Abstract
Glyceroacetonide-Oxyma [(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 2-cyano-2-(hydroxyimino)acetate (1)] displayed remarkable physico-chemical properties as an additive for peptide-forming reactions. Although racemization-free amide-forming reactions have been established for N-urethane-protected α-amino acids with EDCI, 1, and NaHCO3 in water or DMF-water media, amide-forming reactions of N-acyl-protected α-amino acids and segment couplings of oligopeptides still require further development. Diethylphosphoryl-Glyceroacetonide-Oxyma (DPGOx, 3) exhibits relative stability in aprotic solvents and is an effective coupling reagent for N-acyl-protected α-amino acids and oligo peptide segments. The conditions reported here is also effective in lactam-forming reactions. Unlike most of the reported coupling reagents, simple aqueous work-up procedures can remove the reagent and by-products generated in the reactions.
Keywords: Diethylphosphoryl-Glyceroacetonide-Oxyma, Oxyma, segment coupling, lactam-forming reactions, peptide synthesis
Introduction
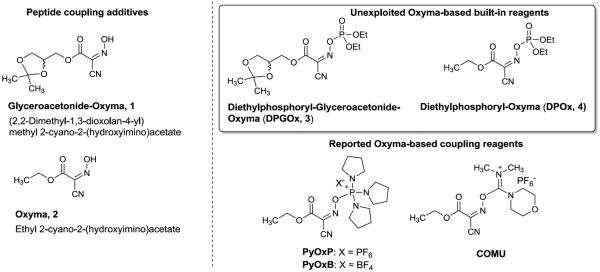
Peptides regulate most physiological functions in intracellular and intercellular processes [1–5]. Due to advances in new formulation systems that prevent rapid enzymatic degradations and enhance systemic delivery of peptide drugs, there has been a rapid expansion in the use of peptides as drugs over the last decade [6–7]. Several technologies have been applied for the productions of peptide drugs; among which chemical and enzymatic (recombinant technology) synthesis play key roles in drug industries [8–10]. In productions of small to medium size of peptide drugs, chemical syntheses via solid- and solution-phase peptide syntheses are still the choice of manufacturing procedure for 9–50 residue peptides [9]. Because short- to oligo-peptide can be synthesized efficiently via solid-phase peptide synthesis (e.g. procedures based on Fmoc-chemistry), assembling purified segment(s) in solution phase or on polymer-supports is one of the most reliable methods for the syntheses of oligo-peptides [11–12]. Although a large number of peptide coupling methods have been reported for syntheses of small peptides with urethane-protected α-amino acids [13], very few conditions have been applied to coupling reactions of oligo-peptide segments. For example, acyl azide and DCC-mediated coupling conditions are the choice of segment coupling to synthesize large peptide molecules [14]. Among the commercially available peptide coupling reagents, O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) and related reagents have been described as useful segment coupling agents [15]. The other coupling reagents such as uronium- and phosphonium-type reagents are considered to be potential peptide-forming reagents for segment couplings; however, no robust protocol has been established using these reagents. In segment coupling reactions, racemization of the C-terminal amino acid is a serious problem; thus far, reported conditions are not considered to be racemization-free segment coupling reactions. In addition, most of the peptide-forming agents and the coupling additives are difficult to remove by standard work-up procedures when applied to peptide coupling reactions in solution. Based on Oxyma [ethyl (hydroxyimino)cyanoacetate (2)]- and Glyceroacetonide-Oxyma 1-promoted peptide-forming reactions studied by us [16–17], it can be speculated that Oxyma-based built-in reagents such as phosphonium-, uronium- and phosphate-forms have high potential for successful couplings of peptide segments and with N-acyl α-amino acids. To date, Oxyma uronium salt, COMU and Oxyma phosphonium salts, PyOxP and PyOxB were successfully prepared and characterized their reactivities with the N-urethane-protected α-amino acids [18–19]. Herein we report convenient syntheses of diethylphosphoryl-Glyceroacetonide-Oxyma, 3 and diethylphosphoryl-Oxyma, 4 and applications of these reagents to N-formyl and N-acetyl α-amino acids, segment couplings, and lactamization reactions.
