Abstract
Epithelial cells are important for organ development and function. To this end, they polarize their plasma membrane into biochemically and physically distinct membrane domains. The apical membrane faces the luminal site of an organ and the basolateral domain is in contact with the basement membrane and neighboring cells. To establish and maintain this polarity it is important that newly synthesized and endocytic cargos are correctly sorted according to their final destinations at either membrane. Sorting takes place at one of 2 major sorting stations in the cells, the trans-Golgi network (TGN) and recycling endosomes (REs). Polarized sorting may involve epithelial cell-specific sorting adaptors like the AP-1B clathrin adaptor complex. AP-1B facilitates basolateral sorting from REs. This review will discuss various aspects of basolateral sorting in epithelial cells with a special emphasis on AP-1B.
Keywords: recylcing endosomes, AP-1B, epithelial cells, cell polarity, basolateral membrane
Abbreviations
- AEEs
apical EEs
- BEEs
basolateral EEs
- EEs
early endosomes
- REs
recycling endosomes.
Introduction
The plasma membrane of eukaryotic cells is in constant exchange with intracellular compartments. During biosynthesis, transmembrane proteins are inserted into ER membranes. From the ER cargos may travel into and through the Golgi apparatus to reach the TGN. Once arrived at the TGN, transmembrane proteins become segregated and packaged into distinct vesicular carriers according to their final destinations: the endosomal/lysosomal system or the apical and basolateral plasma membrane domains. Sorting depends on specific sorting signals encoded in the transmembrane proteins and sorting machineries that recognize these signals. While apical sorting information is often complex and encoded in a protein's ectodomain, basolateral sorting signals are typically short peptide motifs that are recognized by cytosolic adaptor complexes.1,2 Frequently, basolateral sorting signals resemble lysosomal sorting signals and endocytic motifs (but see later sections for a more thorough discussion of basolateral sorting signals and cytosolic adaptors).
It should be noted that many transmembrane proteins cycle between different membrane compartments. For example, mannose 6-phosphate receptors (MPRs) collect lysosomal hydrolases in the Golgi/TGN and deliver them into late endosomes where the hydrolases are released. After release of the hydrolases, MPRs recycle back to the TGN (Fig. 1, routes 1, 5, and 6). In addition, nutrient and signaling receptors localized at the plasma membrane are internalized after binding their ligands and either routed into lysosomes for degradation or recycled back to the plasma membrane from early endosomes (EEs) or REs (Fig. 1, routes 8a, 8b, 9a, 9b, 4a, and 4b).
Figure 1.
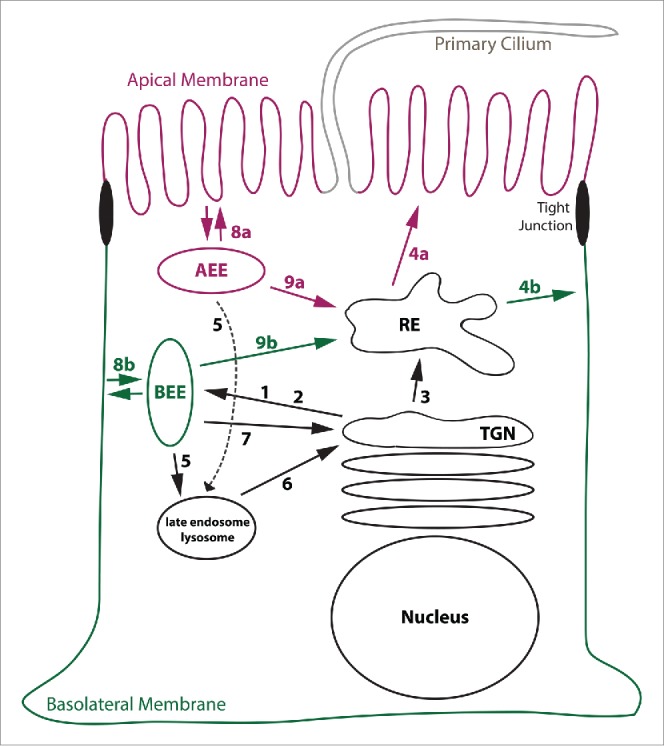
Model illustrating various membrane trafficking routes. This Figure explains basic membrane trafficking routes. (1) TGN exit mediated by AP-1A. (2) TGN exit mediated by AP-4. (3) TGN exit toward REs, regulated by Rab13. (4a) Apical sorting from REs. (4b) AP-1B-mediated sorting to the basolateral membrane. (5) Sorting into late endosomes/lysosomes. (6) Retrieval from late endosomes/lysosomes to the TGN. (7) Retrieval from BEEs to the TGN. (8a) Apical endocytosis and fast recycling from AEEs. (8b) Basolateral endocytosis and fast recycling from BEEs. (9a and 9b) Delivery of internalized cargos to REs. See main text for more details. Note that pathways leading from REs toward the TGN, AEEs, BEEs, and late endosomes are not shown.
