Abstract
Background
Conditioned cues can elicit drug-seeking in both humans and rodents. The majority of preclinical research has employed excitatory conditioned cues (stimuli present throughout the availability of a reinforcer), but oral consumption of alcohol is similar to a conditional stimuli (presence of stimuli is paired with the delivery of the reinforcer) approach. The current experiments attempted to determine the effects of conditional stimuli (both excitatory and inhibitory) on the expression of context-induced ethanol (EtOH)-seeking.
Methods
Alcohol-preferring (P) rats self-administered EtOH and water in standard 2-lever operant chambers. A flavor was added to the EtOH solution (CS+) during the EtOH self-administration sessions. After 10 weeks, rats underwent extinction training (7 sessions), followed by a 2-week home cage period. Another flavor was present during extinction (CS-). Rats were exposed to a third flavor in a non-drug paired environment (CS0). EtOH-seeking was assessed in the presence of no cue, CS+, CS- or CS0 in the dipper previously associated with EtOH self-administration (no EtOH available). Rats were maintained a week in their home cage before being returned to the operant chambers with access to EtOH (flavored with no Cue, CS+, CS- or CS0).
Results
The results indicated that the presence of the CS+ enhanced EtOH-seeking, while the presence of the CS- suppressed EtOH-seeking. Similarly, adding the CS- flavor to 15% EtOH reduced responding for EtOH while the CS+ enhanced responding for EtOH during relapse testing.
Conclusions
Overall, the data indicate that conditional stimuli are effective at altering both EtOH-seeking behavior and EtOH relapse drinking.
Keywords: Alcohol-Preferring (P) rats, EtOH-seeking, EtOH-relapse, conditioned cues, Pavlovian Spontaneous Recovery
Introduction
Alcohol abuse is prevalent in the United States, with approximately 7.2 percent of adults over the age of 18 and 3.2 percent of adolescents between the ages of 12 and 17 meeting criteria for an alcohol use disorder (SAMHSA, 2012). Long term-relapse following remission is common, and has been estimated to occur in as many as 40 percent of treated cases and in as many as 60 percent of untreated cases (Moos and Moos, 2006). Repeated exposure to environmental and drug related cues, preceding and during drug use, is thought to produce learned associations that play a significant role in triggering relapse (O'Brien et al, 1998). In the laboratory setting, exposure to alcohol related cues can lead to increased craving, anxiety, and negative affect (Pomerleau et al., 1983; Davidson et al., 2003; Sinha et al., 2009), as well as physiological alterations, in both abstinent and untreated patients with a history of high alcohol use (Staiger and White, 1991; Stormark et al., 1995). Imaging studies have supported the notion of differential processing of alcohol related cues in heavy drinkers (for a review, see Schacht et al., 2012). Research investigating the ability of environmental and drug paired cues to form learned associations with alcohol intake is essential for developing therapeutic strategies aimed at treating and preventing relapse.
Laboratories have studied cue induced craving using animal models designed to parallel drug craving in the human population. Typically, experiments in the alcohol field have either utilized a reinstatement model of relapse or a renewal model. In the reinstatement model, the learned association between a context or cue and drug availability or intake is extinguished in the original context by removing the cue and reinforcement following operant responses. Reinstatement of operant behavior is then achieved by re-introducing the conditioned cue (CS+) into the original context (Marchant et al., 2013). In the renewal model, also known as the ABA model, cue/drug associations are formed in an original context (context A), and operant behavior is extinguished in a new context (context B). Renewal of operant behavior is achieved by placing the animal back in the original context (Bouton, 2002). The presentation of conditioned cues induces alcohol seeking in both the reinstatement (Katner and Weiss, 1999; Katner et al., 1999; Schroeder et al., 2008; Ciccocioppo et al., 2002; Bachteler et al., 2005) and renewal (Tsiang and Janak, 2008; Chaudhri et al., 2008a,b; Chaudhri et al., 2013; Remedios and Chaudhri, 2014, Sciascia et al., 2014) models. In both cases, labs typically employ the relapse session a day after the last extinction session. In human alcohol abuse, the compulsion to use the drug can occur long after alcohol intake has stopped and long after any evidence of physical dependence or withdrawal symptoms have subsided (O'Brien et al., 1998). Pavlovian Spontaneous Recovery (PSR) refers to reinstatement that occurs in the absence of the trained reward, following a period of rest after the extinction phase (Pavlov, 1927; Bouton, 2002). Our laboratory has reported significant contextual cue-induced ethanol-seeking in alcohol-preferring (P) rats in the spontaneous recovery model (Rodd-Henricks, 2002a,b; Hauser et al., 2011; Hauser et al., 2012;, Deehan et al., 2012).
The majority of cue-induced seeking studies in the alcohol field have utilized olfactory, auditory, or visual cues, or a combination of those. To our knowledge, no group has utilized flavor as a conditional cue for alcohol intake availability in operant tests of cue induced EtOH seeking behavior or relapse in animals. Flavor can serve as a conditioned cue. For example, Sprague-Dawley rats given access to different flavored solutions preceding intraperitoneal injections of either 15 mg/kg morphine or saline show a conditioned aversion for the flavored solution paired with morphine (Nyland and Grigson, 2013), and Sprague-Dawley rats showed a conditioned flavor preference for a flavor cue paired with intragastric infusions of EtOH (Ackroff and Sclafani, 2001). In human alcohol use and abuse, there are a number of flavor-related stimuli that an abstinent alcoholic could be exposed to in every-day life. For example, flavors used to increase the palatability of alcohol are also commonly found in food – i.e. orange juice, sodas, seasonings, and fruits used in wines and mixed drinks. Given the prevalence of potential alcohol related gustatory cues, it is important to consider flavor as a potential source of learned associations in studies of alcohol use and relapse.