Materials and Methods
General
All chemicals were purchased from commercial sources and used without further purification unless otherwise noted. Tetrahydrofuran (THF), methylene chloride (CH2Cl2), and DMF were purified via Innovative Technology's Pure-Solve System. All reactions were performed under an Argon atmosphere. All stirring was performed with an internal magnetic stirrer. Reactions were monitored by thin-layer chromatography (TLC) performed with 0.25 mm coated commercial silica gel plates (EMD, Silica Gel 60F254) using UV light for visualization at 254 nm, or developed with ceric ammonium molybdate or anisaldehyde or copper sulfate or ninhydrin solutions by heating on a hot plate. Reactions were also monitored by using SHIMADZU LCMS-2020 with solvents: A: 0.1% formic acid in water, B: acetonitrile. And reactions were also monitored by SHIMADZU prominence HPLC using Phenomenex Kinetex 1.7 μ XB-C18 100A column (150 × 2.10 mm) and monitoring at 220, 254 nm with solvents: A: 0.05 M ammonium bicarbonate in water, B: acetonitrile. Flash chromatography was performed with SiliCycle silica gel (Purasil 60 Å, 230–400 Mesh). Proton magnetic resonance (1H-NMR) spectral data were recorded on 400, and 500 MHz instruments. Carbon magnetic resonance (13C-NMR) spectral data were recorded on 100 and 125 MHz instruments. Phosphorus magnetic resonance (31P-NMR) spectral data were recorded on 162 MHz instruments. For all NMR spectra, chemical shifts (δH, δC, δP) were quoted in parts per million (ppm), and J values were quoted in Hz. 1H and 13C NMR spectra were calibrated with residual undeuterated solvent (CDCl3: δH =7.26 ppm, δC =77.16ppm; CD3CN: δH=1.94ppm, δC =1.32ppm; CD3OD: δH =3.31ppm, δC =49.00 ppm; DMSO-d6: δH=2.50ppm, δC =39.52ppm; D2O: δH=4.79 ppm) as an internal reference. The following abbreviations were used to designate the multiplicities: s=singlet, d=doublet, dd=double doublets, t=triplet, q=quartet, quin=quintet, hept=heptet, m=multiplet, br=broad. Infrared (IR) spectra were recorded on a Perkin-Elmer FT1600 spectrometer.
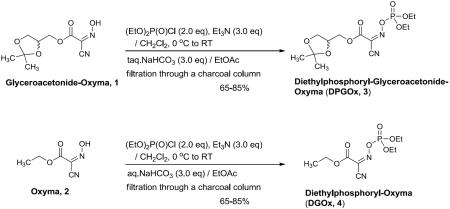
Synthesis of diethylphosphoryl-Glyceroacetonide-Oxyma (DPGOx 3): To a solution of Glyceroacetonide-Oxyma 1 (2.46 g, 10.8 mmol) and triethylamine (3.01mL, 21.6 mmol) in CH2Cl2 (15 mL) at 0 °C was added diethyl chlorophosphate (2.34 mL, 16.2 mmol) dropwisely. After being stirred for 1h, the reaction solution was warmed up to room temperature. After 1h, the reaction mixture was diluted with EtOAc (50 mL) and washed with saturated aqueous solution of NaHCO3 (50mL × 3). The organic layer was washed with brine and dried over Na2SO4. The crude mixture was passed through charcoal column (solvents: EtOAc : hexanes = 2:1) to afford 3 (3.19 g, 81%) as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 4.45 – 4.38 (m, 3H), 4.33 (qd, J = 7.1, 2.1 Hz. 4H), 4.12 (ddd, J = 8.6, 5.0, 1.4 Hz, 1H), 3.83 (ddd, J = 8.7, 3.8, 1.7 Hz, 1H), 1.44 (s, 3H), 1.41 (td, J = 7.1, 1.2 Hz, 6H), 1.37 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 156.34, 132.97, 110.26, 106.35, 72.76, 67.47, 66.57, 66.51, 65.91, 26.61, 25.28, 16.08, 16.02; 31P NMR (162 MHz, CDCl3) δ −2.57; LRMS (EI) calcd for C13H22N2O8P ([M + H]+): 365.11, found: 365.05.