EEs are typically the first endocytic compartment reached by internalized transmembrane proteins. Because cargos may be directly recycled back to the plasma membrane, retrieved to the TGN, delivered into REs, or send into lysosomes for degradation, EEs are often referred to as sorting endosomes.3 Notably, polarized epithelial cells maintain biochemically distinct apical EEs (AEEs) that underlie the apical membrane and basolateral EEs (BEEs) that are positioned underneath the basolateral membrane.4,5 That way, internalized cargos are sent back to their membrane of origin during rapid recycling from EEs (i.e. the apical membrane from AEEs and the basolateral membrane from BEEs, Figure 1, routes 8a and 8b).
Unlike EEs that are positioned in proximity to the plasma membrane, REs are found in the perinuclear region in close proximity to the TGN. They receive internalized cargos from both AEEs and BEEs (Fig. 1, routes 9a and 9b). In addition, newly synthesized cargos such as VSVG may move from the TGN into REs during biosynthetic delivery 6,7 (Fig. 1, route 3). REs contain domains from which cargo is selected for targeting to the apical membrane and regions that facilitate sorting of cargos toward the basolateral membrane 8 (Fig. 1, routes 4a and 4b). These domains may be referred to as apical REs and common REs, respectively.9 Sorting to the basolateral membrane from REs often involves AP-1B (Fig. 1, route 4b). Intriguingly, AP-1B expression in epithelial cells results in the creation of a specialized basolateral sorting domain as will be discussed in later sections.
Basolateral Sorting Signals and Their Recognition by Cytosolic Adaptor Complexes
Basolateral sorting signals are typically small peptide motifs encoded in the cytoplasmic tail of transmembrane proteins. Although non-canonical signals exist, basolateral sorting signals frequently conform to 2 types of sorting motifs: tyrosine-based motifs that conform to YxxØ (in which Ø is a bulky hydrophobic amino acid F, I, L, M, or V) or FxNPxY signals, and dileucine motifs most often in the form of [DE]xxxL[LI] sequences. These signals are frequently recognized by heterotetrameric adaptor protein (AP) complexes AP-1 (γ, β1, µ1, σ1), AP-2 (α, β2, µ2, σ2), AP-3 (δ, β3, µ3, σ3), and AP-4 (ε, β4, µ4, σ4).10-12 Each complex consists of 2 large subunits (γ, α, δ, ε, and β1 − β4), a medium subunit (µ1 – µ4), and a small subunit (σ1 – σ4). AP-1, AP-3, and AP-4 all localize at the TGN or endosomes to facilitate protein sorting within the endosomal system.13 In contrast, AP-2 localizes at the plasma membrane to facilitate endocytosis.10 Whereas the scaffolding proteins that facilitate AP-3 and AP-4 vesicle formation remain elusive, AP-1 and AP-2 complexes interact with clathrin and incorporate their cargos into clathrin-coated vesicles.10,13
YxxØ signals are recognized by the adaptor complex medium subunits.14,15 In contrast, [DE]xxxL[LI] signals are recognized by the σ1/γ, σ2/α, and σ3/δ subunits of AP-1, AP-2, and AP-3, respectively.16-19 Finally, FxNPxY signals are recognized by co-adaptors dab2, numb and autosomal recessive hypercholesterolemia (ARH) protein.20 Both dab2 and numb directly interact with the α subunit of AP-2.20 In contrast, ARH interacts with the β1 and β2 subunits and thus facilitates incorporation of proteins with FxNPxY signals into both AP-1B and AP-2 clathrin-coated vesicles.21,22 So far, only AP-1 and AP-4 complexes were found to support trafficking to the basolateral membrane.23-25 Whereas the role of AP-4 in basolateral sorting is less well studied, the role of AP-1 complexes in basolateral sorting is much clearer. It should be noted that AP-4 recognizes a specialized YxxØ motif (Yx[FYL][FL]E) in a binding site that is non-overlapping with the canonical YxxØ-binding site in µ4.26 Indeed, AP-4 was reported to interact with the YKFFE signal in the amyloid precursor protein (APP), and disruption of this interaction led to a decrease of APP localization in endosomes.26 Interestingly, APP was found to localize to the basolateral membrane in Madin-Darby Canine Kidney (MDCK) cells,27,28 indicating a putative role for AP-4 in basolateral sorting of APP (Fig. 1, routes 2 and 8b). Curiously, the distal targeting determinant of low-density lipoprotein receptor (LDLR), GYSY, is proceeded by an aspartate residue such that this signal resembles an AP-4 signal when read in reverse (YSYGD). Indeed, LDLR was one of the few receptors that have been reported to interact with and rely to some extent on AP-4 for basolateral targeting.25
Epithelial cells express 2 distinct AP-1 complexes, the ubiquitously expressed AP-1A and the tissue specific AP-1B.22 Both complexes share their 2 large γ and β1 subunits as well as the small σ1 subunit (σ1A, σ1B or σ1C), but differ in the incorporation of their respective medium subunits µ1A or µ1B,23 which are responsible for the different functions of AP-1A and AP-1B in epithelial cells. Although AP-1B can substitute for some of AP-1A's functions in mouse embryonic µ1A-/- fibroblasts,29 there are many examples that highlight different functionality of AP-1A and AP-1B in polarized epithelial cells. For example, basolateral sorting from REs in controlled by AP-1B30-32 (Fig. 1, route 4b), whereas furin retrieval from EEs to the TGN strictly depends on AP-1A 33,34 (Fig. 1, route 7). In addition, AP-1A sorts cargos destined for the basolateral membrane at the TGN24 (Fig. 1, routes 1 and 8b). These functions are in agreement with reports proposing localization of AP-1A at the TGN and in EEs and localization of AP-1B in REs,24,30,34 although conflicting data exist (see later sections for further discussion of localization). While AP-1B is so far the only AP complex that sorts proteins from REs to the basolateral membrane, AP-4 may also sort basolateral cargos at the TGN in addition to AP-1A. Because AP-4 and AP-1A sort proteins into the endosomal/lysosomal system it is conceivable that basolateral sorting at the TGN may involve traffic through BEEs. From BEEs cargos may cycle by default through the basolateral membrane (Fig. 1, routes 1 and 2 followed by 8b). Indeed, the lysosomal-associated membrane protein 1 (Lamp1) as well as the cation-dependent mannose 6-phosphate receptor (CD-MPR) were found exclusively at the basolateral membrane in polarized epithelial cells albeit in low amounts.35-37
