. 2c)12,13,24,25.
Figure 2.
Meta-analysis on HbA1c and all-cause mortality in (a) subjects without known diabetes, (b) those without diabetes, and (c) those with normal HbA1c range. The squares represent the hazard ratio per 1% increase in HbA1c level for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary hazard ratio per 1% increase in HbA1c level, with width representing 95% confidence interval.
Association of HbA1c levels with CVD mortality
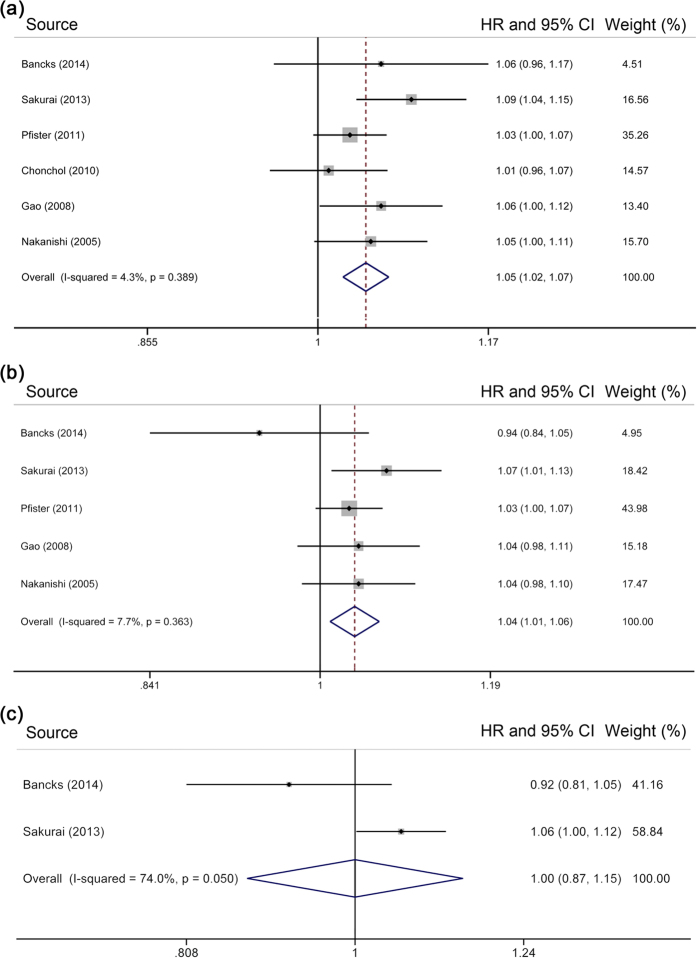
Six individual studies were eligible for the association between HbA1c levels and CVD mortality, with a total of 36,695 participants and 1,713 deaths. The pooled HR per 1% increase in HbA1c level was 1.05 [95% CI = 1.02–1.07] (for every 10 mmol/mol increase, 1.04 [95% CI = 1.02– 1.06]) (Fig. 3a), with evidence of low heterogeneity (I2 = 4.3%, P = 0.39). Meta-analysis of HRs with the least degree of adjustment13,14,31,32 yielded an HR of 1.04 [95% CI = 1.01–1.07]. After excluding participants with undiagnosed diabetes12,13,19,31,32, the result was attenuated slightly, but remained significant [HR = 1.04, 95% CI = 1.01–1.06] (Fig. 3b). However, after further excluding participants with prediabetes12,13, higher HbA1c level was not significantly associated with increased mortality from CVD [HR = 1.00, 95% CI = 0.87–1.15] (Fig. 3c).
Figure 3.
Meta-analysis on HbA1c and cardiovascular disease mortality in (a) subjects without known diabetes, (b) those without diabetes, and (c) those with normal HbA1c range. The squares represent the hazard ratio per 1% increase in HbA1c level for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary hazard ratio per 1% increase in HbA1c level, with width representing 95% confidence interval.