Alcohol addiction is characterized by high rates of relapse following periods of abstinence. The Pavlovian Spontaneous Recovery (PSR) model (Rodd-Henricks et al., 2002a,b) is designed to measure context-induced EtOH-seeking behavior after a drug-free period following extinction. Conditioned cues can either be excitatory (CS+) or inhibitory (CS-). Conditioned cues that are associated with the presence of a reinforcer are excitatory. An inhibitory conditioned cue is a stimulus that is associated with the absence of a reinforcer, and becomes capable of reducing some behavioral change which is normally attributed to excitation (Rescorla, 1969). The current series of experiments were designed to determine the influence of excitatory and inhibitory conditional flavor cues on context-induced EtOH seeking in the PSR model, and the influence of (CS+) and (CS-) on relapse drinking behavior in alcohol-preferring (P) rats. It was hypothesized that excitatory conditional flavor cues would enhance EtOH seeking and relapse responding, and inhibitory conditional flavor cues would reduce EtOH seeking and relapse responding.
Materials and Methods
Animals
Adult female P rats from the 65th – 66th generations weighing 250-325g at the start of the experiment were used. Rats were maintained on a 12-hr reversed light-dark cycle (lights off at 0900 hr). Food and water were available ad libitum throughout the experiment, except during operant testing. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 2011).
Operant Apparatus
EtOH self-administration procedures were conducted in standard two-lever experimental chambers (Coulbourn Instruments, Whitehall, PA) contained within ventilated, sound-attenuated enclosures. Two-operant levers, located on the same wall, were 15 cm above a grid floor and 13 cm apart. A trough was located directly beneath each lever, from which a dipper cup could raise to present fluid. Upon a reinforced response on the respective lever, a small light cue was illuminated in the drinking trough and 4 seconds of dipper cup (0.1 ml) access was presented.
Operant Training
P rats were placed into the operant chamber, without prior training. Operant sessions were 60 min in duration and occurred daily for 10 weeks (Rodd et al., 2006). The EtOH (purchased from Decon Labs, Inc., King of Prussia, PA) concentration used during self-administration was 15% (vol/vol), diluted from 95% EtOH stock. During the initial 4 weeks of daily operant access, both solutions (water and EtOH) were reinforced on an FR1 schedule. The response requirement for EtOH was increased to an FR3 schedule for 3 weeks, and then to FR5 schedule for 3 weeks. From this point forward, the lever associated with the delivery of EtOH was maintained on an FR5 schedule (extinction, seeking, and relapse). After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, they underwent 7 sessions of extinction (60 min/session), when EtOH was not available (Hauser et al., 2012; Rodd et al., 2006).
Conditional Cues
There were 3 conditioned flavor cues (purchased from Faerie's Finest, Hawaiian Gardens, CA used (all non-alcoholic base: blueberry, vanilla (imitation flavor), and raspberry). These non-alcohol based flavors were used in concentrations that reflected the sensitivity of the human tongue (an experimenter tasted all possible solutions; blueberry – 0.4 ml/L, vanilla 0.2 ml/L, and raspberry 0.2 ml/L). The concentration of flavors employed were chosen because of the mild flavor experienced by the experimenter (the different flavors were just barely discernable at these concentrations), as well as by the fact that in the operant chambers there was no noticeable odor from the flavors to human noses. It is important to note that although odors from the flavor concentrations employed were not detectable by the human nose, the possibility that the rats in the current experiments were able to detect an odor/taste component to the flavors cannot be ruled out. The use of the flavors for conditioned cues (CS+, CS- or CS0) was counterbalanced among rats. For the CS+, the assigned flavor was added to the 15% EtOH solution on all sessions during the 10 week alcohol exposure period. During extinction training, the trough formerly associated with the delivery of the flavored EtOH solution was substituted for a flavored water solution (CS-). During the last 2 weeks of EtOH access (sessions 57-70) and during extinction training (sessions 71-77), all rats were exposed to an additional flavor water solution in a non-drug paired environment (CS0); at least 3 hours separated the CS0 from the operant training session. The CS0 flavor was presented to the rats in a water solution held in a 30 ml syringe drinking tube. On average, the rats consumed 0.8 ml of the CS0 flavor during this exposure period. In contrast, P rats typically consumed 4.0 + 0.4 ml during the last 3 weeks of EtOH self-administration. During the 1st extinction session, rats consumed approximately 3.1 + 0.5 ml of the CS- flavor in water, this level of intake was greatly reduced throughout the extinction training.
Effects of Conditioned Stimuli on Pavlovian Spontaneous Recovery (PSR)
After extinction training, all rats were maintained in the home cages for 14 days, before being returned to the operant chambers for PSR testing (no EtOH present) for 8 consecutive sessions. There were 8 consecutive PSR sessions because previous studies showed that exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al., 2002a, b) and some drugs (Hauser et al., 2014) can enhance PSR responding for more than one session. There were 4 groups of rats (n = 47 total; n = 9-14/group). In the trough previously associated with the delivery of EtOH, rats were able to self-administer water (no cue) or water plus a conditioned cue (CS+, CS- or CS0). In the other trough (original water trough), water was available.
