Abstract
Aim:
To examine how the endogenous CYP3A4 phenotype and CYP3A5*3 genotype of Chinese renal transplant recipients influenced the dose-corrected trough concentration (C0/D) and weight-corrected daily dose (D/W) of tacrolimus.
Methods:
A total of 101 medically stable kidney transplant recipients were enrolled, and their blood and urine samples were gathered. The endogenous CYP3A4 phenotype was assessed by the ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urine. CYP3A5*3 genotype was determined using PCR-RELP.
Results:
In overall renal transplant recipients, a multiple regression analysis including the endogenous CYP3A4 phenotype, CYP3A5*3 genotype and post-operative period accounted for 60.1% of the variability in C0/D ratio; a regression equation consisting of the endogenous CYP3A4 phenotype, post-operative period, body mass index, CYP3A5*3 genotype, gender, total bilirubin and age explained 61.0% of the variability in D/W ratio. In CYP3A5*3/*3 subjects, a combination of the endogenous CYP3A4 phenotype, post-operative period and age was responsible for 65.3% of the variability in C0/D ratio; a predictive equation including the endogenous CYP3A4 phenotype, post-operative period, body mass index, gender and age explained 61.2% of the variability in the D/W ratio. Base on desired target range of tacrolimus trough concentrations, individual daily dosage regimen was calculated, and all the observed daily doses were within the predicted range.
Conclusion:
This study provides the equations to predict tacrolimus metabolism and dosage requirements based on the endogenous CYP3A4 phenotype, CYP3A5*3 genotype and other non-genetic variables.
Keywords: tacrolimus, CYP3A phenotype, CYP3A5*3 genotype, Chinese renal transplant recipients
Introduction
Tacrolimus (Tac) is a potent immunosuppressive agent that has been widely used to improve the outcome of organ transplantation. The need for frequent and specific monitoring of drug concentrations remains essential because the therapeutic dosing and pharmacokinetics of Tac show great variability among recipients1. Tac is known to be extensively metabolized by cytochrome P450 (CYP) 3A4 and CYP3A5. Intestinal P-glycoprotein (P-gp) is a product of the multidrug resistant 1 (MDR1) gene in humans and limits the bioavailability of Tac via active efflux2,3. Importantly, most single nucleotide polymorphisms (SNPs) for CYP3A4 are unable to fully explain the inter-individual variability in CYP3A enzymatic activity4. In contrast, the CYP3A5*3 SNP (A6896G) in exon 3 is strongly associated with CYP3A5 expression5, and the effect of the CYP3A5*3 allele on the pharmacokinetics of oral Tac has been confirmed by consistently positive results1,6. Other reports have indicated that the most common SNPs for MDR1, including the synonymous SNPs C1236T in exon 12, C3435T in exon 26 and the nonsynonymous SNP G2677T/A in exon 217, play a minor role in the metabolism of Tac1. Hence, CYP3A5*3 polymorphisms may be the only useful SNP for Tac dosage adjustment6. However, such genetic information is unlikely to be responsible for the residual inter-individual variability in the pharmacokinetics of Tac among CYP3A5 expressers or CYP3A5 non-expressers. Therefore, accurately formulating an individual's dosing regimen remains limited6. As a complementary or alternative strategy, determination of a patient's CYP3A4 phenotype may help to overcome the limitations of genetic assays and optimize Tac dosage regimens.
Currently, the ratio of 6β-hydroxycortisol to free cortisol (6β-OHF/F) in urine is thought to be a useful marker of both the induction and the inhibition of hepatic CYP3A4 activity8. The interconversion of cortisol (F) to cortisone (E) and 6β-hydroxycortisol (6β-OHF) to 6β-hydroxycortisone (6β-OHE) by 11β-hydroxysteroid dehydrogenase (11β-HSD) plays an important role in cortisol metabolism; thus, using cortisol as a CYP3A4 phenotyping probe may be confounded due to the regulation of cortisol feedback9,10. Our previous experiments confirmed that the combined ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urinary MR reflects CYP3A4 catalytic ability and not the total activity of CYP3A4 and CYP3A5 isoforms (unpublished data). Another previous study completed by our laboratory showed that the MR was significantly related with the dose-corrected trough concentrations (C0/D) of Tac in renal transplant recipients (r= -0.824, P<0.05)11.