Synthesis of diethylphosphoryl-Oxyma (DPOx 4): To a solution of Oxyma 2 (2.84 g, 20.0 mmol) and triethylamine (5.58mL, 40.0 mmol) in CH2Cl2 (20 mL) at 0 °C was added diethyl chlorophosphate (4.34 mL, 30.0 mmol) dropwisely. After being stirred for 1h, the reaction mixture was warmed to room temperature. After 1h, the reaction mixture was diluted with EtOAc (100 mL) and washed with saturated aqueous solution of NaHCO3 (100mL × 3). The organic layer was washed with brine and dried over Na2SO4. The crude mixture was passed through charcoal column (solvents: EtOAc : hexanes = 2:1) to afford 4 (3.88 g, 70%) as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 4.45 (q, J = 7.1 Hz, 2H), 4.34 (qd, J = 7.1, 1.7 Hz, 2H), 4.32 (qd, J = 7.1, 1.4 Hz, 2H), 1.41 (td, J = 7.1, 1.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 156.46, 133.59, 106.49, 66.51, 66.44, 64.36, 16.07, 16.00, 13.92; 31P NMR (162 MHz, CDCl3) δ −2.48; LRMS (EI) calcd for C9H16N2O6P ([M + H]+): 279.07, found: 279.14.
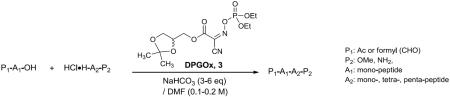
Typical procedure for peptide coupling reactions: All reactions were performed with 1.5–3 equivalents of DPGOx 3 or DPOx 4 in DMF (0.0006–0.2 M). The concentrations are 0.1–0.2 M for intermolecular and 0.0006 M for intramolecular reactions. To a stirred solution of starting material(s) in DMF, NaHCO3 (6 eq to carboxylic acid) and 3 or 4 (1.5–3 eq) were added. The reaction mixture was stirred for 1–12 h and quenched with water (1/5 of DMF). Hydrolysis of excess reagent was completed within 1h. In macrolacatamization reactions, DMF was distilled off at 60 °C (bath temperature) under high vacuum (0.1 mmHg), and then, partition between water and EtOAc was conducted. In the reactions at 0.1–0.2 M concentrations, partition between water and EtOAc was conducted. The combined EtOAc extracts were washed with aq. NaHCO3, 1N HCl, brine, dried over Na2SO4, and evaporated. The crude product was passed through a short SiO2 column (a CHCl3 / CH3OH system) to afford pure product.
Results and Discussion
Glyceroacetonide-Oxyma 1 displayed remarkable physico-chemical properties as a peptide-coupling additive in water media [16]. Short- to oligo-peptides could be synthesized by using 1, EDCI, and NaHCO3 in water without measurable racemization. Significantly, a simple basic and acidic aqueous work-up procedure can remove all reagents utilized in the reactions to afford only coupling products in consistently excellent yields. In general, Glyceroacetonide-Oxyma/EDCI-mediated reactions are suitable for the coupling with N-urethane-protected α-amino acids in water or DMF-water systems. However, under these conditions peptide-forming reactions of the N-acetyl-protected α-amino acids required over 7 equivalents of the N-acetyl substrates to achieve the reactions with >80% yield. Under these conditions peptide-forming reactions of N-formyl α-amino acids were failed to produce the corresponding coupling products. Albeit N-formylpeptides are important biological tools for investigation of cell functions and are versatile building blocks for syntheses of bioactive molecules including drugs [20–22], step-wise synthesis (deprotection-formylation approach) is the standard for chemical syntheses of N-formylpeptides [23]. In this regard, a mild peptide-forming reaction that is amenable to coupling reactions with N-formyl-α-amino acids is very useful asset in peptide sciences. Such reaction conditions will also be applicable to a wide range of peptide segment couplings to form oligo to large peptides via convergent approaches.