Association of HbA1c levels with cancer mortality
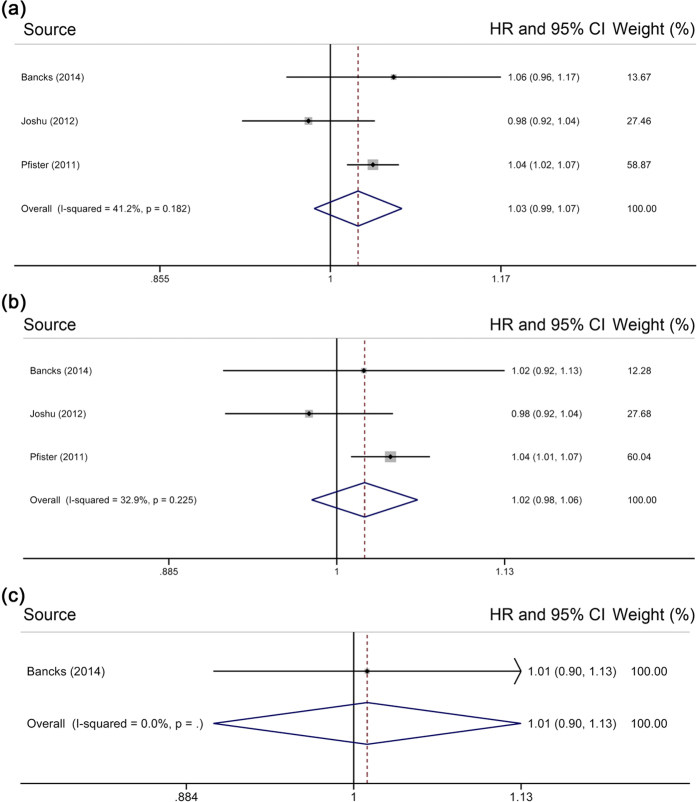
Only 3 studies were included in the two-stage dose–response analysis of HbA1c levels and cancer mortality, involving a total of 36,252 individuals and 2,115 deaths. Random-effects meta-analysis revealed an HR of 1.03 [95% CI = 0.99–1.07] per 1% increase in HbA1c level (for every 10 mmol/mol increase, 1.02 [95% CI = 0.98–1.06]) (Fig. 4a), with evidence of moderate heterogeneity (I2 = 41.2%, P = 0.18). With 2 studies25,31 reporting minimally adjusted HRs, a pooled HR of 1.04 [95% CI = 1.01–1.06] was obtained. Our analysis produced a non-significant pooled risk estimate [HR = 1.02, 95% CI = 0.98–1.06] (Fig. 4b) after excluding participants with undiagnosed diabetes12,25,31. Further excluding participants with prediabetes12 resulted in an HR of 1.01 [95% CI = 0.90–1.13] (Fig. 4c).
Figure 4.
Meta-analysis on HbA1c and cancer mortality in (a) subjects without known diabetes, (b) those without diabetes, and (c) those with normal HbA1c range. The squares represent the hazard ratio per 1% increase in HbA1c level for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% confidence interval. The diamond represents the summary hazard ratio per 1% increase in HbA1c level, with width representing 95% confidence interval.
Non-linear dose–response analyses
Using restricted cubic spline function, we found the evidence of non-linear associations between HbA1c levels and risks of death from all causes (Pnon-linearity < 0.0001) (Fig. 5a), CVD (Pnon-linearity < 0.0001) (Fig. 5b) and cancer (Pnon-linearity = 0.0001) (Fig. 5c). The curves were relatively flat when HbA1c levels were less than approximately 5.7% (39 mmol/mol), and rose steeply thereafter. The curvilinear slope reached the maximal value at HbA1c level of around 6.5% (48 mmol/mol). Similar increasing patterns were observed for CVD and cancer mortality.
Figure 5.