Although the analysis of cargo trafficking has greatly benefited from introducing small epitope tags into the proteins of interest, it should be noted that some common tags contain basolateral sorting information (Table 1). For example, the c-myc tag contains a bona fide LIxxxD signal that binds to the dileucine binding pocket of σ2,38 while the VSVG tag contains the known basolateral sorting signal YTDI of the VSVG protein.39 Furthermore, the HA tag contains 3 tyrosine residues that may conform to a weak YxxØ signal, especially when used as a triple tag. Thus great care should be used when introducing epitope tags to the N- and C-termini of proteins to avoid non-physiological interactions of the tagged protein with cytosolic adaptor complexes.
Table 1.
Commonly used small protein tags
| c-myc* | EQKLISEEDL |
| HA | YPYDVPDYA |
| V5 | GKPIPNPLLGLDST |
| T7 | MASMTGGQQMG |
| Flag | DYKDDDDK |
| VSVG* | YTDIEMNRLGK |
| HSV | QPELAPEDPEDC |
Denotes tags for which direct interaction with APs were shown (c-myc) or that contain known sorting information (VSVG) as highlighted in red. Other tyrosine residues are also highlighted in red. See text for details.
Subcellular Localization of AP-1B: Dependence on PtdIns(3,4,5)P3 in REs
Although membrane recruitment of AP-1B onto REs is not yet understood on a structural level, we can make predictions based on crystallography studies performed with AP-1A and AP-2 complexes in combination with cell biological studies performed with AP-1B. It is generally assumed that recruitment of adaptor complexes to membranes is cooperative involving multiple binding events between the adaptor complex, cargo proteins and either small GTPases of the Arf family or specific lipids. Activation of AP complexes is believed to involve a conformational change such that the C-terminus of the µ subunit rotates away from the complex enabling it to bind to sorting signals.40-42 Indeed, recent in depth studies with AP-1A confirmed that cargo binding was activated by binding of AP-1A to GTP-loaded Arf1.43 Stable membrane association of AP-1A further depended on PtdIns(4)P at the TGN,44 and γ-adaptin was shown to bind to PtdIns(4)P.45 In contrast, it is well established that membrane recruitment of AP-2 strictly depends on PtdIns(4,5)P2, but is independent of direct binding to Arf family members.46 Structural studies revealed multiple PtdIns(4,5)P2 binding sites in AP-2.40 It is suspected that the strong binding to PtdIns(4,5)P2 is sufficient to selectively and robustly recruit AP-2 to the plasma membrane.40,42 Importantly, µ2 has a PtdIns(4,5)P2-binding site in its C-terminus that becomes exposed after activation of the complex and contributes to membrane binding.40,47 This lipid binding site is not conserved in µ1A.
Membrane recruitment of AP-1B seems to rely on both binding to Arf6 and PtdIns(3,4,5)P3. Cell biological studies showed that depletion of Arf6 with shRNA selectively inhibited sorting of AP-1B-dependent cargos to the basolateral membrane.48 Furthermore, basolateral sorting along the AP-1B pathway was perturbed by the expression of dominant-negative and dominant-active Arf6 mutant proteins Arf6D125N and Arf6Q67L, respectively. In contrast, Arf1 mutant proteins had no selective dominant negative effect on AP-1B-dependent sorting. Because the switch I and switch II domains of Arf1 and Arf6 present almost identical surfaces in the GTP-loaded forms,49 and because it was shown that Arf1 binds to the γ and β1 subunits of AP-1A,43 it seems fair to speculate that Arf6 binding may involve similar surfaces in AP-1B. In addition, AP-1B also depends on a signature lipid for membrane recruitment. We found that the presence of PtdIns(3,4,5)P3 in REs is necessary for AP-1B recruitment. Specific dephosphorylation of PtdIns(3,4,5)P3 to PtdIns(4,5)P2 by overexpressed PTEN led to a loss of AP-1B in REs and missorting of AP-1B-dependent cargos to the apical domain.47 Because membrane recruitment onto REs was also inhibited when a µ1B mutant, µ1Bloca, was incorporated into AP-1B, specific binding of AP-1B to PtdIns(3,4,5)P3 is most likely mediated through its µ1B subunit. To create µ1Bloca, residues R338, N339 and V340 were changed to the corresponding NSE residues in µ1A (Table 2), Interestingly, mutations made in µ1Bloca are located in an area that corresponds to the PtdIns(4,5)P2-binding area in µ2.40,47 Thus, putative lipid binding by µ1B may be analogous to lipid binding by µ2. In summary, membrane recruitment of AP-1B may start with binding to Arf6 followed by lipid and cargo binding leading to stable recruitment of AP-1B (Fig. 2). Regardless of the exact molecular mechanisms that lead to AP-1B recruitment, the reliance of AP-1B on PtdIns(3,4,5)P3 is probably the reason for its selective recruitment to REs. It is assumed that the putative PtdIns(3,4,5)P3 binding by µ1B overrides the PtdIns(4)P binding by γ-adaptin. Alternatively, PtdIns(4)P binding by γ-adaptin may help recruit AP-1B onto REs membranes assuming that REs contain PtdIns(4)P in addition to PtdIns(3,4,5)P3. Therefore, although highly similar, membrane recruitment of AP-1A and AP-1B is regulated in slightly different manners.