The major aim of our current study was to investigate the relationship between the endogenous CYP3A4 phenotype (assessed by urinary MR) and Tac metabolism and dosage requirements in Chinese renal transplant recipients. We also investigated the influence of CYP3A5*3 genotype and other potential determinants of Tac disposition.
Materials and methods
Ethics statement
This study was performed in accordance with the declaration of Helsinki and its amendments. The experimental protocol was approved by the Ethical Committee of the School of Pharmaceutical Sciences at Central South University. The study is a part of registered clinical trial in ClinicalTrials.gov and the identifier is NCT01699360 (http://clinicaltrials.gov/show/NCT01699360). Written informed consents were obtained from all subjects before commencing the study.
Materials
The 6β-OHF, 6β-OHE, F, E and dexamethasone were purchased from Sigma-Aldrich (St Louis, MO, USA). They were of at least 98% purity. Acetonitrile, methanol and formic acid were of LC grade and purchased from Tedia (Tedia Company Inc, Fairfield, CT, USA). All other chemicals were of AR grade and obtained from commercial sources (Sinopharm Chemical Reagent Co Ltd, Shanghai, China).
Human subject study
The study was comprised of 101 medically stable kidney transplant recipients between 1 and 2 years after transplantation. A total of 231 specimens were collected at the 3rd Affiliated Hospital of Xiangya Medical Institute at Central South University. Patients who met the following criteria were included: receiving an immunosuppressive regimen containing Tac (tacrolimus capsules, Prograf®, Astellas Ireland Co Ltd, Ireland), mycophenolate mofetil (CellCept®, Roche, Shanghai, China) and prednisolone; oral administration of Tac at twice the daily dose at least 5 d prior to the study; undergone no more than one renal transplant; normal liver and renal function; and older than 18 years of age. The following exclusion criteria were used for this study: an acute rejection episode or infection; multiple organ transplantation; use of any other medications known to induce or inhibit the CYP3A enzyme or interact with immunosuppressive agents, with the exception of amlodipine; or any abnormal findings on physical examinations or laboratory tests.
At the time of investigation, the dose of Tac ranged between 1.0 and 8.0 mg/day. The doses of mycophenolate mofetil and prednisolone were 0.36–1.5 mg/day and 2.0–30.0 mg/day, respectively. On the morning of the study, urine specimens were collected between 8:00 AM and 10:00 AM. Blood samples were obtained before Tac administration. Blood samples were immediately sent to the clinical laboratory for Tac analysis and CYP3A5*3 genotyping, and urine samples were stored at -20 °C for subsequent analysis of 6β-OHF, 6β-OHE, F and E. All subjects underwent routine laboratory tests, including hematology, blood chemistries and urinalysis. The demographics of each subject and their co-medications were also recorded (Table 1). Each patient was instructed to eat his or her usual breakfast, excluding caffeine- and grapefruit-containing foods, and to take any other morning medication except Tac.
Table 1. The data of renal transplant recipients. Mean±SD.