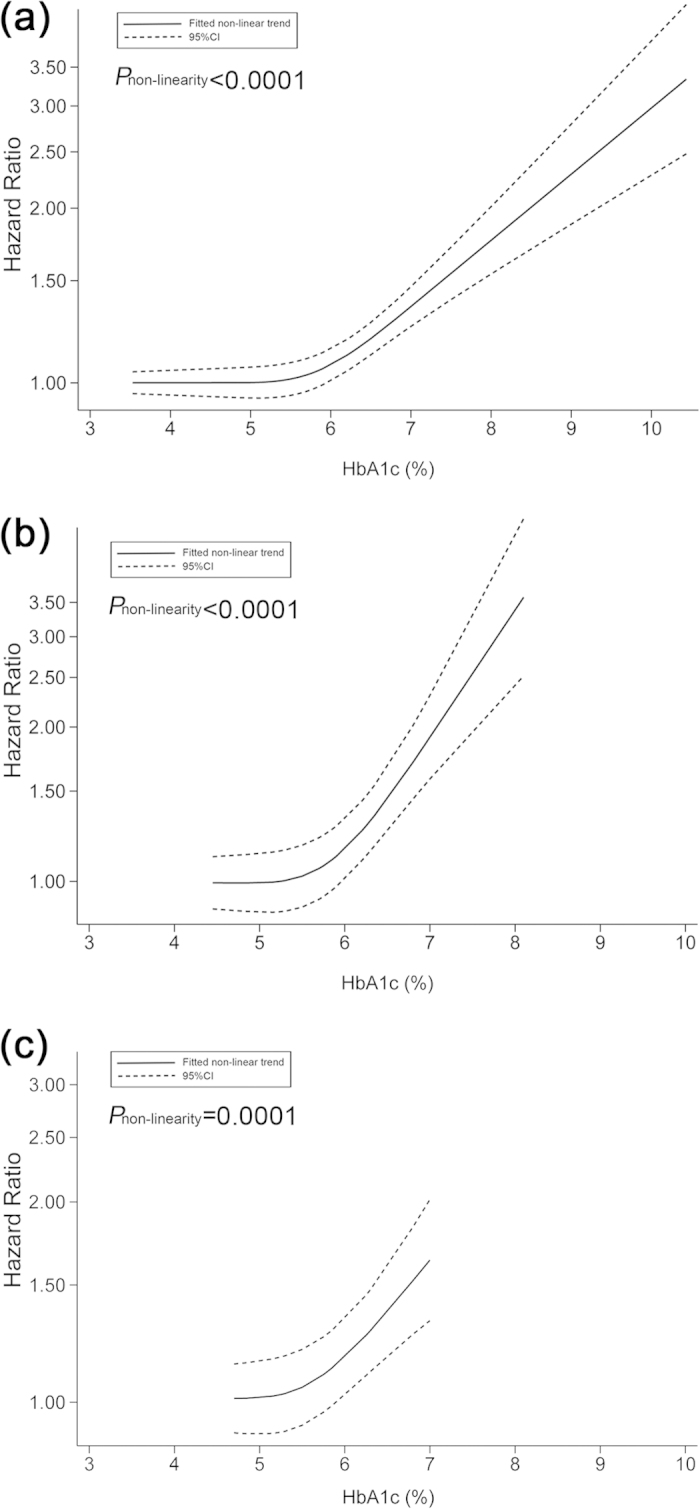
Non-linear dose–response analyses on the association of HbA1c level with mortality from (a) all causes, (b) cardiovascular disease, and (c) cancer.
Subgroup and sensitivity analyses
The significant association between HbA1c levels and all-cause mortality remained in all subgroups (see Supplementary Table S2). Pooled risk estimates from the random-effects model and fixed-effects model were virtually identical. Omitting a single study in turn did not significantly change the summary risk estimate of either all-cause or CVD mortality. Repeating meta-analyses according to various eligibility criteria did not change our pooled results, either (see Supplementary Table S3).
Publication bias
There was no evidence of publication bias for any association as revealed by Begg’s test and Egger’s test (all P > 0.1).
Discussion
The findings from the two-stage dose–response analyses suggested that in subjects without known diabetes, higher HbA1c level is a predictor of increased all-cause and CVD mortality but not of cancer mortality. In subjects without diabetes, higher HbA1c level is a predictor of increased CVD mortality only; in those with normal HbA1c level, higher HbA1c level is not a predictor of any studied mortality outcome.
We found evidence of a non-linear association between HbA1c and mortality from all causes, CVD and cancer in this meta-analysis. The dose-response curves were relatively flat for HbA1c less than around 5.7%, and rose steeply thereafter. This fact reveals a clear threshold effect for the association of HbA1c levels with mortality. In addition, from the perspective of mortality benefit and health care burden, it suggests that the most appropriate HbA1c level of initiating intervention is approximately 5.7%. Considering the essential requirement for HbA1c to maintain normal metabolism of our body, it is reasonable that normal HbA1c levels are not significantly associated with increased mortality, which was also clearly shown in our two-stage dose–response analyses on HbA1c levels and mortality. However, mounting evidence shows that people are at high risk of some health conditions that could significantly increase mortality risk when HbA1c level increases abnormally. For example, hyperglycemia measured by HbA1c has been found to be associated with increased risks of stroke39, coronary heart disease40, colorectal cancer41, as well as hearing impairment42 in people without a history of diabetes.