Table 2.
Alignment of “loca” region in µ1 genes from various species (corresponding to residues 333 – 346 in µ1B)
| µ1 homolog | “loca” sequence | Net charge |
|---|---|---|
| µ1A H. sapiens | KWVPENSEIVWSIK | 0 |
| µ1B H. sapiens | KYVPERNVVIWSIK | +2 |
| µ1A D. rerio | KWVPENSEIVWSIK | 0 |
| µ1B D. rerio | KYVPEKNLVVWSIK | +2 |
| µ1C D. rerio | KWVPEKSAVEWNIK | +1 |
| Unc-101 C. elegans | KYTPEQSAFVWTIK | +1 |
| Apm-1 C. elegans | KYVPELNAIVWSIR | +1 |
| µ1 D. melanogaster | KYAPEQNAIIWTIK | +1 |
| APM1 S. cerevisiae | KYVPEKSAILWKIR | +3 |
Basic residues are shown in blue, acidic residues are shown in red, and underlined residues correspond to those that were mutated in µ1Bloca.
Figure 2.
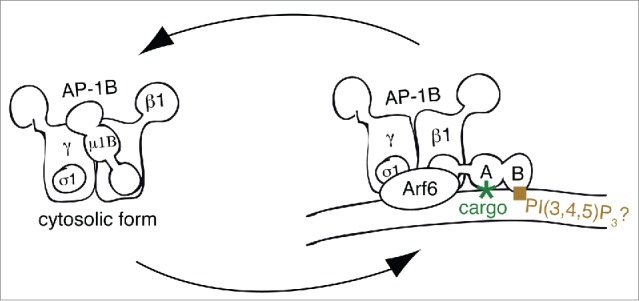
Model for Membrane recruitment of AP-1B. This figure represents a schematic drawing of the putative membrane recruitment of AP-1B indicating the rotation of the µ1B subunit upon membrane recruitment. Note that the C-terminus of µ subunits is organized in subdomains A and B as illustrated in the membrane-bound form. Whereas subdomain A binds to YxxØ sorting signals and thus cargo proteins, subdomain B probably binds to PtdIns(3,4,5)P3. Finally, Arf6 most likely binds to both γ and β1 adaptin. Abbreviations PI(3,4,5)P3, PtdIns(3,4,5)P3
Although many research papers described differential functions and localizations of AP-1A and AP-1B, a recent publication challenged that dogma. In that study, mouse µ1B or µ1A with triple c-myc or triple HA-tags at their C-termini were stably expressed in MDCK cells resulting in colocalization of both complexes mostly at the TGN and to a lower degree in REs.50 The same colocalization was observed between AP-1B and AP-1A when µ1B was tagged at its C-terminus with mCherry and µ1A was tagged with GFP.50 In contrast, colocalization of AP-1B and AP-1A was not observed with internally tagged constructs.24,30 Perhaps the tagging of µ1B at its C-terminus sterically interferes with its putative binding to PtdIns(3,4,5)P3. In addition, the negatively charged c-myc tag might repel AP-1B from PtdIns(3,4,5)P3-positive REs, and furthermore AP-1A and AP-1B might bind to each other through recognition of sorting signals in the C-terminal HA and c-myc tags (see Table 1). These scenarios might result in displacement of AP-1B from REs and localization of AP-1B at the TGN. Indeed, AP-1B localization in that study was observed to be dependent on Arf1,50 which is in contrast to the functional studies done in MDCK cells that implicated Arf6 in the AP-1B pathway as discussed above.48
Although the strong colocalization of AP-1A and AP-1B observed by Guo et al. might be artificial, the question if AP-1B can localize to the TGN and AP-1A in REs remains valid. Indeed, in mouse embryonic fibroblasts in the absence of AP-1A, AP-1B seems to operate and localize at the TGN,34,51 and AP-1B function at the TGN was suggested in MDCK cells that were depleted of AP-1A.24 Moreover, AP-1A has been reported to localize in REs in HeLa cells that do not express AP-1B, but have some accumulation of PtdIns(3,4,5)P3 in REs nonetheless.47,52 Thus, although AP-1B may have a lesser affinity to the TGN as opposed to REs and AP-1A may have a higher affinity to the TGN than REs, they may be able to be recruited to the non-favorable location if the competing complex is missing.