Effects of Conditioned Stimuli on Alcohol Relapse Drinking
Following the PSR phase of the experiment, all rats (there was no loss of animals) were maintained in the home cages for 7 days. Rats were then transferred to the operant chambers with both 15% EtOH and water available for the 60 minute sessions, as previously described (Hauser et al., 2012; Rodd et al., 2006). Rats were assigned to groups counterbalanced from the PSR experiment phase to one of 4 conditions. Rats were allowed to self-administer unadulterated EtOH (no cue) or EtOH flavored with one of the condition cues (CS+, CS- or CS0) for daily sessions over a period of 7 days.
Separating Context-Induced and Conditioned Stimuli EtOH-Seeking
In order to determine if a conditioned stimulus could elicit EtOH-seeking following the expression of context-induced EtOH-seeking the following experiment was conducted. All rats (n = 14) were given 10 weeks of daily operant access to a flavored 15% EtOH solution (CS+), followed by 7 extinction sessions (CS-) and a 14 day home cage period. During the first 2 PSR test sessions, only water was available in the trough previously associated with the delivery of EtOH. During PSR test session 3-10, the water was treated with the CS+ flavor.
Flavored Water Control
It was possible that the flavors added to the solutions had positive properties to the rats. Therefore, an additional group of rats (n = 12) were conducted identical to the initial experiment except that EtOH was never available in the operant chambers. These rats were exposed to all conditioned cues outlined above (CS+, CS- or CS0). For example, 4 rats were given vanilla flavored water as the ‘CS+’ (10 weeks), blueberry flavored water during extinction (‘CS-’; 7 sessions), and raspberry flavored water in the non-drug paired environment (‘CS0’; 21 exposure periods). Other groups of 4 rats received blueberry or raspberry as the ‘CS+’, vanilla or raspberry as the ‘CS-“, and vanilla or blueberry as the ‘CS0’. However, all rats were exposed to the 3 unique flavors. The rats were tested for PSR responding and ‘relapse’ drinking identically as the rats in the initial experiment.
Statistical Analyses
Overall operant responding (60 min) on EtOH and water levers data were analyzed with a mixed factorial ANOVA with a between subject factors of ‘conditioned cue’ and a repeated measure of ‘session’. For the PSR experiments, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH (or water) lever for the last 3 extinction sessions. For the relapse studies, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH (or water) lever for the 3 sessions immediately prior to extinction training. Post-hoc Tukey's b tests were performed to determine individual differences. Within subject analyses (determining significant alterations in responding compared to baseline levels) were performed by paired t-tests and orthogonal contrasts. The number of t-tests utilized was limited, and at best would have statistically resulted in 1 false positive finding at an occurrence rate of 52%. We did not utilize a correction method because of the low sample size, a priori hypotheses, and that orthogonal contrasts replicated all findings obtained by t-tests.
Results
Effects of Conditioned Stimuli on Pavlovian Spontaneous Recovery (PSR)
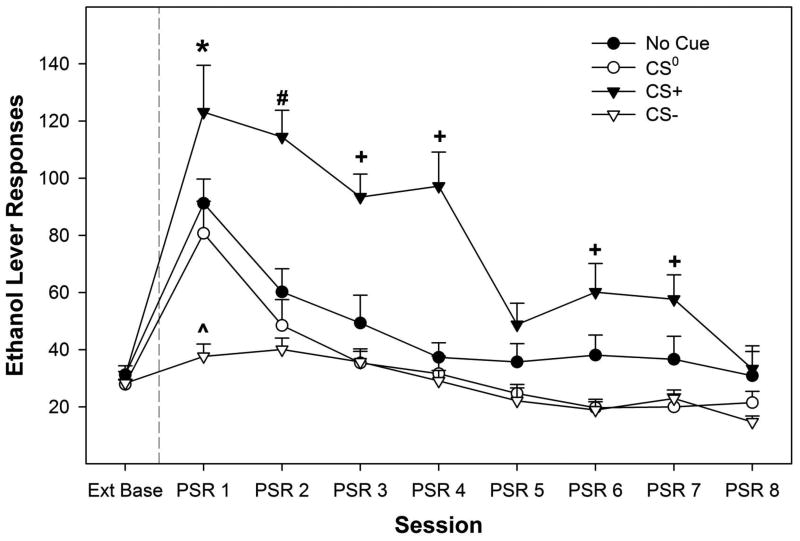
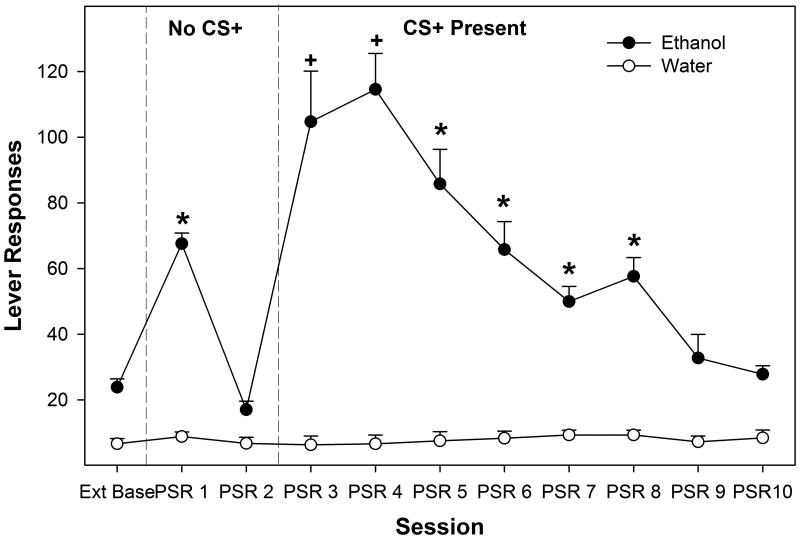
Examining the responses on the lever previously associated with the delivery of EtOH indicated a significant ‘session’ by ‘conditioned cue’ interaction term (F21, 117 = 2.81; p < 0.0001). The significant interaction term was decomposed by holding ‘session’ constant and examining the differences between the ‘conditioned cue’ groups for each individual day (Fig. 1). There was no significant effect of ‘conditioned cue’ group on the amount of responding during the last 3 extinction days (Ext Base; F3,43 = 0.89; p = 0.45). There were significant differences between the ‘conditioned cue’ groups during the first 7 PSR test sessions (F3,43 values > 3.9; p values < 0.015). Post-hoc comparisons revealed that during the 1st PSR test session responding on the lever previously associated with the delivery of EtOH was significantly higher in the CS+ group compared to all other groups and responding in the No Cue or CS0 was significantly greater than observed in the CS- group. During the 2nd PSR test session, EtOH lever responding was significantly higher in the CS+ group compared to all other groups and responding and the No Cue group was greater than the CS- group. During the 3rd, 4th, 6th, and 7th PSR test session, responding by the CS+ group was significantly higher than all other groups.