In this study, our two-stage dose–response analyses did not find any significant associations with cancer mortality in the whole studied population as well as in subjects with HbA1c < 6.5%. Considering limited studies and relatively wide CIs for risk estimates, the failure to detect significant associations was possibly caused by lack of power. In addition, if using a fixed-effects model to achieve data pooling, significant risk estimates could be obtained (for the whole studies population, HR = 1.03, 95% CI = 1.01–1.06, P < 0.01; for subjects with HbA1c < 6.5%, HR = 1.03, 95% CI = 1.00–1.05, P = 0.03). With those considerations above, the results for cancer mortality in these two groups should be treated with caution, and more studies on HbA1c and cancer mortality are needed.
Previous studies found that hyperglycemia measured by HbA1c was significantly related to increased all-cause mortality in diabetic subjects10. Our study extended this association into subjects without known diabetes. The deleterious effect of hyperglycemia on mortality is biologically plausible. Ample evidence shows that hyperglycemia can result in oxidative stress (OS)43,44 through several potential mechanisms involving generation of reactive oxygen species, non-enzymatic glycation of proteins, and auto-oxidation of glucose43. OS then results in vascular endothelial dysfunction, which contributes to the presence of CVD45. OS can induce DNA damage46, protein carbonylation47, actions on signaling pathways48, possibly resulting in gene mutation or cell proliferation48. Therefore, OS is also a candidate contributor for cancer development49. Taken together, hyperglycemia-induced OS plays a major role in the underlying mechanisms for the association of hyperglycemia with increased mortality.
In the present meta-analysis, most of included studies ascertained non-diabetic status through self-reports. Consequently, our study population comprised a part of subjects with undiagnosed diabetes in the context of a large proportion of diabetic cases being not captured by self-reports50. Several epidemiological studies have demonstrated increased mortality in subjects with undiagnosed diabetes51,52. Considering the aforementioned facts, the increased all-cause and CVD mortality observed in the two-stage dose–response analyses might be resulted from inclusion of those with undiagnosed diabetes. Therefore, whether higher HbA1c level is still predictive of increased mortality among subjects with HbA1c < 6.5% is unclear. For addressing this critical concern, we repeated our meta-analyses by excluding subjects with undiagnosed diabetes. Corresponding results showed that the positive association with higher HbA1c level remained for CVD mortality only, suggesting that higher HbA1c level is still a predictor of increased CVD mortality but not of all-cause mortality in subjects with HbA1c < 6.5%. The underlying reasons for the above phenomenon are unclear. A possible explanation is that the association between higher HbA1c level and mortality from non-CVD is of extreme non-significance, making the contribution of CVD mortality to all-cause mortality insubstantial.
Several cross-sectional studies consistently indicate that HbA1c levels increase with age in individuals without known diabetes53,54,55. Taking into account the above fact in combination with the dose–response patterns observed in our study, age is a possible effect modifier of the HbA1c-mortality association. However, the result of our subgroup analysis stratified by age did not provide evidence to support this hypothesis. Another candidate effect modifier is sex, considering that a sex difference in HbA1c levels has been reported despite inconclusive results53,56. Of included studies, only 312,13,25 investigated this potential effect modifier. Two of them12,13 consistently revealed no modification effect by sex for all-cause and CVD mortality, whereas the remaining one25 found that the significant positive association of HbA1c levels with increased cancer mortality was only seen in women. Limited studies precluded our attempts to investigate this potential effect modifier through subgroup analysis12,13,25. Therefore, more studies are warranted to identify whether sex can modify the HbA1c-mortality association.
