Abstract
Background
Recent evidence suggests that living kidney donors are at an increased risk of end-stage renal disease. However, predicting which donors will have renal dysfunction remains challenging, particularly among those with no clinical evidence of disease at the time of donation. Although renal biopsies are not routinely performed as part of the donor evaluation process, they may yield valuable information that improves the ability to predict renal function in donors.
Methods
We used implantation protocol biopsies to evaluate the association between histological abnormalities in the donated kidney and postdonation renal function (estimated glomerular filtration rate, eGFR) of the remaining kidney in living kidney donors. Longitudinal analysis using mixed-effects linear regression was used to account for multiple eGFR measures per donor.
Results
Among 310 donors between 1997 and 2012, median (IQR) follow-up was 6.2 (2.5–8.7; maximum 14.0) years. In this cohort, the overall prevalence of histological abnormalities was 65.8% (19.7% abnormal glomerulosclerosis, 23.9% abnormal interstitial fibrosis and tubular atrophy (IFTA), 4.8% abnormal mesangial matrix increase, 32.0% abnormal arteriolar hyalinosis, and 32.9% abnormal vascular intimal thickening). IFTA was associated with a 5-mL/min/1.73m2 decrease of postdonation eGFR after adjusting for donor age at donation, sex, race, preoperative systolic blood pressure, preoperative eGFR, and time since donation (p<0.01).
Conclusions
In this single-center study, among healthy individuals cleared for living donation, IFTA was associated with decreased postdonation eGFR, while no other subclinical histological abnormalities provided additional information.
INTRODUCTION
Despite being rigorously screened for kidney disease,1 living kidney donors may develop end-stage renal disease (ESRD) as early as 5–10 years postdonation.2–6 Since it typically takes decades for a healthy, screened individual to develop de novo disease, and to progress from chronic kidney disease (CKD) to ESRD,7 the occurrence of ESRD in the early postdonation period may point to subtle subclinical, undiagnosed kidney disease at donation. Indeed, 4–50% of implantation biopsies have been found to harbor moderate-to-severe histological abnormalities.8–13 However, there are conflicting reports about the association between these histological abnormalities and postdonation renal function (estimated glomerular filtration rate, eGFR) in living kidney donors.8–10,12,14
In studies that found an association between histological abnormalities and postdonation renal function,12,14 the difference in renal function between donors with and without histological abnormalities (0.2–1.4% difference in the percent of eGFR recovery at 0.5–2 years postdonation) was not clinically significant. The studies that found no association between histological abnormalities and postdonation renal function were notable for insufficient follow-up (<1 year) for the evolution of clinically salient CKD.8,9 Also, no study to date, to our knowledge, has accounted for the hierarchical structure of eGFR data (repeated measurements of eGFR for an individual); as such, even the significant association found in some studies might represent statistical artifacts.
In order to gain more insight into the relationship between histological abnormalities and postdonation renal function in living kidney donors, we linked implantation biopsy data (ie donor histology) to postdonation medical records. We conducted a retrospective cohort analysis that aimed to characterize the prevalence of histological abnormalities in donated kidneys at time of donation, and the association between these abnormalities and long-term postdonation eGFR trajectories of the remaining kidneys in living kidney donors. To account for multiple eGFR measures per donor, we used longitudinal data analysis (hierarchical) methods.
METHODS
Study population
We identified 310 individuals who underwent living donor nephrectomy at our center between February 1997 and June 2012 and had an implantation biopsy of the donated kidney performed at the time of donation as part of a recipient study protocol. Selection for implantation biopsy was related to recipient involvement in research studies, and not based on any donor characteristics (see Table 1). This study was approved by our institution's Institutional Review Board (NA_00044282).
Table 1. Donor characteristics stratified by biopsy availability.