| Parameter | Value |
|---|---|
| Age (year) | 37.4±9.9 |
| Gender (male/female) | 77/24 |
| Body weight (kg) | 60.2±10.5 |
| Height (cm) | 165.7±6.4 |
| Body mass index (kg/m2) | 21.8±3.2 |
| Postoperative periods (h) | 11 887.6±2032.6 |
| Glutamic-pyruvic transaminase (U/L) | 25.2±16.8 |
| Glutamic oxalacetic transaminase (U/L) | 21.2±12.3 |
| Total bilirubin (μmol/L) | 13.2±4.3 |
| Blood urea nitrogen (mmol/L) | 6.1±2.0 |
| Serum creatinine (μmol/L) | 97.3±47.6 |
| Serum uric acid (μmol/L) | 337.4±70.1 |
| Comedication of amlodiping (with/without) | 50/51 |
CYP3A5*3 genotyping
Genomic DNA was extracted from peripheral lymphocytes according to the instructions provided with the Genomic DNA Purification Kit (Promega, Madison, WI, USA). Identification of CYP3A5*3 genotypes were performed using the previously reported polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay12. The forward primer was 5′-CATGACTTAGTAGACAGATGAC-3′ and the reverse primer was 5′-GGTCCAAACAGGGAAGAAATA-3′. The amplified DNA fragment was 293 bp and was digested with Ssp I (Fermentas, Vilnius, Lithuania) at 37 °C for 4 h. The genotype for each individual was further validated by a sequencing assay (data not shown).
Analytical methods
6β-OHF, 6β-OHE, F and E in urine
The 6β-OHF, 6β-OHE, F and E in urine were measured using the HPLC-UV method developed in our laboratory11. The lower limit of quantification was 5 μg/L for all of the compounds in the urine. The accuracy was determined at three concentrations and ranged between 98.2% and 115.5%.
Trough concentrations of tacrolimus in whole blood
Trough concentrations of Tac in whole blood were assayed by the Microparticle Enzyme Immunoassay (MEIA, Abbott, Princeton, NJ, USA) using blood samples taken on the morning of the study. The assay was performed according to the instructions supplied with the Tacrolimus II monoclonal antibody kit (Abbott, Princeton, NJ, USA).
Data and statistical analysis
The normality distribution of parameters was assessed using the Kolmogorov-Smirnov test. The C0/D ratio and weight-corrected daily dosage (D/W) of Tac between CYP3A5*3 genotypes was compared using a one-way ANOVA followed by post-hoc Bonferroni corrected t-tests. The relationship between the C0/D and D/W ratios of Tac and urinary MR were analyzed by linear regression. Correlations of the C0/D and D/W ratios of Tac with each factor (as shown in Table 1) were analyzed with an α=0.05. Factors that were not statistically significant were rejected. A stepwise multiple regression analysis with statistically significant factors was used to obtain the coefficient of determination (R2). The models for the prediction of the C0/D and D/W ratios of Tac were constructed based on the multiple regression equation. All statistical analysis was performed using SPSS 17.0 statistics software, and a P value <0.05 was considered significant.
Results
Effect of CYP3A5*3 genotype on the C0/D and D/W ratios of Tac
The Kolmogorov-Smirnov test illustrated that the logarithm of C0/D and D/W ratios of Tac and the logarithm of urinary MR, postoperative periods, body mass index and total bilirubin were normally distributed. The values of all other continuous variables (as shown in Table 1) showed a normal distribution in Chinese renal transplant recipients.
The C0/D ratios of Tac in the CYP3A5*3/*3 group were significantly higher when compared with those in the CYP3A5*1/*1 and CYP3A5*1/*3 groups (Supplementary Table S1). Consistent with the relationship between CYP3A5 genotype and phenotype, the D/W ratios of Tac in CYP3A5*3/*3 subjects were markedly lower when compared with CYP3A5*1 carriers (CYP3A5*1/*1 and CYP3A5*1/*3 genotypes) (Supplementary Table S1).