Figure 1.
Depicts the mean (+ SEM) responses on the lever previously associated with the delivery of EtOH influenced by the presences of conditioned cues. * indicates that the CS+ > No Cue and CS0 > CS-, and CS+, No Cue and CS0 groups responded more than extinction baseline. The (x0005E) symbol indicates that the CS- group did not respond more than extinction baseline, and was significantly lower compared to all other groups. # indicates that the CS+ > No Cue > CS-, and CS+ and No Cue groups responded more than baseline. + indicates that the CS+ > No Cue, CS- and CS0 , and CS+ group responded more than baseline.
Decomposing the interaction term by examining differences in responding between sessions in each ‘conditioned cue’ group revealed that there was no significant effect of ‘session’ in the CS- group (p = 0.56). In contrast, there was a significant effect of ‘session’ in the CS+, CS0, and No Cue groups (p values < 0.023). Since ‘session’ is a within subject factor, paired t-tests were used to contrast responding during PSR test session days and extinction baseline. In the CS0 group, responding only during the 1st PSR test session was significantly higher than that observed during the last 3 sessions of extinction training (p < 0.001). In the ‘No Cue’ group, responded was elevated compared to extinction baseline during the 1st and 2nd PSR test sessions (p values < 0.026). In rats exposed to the CS+, responding on the EtOH lever was elevated during the 1st, 2nd, 3rd, 4th, 6th, and 7th PSR test sessions (p values < 0.013).
In contrast, there was no significant alteration in responding on the water lever during PSR testing (session F7,37 = 1.4; p = 0.221: conditioned cue F3,43 = 0.395; p = 0.757: session x conditioned cue F21,117 = 1.046; p = 0.416). In general, water lever responding hovered around 20 responses per session for all groups throughout testing (Fig. 2).
Figure 2.
Depicts the mean (+ SEM) responses on the lever previously associated with the delivery of water influenced by the presences of conditioned cues. There were no significant effects.
Effects of Conditioned Stimuli on Alcohol Relapse Drinking
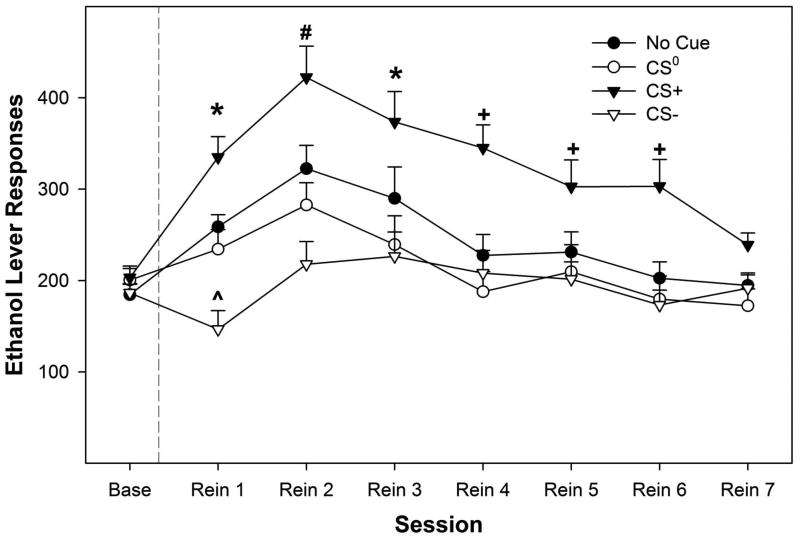
The presences of conditioned stimuli significantly altered EtOH self-administration during relapse drinking conditions (Fig. 3). Statistically, there was a significant ‘session’ by ‘conditioned cue’ interaction F21, 117 = 1.935; p = 0.014). Decomposing the significant interaction term by holding ‘session’ constant allowed for the examination of differences between ‘conditioned cue’ groups for each session. There were no significant differences between the ‘conditioned cue’ groups on baseline responding (F3,43 = 1.633; p = 0.196). There were significant differences between the ‘conditioned cue’ groups during relapse sessions 1-6 (F3,43 values > 3.557; p values < 0.022). Post-hoc comparisons indicated that, during the 1st relapse session, EtOH self-administration was significantly higher in the CS+ group compared to all other groups, while responding by the No Cue and CS0 groups was significantly higher than the CS- group. On the 2nd relapse session, EtOH self-administration was increased in the CS+ group compared to all other groups and responding in the No Cue group was higher than the CS- group. During the 3rd-6th relapse sessions, responding in the CS+ group was significantly higher compared to all other groups.