To date, a few Oxyma-based phosphonium and uronium salts have been reported. Subirós-Funosas et al. studied the chemical properties of PyOxP, PyOxB, and COMU (Figure 1) in [1+1], [2+1] and [3+3] segment couplings [18]. Under their conditions, dramatically improvement of the suppression of racemization of the linier peptides in the segment couplings was not observed compared to the same reactions with the benzotriazole-based coupling agents (e.g. PyAOP). Although phosphates of the peptide coupling promoters (additives) have the high potential of performing a wide range of peptide-forming reactions, a very few phosphate peptide coupling reagents have been successfully demonstrated for segment couplings [e.g. 3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one (DEPBT), diphenylphosphoryl azide (DPPA)] [24–25]. This is due to the fact that most of the phosphate coupling reagents are hydrolytically unstable and are difficult to isolate as their pure forms except for a few cases. Diethylphosphoryl-Glyceroacetonide-Oxyma (DPGOx 3) synthesized by the reaction of 1 with (EtO)2P(O)Cl and Et3N in CH2Cl2 was hydrolyzed during silica gel chromatography. DPGOx 3 was not tolerated for a purification by distillation at over 200 °C. It was found that the by-product(s) from (EtO)2P(O)Cl and Et3N were removed via water-work up and a charcoal filtration, respectively. Thus, according to the procedures illustrated in Table 1, DPGOx 3 and DPOx 4 could be synthesized in 65–85% yield.
Figure 1.
Reported Oxyma-based coupling agents and unexploited Oxyma-based coupling reagents.
Table 1.
Syntheses of the dethylphosphoryl Oxyma derivatives, 3 and 4, and their half-lives in common organic solvents.
| entry | Molecule | Solvent | Half-life (t1/2)a |
|---|---|---|---|
| 1 | 3 | DMF | >5 days |
| 2 | 4 | DMF | >5 days |
| 3 | 3 | CH3CN | > 5 days |
| 4 | 4 | CH3CN | > 5 days |
| 5 | 3 | DMSO | 12 h |
| 6 | 4 | DMSO | 12 h |
| 7 | 3 | H2O | < 3h |
| 8 | 4 | H2O | < 3h |
| 9 | 3 | -b | >30 days |
| 10 | 4 | -b | >30 days |
t1/2 at room temperatures, t1/2 was determined via 1H-NMR (CDCl3);
neat conditions;
Hydrolytic stability of DPGOx 3 and DPOx 4 were examined in several common organic solvents, and their stabilities at room temperatures were summarized in Table 1. DPGOx 3 and DPOx 4 were readily hydrolyzed in water; the half-lives of 3 and 4 in water were shorter than 3h. DPGOx 3 and DPOx 4 were slowly reacted with DMSO, producing CH3SCH3 through the formation of ((diethoxyphosphoryl)oxy)dimethylsulfonium species. Diethylphosphoryl-Oxyma derivatives were stable in DMF and CH3CN; their half-lives were over 5 days (Entries 1–4 in Table 1). No detectable hydrolysis of 3 and 4 was observed in anhydrous DMF in the presence of NaHCO3 even after 24h. These studies indicated that 3 and 4 exhibit appropriate stability in DMF, and 3 and 4 can conveniently be stored at room temperatures over 30 days without measurable decomposition.
In order to examine the effectiveness of DPGOx 3 in peptide segment couplings, our standard peptide coupling conditions using NaHCO3 in DMF were applied to the syntheses of di-, penta- and hexa-peptides with N-acyl α-amino acids. It is noteworthy that racemization-free couplings of N-formyl α-amino acids are considered to be one of the most challenging peptide-forming reactions. To the best of our knowledge, there is no useful coupling reagent that can perform couplings with N-formyl α-amino acids with high yield. Selected examples are summarized in Table 2. Coupling reaction of 1.5 equivalents of Ac-L-Phe-OH and HCl•H-L-Ala-OMe with 3 provided the desired N-acetyl dipeptide in 85% yield without detectable diastereomers (analyzed by HPLC) (entry 1 in Table 2). Epimerization-prone Ac-L-Tyr-OH was successfully coupled with HCl•H-L-Ala-OMe to afford Ac-L-Tyr-L-Ala-OMe in 75% yield with >99% de (entry 2). The same reaction with 3 equivalents of Ac-L-Tyr-OH improved the isolation yield of Ac-L-Tyr-L-Ala-OMe (entry 3). Coupling of Ac-L-Ser-OH with HCl•H-L-Ala-OMe afforded Ac-L-Ser-L-Ala-OMe in 85% yield with >99% de (entry 4). DPGOx 3 could promote coupling reactions of the N-formyl α-amino acids, CHO-L-Leu-OH and CHO-L-Phe-OH with HCl•H-L-Ala-OMe to furnish the corresponding N-formyl dipeptides in greater than 70% yields with 1.5 equivalents of N-formyl α-amino acids (entries 5 and 6). Similarly, the isolation yields of N-formyl dipeptides were dramatically improved by using excess of N-formyl α-amino acids (entries 7 and 8). These results are the first observation that N-formyl α-amino acids are applied to peptide-forming reactions with high yield and no measurable epimerization. A few examples of [4+1] and [5+1] coupling reactions with N-acetyl and N-formyl lysines are summarized in entries 9–12 in Table 2. These reactions were efficiently promoted by using DPGOx 3 to afford the corresponding penta- and hexa-peptides in over 80% yields with >92% de (determined by 1HNMR analyses). All reactions summarized in Table 2 could be performed with DPOx 4; the isolation yields and des are comparable to those summarized in Table 2. In DPGOx 3-mediated coupling reactions, 3 and the generated by-product 1 can readily be removed by basic aqueous-workup, whereas, removal of the by-product, Oxyma 2 after the reactions using DPOx 4 requires extensive work-ups; in many cases, a few percent of Oxyma were remained in the crude products that require purification by chromatography. On the other hands, the oligopeptides synthesized via the conditions applied in Table 2 can be isolated with high purity by simple basic work-up [16].