Our study has several limitations. First, all included studies measured HbA1c level on a single occasion at baseline. Considering the within-subject variation of HbA1c level and measurement error, our results might be subject to regression dilution bias57, indicating an underestimated effect size for the association of HbA1c levels with mortality. Nonetheless, within-subject variation of HbA1c in the healthy population has been found to be minimal58. Second, several included studies used death certificate to ascertain the causes of death14,19,25,31,32. Information from death certificate is inaccurate in some conditions59. Therefore, misclassification bias possibly affected our results on the associations between HbA1c levels and risks of death from CVD and cancer. Third, although we extracted the most fully adjusted risk estimates, our results might be still biased by residual confounding. Nevertheless, the pooled results of maximally and minimally adjusted risk estimates are similar, indicating that the observed associations in our study cannot be explained predominantly by confounding. Fourth, we cannot calculate summary risk estimates for groups with undiagnosed diabetes as well as those with prediabetes because of diverse categorization across studies, although these data are important for medical practitioners. Fifth, our results may be influenced by language bias, because we restricted our search to studies published in English or Chinese. Nevertheless, it has been found that the effect of language restriction on the results of systematic reviews is non-significant60. Finally, although not indicated by both Begg’s test and Egger’s test, our results may be still driven by publication bias because above two tests have insufficient power when including limited studies. Moreover, exclusion of several studies where necessary data for dose–response analysis were not reported possibly results in publication bias.
In conclusion, higher HbA1c level is associated with increased mortality from all causes, CVD, and cancer among subjects without known diabetes. However, this association is influenced by those with undiagnosed diabetes or prediabetes. Because of limited studies, the results in relation to cancer mortality should be treated with caution, and more studies are therefore warranted to investigate whether higher HbA1c level is associated with increased cancer mortality.
Additional Information
How to cite this article: Zhong, G.-C. et al. HbA1c and Risks of All-Cause and Cause-Specific Death in Subjects without Known Diabetes: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Sci. Rep. 6, 24071; doi: 10.1038/srep24071 (2016).
Supplementary Material
Acknowledgments
No funding or financial support was received for this study. The authors thank Dr. Elizabeth Selvin (Johns Hopkins Bloomberg School of Public Health) for providing original data.
Footnotes
Author Contributions G.C.Z. and J.P.G. conceived the study idea. G.C.Z. and J.H.C. performed literature search, study selection, and data extraction. M.X.Y. and Y.Z. performed statistical analyses and interpretation of corresponding results. G.C.Z. drafted the initial manuscript. J.P.G. had primarily responsibility for final content. All authors made critical comment for the initial manuscript.
References
- Lenters-Westra E., Schindhelm R. K., Bilo H. J. & Slingerland R. J. Haemoglobin A1c: Historical overview and current concepts. Diabetes Res Clin Pract. 99, 75–84, doi: 10.1016/j.diabres.2012.10.007 (2013). [DOI] [PubMed] [Google Scholar]
- Paprott R. et al. Association Between Hemoglobin A1c and All-Cause Mortality: Results of the Mortality Follow-up of the German National Health Interview and Examination Survey 1998. Diabetes Care. 38, 249–256, doi: 10.2337/dc14-1787 (2015). [DOI] [PubMed] [Google Scholar]
- Committee I. E. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 32, 1327–1334, doi: 10.2337/dc09-9033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 37 Suppl 1, S81–90, doi: 10.2337/dc14-S081 (2014). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011. Geneva : World Health Organization (2013). [PubMed] [Google Scholar]
- Pandey R. et al. Emerging trends in optical sensing of glycemic markers for diabetes monitoring. Trends in analytical chemistry: TRAC. 64, 100–108, doi: 10.1016/j.trac.2014.09.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. & Anzai J.-i. Recent Progress in Electrochemical HbA1c Sensors: A Review. Materials. 8, 1187–1203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. et al. Recent advances in electrochemical glucose biosensors: a review. Rsc Advances. 3, 4473–4491 (2013). [Google Scholar]