Figure 3.
Depicts the mean (+ SEM) responses on the EtOH lever during relapse conditions influenced by the presences of conditioned cues. * indicates that the CS+ > No Cue and CS0 > CS-, and CS+ group responded more, while the CS- group responded less, than baseline. (x0005E) indicates that the CS- group did not respond more than baseline and was significantly lower compared to all other groups. # indicates that the CS+ > No Cue, CS0 and CS-, and CS+ and No Cue groups responded more than baseline. + indicates that the CS+ > No Cue, CS- and CS0 , and CS+ group responded more than baseline.
Decomposing the interaction term by examining the effect of ‘session’ in each ‘conditioned cue’ group revealed that there was no significant effect of ‘session’ in the CS0 group (p = 0.11). In the CS+, CS-, and No cue groups there was a significant effect of ‘session’ (p values < 0.048). Paired t-tests revealed that, in the CS- group, EtOH self-administration was reduced during the 1st relapse session compared to baseline responding. In the No Cue group, paired t-tests indicated that EtOH self-administration was increased only during the 2nd relapse session compared to baseline levels of responding. EtOH self-administration was increased in the CS+ group for the initial 6 relapse sessions.
Responding on the water lever was not altered during reinstatement testing (Fig. 4). Statistically, there was no effect of ‘session’, ‘conditioned cue’, or a ‘session’ by ‘conditioned cue’ interaction (p values > 0.34). In general, water responding was consistently around 20 responses/session and did not alter during testing.
Figure 4.
Depicts the mean (+ SEM) responses for water influenced by the presences of conditioned cues during EtOH relapse conditions. There were no significant effects.
Separating Context-Induced and Conditioned Stimuli EtOH-Seeking
The ability of context to induce EtOH-seeking in the PSR model typically lasts for a single session (Hauser et al., 2012; Rodd et al., 2006). The current experiment examined the ability of a CS+ conditioned stimuli to re-establish EtOH-seeking after the cessation of context-induced EtOH-seeking (Fig. 5). A within subject analysis (‘session’) indicated that there was a significant effect of ‘session’ (F9,6 = 13.667; p = 0.002). The significant within subject effect was further analyzed using paired t-tests. Responding during the 1st PSR test session (No CS+ present) was significantly higher than during extinction baseline (p < 0.001), but was not apparent during the 2nd PSR test session (p = 0.68). EtOH-seeking was re-established with the introduction of the CS+ during PSR test sessions 3-8. Statistically, responding during PSR test sessions 3 and 4 were significantly higher than extinction baseline and that observed during the 1st PSR test session (p values < 0.031). During the 5th-8th PSR test session, EtOH lever responding was significantly higher than extinction baseline (p values < 0.022). Responding on the water lever was not affected by ‘session’ (p = 0.57; data not shown).
Figure 5.
Depicts the mean (+ SEM) responses on the lever previously associated with the delivery of EtOH during both context-induced (PSR sessions 1 and 2) and conditioned stimuli-induced (PSR sessions 3-10) EtOH-seeking. * indicates significantly higher level of responding compared to extinction baseline. + indicates significantly higher level of responding compared to extinction baseline and during the 1st PSR test session.
Flavored Water Control
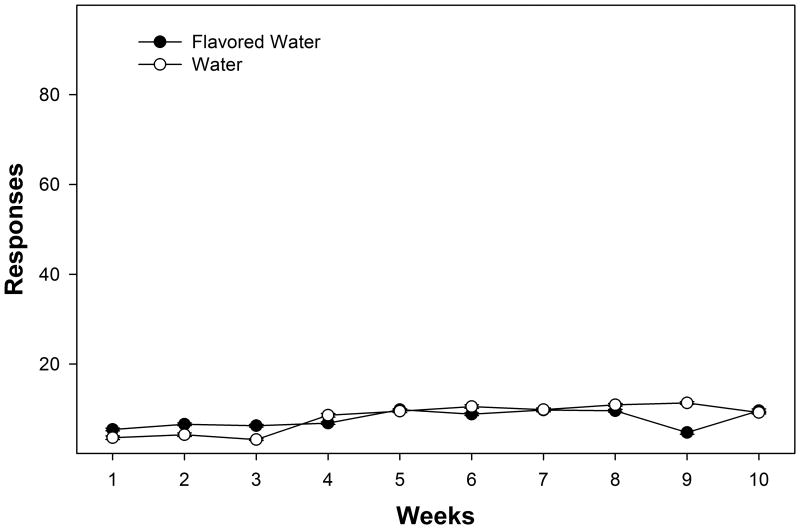
P rats did not prefer the flavored water over water during the initial 10 weeks of operant access (Fig. 6; p values > 0.65). In general, responding for both water and flavored water was relatively low (approximately 10 responses/session each) and was equivalent to that consistently observed for P rats responding for water with EtOH available (20 total responses, see above experiments). There was no context-induced flavored water seeking (p values > 0.76) or relapse drinking for flavored water (p values > 0.53; data not shown). Additionally, responding for the raspberry, blueberry and vanilla flavored water solutions was equivalent (responding for the 3 flavors were equal when employed as the ‘CS+’ for 10 weeks, in addition there was no alteration in responding during flavor substitution in the ‘CS-’ period).