The relationship between the endogenous CYP3A4 phenotype and tacrolimus metabolism
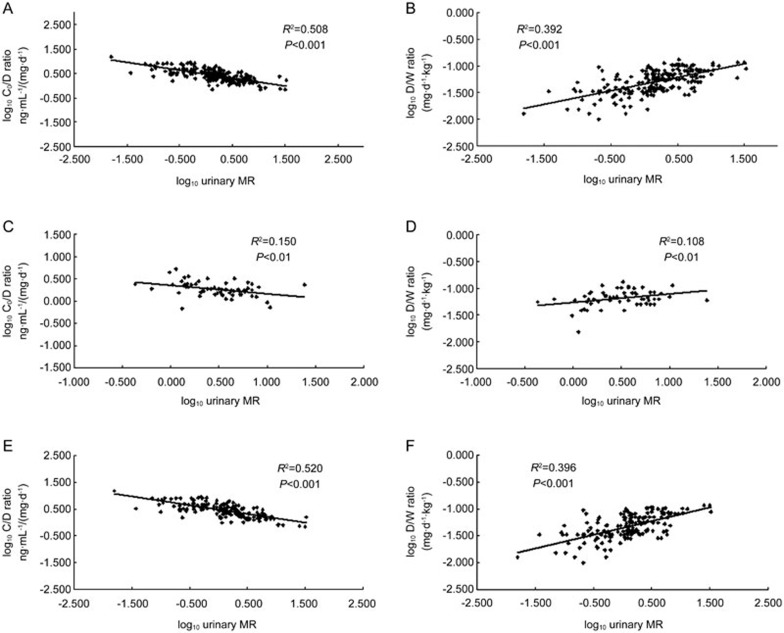
As shown in Figure 1, the endogenous CYP3A4 phenotype (assessed by urinary MR) significantly correlated with the C0/D and D/W ratios of Tac. Within CYP3A5*3/*3 subjects, urinary MR was related to the C0/D and D/W ratios; however, only weak correlations were observed in CYP3A5*1 carriers (CYP3A5*1/*1 and CYP3A5*1/*3 genotypes).
Figure 1.
Linear regression relationships between urinary MR and the C0/D and D/W ratios of tacrolimus across the different CYP3A5*3 genotypes: (A) with the C0/D ratio in overall subjects (n=231); (B) with the D/W ratio in overall subjects (n=231); (C) with the C0/D ratio in CYP3A5*1 carriers (n=58); (D) with the D/W ratio in CYP3A5*1 carriers (n=58); (E) with the C0/D ratio in CYP3A5*3/*3 genotype (n=173); (F) with the D/W ratio in CYP3A5*3/*3 genotype (n=173).
Multiple regression analysis for the prediction of tacrolimus metabolism and dosage requirements
Final regression models were established to predict the C0/D and D/W ratios of Tac (Table 2). The coefficients of determination (R2) were 0.601 and 0.610 for the C0/D and D/W ratios, respectively. Within the CYP3A5*3/*3 genotype, the values of R2 were 0.653 and 0.612 for the C0/D and D/W ratios, respectively (Table 3). According to equations (1) and (3), the daily dosage regimen was determined:
Table 2. The regression analysis for the prediction of the C0/D and D/W ratios of Tac in overall renal transpant recipients (n=231).
| No | Predicted value | Regression equation | R2 | P |
|---|---|---|---|---|
| Equation (1) | C0/D ratio ng·mL−1/(mg·d−1) | Log10 C0/D=0.331−0.338*Log10 MR−0.083*CYP3A5+0.046*Log10 POT | 0.601 | <0.001 |
| Equation (2) | D/W ratio (mg·d−1·kg−1) | Log10 D/W=0.303+0.230*Log10 MR−0.133*Log10 POT−0.750* Log10 BMI +0.081* CYP3A5+0.066* gender−0.133*Log10TBIL−0.002* age | 0.610 | <0.001 |
C0/D, dose-corrected trough concentration of Tac; D/W, weight-corrected stable dose of Tac; Log10 MR, the logarithm of the ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urine; CYP3A5*1/*1 and CYP3A5*1/*3 genotypes were set at the value of "1"; CYP3A5*3/*3 genotype was set at the value of "0"; Log10 POT, the logarithm of post-operative period; Log10 BMI, the logarithm of body mass index; gender, male was defined as the value of "0"; female was defined as the value of "1"; Log10 TBIL, the logarithm of total bilirubin; R2, coefficient of determination; P, probability.