- Spegazzini N. et al. Spectroscopic approach for dynamic bioanalyte tracking with minimal concentration information. Scientific Reports. 4, 7013, doi: 10.1038/srep07013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hu G., Yuan Z. & Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 7, e42551, doi: 10.1371/journal.pone.0042551 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L. W. & Wang Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. The review of diabetic studies: RDS. 11, 138–152, doi: 10.1900/rds.2014.11.138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancks M. P. et al. Glycated hemoglobin and all-cause and cause-specific mortality in Singaporean Chinese without diagnosed diabetes: The Singapore Chinese Health Study. Diabetes Care. 37, 3180–3187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. et al. HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care. 36, 3759–3765, doi: 10.2337/dc12-2412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonchol M. et al. Glycosylated hemoglobin and the risk of death and cardiovascular mortality in the elderly. Nutrition, metabolism, and cardiovascular diseases: NMCD. 20, 15–21, doi: 10.1016/j.numecd.2009.02.007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydah S., Tao M., Imperatore G. & Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care. 32, 1440–1446, doi: 10.2337/dc09-0117 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Oliveira R. et al. Haemoglobin A1c levels and subsequent cardiovascular disease in persons without diabetes: a meta-analysis of prospective cohorts. Diabetologia. 54, 1327–1334, doi: 10.1007/s00125-011-2078-8 (2011). [DOI] [PubMed] [Google Scholar]
- Singer D. E., Nathan D. M., Anderson K. M., Wilson P. W. & Evans J. C. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 41, 202–208 (1992). [DOI] [PubMed] [Google Scholar]
- Khaw K. T. et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 141, 413–420 (2004). [DOI] [PubMed] [Google Scholar]
- Gao L., Matthews F. E., Sargeant L. A. & Brayne C. An investigation of the population impact of variation in HbA 1clevels in older people in England and Wales: From a population based multi-centre longitudinal study. BMC Public Health. 8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vegt F. et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 42, 926–931, doi: 10.1007/s001250051249 (1999). [DOI] [PubMed] [Google Scholar]
- Corpus R. A., O’Neill W. W., Dixon S. R., Timmis G. C. & Devlin W. H. Relation of hemoglobin A1c to rate of major adverse cardiac events in nondiabetic patients undergoing percutaneous coronary revascularization. The American journal of cardiology. 92, 1282–1286 (2003). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed.). 339, b2535, doi: 10.1136/bmj.b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanas R. & John G. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care. 33, 1903–1904, doi: 10.2337/dc10-0953 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E. B. et al. HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia. 51, 267–275, doi: 10.1007/s00125-007-0882-y (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshu C. E. et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990–2006. International Journal of Cancer. 131, 1667–1677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.). 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 21, 1539–1558, doi: 10.1002/sim.1186 (2002). [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R. & Greenland S. Generalized least squares for trend estimation of summarized dose–response data. The Stata Journal. 6(1), 40–57 (2006). [Google Scholar]
- Hartemink N., Boshuizen H. C., Nagelkerke N. J., Jacobs M. A. & van Houwelingen H. C. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol. 163, 1042–1052, doi: 10.1093/aje/kwj141 (2006). [DOI] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R. & Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Statistics in medicine. 27, 954–970, doi: 10.1002/sim.3013 (2008). [DOI] [PubMed] [Google Scholar]
- Pfister R., Sharp S. J., Luben R., Khaw K. T. & Wareham N. J. No evidence of an increased mortality risk associated with low levels of glycated haemoglobin in a non-diabetic UK population. Diabetologia. 54, 2025–2032 (2011). [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Yamada M., Hattori N. & Suzuki G. Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia. 48, 230–234, doi: 10.1007/s00125-004-1643-9 (2005). [DOI] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 175, 66–73, doi: 10.1093/aje/kwr265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L. & Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine. 29, 1037–1057, doi: 10.1002/sim.3841 (2010). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.). 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E. et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New England Journal of Medicine. 362, 800–811 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver M. V., Borch-Johnsen K., Lauritzen T. & Sandbaek A. HbA1c as predictor of all-cause mortality in individuals at high risk of diabetes with normal glucose tolerance, identified by screening: a follow-up study of the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION), Denmark. Diabetologia. 53, 2328–2333, doi: 10.1007/s00125-010-1867-9 (2010). [DOI] [PubMed] [Google Scholar]