Figure 6.
Depicts the weekly average responses (+ SEM) for water and flavored water for the initial 10 weeks of operant access to the solutions. There were no significant effects.
Discussion
The current experiments present a number of important findings. First, we report that oral stimuli can serve as a conditional cue to enhance (CS+) or inhibit (CS-) EtOH seeking in P rats in the PSR model (Fig.1), and can enhance or inhibit EtOH relapse responding (Fig. 3). The PSR paradigm is designed to provide a period of abstinence following extinction, which is frequently seen in human alcohol use prior to relapse (O'Brien et al., 1998). When exposed to the CS+, subjects exhibited elevated EtOH seeking that persisted for more PSR sessions than was seen following exposure to context alone (e.g., compare figs. 1 and 5), suggesting that the learned association formed with a conditional cue may have been stronger than associations formed by contextual cues. Finally, exposure to an excitatory conditional flavor cue (CS+) induced EtOH-seeking in the PSR model after context-induced EtOH seeking had returned to extinction baseline levels (Fig. 5).
Most animal studies of operant alcohol consumption required experimental manipulations (EtOH prime, stress, sucrose fading) before voluntary consumption was achieved (Katner and Weiss, 1999; Katner et al., 1999). The experiments in the current study utilized alcohol-preferring (P) rats, which will orally self-administer EtOH under free choice conditions, obtain pharmacologically relevant EtOH BAC levels, and following chronic consumption, develop metabolic and functional tolerance and exhibit signs of withdrawal (reviewed in McBride et al., 2014). Accordingly, the P rat is thought to represent a useful animal model of problematic human alcohol consumption. The majority of context and cue-induced studies of EtOH seeking following extinction employ relapse or reinstatement testing a day after the last extinction session. In human alcohol abuse, periods of abstinence following remission are common prior to relapse. Spontaneous recovery is described as the partial recovery of responding following a delay between extinction and recovery testing (Rescorla, 2004). In the current study, P rats tested in the PSR model exhibited robust cue and context induced EtOH seeking following a two week post-extinction abstinent period, which parallels past findings from our laboratory in the PSR paradigm (Rodd-Henricks et al., 2002a,b; Rodd et al., 2006; Hauser et al., 2011, 2012), and has been shown to occur following as many as five months between extinction and reinstatement testing (Jupp et al., 2011).
The environment presents a combination of discriminative (odors, alcoholic beverage bottles, bar sounds) and conditional (flavors associated with mixed or other alcoholic beverages) cues associated with human EtOH exposure. Previous experiments investigating context or cue-induced EtOH-seeking following extinction have utilized cues of various modalities, e.g. discriminative odor or auditory cues (Katner et al., 1999; Katner and Weiss, 1999; Ciccocioppo et al., 2002; Chaudhri et al., 2008a,b), and compound auditory/visual conditional cues (Tsiang and Janak, 2008), but to our knowledge, no one has assessed the ability of non-alcoholic flavors paired with EtOH to induce EtOH-seeking following a prolonged EtOH abstinence period. Flavors are capable of producing conditioned preferences or aversions when paired with EtOH, depending on factors including the dose, method of administration, and satiety state of the animal (Deems et al., 1986; Ackroff and Sclafini, 2001). It has recently been reported that flavors, associated with alcohol, induce increased striatal dopamine release in subjects with a first degree relative with a history of alcoholism (Oberlin et al., 2013), suggesting that alcohol associated flavor cues are capable of activating reward associated brain regions. Subjects in the current experiments did not prefer the conditional flavor cues (mixed with water) over water alone, and there were no differences in response levels for each of the three flavors (Fig. 6).
In the present study, exposure to an excitatory conditioned flavor cue (CS+) induced robust EtOH seeking two weeks after extinction training (Fig. 1). Similar to what was seen in PSR testing, subjects exposed to the CS+ exhibited higher cue induced relapse responding relative to baseline response levels during the first 6 relapse sessions (Fig. 3). To our knowledge, this is the first direct evidence that flavor can serve as a conditional cue capable of inducing both EtOH-seeking and relapse responding. These findings parallel human alcohol abuse where relapse following remission is common (Moos and Moos, 2006), and suggest that exposure to flavors associated with EtOH may be sufficient to induce craving or drug-seeking in human alcoholics. Conditional cues are thought to produce stronger learned associations than discriminative cues (Macintosh, 1977). As flavor is the primary conditional cue associated with EtOH intake in human drinking, conditioning based treatment strategies for reducing craving and relapse should address flavor-related EtOH associations.