During recruitment, AP-1B may directly interact with its cargo proteins containing YxxØ or LL-based sorting signals, while ARH may select cargos with FxNPxY sorting motifs for incorporation into nascent AP-1B vesicles. Note that ARH selectively cooperates with AP-1B in REs, but not AP-1A at the TGN.22 Cargos found associated with isolated AP-1B vesicles included TfnR and CI-MPR, and LDLR was found cross-linked to AP-1B in Lilly Laboratories cell porcine kidney (LLC-PK1) cells that expressed exogenous copies of µ1B (= LLC-PK1::µ1B cells).30,34 Vesicle formation is most likely concluded with the recruitment of clathrin.30,53 Furthermore, members of the targeting and fusion machinery such as the v-SNARE cellubrevin and the exocyst subunits Sec8 and Exo70 are included into AP-1B vesicles.30,54 Transport of AP-1B vesicles to the basolateral membrane may at least partially depend on myosin VI,55 and correct fusion at the basolateral membrane depends on the exocyst complex and the t-SNARE syntaxin 4.54,56 Notably, syntaxin 4 itself depends on AP-1B expression for basolateral targeting.57 The putative factors involved in AP-1B vesicle formation are illustrated in Figure 3.
Figure 3.
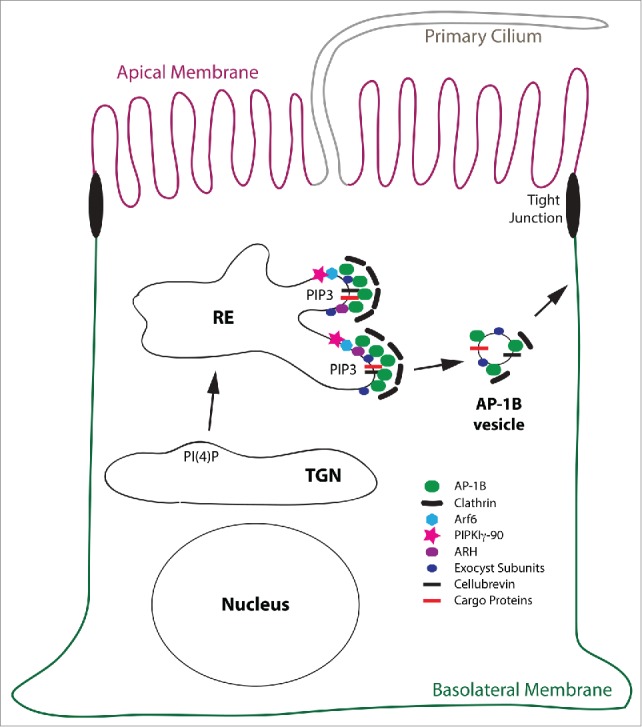
Model for the AP-1B domain in REs. This is a schematic depiction of the AP-1B domain in a RE leading to the generation of AP-1B vesicles that are targeted to the basolateral membrane. Proteins depicted are AP-1B, clathrin, Arf6, PIPKIγ-90, ARH, exocyst subunits, cellubrevin and cargo proteins. Note, that cellubrevin, exocyst subunits, and clathrin are confirmed components of AP-1B vesicles.30,54 For simplicity reasons, the early secretory pathway including the Golgi apparatus, AEEs, BEEs, and other endocytic organelles as well as endocytic trafficking are not depicted. Abbreviation: PIP3, PtdIns(3,4,5)P3.