This table compares the donor characteristics of our study population (biopsied donors) and the general living donor pool (nonbiopsied donors) at our center
| Characteristics | Biopsied donors (n=310) |
Non-biopsied donors (n=522) |
p-value |
|---|---|---|---|
| Age at donation (y), median (IQR) |
47.0 (39.2–54.1) | 46.4 (37.3–54.3) | 0.2 |
| African American, n(%) | 23 (7.4%) | 54 (10.3%) | 0.4 |
| Female, n(%) | 203 (65.5%) | 332 (63.6%) | 0.6 |
| Preoperative systolic blood pressure (mmHg), median (IQR) |
120 (110–131) | 124 (112–133) | 0.3 |
| Preoperative eGFR (mL/min/1.73m2), median (IQR) |
97.0 (86.1–107.0) | 97.3 (84.8–107.6) | 0.9 |
Histological Abnormalities
Core biopsies from the donor kidney were fixed in buffered formalin, embedded in paraffin, and cut sections were stained with hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, and Jones’ silver methenamine. Evaluation was performed by light microscopy. Biopsies were reviewed by 1 of 5 experienced nephropathologists at our center at the time of donation, who were blinded to donor demographics, except for donor sex. They were also blinded to the research question, since this is a retrospective analysis of their clinical reads.
Biopsies were evaluated for glomerulosclerosis (gs), interstitial fibrosis and tubular atrophy (IFTA), mesangial matrix increase (mm), arteriolar hyalinosis (ah), and vascular fibrous intimal thickening (cv). gs was quantified as the percentage of observed glomeruli that were globally sclerotic. IFTA was assessed as the observed percentage of cortical area that had IFTA and categorized as minimal (≤5%), mild (6–25%), moderate (26–50%), or severe (>50%); corresponding to Banff IFTA0, IFTA1, IFTA2, and IFTA3, respectively.15–17 mm was quantified as increase versus no increase; corresponding to Banff mm1–3 and mm0. ah was categorized as no ah, mild-to-moderate, moderate-to-severe, or severe; corresponding to Banff ah0, ah1, ah2, and ah3, respectively. cv was categorized as no cv, mild-to-moderate, moderate-to-severe, or severe; corresponding to Banff cv0, cv1, cv2, and cv3, respectively. If the pathology report did not mention the presence of vasculature, we assumed that there was none observed in the biopsy.
We defined a donor as having any histological abnormalities if he/she had at least 1 of the following types of abnormalities: abnormal gs (≥10%), abnormal IFTA (≥IFTA1), abnormal mm (≥mm1), abnormal ah (≥ah1), and/or abnormal cv (≥cv1).
Postdonation eGFR
We used postdonation medical records to estimate GFR, based on serum creatinine measurements obtained over the course of the donor's postdonation medical care, using the CKD-EPI creatinine equation.18 We used boxplots to visualize eGFR trajectories over time. We also used multilevel mixed-effects linear regression to perform longitudinal data analysis. Based on the Akaike information criterion and likelihood ratio tests, we selected an optimal hierarchical regression model that allowed for random intercepts (starting eGFR) and slopes (eGFR trajectory). This model was then used to study the unadjusted and adjusted relationships between histological abnormalities and eGFR trajectories, adjusting for time since donation, preoperative donor age at donation, sex, African American race, preoperative systolic blood pressure, and preoperative eGFR.
Statistical analysis
For continuous variables, the median and interquartile range (IQR) are reported. For categorical variables, the number and percentage are reported. We used t-tests and Fisher exact tests as appropriate to compare our study population (biopsied donors) to the general living kidney donor pool at our center (nonbiopsied donors). We also used t-tests and Fisher exact tests as appropriate to compare baseline characteristics of donors with and without any histological abnormalities within our study population. Statistical significance was established at a p-value less than 0.05 (2-tailed). All analyses were performed using STATA 13.0 for Mac (College Station, TX). Confidence intervals are reported as per the Louis and Zeger method.19
RESULTS
Study population
The median (IQR) age at donation of our study population was 47.0 (39.2–54.1) years; 7% were African American and 66% were female (Table 1). The median (IQR) preoperative systolic blood pressure was 120 (110–131) mmHg, and the median (IQR) preoperative eGFR was 97.0 (86.1–107.0) mL/min/1.73m2 (Table 1). Our study population (biopsied donors) did not differ from the general living kidney donor pool (nonbiopsied donors) at our center, in terms of donor age at donation (p=0.2), sex (p=0.6), African American race (p=0.4), preoperative systolic blood pressure (p=0.3), and preoperative eGFR (p=0.9) (Table 1).