Within the current experimental protocol, exposure to the different types of CS's is not equivalent. An intuitive assumption would be that repeated pairings between the US and the CS+ would have resulted in a strong association between these two factors, which would enhance the efficacy of the CS+. A long established, replicated phenomenon in the learning field is that the associative strength between a US and CS as a factor of the number of pairings is not linear (Resorla, 2004). Stimulus habituation was first noted by Pavlov (1927). Stimulus habituation can be briefly described as a decrease in associative strength between factors after extensive pairing (McSweeney and Swindell, 1999). Stimulus habituation is influenced by the saliency of the US, nature of conditioning, and past experience (McSweeney and Swindell, 1999). In reality, the associative strength between a CS and a US is not linear, but a modified inverted U (with the descending tail being truncated to indicate the remaining association). In the current experimental protocol, it is likely that the extensive US-CS+ pairings have extended past the zenith of the associative strength. In contrast, given the nature of CS- conditioning, it is possible that the current protocol resulted in a potential maximum associative strength between US and CS- being established. The extensive US-CS+ was chosen because of the desire to have consistent EtOH consumption in subjects and to reflect the persistent nature of alcohol consumption being paired with conditioned cues in alcoholics.
This issue of familiarity to the flavor solutions could be inappropriately applied to the results. In general, an interpretation of the data is that past higher rate of exposure to the CS+ cue resulted in the subjects responding more for this familiar solution during PSR testing. However, that can be refuted by the data and by the established literature. First, the no flavor group was given access to water during PSR testing. The subjects were most familiar to this solution (which does have a flavor) but did not respond more for this solution compared to the CS+. Second, if familiarity was generating the increase in responding produced by the CS+ during PST testing, the enhanced responding should have never extinguished. Basically, the familiarity to the CS+ should have only been enhanced (compared to the other flavor groups) during PSR testing, and this should have further enhanced/continued the heightened level of responding. As indicated by the data, this is not the case. Third, when the ability of the CS+ to elicit seeking was tested following the extinction of context-induced EtOH-seeking (Fig. 5), the CS+ was again only able to stimulate seeking for a limited number of test sessions. This stance also fails to consider the control experiments of testing the flavor cues in the absence of EtOH self-administration (Fig. 6). This control experiment clearly indicates that the flavor cues selected were neutral stimuli (no development of responding above water). Given that the null-CS+ was self-administered for more test sessions than either the null-CS- or null-CS0 , that familiarity position would assert that more ‘seeking’ for the null-CS+ should occur during null-PSR testing. This clearly did not occur.
Rats do prefer to drink familiar solutions over completely novel solutions (neophobia; DeMatte et al., 2014). Neophobia is a very short lived phenomenon in rodents, and is reduced by exposure to previous novel stimuli (Sclafani, 2013). Although not specifically tested, most recent research would suggest that neophobia to the CS- or CS0 was most likely overcame within the 2nd exposure session (Scalfani, 2013). Unlike humans, familiarity does not mean favorability in rodents. Specifically, past consumption of a neutral stimulus does not increase the likelihood of consumption of that neutral solution in the future (Swithers, 1996). Similar to stimulus habituation, the field of ingestive behaviors has consistently reported that rodents habituate to oral neutral stimuli (Swithers, 1996; Swithers et al., 2009; Davidson et al., 2014). So in contrast to the alternative hypothesis of flavor familiarity, well established learning and ingestive behavior research would indicate that repeated exposure to a neutral stimuli in rodents does not produce favoritism but indifference. The present results (Figs. 1 and 3) indicate that an excitatory conditional flavor cue (CS+) is able to induce increased EtOH-seeking and relapse relative to context, and this effect persists for multiple sessions. Cue induced augmentation of contextual EtOH seeking has been reported previously (Tsiang and Janak, 2008). However, the present experiment presents evidence that this can occur following a two week abstinent period after extinction training, and not just immediately following extinction.
In the current study, subjects exposed to a (CS-) showed decreased responding relative to all other groups during the 1st session of EtOH-seeking (Fig. 1) and relapse (Fig. 3). Furthermore, the group exposed to the (CS-) responded significantly less than baseline responding during the first relapse session (Fig. 3). Previous research has reported conditioned inhibition of operant responding for food (Lombas et al., 2008) and cocaine (Kearns et al., 2005), but the current study presents evidence that a (CS-) can effectively inhibit EtOH seeking and relapse responding compared to all other groups 2 weeks after extinction training.
Clinical research has attempted to apply conditioning principles to the treatment of addiction. Typically, the conditioning strategies employ the use of cue-exposure treatment (systematic, gradual exposure to EtOH or drug related cues), with the goal of extinguishing cue reactivity (O'Brien et al., 1998). Unfortunately, extinction based cue-exposure treatments have been characterized by limited success in increasing long term abstinence in drug-dependent patients (Conklin and Tiffany, 2002). One potential reason for the relative ineffectiveness of extinction based treatments is that it is impossible to expose a patient to extinction training for every drug-related cue that one might encounter in the environment. Extinction of a learned association between a specific drug-related cue and the drug is not likely to alter associations between the drug and other cues. A CS-, on the other hand, should be able to attenuate or block conditioned responses induced by a drug related stimulus, and could be used to reduce craving in a situation where drug-related stimuli are present. Here, we show that in both PSR and relapse testing, CS- exposure is able to inhibit seeking and relapse induced by EtOH related contextual cues, that is, by reducing responding relative to subjects only exposed to contextual but not conditional excitatory or inhibitory cues.
In a separate experiment, we tested the ability of conditional flavor cues to induce EtOH-seeking following context-induced seeking in the PSR model (Fig. 5). In parallel with past findings from our laboratory, context-induced increases in EtOH seeking were seen in the first PSR session but did not persist to the second session (Rodd et al., 2006; Hauser et al., 2012). When the CS+ was made available (sessions 3-8), subjects responded significantly more on the EtOH lever than extinction baseline responding. Moreover, responses on the EtOH lever were higher in sessions 3 and 4 than in session 1. The results of this experiment suggest that a conditional flavor cue is capable of restoring EtOH seeking in the PSR model following a reduction in context-induced seeking.