Fusion at the basolateral membrane may be regulated by RalA. In depth structural analysis showed that GTP-loaded RalA bound to the exocyst subunit Sec5.58,59 The binding surface for Sec5 on RalA was further shown to partially overlap with the RalA binding site for the exocyst subunit Exo84. Moreover, Sec5 and Exo84 competitively bound to RalA suggesting that RalA might regulate the assembly of the exocyst complex and therefore basolateral secretion.60-62 Indeed, depletion of RalA in Caco2 cells resulted in apical missorting of an AP-1B-dependent cargo, the epidermal growth factor receptor EGFR.61,63
Regulation of AP-1B Function and The Creation of An AP-1B-Positive Domain in REs
We identified PtdIns(3,4,5)P3 as a signature lipid of REs in AP-1B-expressing epithelial cells.47 Depletion of µ1B in MDCK cells resulted in a loss of PtdIns(3,4,5)P3 in REs, and expression of µ1B in LLC-PK1 cells led to PtdIns(3,4,5)P3 accumulation in REs.47 These data indicated that AP-1B triggers the accumulation of PtdIns(3,4,5)P3. Although the mechanisms are not understood as yet, the interaction between AP-1B and the PtdIns(4)P 5-kinase PtdInsPKIγ-90 may be involved in generating the PtdIns(3,4,5)P3 precursor PtdIns(4,5)P2 from PtdIns(4)P.64 PtdIns(4)P may be delivered to REs with vesicles originating at the TGN.7 Notably, Arf6 also recruits and activates PtdInsPKIγ-90 providing additional hints that PtdInsPKIγ-90 may play a role in PtdIns(3,4,5)P3 formation in REs.46 Indeed, overexpression of mutant Arf6 in MDCK cells led to a mild albeit significant reduction in PtdIns(3,4,5)P3 levels in REs.48 Phosphorylation at the 3 positions might then be performed by PtdIns 3-kinases perhaps recruited by active Cdc42.65 Cdc42 is known to control basolateral sorting of AP-1B cargos VSVG and LDLR.66-68 In addition, Cdc42 controls basolateral sorting of an AP-1B-independent cargo, the β subunit of the Na/K-ATPase gp58.23,67-69 However, basolateral secretion of soluble proteins or the delivery of proteins to the apical membrane is not controlled by Cdc42.68 It was suggested that mutant forms of Cdc42 might inhibit the exit of basolateral proteins from the TGN while stimulating the exit of apically targeted proteins.70 Thus, Cdc42 may control polarized secretion through multiple mechanisms and its putative involvement in PtdIns(3,4,5)P3 formation may be only one aspect. Regardless, because AP-1B depends on PtdIns(3,4,5)P3 for membrane recruitment, the fact that AP-1B triggers the formation of this lipid most likely establishes a positive feedback loop. This positive feedback loop probably also includes activation of Arf6 through GEFs such as Grp1 that bind to PtdIns(3,4,5)P3-positive membranes.71,72
The creation of a PtdIns(3,4,5)P3-positive AP-1B domain in REs changes their biochemical properties. This is probably part of the reason why some proteins that are otherwise known for their role in endocytosis, are now found to colocalize with AP-1B and to control its function. Prime examples for this are Arf6 and ARH that colocalize with AP-1B in REs.22,48 It is suspected that both Arf6 and ARH specifically cooperate with AP-1B because they are not found at the TGN and thus unavailable for cooperation with AP-1A. In addition to Arf6 and ARH, we know of at least 2 other factors that specifically cooperate with AP-1B namely PtdInsPKIγ-90 and the exocyst complex (Fig. 3). PtdInsPKIγ-90 was shown to bind to AP-1B but not AP-1A in MDCK cells.64 This interaction was necessary for basolateral targeting of E-cadherin.64 Moreover, the exocyst is recruited onto REs only when AP-1B is expressed, but not in its absence,30 and future work is needed to determine why that is the case.
Many small GTPases of the Rab family have been described to regulate the AP-1B pathway at various steps. Among these are the RE-localized Rab8 and Rab10,66,73 and the TGN and RE localized Rab13.74 Rab13 was shown to affect all cargos that traffic from the TGN into REs during biosynthetic delivery to the apical and basolateral plasma membrane.74 Thus Rab13 functions in cargo delivery to REs. In contrast, Rab8 and Rab10 may negatively control AP-1B function. We showed that the dominant negative allele of Rab8, Rab8T22N, had no effect on basolateral sorting.66 However, the activated mutant Rab8Q67L led to dramatic missorting of the AP-1B cargos VSVG and LDLR along the biosynthetic pathway.66 However, endocytic recycling of TfnR was not affected by Rab8Q67L expression.75 Notably, expression of Rab8Q67L in LLC-PK1::µ1B cells led to a dispersal of AP-1 staining in the perinuclear region that was not observed in the control LLC-PK1::µ1A cells.66 Like the results with Rab8T22N, knock out of both Rab8a and Rab8b in mice as well as depletion of Rab8 in MDCK cells had no discernable effect on basolateral sorting.73,76,77 Thus, Rab8 activity is not needed for basolateral sorting by AP-1B, whereas hyperactivation of Rab8 leads to apical missorting of AP-1B cargos. Therefore, Rab8 seems to negatively regulate AP-1B. The same may be true for Rab10. Whereas Rab10Q68L overexpression led to apical missorting of VSVG, Rab10T23N had no discernible effect on VSVG trafficking.73 Furthermore, Rab10 depletion in MDCK cells hardly affected basolateral trafficking of VSVG; however, simultaneous depletion of Rab8 and Rab10 together had a synergistic effect leading to mild missorting of VSVG to the apical membrane.73 Interestingly, the Arf6 GAPs ACAP-1 and ACAP-2 are both effectors of Rab10 and possibly Rab8,78 suggesting that perhaps activated Rab8 and Rab10 may lead to inactivation of Arf6 and thus impairment of the AP-1B pathway. However, more work is needed to validate this putative scenario.
Expression Pattern of AP-1B
AP-1B expression was mainly found in columnar epithelial cells. Examples of tissues with AP-1B expression include but are not limited to the placenta, kidney, lung, colon, stomach, ovaries, testes, and various glands.79 Thus it appears that AP-1B is an adaptor complex expressed in cells and tissues that depend on polarized membrane trafficking. However, there are notable exceptions. For example, although neurons in the brain and hepatocytes in the liver are highly polarized cells, they lack AP-1B expression.79 Furthermore, AP-1B expression is absent in columnar retinal pigment epithelial (RPE) and LLC-PK1 cell lines.79,80 LLC-PK1 cells most likely derived from the proximal kidney tubules that naturally lack AP-1B expression.81 Nevertheless, mainly expressed in polarized tissues, AP-1B seems to help with establishing or maintaining cell polarity. Indeed, LLC-PK1 cells pile up on each other when grown in 2D culture.23 Interestingly, exogenous expression of AP-1B restored monolayer growth in addition to basolateral sorting from recycling endosomes.23 Importantly, histological studies suggested that more than 80% of kidney cancers arise from the proximal kidney tubules,82-84 and AP-1B expression is down regulated in mouse models for colon cancer.85 Furthermore, AP-1B was also found to be reduced in the colon of Crohn's disease patients,86 and µ1B knock out mice suffer from proliferation defects and hyperplasia in their intestine.87 These observations highlight the importance of AP-1B in epithelial cell maintenance.