Histological abnormalities
The prevalence of donors with any histological abnormalities was 65.8% (Table 2). Specifically, 19.7% had abnormal gs, 23.9% had abnormal IFTA, 4.8% had abnormal mm, 32.0% had abnormal ah, and 32.9% had abnormal cv (Table 2). Among 204 donors with any histological abnormalities, 105 (51.5%) had only 1 type of abnormality; 63 (30.9%) had a combination of 2 types of histological abnormality; 25 (12.3%) had a combination of 3 types of histological abnormality; 10 (4.9%) had a combination of 4 types of histological abnormality; and 1 (0.5%) donor had histological abnormalities in all 5 categories.
Table 2. Prevalence of histological abnormalities detected at time of kidney donation among living kidney donors.
Five types of histological abnormalities were identified at time of kidney donation: glomerulosclerosis (gs), interstitial fibrosis and tubular atrophy (IFTA), mesangial matrix increase (mm), arteriolar hyalinosis (ah), and vascular fibrous intimal thickening (cv).
| Histological Abnormalities (n=310) | n (%) |
|---|---|
| glomerulosclerosis (gs) | |
| <10% | 200 (64.5) |
| ≥10% | 61 (19.7) |
| missing | 49 (15.8) |
| interstitial fibrosis and tubular atrophy (IFTA) | |
| IFTA0 - Minimal | 234 (75.5) |
| IFTA1 - Mild | 70 (22.6) |
| IFTA2 - Moderate | 4 (1.3) |
| IFTA3 - Severe | 0 (0.0) |
| missing | 2 (0.7) |
| mesangial matrix increase (mm) | |
| mm0 - No increase | 280 (90.3) |
| mm1–3 - Increase | 15 (4.8) |
| missing | 15 (4.8) |
| arteriolar hyalinosis (ah) | |
| ah0 - No ah | 188 (60.7) |
| ah1 - Mild-to-moderate | 96 (31.0) |
| ah2 - Moderate-to-severe | 2 (0.7) |
| ah3 - Severe | 1 (0.3) |
| missing | 23 (7.4) |
| vascular fibrous intimal thickening (cv) | |
| cv0 - No cv | 185 (59.7) |
| cv1 - Mild-to-moderate | 92 (29.7) |
| cv2 - Moderate-to-severe | 9 (2.9) |
| cv3 - Severe | 1 (0.3) |
| missing | 23 (7.4) |
| any histological abnormalities | |
| present | 204 (65.8) |
| absent | 106 (34.2) |
Donors with any histological abnormalities were slightly older than donors without any histological abnormalities (median (IQR) 48.1 (40.4–55.2) versus 45.9 (37.4–53.1) years, p=0.04). Preoperative systolic blood pressure (p=0.1), preoperative eGFR (p=0.1), percent African American (p=0.7), percent female (p=0.5), and follow-up time (p=0.8) did not differ between donors with versus without any histological abnormalities (Table 3).
Table 3. Study population characteristics stratified by any histological abnormalities.
A donor was considered to have any histological abnormalities if he/she had at least 1 abnormality in any of: glomerular sclerosis, interstitial fibrosis and tubular atrophy, mesangial matrix increase, arteriolar hyalinosis, and/or vascular fibrous intimal thickening.