Table 2.
| entry |
N-protected α-amino acid |
C-protected α-amino acid |
conditionsa | time (h) |
product | yield (%) |
de (%) |
|---|---|---|---|---|---|---|---|
| [1+1] | |||||||
| 1 | Ac-L-Phe-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Phe-L-Ala-OMe | 85 | >99b |
| 2 | Ac-L-Tyr-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Tyr-L-Ala-OMe | 75 | >99b |
| 3 | Ac-L-Tyr-OH | HCl•H-L-Ala-NH2 | B | 2 | Ac-L-Tyr-L-Ala-OMe | 95 | >92c |
| 4 | Ac-L-Ser-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Ser-L-Ala-OMe | 85 | >99b |
| 5 | CHO-L-Leu-OH | HCl•H-L-Ala-OMe | A | 3 | CHO-L-Leu-L-Ala-OMe | 70 | >99b |
| 6 | CHO-L-Leu-OH | HCl•H-L-Ala-OMe | B | 3 | CHO-L-Leu-L-Ala-OMe | 95 | >99b |
| 7 | CHO-L-Phe-OH | HCl•H-L-Ala-OMe | A | 3 | CHO-L-Phe-L-Ala-OMe | 73 | >99b |
| 8 | CHO-L-Phe-OH | HCl•H-L-Ala-OMe | B | 3 | CHO-L-Phe-L-Ala-OMe | 95 | >99b |
| [4+1] | |||||||
| 9 | Ac-L-Lys(Z)-OH | HCl•H-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 2 | Ac-L-Lys(Z)-L-Ala-L-Lys(Z)-Lys(Z)-L-Phe-OMe | 80 | >92c |
| 10 | CHO-L-Lys(Z)-OH | HCl•H-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 2 | CHO-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 81 | >92c |
| [5+1] | |||||||
| 11 | Ac-L-Lys(Z)-OH | HCl•H-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 3 | Ac-L-Lys(Z)-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 80 | >92c |
| 12 | CHO-L-Lys(Z)-OH | HCl•H-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 3 | CHO-L-Lys(Z)-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 80 | >92c |
A: N-acyl-α-amino acid (1.5 equiv), C-protected α-amino acid (1.0 eqiv), 3 (1.5–2.0 equiv), NaHCO3 (3 equiv), DMF (0.2 M); B: N-acyl-α-amino acid (3.0 equiv), C-protected α-amino acid (1.0 eqiv), 3 (3.0 equiv), NaHCO3 (3 equiv), DMF (0.2 M);
de was determined by HPLC;
de was determined by 1H-NMR analysis;
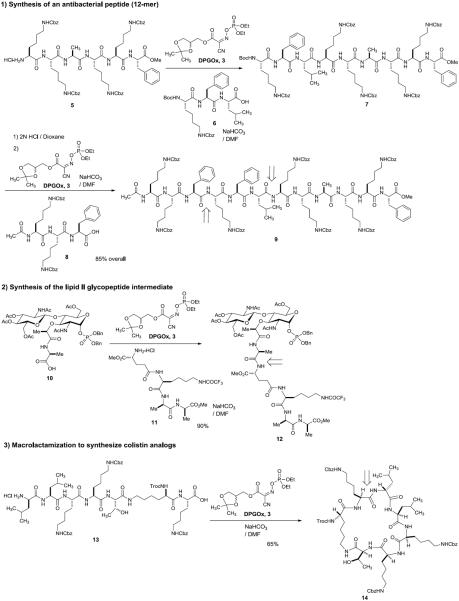
To date, we have applied DPGOx 3 to the syntheses of complex glycopeptides, 18-mers of linier oligopeptides, and cyclic-peptides [26–29]. The highlights of representative segment couplings of these molecules are illustrated in Scheme 1. [6+3] coupling between 5 and 6 afforded the nonapeptide 7 with over 92% purity after aq. NaHCO3 work-up. Boc deprotection of 7 followed by [3+9] coupling with 8 furnished the dodecapeptide 9 in 85% overall yield from 5. Coupling of α-phosphoryl GlcNAc-MurNAc-monopeptide 10 with the tetrapeptide 11 provided the acid-labile α-phosphoryl GlcNAc-MurNAc-pentapeptide in 90% yield after column chromatography. DPGOx 3 has been demonstrated for several macrolactamizations in our group [29]. The linier heptapeptide 13 was cyclized at 0.0006 M concentration within 5h to furnish the cyclic peptide 14 having a 25-membered ring in 65% yield. The same macrolactamization of 13 with diphenylphosphoryl azide (DPPA) required over 48h to furnish 14 in 45% yield.
Scheme 1.
DPGOx 3 promoted-peptide segment couplings and macrolactamization.
Conclusion