Taken together, the results of the current experiments show that oral conditional cues can excite or inhibit EtOH seeking and relapse. These findings may be relevant to clinical goals in the treatment of EtOH craving and relapse. As a stand-alone therapy, cue exposure treatment is not likely to alter craving induced by exposure to all EtOH related cues. The results presented here suggest that conditioned inhibition strategies may provide a useful supplement to cognitive behavioral and pharmacological treatment plans. Here, we show that flavor paired with the absence of EtOH is able to serve as a conditional cue to reduce context-induced EtOH-seeking behavior and relapse drinking. The use of (CS-) cues may provide a useful strategy for reducing craving in situations where EtOH related cues are encountered.
Acknowledgments
The skillful technical assistance of Tylene Pommer and Victoria McQueen is acknowledged. This research was supported by NIAAA grants AA07611, AA022287, AA07462, and AA020908. None of the authors of this manuscript have conflicts of interest to report.
Sources of support: Grants AA07611, AA022287, AA07462, and AA020908
References
- Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric infusion of ethanol in rats. Pharmacol Biochem Behav. 2001;68:327–338. doi: 10.1016/s0091-3057(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque, LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008a;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008b;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci. 2013;38:2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of μ1 or δ opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Davidson D, Tiffany ST, Johnston W, Flury L, Li TK. Using the cue availability paradigm to assess cue reactivity. Alcohol Clin Exp Res. 2003;27:1251–1256. doi: 10.1097/01.ALC.0000080666.89573.73. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem. 2014;108:172–84. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, McKinzie DL, Carroll FI, McBride WJ, Rodd ZA. The long lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2012;101:581–587. doi: 10.1016/j.pbb.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deems DA, Oetting RL, Sherman JE, Garcia J. Hungry, but not thirsty, rats prefer flavors paired with ethanol. Physiol Behav. 1986;36:141–144. doi: 10.1016/0031-9384(86)90087-9. [DOI] [PubMed] [Google Scholar]
- Dematte ML, Endrizzi I, Gasperi F. Food neophobia and its relation with olfaction. Front Psychol. 2014;17:5–127. doi: 10.3389/fpsyg.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Wilden JA, Deehan GA, Jr, McBride WJ, Rodd ZA. Cocaine influences alcohol-seeking behavior and relapse drinking in alcohol preferring (P) rats. Alcohol Clin Exp Res. 2014;38:2678–2686. doi: 10.1111/acer.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin1 receptors. Br J Pharmacol. 2011;162:880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Schindler CW, Panlilio LV. Conditioned inhibition of cocaine seeking in rats. J Exp Psychol Anim Behav Process. 2005;31:247–253. doi: 10.1037/0097-7403.31.2.247. [DOI] [PubMed] [Google Scholar]
- Lombas AS, Kearns DN, Weiss SJ. A comparison of the effects of discriminative and pavlovian inhibitors and excitors on instrumental responding. Behav Processes. 2008;78:53–63. doi: 10.1016/j.beproc.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Macintosh JJ. Stimulus control: Attentional factors. In: Honig WK, Staddon JER, editors. Handbook on operant behavior. Prentice-Hall; NJ: 1977. pp. 162–241. [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high alcohol-drinking (HAD) rats – animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: the role of habituation. Psychol Bull. 1999;125:437–457. [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyland JE, Grigson PS. A drug-paired flavor cue elicits withdrawal and predicts cocaine self-administration. Behav Brain Res. 2013;240:87–90. doi: 10.1016/j.bbr.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: implications for a stimulus control analysis of drinking. Addict Behav. 1983;8:1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Remedios J, Woods C, Tardif C, Janak PH, Chaudhri N. Pavlovian-conditioned alcohol-seeking behavior in rats is invigorated by the interaction between discrete and contextual alcohol cues: implications for relapse. Brain Behav. 2014;4:278–289. doi: 10.1002/brb3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychol Bull. 1969;72:77–94. [Google Scholar]
- Rescorla RA. Spontaneous Recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- SAMHSA; National Survey on Drug Use and Health (NSDUH) Substance dependence or abuse in the past year, by demographic characteristics: numbers in thousands, 2011 and 2012. [Accessed January 15, 2015];2012 [SAMHSA Web site] Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2012/NSDUH-DetTabs2012/HTML/NSDUH-DetTabsSect5peTabs1to56-2012.htm.
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia JM, Mendoza J, Chaudhri N. Blocking dopamine d1-like receptors attenuates context-induced renewal of pavlovian-conditioned alcohol-seeking in rats. Alcohol Clin Exp Res. 2014;38:418–427. doi: 10.1111/acer.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Gut-brain nutrient signaling, Appetition vs. satiation. Appetite. 2013;71:454–8. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger PK, White JM. Cue reactivity in alcohol abusers: stimulus specificity and extinction of the responses. Addict Behav. 1991;16:211–221. doi: 10.1016/0306-4603(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Effects of oral experience on rewarding properties of oral stimulation. Neurosci Biobehav Rev. 1996;20:27–32. doi: 10.1016/0149-7634(95)00031-9. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweetners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123:772–80. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Bjerland T, Nordby H, Hugdahl K. Autonomic cued reactivity in alcoholics: the effect of olfactory stimuli. Addict Behav. 1995;20:571–584. doi: 10.1016/0306-4603(95)00017-7. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–88. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]