AP-1B is expressed in vertebrate species, but absent in invertebrates. Whereas flies express only one µ1 chain, worms express 2 different µ1 chains (unc-101 and apm-1); however, both lack clear homology to µ1B.88,89 Thus, though invertebrate models are invaluable for many applications, they cannot serve as models for AP-1B function. In addition, another favorite model organism, zebrafish, expresses 3 µ1 chains, and although zebrafish µ1A and µ1B have clear homologies to µ1A and µ1B of higher vertebrates, their expression profile differs.90,91 For example, whereas µ1B expression is missing in the liver of mice, zebrafish expresses µ1B in its liver. Furthermore, unlike in mice, µ1A is not ubiquitously expressed in zebrafish and missing in its pancreas, liver, and kidneys.91 This fact together with the expression of the unique µ1C makes zebrafish a less ideal model organism to study µ1B function.
Interestingly, comparison of the localization patch in human µ1A and µ1B and invertebrate µ1s reveals an intriguing observation: Both µ1 isoforms in C. elegans and the one µ1 variant in Drosophila have a net charge of +1, placing them in between µ1A (net charge 0) and µ1B (net charge +2, Table 2). Because changing the net charge in µ1B from +2 to 0 in the µ1Bloca mutant resulted in displacement of the corresponding AP-1Bloca complex from REs,47 it is assumed that the positive net charge confers the ability to bind to PtdIns(3,4,5)P3 in REs. Does this mean invertebrate µ1s with a net charge of +1 may function at the TGN and in REs, perhaps making the need for an AP-1B complex obsolete? Indeed, Drosophila AP-1 has been shown to localize and function at both TGN and REs.92 Moreover, AP-1 is essential for the generation of secretory granules in Drosophila,93 and basolateral targeting of DISCS large depends on AP-1 expression in Drosophila salivary glands.94 Thus, AP-1 in Drosophila seems to be important for the basolateral localization of a subset of proteins reminiscent of AP-1A and AP-1B function in mammalian cells.92,94 Of interest, whereas zebrafish µ1A and µ1B are well conserved especially with regard to the net charge of the loca patch, zebrafish µ1C's net charge is +1 like the invertebrate µ1. Thus zebrafish µ1C may localize to both TGN and REs as well.
Importantly, because PtdIns(3,4,5)P3 accumulation in REs as described above is dependent on AP-1B expression, it is assumed that REs in invertebrates may not accumulate PtdIns(3,4,5)P3, just like fibroblasts or epithelial cells lacking AP-1B.47 Thus, the AP-1B domain in REs may be specific to vertebrate epithelial cells and not translatable as such into invertebrate systems. Indeed, REs in C. elegans seem to be enriched in PtdIns(4,5)P2 and not PtdIns(3,4,5)P3,78 perhaps correlating with the net charge of the lipid binding patch in their µ1s.
Future Questions
Since its discovery in 1999, AP-1B has proven itself as an important player in epithelial cell polarity. Although its role in basolateral sorting is undisputed and well documented, we are only beginning to understand how AP-1B is recruited onto REs and how vesicle formation is regulated. Some of the players cooperating with AP-1B such as clathrin, Arf6, ARH, cellubrevin, the exocyst complex subunits Sec8 and Exo70, and PtdInsPKIγ-90 are already known, but other factors probably remain to be discovered. It will be interesting to learn how the interacting proteins cooperate with AP-1B in space and time. This should lead to a detailed mechanism of AP-1B function and thus deeper understanding of how the absence of AP-1B or its deregulation may contribute to diseases such as metastatic cancer and Crohn's disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in the Fölsch laboratory was funded by a grant from the National Institutes of Health (GM070736).