In summary, diethylphosphoryl-Glyceroacetonide-Oxyma (DPGOx 3) and diethylphosphoryl-Oxyma (DPOx 4) can be synthesized via a standard phosphorylation condition and a purification using charcoal. Diethylphosphoryl-Oxyma derivatives 3 and 4 exhibit good relative stability in anhydrous DMF in the presence of NaHCO3 that promote peptide coupling reactions with N-acetyl and N-formyl α-amino acids, yielding the desired dipeptides in excellent yields without measurable racemization. Segment couplings of [6+3] and [3+9] have been demonstrated to synthesize series of antibacterial peptides. A high-yielding segment coupling of an acid labile α-phosphoryl GlcNAc-MurNAc-peptide 10 implies that the DPGOx-promoted reactions are applicable to a wide range of substrates possessing acid labile groups. DPGOx is also effective for macrolactamizations to form medium ring size of lactams. Reaction rate of DPGOx-promoted macrolactamizations is much faster than that with diphenylphosphoryl azide (DPPA). The segment coupling conditions reported here are operationally very simple and basic aqueous work-ups can remove the reagents utilized in the reactions to afford coupling products in high yield with excellent purity.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIAID grants AI084411 and AI119796) and University of Tennessee for generous financial support. NMR and MS data were obtained on instruments supported by the NIH Shared Instrumentation Grants.
Footnotes
Supporting Information Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- 1.Iwaniak A, Minkiewic P. Biologically active peptide derivatives from proteins – A review. Pol. J. Food Nutr. Sci. 2008;58:289–294. [Google Scholar]
- 2.Sharma S, Singh R, Rana S. Bioactive peptides: A review. Int. J. Bio. Automation. 2011;15:223–250. [Google Scholar]
- 3.Salas CE, Badillo-Corona JA, Ramírez-Sotelo G, Oliver-Salvador C. Biologically active and antimicrobial peptides from plants. Bio. Med Res. Int. 2015;2015:1–11. doi: 10.1155/2015/102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hills CE, Brunskill NJ. C-Peptide and its intracellular signaling. Rev. Diabet. Stud. 2009;6:138–147. doi: 10.1900/RDS.2009.6.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogenesch JB, Herzog ED. Intracellular and intercellular processes determine robustness of the circadian clock. FEBS Lett. 2011;58:1427–1434. doi: 10.1016/j.febslet.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK. Drug delivery systems: An updated review. Int J. Pharm. Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaji J, Patole V. Protein and peptide drug delivery: Oral approaches. Indian J. Pharm. Sci. 2008;70:269–277. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Faham A, Albericio F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011;9:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]
- 9.Bray BL. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nature Rev. Drug Disc. 2003;2:587–593. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 10.Guzmán F, Barberis S, Illanes A. Peptide synthesis: chemical or enzymatic. Electr. J. Biotechnol. 2007;10:279–314. [Google Scholar]
- 11.Gutte B, Merrifield RB. The synthesis of ribonuclease A. J. Biol. Chem. 1971;246:1922–1941. [PubMed] [Google Scholar]
- 12.Story SC, Aldrich JV. Preparation of protected peptide amides using the Fmoc chemical protocol. Comparison of resins for solid phase synthesis. Int. J. Pept. Protein Res. 1992;39:87–92. doi: 10.1111/j.1399-3011.1992.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 13.Han S-Y, Kim Y-A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron. 2004;60:2447–2467. [Google Scholar]