Table 3. The regression analysis for the prediction of the C0/D and D/W ratios of Tac in renal transpant recipients with CYP3A5*3/*3 genotype (n=173).
| No | Predicted value | Regression equation | R2 | P |
|---|---|---|---|---|
| Equation (3) | C0/D ratio ng·mL−1/(mg·d−1) | Log10 C0/D=0.132−0.357*Log10 MR+0.074*Log10 POT+0.003*age | 0.653 | <0.001 |
| Equation (4) | D/W ratio (mg·d−1·kg−1) | Log10 D/W=0.333+0.251*Log10 MR−0.151* Log10 POT−0.820*Log10 BMI+0.061*gender−0.002*age | 0.612 | <0.001 |
C0/D, dose-corrected trough concentration of Tac; D/W, weight-corrected stable dose of Tac; Log10 MR, the logarithm of the ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urine; Log10 POT, the logarithm of post-operative period; Log10 BMI, the logarithm of body mass index; gender, male was defined as the value of "0"; female was defined as the value of "1"; R2, coefficient of determination; P, probability.
Daily dosage regimen for all subjects=C0/Power [10, (0.331– 0.338*Log10 MR–0.083*CYP3A5+0.046*Log10POT)] Equation (5)
C0, trough concentration of Tac; Log10 MR, the logarithm of the ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urine; CYP3A5*1/*1 and CYP3A5*1/*3 genotypes were set at “1” the CYP3A5*3/*3 genotype was set at “0” Log10 POT, the logarithm of post-operative period.
Daily dosage regimen for CYP3A5*3/*3 subjects=C0/Power [10, (0.132–0.357*Log10MR+0.074*Log10 POT+0.003* age)] Equation (6)
C0, trough concentration of Tac; Log10MR, the logarithm of the ratio of 6β-hydroxycortisol and 6β-hydroxycortisone to cortisol and cortisone in urine; Log10 POT, the logarithm of post-operative period.
Based on the desired target range of Tac C0 levels, an individual's daily dosage regimen was directly calculated by equation (5) and (6). The results illustrate that all of the observed doses were within the predicted dosage range (Table 4).
Table 4. The desired target range of Tac concentration, the predicted daily dosage range and the observed daily dose of Tac in overall (n=231) and CYP3A5*3/*3 subjects (n=173). Mean±SD.
| Subjects | Desired target range of concentration (ng·mL−1)a | Predicted daily dosage range (mg·d−1) |
Observed daily dose (mg·d−1) | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Overall | 3–7 | 1.0±0.4 | 3.7±1.5 | 3.4±1.5 |
| CYP3A5*3/*3 genotype | 3–7 | 0.9±0.4 | 3.2±1.6 | 3.1±1.5 |
aThe desired target range of concentration was set according to the clinical practice in the 3rd Affiliated Hospital of Xiangya Medical Institute, Central South University;
Discussion
Therapeutic drug monitoring (TDM) and CYP3A5*3 genotyping are common tools for the adjustment of an individual's Tac dosage in the clinic. However, TDM can be labor intensive because this method requires the frequent monitoring of drug concentrations in blood. Notably, TDM cannot predict an individual's dosage; thus, many patients can have adverse reactions before drug monitoring begins1. CYP3A5*3 genotyping can help to optimize the Tac dose regimen between CYP3A5 expressers and CYP3A5 non-expressers. Our results confirm previous reports showing that CYP3A5*3 polymorphisms play an important role in Tac metabolism1,6. CYP3A5 non-expressers (CYP3A5*3/*3) displayed higher C0/D ratios and lower D/W ratios when compared with CYP3A5 expressers (CYP3A5*1/*1 and CYP3A5*1/*3) (Supplementary Table S1). Within CYP3A5 expressers or CYP3A5 non-expressers, there was a residual inter-individual variability in the dosage requirements and pharmacokinetics of Tac. Therefore, we hypothesized that in vivo CYP3A4 phenotyping was an independent predictor of Tac disposition.