| Characteristics | Donors with any histological abnormalities (n=204) |
Donors without any histological abnormalities (n=106) |
p-value |
|---|---|---|---|
| Age at donation (y), median (IQR) |
48.1 (40.4–55.2) | 45.9 (37.4–53.1) | 0.04 |
| African American, n(%) |
16 (7.8) | 7 (6.6) | 0.7 |
| Female, n(%) | 131 (64.2) | 72 (67.9) | 0.5 |
| Preoperative systolic blood pressure (mmHg), median (IQR) |
124 (110–132) | 118 (110–130) | 0.1 |
| Preoperative eGFR (mL/min/1.73m2), median (IQR) |
96.3 (85.7–107.6) | 99.0 (87.5–105.1) | 0.5 |
| Follow-up time (y), median (IQR) |
6.3 (2.5–8.7) | 6.1 (2.6–8.7) | 0.8 |
| Post-donation eGFR, (mL/min/1.73m2), median (IQR) |
58.6 (50.8–69.0) | 59.8 (52.5–69.4) | 0.1 |
| Number of post- donation eGFR measurements per donor, median (IQR) |
5 (3–8) | 5 (3–9) | 0.2 |
Postdonation eGFR
Donors had a median of 5 postdonation eGFR measurements (IQR 3–8, range 1–30). The median (IQR) postdonation eGFR was 60.5 (53.7–70.1) mL/min/1.73m2 over a median follow-up time of 6.2 (IQR 2.5–8.7; maximum 14.0) years. The percent of donors who had a post-donation mean eGFR below 60 mL/min/1.73m2 was 46.5%. The median (IQR) postdonation eGFR was similar among donors with and without any histological abnormalities (58.6 (50.8–69.0) versus 59.8 (52.5–69.4) mL/min/1.73m2, p=0.1) (Table 3).
In an adjusted hierarchical regression model, postdonation eGFR increased with time (0.180.410.65 mL/min/1.73m2 per year, p<0.001), decreased with donor age (−4.55−3.10−1.65 mL/min/1.73m2 per 10 years, p<0.001), and increased with preoperative eGFR (3.023.964.90 mL/min/1.73m2 per 10 mL/min/1.73m2, p<0.001) (Table 4). Moreover, abnormal IFTA was associated with decreased postdonation eGFR (−8.13−4.77−1.41 mL/min/1.73m2 per year, p<0.01) (Table 4, Figure 1). However, there was no statistically significant association between the other types of histological abnormalities (gs, mm, ah, and cv) and post-donation eGFR. There was also no evidence of interaction between IFTA and time since donation (p=0.4).
Table 4. Multilevel mixed-effects linear regression models of postdonation eGFR trajectory using individual histological abnormalities.
The unadjusted model included time since donation (per 1-year increase), glomerulosclerosis (gs per 10% increase in globally sclerotic glomeruli), interstitial fibrosis and tubular atrophy (abnormal IFTA≥1 vs normal IFTA<1), mesangial matrix increase (abnormal mm≥1 vs normal mm<1), arteriolar hyalinosis (abnormal ah≥1 vs normal ah<1), and vascular fibrous intimal thickening (abnormal cv≥1 vs normal <1). The adjusted model includes all of the above plus age at donation (per 10 years increase), sex (females vs males), race (African American vs other), preoperative systolic blood pressure (per 10 mmHg increase), and preoperative eGFR (per 10 mL/min/1.73m2).
| Difference in eGFR (mL/min/1.73m2)C | ||
|---|---|---|
| Unadjusted Model | Adjusted ModelA | |
| Per year | 0.280.500.72 | 0.180.410.65 |
| gs (per 10%) | −3.63−1.291.05 | −0.031.843.70 |
| Abnormal IFTA (vs normal) | −8.96−4.74−0.53 | −8.13−4.77−1.41 |
| Abnormal mm (vs normal) | −7.55−0.436.70 | −5.82−0.015.81 |
| Abnormal ah (vs normal) | −0.692.766.20 | −1.541.223.99 |
| Abnormal cv (vs normal) | −2.081.515.10 | −1.391.464.31 |
| Age at donation (per 10y) | - | −4.55−3.10−1.65 |
| Female (vs. male) | - | −2.440.232.90 |
| African American (vs. other) | - | −5.230.666.54 |
| Preoperative systolic blood pressure (per 10 mmHg) |
- | −1.62−0.770.07 |
| Preoperative eGFR (per 10 mL/min/1.73m2) |
- | 3.023.964.90 |
Notes: Bolded p-value statistically significant at α =0.05.