- 14.Chandrudu S, Simerska P, Toth I. Chemical methods for peptide and protein production. Molecules. 2013;18:4373–4388. doi: 10.3390/molecules18044373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khattab SN. OxymaPure: An efficient and convenient additive used with tetramethylfluoroformamidinium hexafluorophosphate (TFFH) to replace 1-hydroxybenzotriazole (HOBt) and 1-hydroxy-7-azabenzotriazole (HOAt) during peptide synthesis. Bull. Chem. Soc. Jpn. 2010;83:1374–1379. [Google Scholar]
- 16.Wang Q, Wang Y, Kurosu M. A New oxyma derivative for non-racemizable amide-forming reactions in water. Org. Lett. 2012;14:3372–3375. doi: 10.1021/ol3013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Aleiwi BA, Wang Q, Kurosu M. Selective esterifications of primary alcohols in a water-containing solvent. Org. Lett. 2012;14:4910–4913. doi: 10.1021/ol3022337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Faham A, Subirós-Funosas R, Albericio F. A novel family of onium salts based upon isonitroso Meldrum's acid proves useful as peptide coupling reagents. Chem. Eur. J. 2010;19:3641–3649. [Google Scholar]
- 19.Subirós-Funosas R, Prohens R, Barbas R, El-Faham A, Albericio F. Oxyma: An efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009;15:9394–9403. doi: 10.1002/chem.200900614. [DOI] [PubMed] [Google Scholar]
- 20.Zhang1 Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo I, Rattazzi L, Piras G, Gobbetti T, Panza E, Perretti M, Dalley JW, D'Acquisto F. Formyl peptide receptor as a novel therapeutic target for anxiety-related disorders. PLoS One. 2014;9:e114626. doi: 10.1371/journal.pone.0114626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabiet M-J, Huet E, Bouley F. Human mitochondria-derived N-formylated peptides are novel agonists equallyactive on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 2005;35:2486–2249. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 23.Aleiwi BA, Mitachi K, Kurosu M. Mild and convenient N-formylation protocol in water-containing solvents. Tetrahedron Lett. 2013;54:2077–2081. doi: 10.1016/j.tetlet.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Jiang X, Ye YH, Fan C, Romoff T, Goodman M. 3-(Diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one (DEPBT): a new coupling reagent with remarkable resistance to racemization. Org. Lett. 1999;1:91–94. doi: 10.1021/ol990573k. [DOI] [PubMed] [Google Scholar]
- 25.Goodman M. Synthesis of peptides and peptidomimetics. Methods of Org. Chem (Houben-Weyl) 2002;E22a:425–888. [Google Scholar]
- 26.Mitachi K, Mohan P, Siricilla S, Kurosu M. One-pot protection-glycosylation reactions for synthesis of lipid II analogues. Chem. Eur. J. 2014;20:4554–4558. doi: 10.1002/chem.201400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurosu M, Mahapatra S, Narayanasamy P, Crick DC. Chemoenzymatic synthesis of Park's nucleotide: toward the development of high-throughput screening for MraY inhibitors. Tetrahedron Lett. 2007;48:799–803. [Google Scholar]
- 28.Mitachi K, Siricilla S, a Klaic L, Clemons WM, Kurosu M. Chemoenzymatic syntheses of water-soluble lipid I fluorescent probes. Tetrahedron Lett. 2015;56:3441–3446. doi: 10.1016/j.tetlet.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosu YE, Mitachi K, Kurosu M. Manuscript in preparation. Antibacterial activities of new colistin analogs against Gram-negative bacteria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.