A previous study suggested that 56%–59% of the variability in Tac dose requirements and clearance could be explained by in vivo CYP3A4 activity assessed by midazolam clearance and CYP3A5 genotype6. Administration of midazolam is not convenient because of its pharmacological effects as a central nervous system depressant; thus, taking advantage of endogenous substances, such as markers of the CYP3A4 phenotype, can avoid unnecessary administration of probe drugs to humans. In our study, a non-invasive CYP3A4 phenotype was used by determining the metabolic ratio of endogenous 6β-hydroxymetabolites to cortisol and cortisone. No serial blood sampling for the CYP3A4 phenotype was needed. The findings showed that this endogenous CYP3A4 probe explained 50.8% and 39.2% of variability in the C0/D and D/W ratios, respectively, of all subjects (Figure 1). The multiple regression analysis, including CYP3A4 phenotype assessed by urinary MR, CYP3A5*3 genotype and post-operative period, accounted for 60.1% of the variability in the C0/D ratio (Table 2). Combination of the in vivo CYP3A4 phenotype, post-operative period, body mass index, CYP3A5*3 genotype, gender, total bilirubin and age explained 61.0% of variability in the D/W ratio (Table 2). These results are similar to a previous report on midazolam clearance and CYP3A5*3 genotype6.
Within CYP3A5 non-expressers (CYP3A5*3/*3), urinary MR was responsible for 52.0% and 39.6% of the variability in the C0/D and D/W ratios of Tac, respectively; however, only a 15.0% and 10.8% contribution was found among CYP3A5 expressers (CYP3A5*1/*1 and CYP3A5*1/*3) (Figure 1). Furthermore, 65.3% and 61.2% of the variance in the C0/D and D/W ratios, respectively, was explained by the multiple regression equations presented in Table 3. Our previous study suggested that urinary MR reflects CYP3A4 activity (unpublished data), and the metabolism of Tac depends on a total of CYP3A4 and CYP3A5 activities13. Among CYP3A5*3/*3 subjects, we assumed that all compounds were mainly catalyzed by the CYP3A4 isoform, and the absence of the CYP3A5 enzyme may contribute to the marked correlation between urinary MR and Tac disposition.
The recommended blood concentration of Tac is 3–7 ng/mL at least 3 months after transplantation. The dosage requirements should be carefully controlled in order to achieve the desired concentration of Tac. Using equations (5) and (6), the desired daily dosage was predicted, and all of the observed doses were within the predicted range (Table 4). Our findings suggest that equations (5) and (6) can provide additional valuable information for the use of Tac in the clinic.
In our study, all patients received an immunosuppressive regimen containing Tac, mycophenolate mofetil and prednisolone. Prednisolone can be converted to prednisone, which is a weak inducer of CYP3A414. However, we found that a varying dosage of prednisolone had no significant effect on Tac disposition and CYP3A4 phenotype (data not shown). Furthermore, calcium-channel blockers were more commonly used after transplantation. Amlodipine does not interact with the CYP3A enzyme at therapeutic doses14 and was included in the study population. Our results suggest that co-medication with amlodipine did not affect the C0/D and D/W ratios of Tac in Chinese renal transplant recipients (data not shown).
Tac is metabolized by the CYP3A enzyme, but it can also be influenced by the patient's physical condition after transplantation. Thus, only patients with a similar postoperative period were selected. In our study, patients at a stable stage (between 1–2 years after transplantation) were enrolled. As a result, only stable doses were investigated, and further studies should focus on the prediction of initial dosage requirements in the early post-operative period.
In summary, we developed equations that describe the association between the endogenous CYP3A4 phenotype and Tac metabolism and dosage requirements. These equations take into account the CYP3A5*3 genotype and other potential variables. Within CYP3A5*3/*3 genotypes, 65.3% and 61.2% of the inter-individual variability in Tac metabolism and dosage requirements were explained by the endogenous CYP3A4 phenotype and other non-genetic variables. These findings provide a potential basis for estimating a Tac dosing regimen using a non-invasive CYP3A4 probe, rather that the administration of probe drugs or serial blood sampling. Furthermore, our predictive algorithm based on phenotype and genotype can be used prior to treatment.