Adjusted for time since donation, donor age at donation, sex, race, preoperative systolic blood pressure, and preoperative eGFR.
95% confidence intervals are reported as per the Louis and Zeger method.19
Figure 1.
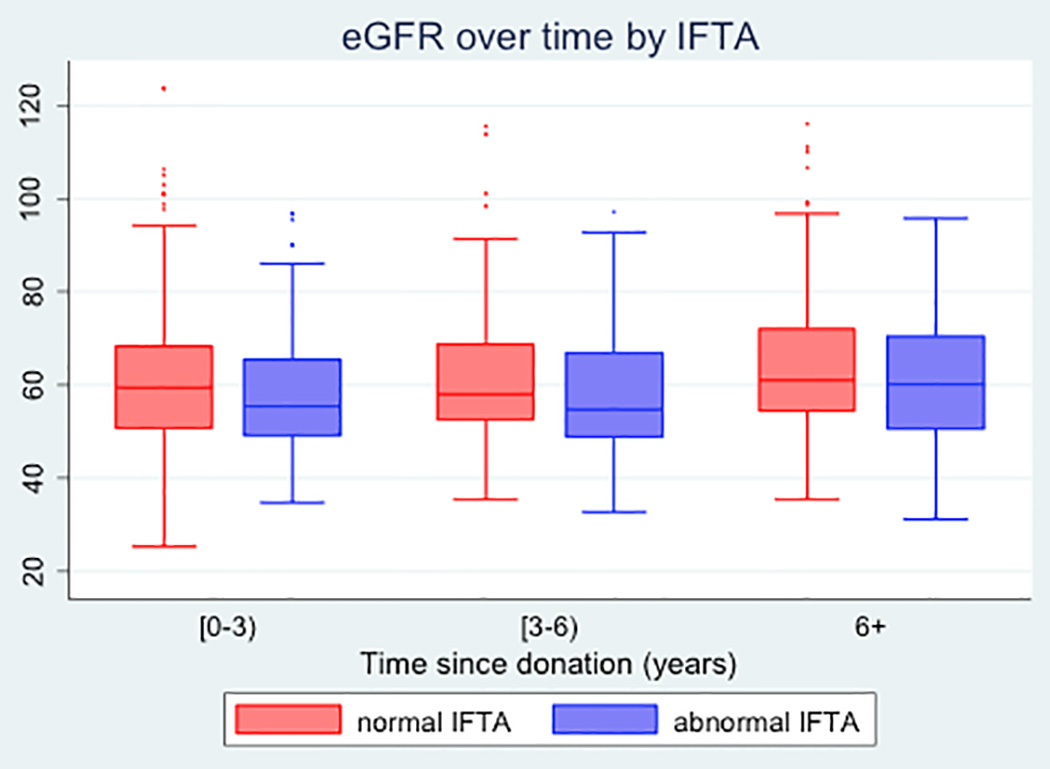
Boxplots of post-donation eGFR of living kidney donors over time, by status of interstitial fibrosis and tubular atrophy (IFTA). Time since donation is categorized into three bins: (0–3) years, (3–6) years, and 6+ years. IFTA is categorized as abnormal (IFTA≥1) vs normal (IFTA<1)
DISCUSSION
In this retrospective single-center study of 310 living kidney donors, the prevalence of donors with any histological abnormalities was 65.8%. After adjusting for donor clinical characteristics and time since donation, we found that subclinical IFTA was associated with a 5-mL/min/1.73m2 decrease of postdonation eGFR.