- Selvin E., Rawlings A. M., Bergenstal R. M., Coresh J. & Brancati F. L. No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care. 36, 2995–3001, doi: 10.2337/dc12-2715 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N. et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 7, e1000278, doi: 10.1371/journal.pmed.1000278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S. et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 17, 3108–3115, doi: 10.1158/1055-9965.epi-08-0495 (2008). [DOI] [PubMed] [Google Scholar]
- Michikawa T., Mizutari K., Saito H., Takebayashi T. & Nishiwaki Y. Glycosylated hemoglobin level is associated with hearing impairment in older Japanese: the Kurabuchi Study. J Am Geriatr Soc. 62, 1231–1237, doi: 10.1111/jgs.12906 (2014). [DOI] [PubMed] [Google Scholar]
- Blaak E. E. et al. Impact of postprandial glycaemia on health and prevention of disease. Obesity reviews: an official journal of the International Association for the Study of Obesity. 13, 923–984, doi: 10.1111/j.1467-789X.2012.01011.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains J. L. & Jain S. K. Oxidative stress, insulin signaling, and diabetes. Free radical biology & medicine. 50, 567–575, doi: 10.1016/j.freeradbiomed.2010.12.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah E. & Bruno R. S. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutrition research (New York, N.Y.). 32, 727–740, doi: 10.1016/j.nutres.2012.08.002 (2012). [DOI] [PubMed] [Google Scholar]
- Loft S. et al. Biomarkers of oxidative damage to DNA and repair. Biochem Soc Trans. 36, 1071–1076, doi: 10.1042/bst0361071 (2008). [DOI] [PubMed] [Google Scholar]
- Rao R. S. & Moller I. M. Pattern of occurrence and occupancy of carbonylation sites in proteins. Proteomics. 11, 4166–4173, doi: 10.1002/pmic.201100223 (2011). [DOI] [PubMed] [Google Scholar]
- Mates J. M., Segura J. A., Alonso F. J. & Marquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Archives of toxicology. 82, 273–299, doi: 10.1007/s00204-008-0304-z (2008). [DOI] [PubMed] [Google Scholar]
- Thanan R. et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 16, 193–217, doi: 10.3390/ijms16010193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A., Dasgupta K., Chiasson J. L. & Rahme E. Estimating the population prevalence of diagnosed and undiagnosed diabetes. Diabetes Care. 36, 3002–3008, doi: 10.2337/dc12-2543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes S., Botas P., Delgado E. & Diaz Cadorniga F. Mortality risk in spanish adults with diagnosed diabetes, undiagnosed diabetes or pre-diabetes. The Asturias study 1998-2004. Revista espanola de cardiologia. 62, 528–534 (2009). [DOI] [PubMed] [Google Scholar]
- Wild S. H., Smith F. B., Lee A. J. & Fowkes F. G. Criteria for previously undiagnosed diabetes and risk of mortality: 15-year follow-up of the Edinburgh Artery Study cohort. Diabetic medicine: a journal of the British Diabetic Association. 22, 490–496, doi: 10.1111/j.1464-5491.2004.01433.x (2005). [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Lu F. H., Wu J. S. & Chang C. J. Age and sex effects on HbA1c. A study in a healthy Chinese population. Diabetes Care. 20, 988–991 (1997). [DOI] [PubMed] [Google Scholar]
- Yates A. P. & Laing I. Age-related increase in haemoglobin A1c and fasting plasma glucose is accompanied by a decrease in beta cell function without change in insulin sensitivity: evidence from a cross-sectional study of hospital personnel. Diabetic medicine: a journal of the British Diabetic Association. 19, 254–258 (2002). [DOI] [PubMed] [Google Scholar]
- Davidson M. B. & Schriger D. L. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract. 87, 415–421, doi: 10.1016/j.diabres.2009.12.013 (2010). [DOI] [PubMed] [Google Scholar]
- Bae J. C. et al. Hemoglobin A1c values are affected by hemoglobin level and gender in non-anemic Koreans. J Diabetes Investig. 5, 60–65, doi: 10.1111/jdi.12123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon S. et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 335, 765–774 (1990). [DOI] [PubMed] [Google Scholar]
- Lenters-Westra E., Roraas T., Schindhelm R. K., Slingerland R. J. & Sandberg S. Biological variation of hemoglobin A1c: consequences for diagnosing diabetes mellitus. Clin Chem. 60, 1570–1572, doi: 10.1373/clinchem.2014.227983 (2014). [DOI] [PubMed] [Google Scholar]
- Kircher T., Nelson J. & Burdo H. The autopsy as a measure of accuracy of the death certificate. The New England journal of medicine. 313, 1263–1269, doi: 10.1056/nejm198511143132005 (1985). [DOI] [PubMed] [Google Scholar]
- Moher D., Pham B., Lawson M. L. & Klassen T. P. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health technology assessment (Winchester, England). 7, 1–90 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.