Author contribution
Xi LUO and Ze-neng CHENG designed the research; Xi LUO, Li-jun ZHU and Li-yun ZHENG performed the research; Li-jun ZHU and Ze-neng CHENG contributed analytic tools; Xi LUO, Ning-fang CAI and Li-yun ZHENG analyzed the data; and Xi LUO and Ze-neng CHENG wrote the paper.
Acknowledgments
The research was funded by the National Natural Science Foundation of China (No 81072700/H3110). The project was funded by the China Postdoctoral Science Foundation (No 2014M562140), Research Fund for Young Scholars of Fujian Province Health and Family Planning Commission (No 2014-1-79) and Scientific Research Foundation for Postdoctoral of Central South University (No 31000-160320068). We gratefully thank Wen-zhao XIE and Ya GONG for gathering the biological samples. We also thank Qing LIU for her kind help with the data analysis.
Footnotes
Supplementary Table S1 is available at the Acta Pharmacologica Sinica's website.
Supplementary Information
The C0/D and D/W ratios of Tac across the three CYP3A5*3 genotypes in Chinese renal transplant recipients.
References
- Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther 2006; 112: 184–98. [DOI] [PubMed] [Google Scholar]
- Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev 1997; 27: 201–14. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang S, Poloyac S, Strom S, Venkataramanan R. Determination of 13-O-demethyl tacrolimus in human liver microsomal incubates using liquid chromatography–mass spectrometric assay (LC–MS). J Chromatogr B Analyt Technol Biomed Life Sci 2005; 821: 31–7. [DOI] [PubMed] [Google Scholar]
- Oleson L, von Moltke LL, Greenblatt DJ, Court MH. Identification of polymorphisms in the 3′-untranslated region of the human pregnane X receptor (PXR) gene associated with variability in cytochrome P450 3A (CYP3A) metabolism. Xenobiotica 2010; 40: 146–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001; 27: 383–91. [DOI] [PubMed] [Google Scholar]
- de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance inrenal transplant patients. Clin Pharmacol Ther 2012; 92: 366–75. [DOI] [PubMed] [Google Scholar]
- Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 2001; 69: 169–74. [DOI] [PubMed] [Google Scholar]
- Galteau MM, Shamsa F. Urinary 6β-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur J Clin Pharmacol 2003; 59: 713–33. [DOI] [PubMed] [Google Scholar]
- Peng CC, Templeton I, Thummel KE, Davis C, Kunze KL, Isoherranen N. Evaluation of 6β-hydroxycortisol, 6β-hydroxycortisone and their combination as endogenous probes for inhibition of CYP3A4 in vivo. Clin Pharmacol Ther 2011; 89: 888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Li XM, Hu ZY, Cheng ZN. Evaluation of CYP3A activity in humans using three different parameters based on endogenous cortisol metabolism. Acta Pharmacol Sin 2009; 30: 1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Luo X, Zhu L, Xie W, Liu S, Cheng Z. Simultaneous determination of cortisol, cortisone, 6β-hydroxycortisol and 6β-hydroxycortisone by HPLC. J Chromatogr Sci 2015; 53: 451–5. [DOI] [PubMed] [Google Scholar]
- Katz DA, Grimm DR, Cassar SC, Gentile MC, Ye X, Rieser MJ, et al. CYP3A5 genotype has a dose-dependent effect on ABT-773 plasma levels. Clin Pharmacol Ther 2004; 75: 516–28. [DOI] [PubMed] [Google Scholar]
- Renders L, Frisman M, Ufer M, Mosyagin I, Haenisch S, Ott U, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther 2007; 81: 228–34. [DOI] [PubMed] [Google Scholar]
- Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant 2003; 18: 2409–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The C0/D and D/W ratios of Tac across the three CYP3A5*3 genotypes in Chinese renal transplant recipients.