Our findings are consistent with the findings of Ohashi et al12 that study found that the presence of chronic histological changes (defined as having at least 2 of the following: >5% gs, any IFTA, and any arteriosclerosis) was associated with a 0.23% decrease in the percent eGFR recovery from immediate post-donation to 2-year postdonation, after adjusting for donor age and change of eGFR from baseline to immediate postdonation (n=110).12 However, our findings are in contrast with the findings of Chauhan et al,8 Choi et al,9 and Elsherbiny et al.14 This difference could arise due to several reasons, including our longer follow-up time, differences in patient casemix, covariate adjustment, our robust statistical methods to account for the correlated nature of the longitudinal data (multiple eGFR measurements per donor), and/or the types of histological abnormalities studied and their cut-offs.
Chauhan et al (n=1600) reported that 4-month postdonation eGFR was similar in donors with and without moderate-to-severe histological changes (a composite score of interstitial fibrosis, ah, cv, tubular atrophy, and allograft glomerulopathy), after adjusting for donor clinical characterisics;8 given the very low prevalence (4%) of moderate-to-severe histological changes in living kidney donors in their study, their study may have lacked statistical power to identify the association seen in our study.8 Choi et al (n=121) reported that 12-month postdonation eGFR was not affected by tubular atrophy, gs, or ah at time of donation, after adjusting for clinical characteristics;9 they do not include interstitial fibrosis in their analysis that could again explain the discrepancy in our findings.9 Elsherbiny et al (n=1,395) reported a 1.4% decrease in the percent of GFR recovery, from baseline to a mean follow-up of 6.2 months postdonation, per 1 mm3 of globally sclerotic density, after adjusting for other renal morphometric measures at donation;14 differences in our findings could be due to the differences in casemix and covariate adjustment.
We also found that, after adjusting for other donor clinical characteristics, postdonation eGFR increased with time (0.4 mL/min/1.73m2 per year), indicating that the remaining donor kidney might be hyperfiltrating as a compensation mechanism for the insult sustained during donation. Postdonation eGFR also increased with preoperative eGFR (4 mL/min/1.73m2 per 10 mL/min/1.73m2), and decreased with donor age at donation (-3 mL/min/1.73m2 per 10 years), suggesting that preoperative eGFR and age are 2 important clinical characteristics in quantifying donor healthiness and ability to recover postdonation.
Our study is limited by sample size, the retrospective nature of the data, and the use of eGFR to quantify renal function.20 Nevertheless, our study has longitudinal follow-up (multiple measures over time for many patients), with relatively longer postdonation follow-up time compared to previous studies; our median postdonation follow-up was 6.2 years, whereas other studies reported mean follow-up times of 0.3–2 years.8,9,12,14 Another strength of our study is that we accounted for the hierarchical structure (repeated measurements of eGFR for an individual) of postdonation eGFR data. Moreover, in our study we separately investigated each type of histological abnormality. This allowed us to investigate how each type of abnormality is associated with postdonation eGFR.
Our study was conducted on healthy individuals who have undergone rigorous living donor evaluation and were cleared for donation. Most abnormalities observed in our study were mild. Therefore, our results may not generalize to abnormalities more serious than those observed in our study, or observed in individuals with poorer baseline health. While our study shows decreased eGFR in donors with mild to moderate IFTA, biopsies are invasive processes. Even if a biopsy may yield increased information about postdonation eGFR in a potential donor, any benefit from this information may be outweighed by the harm of the biopsy. Moreover, the decline in postdonation eGFR among donors with IFTA is modest; the human kidney is redundant by design and may compensate for minor damage. Therefore, we do not recommend incorporating biopsies into the donor screening process. However, if biopsy data are available, they may be useful in counseling donors postdonation.
In conclusion, in our single-center study, among healthy individuals cleared for living kidney donation, subclinical IFTA was associated with decreased postdonation eGFR, after adjusting for other donor characteristics. These results may help guide the direction of future research of postdonation renal function in living kidney donors.
Acknowledgments
FUNDING
This work was supported by grant numbers R01DK096008 and K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
ABBREVIATIONS
- ah
arteriolar hyalinosis
- CKD
chronic kidney disease
- cv
vascular fibrous intimal thickening
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- gs
glomerular sclerosis
- IFTA
interstitial fibrosis and tubular atrophy
- IQR
interquartile range
- mm
mesangial matrix increase
Footnotes
AUTHORSHIP PARTICIPATION
Lara M. Fahmy, ScM: Conception and design, statistical analysis, interpretation of results, drafting of manuscript
Allan B. Massie, PhD MHS: Conception and design, statistical analysis, interpretation of results, revision of manuscript
Abimereki D. Muzaale, MD MPH: Interpretation of results, revision of manuscript
Serena M. Bagnasco, MD: Interpretation of data, revision of manuscript
Babak J. Orandi, MD PhD: Acquisition of data, revision of manuscript
Jennifer L. Alejo, BA: Acquisition of data, revision of manuscript
Brian J. Boyarsky, BA: Acquisition of data
Saad K. Anjum, BA: Acquisition of data, revision of manuscript
Robert A. Montgomery, MD DPhil: Acquisition of data
Nabil N. Dagher, MD: Acquisition of data
Dorry L. Segev, MD PhD: Conception and design, interpretation of results, revision of manuscript
DISCLOSURE
The authors of this manuscript have no conflicts of interest.
References
- 1.Mandelbrot DA, Pavlakis M. Living donor practices in the United States. Adv Chronic Kidney Dis. 2012;19(4):212–219. doi: 10.1053/j.ackd.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11(8):1650–1655. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mjøen G, Hallan S, Hartmann A, et al. Long-term risk for kidney donors. Kideny Int. 2013;86:162–167. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 5.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. Jama. 2014;311(6):579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wafa EW, Refaie AF, Abbas TM, et al. End-stage renal disease among living-kidney donors: single-center experience. Exp Clin Transplant. 2011;9(1):14–19. [PubMed] [Google Scholar]
- 7.Steiner RW, Ix JH, Rifkin DE, Gert B. Estimating risks of de novo kidney diseases after living kidney donation. Am J Transplant. 2014;14(3):538–544. doi: 10.1111/ajt.12625. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan A, Diwan TS, Franco Palacios CR, et al. Using implantation biopsies as a surrogate to evaluate selection criteria for living kidney donors. Transplantation. 2013;96(11):975–980. doi: 10.1097/TP.0b013e3182a2b455. [DOI] [PubMed] [Google Scholar]
- 9.Choi KH, Yang SC, Joo DJ, et al. Do the abnormal results of an implantation renal biopsy affect the donor renal function? Transplant Proc. 2014;46(2):359–362. doi: 10.1016/j.transproceed.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 10.Goecke H, Ortiz AM, Troncoso P, et al. Influence of the kidney histology at the time of donation on long term kidney function in living kidney donors. Transplant Proc. 2005;37(8):3351–3353. doi: 10.1016/j.transproceed.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 11.Mancilla E, Avila-Casado C, Uribe-Uribe N, et al. Time-zero renal biopsy in living kidney transplantation: a valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation. 2008;86(12):1684–1688. doi: 10.1097/TP.0b013e3181906150. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi Y, Thomas G, Nurko S, et al. Association of metabolic syndrome with kidney function and histology in living kidney donors. Am J Transplant. 2013;13(9):2342–2351. doi: 10.1111/ajt.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sund S, Reisaeter AV, Scott H, et al. Morphological studies of baseline needle biopsies from living donor kidneys: light microscopic, immunohistochemical and ultrastructural findings. APMIS. 1998;106(11):1017–1034. doi: 10.1111/j.1699-0463.1998.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 14.Elsherbiny HE, Alexander MP, Kremers WK, et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol. 2014;9(11):1892–1902. doi: 10.2215/CJN.02560314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12(3):563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 17.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Nephrol Dial Transplant. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CY, Bansal N. Measured GFR as "gold standard"--all that glitters is not gold? Clin J Am Soc Nephrol. 2011;6(8):1813–1814. doi: 10.2215/CJN.06040611. [DOI] [PubMed] [Google Scholar]