References
- 1.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 2009; 122:4253-66; PMID:19923269; http://dx.doi.org/ 10.1242/jcs.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fölsch H. Regulation of membrane trafficking in polarized epithelial cells. Curr Opin Cell Biol 2008; 20:208-13; PMID:18282697; http://dx.doi.org/ 10.1016/j.ceb.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev 2005; 6:233-47; PMID:15738988; http://dx.doi.org/ 10.1038/nrm1593 [DOI] [PubMed] [Google Scholar]
- 4.Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 1990; 62:719-31; PMID:2143699; http://dx.doi.org/ 10.1016/0092-8674(90)90117-W [DOI] [PubMed] [Google Scholar]
- 5.Sheff DR, Kroschewski R, Mellman I. Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol Biol Cell 2002; 13:262-75; PMID:11809838; http://dx.doi.org/ 10.1091/mbc.01-07-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 2004; 167:531-43; PMID:15534004; http://dx.doi.org/ 10.1083/jcb.200408165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fölsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic (Copenhagen, Denmark) 2009; 10:972-81; PMID:19453969; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang SF, Fölsch H. The role of secretory and endocytic pathways in the maintenance of cell polarity. Essays Biochem 2012; 53:29-39; PMID:22928506; http://dx.doi.org/ 10.1042/bse0530029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol 2003; 5:287-93; PMID:12669082; http://dx.doi.org/ 10.1038/ncb0403-287 [DOI] [PubMed] [Google Scholar]
- 10.Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell 2001; 12:2907-20; PMID:11598180; http://dx.doi.org/ 10.1091/mbc.12.10.2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta 1998; 1404:173-93; PMID:9714795; http://dx.doi.org/ 10.1016/S0167-4889(98)00056-1 [DOI] [PubMed] [Google Scholar]
- 12.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol 2001; 17:517-68; PMID:11687498; http://dx.doi.org/ 10.1146/annurev.cellbio.17.1.517 [DOI] [PubMed] [Google Scholar]
- 13.Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 2003; 28:419-29; PMID:14745134; http://dx.doi.org/ 10.1247/csf.28.419 [DOI] [PubMed] [Google Scholar]
- 14.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Sci (New York, N.Y) 1995; 269:1872-5; PMID:7569928; http://dx.doi.org/ 10.1126/science.7569928 [DOI] [PubMed] [Google Scholar]
- 15.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science (New York, N.Y) 1998; 282:1327-32; PMID:9812899; http://dx.doi.org/ 10.1126/science.282.5392.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol 2003; 163:1281-90; PMID:14691137; http://dx.doi.org/ 10.1083/jcb.200307157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 2011; 286:2022-30; PMID:21097499; http://dx.doi.org/ 10.1074/jbc.M110.197178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell 2007; 18:1887-96; PMID:17360967; http://dx.doi.org/ 10.1091/mbc.E07-01-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Fuente-Ortega E, Gravotta D, Perez Bay A, Benedicto I, Carvajal-Gonzalez JM, Lehmann GL, Lagos CF, Rodríguez-Boulan E. Basolateral sorting of chloride channel 2 is mediated by interactions between a dileucine motif and the clathrin adaptor AP-1. Mol Biol Cell 2015; 26:1728-42; PMID:25739457; http://dx.doi.org/ 10.1091/mbc.E15-01-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol 2003; 163:203-8; PMID:14581447; http://dx.doi.org/ 10.1083/jcb.200309175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra SK, Keyel PA, Edeling MA, Dupin AL, Owen DJ, Traub LM. Functional dissection of an AP-2 beta2 appendage-binding sequence within the autosomal recessive hypercholesterolemia protein. J Biol Chem 2005; 280:19270-80; PMID:15728179; http://dx.doi.org/ 10.1074/jbc.M501029200 [DOI] [PubMed] [Google Scholar]
- 22.Kang RS, Fölsch H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J Cell Biol 2011; 193:51-60; PMID:21444685; http://dx.doi.org/ 10.1083/jcb.201012121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 1999; 99:189-98; PMID:10535737; http://dx.doi.org/ 10.1016/S0092-8674(00)81650-5 [DOI] [PubMed] [Google Scholar]
- 24.Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 2012; 22:811-23; PMID:22516199; http://dx.doi.org/ 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol 2002; 4:154-9; PMID:11802162; http://dx.doi.org/ 10.1038/ncb745 [DOI] [PubMed] [Google Scholar]
- 26.Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 2010; 18:425-36; PMID:20230749; http://dx.doi.org/ 10.1016/j.devcel.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haass C, Koo EH, Teplow DB, Selkoe DJ. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci U S A 1994; 91:1564-8; PMID:8108445; http://dx.doi.org/ 10.1073/pnas.91.4.1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Strooper B, Craessaerts K, Dewachter I, Moechars D, Greenberg B, Van Leuven F, Van den Berghe H. Basolateral secretion of amyloid precursor protein in Madin-Darby canine kidney cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimer's disease. J Biol Chem 1995; 270:4058-65; PMID:7876155; http://dx.doi.org/ 10.1074/jbc.270.51.30310 [DOI] [PubMed] [Google Scholar]
- 29.Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. µ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 2000; 19:2193-203; PMID:10811610; http://dx.doi.org/ 10.1093/emboj/19.10.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol 2003; 163:351-62; PMID:14581457; http://dx.doi.org/ 10.1083/jcb.200309020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol 2002; 4:605-9; PMID:12105417 [DOI] [PubMed] [Google Scholar]
- 32.Traub LM, Apodaca G. AP-1B: polarized sorting at the endosome. Nat Cell Biol 2003; 5:1045-7; PMID:14647300; http://dx.doi.org/ 10.1038/ncb1203-1045 [DOI] [PubMed] [Google Scholar]
- 33.Meyer C, Eskelinen EL, Guruprasad MR, von Figura K, Schu P. Mu 1A deficiency induces a profound increase in MPR300/IGF-II receptor internalization rate. J Cell Sci 2001; 114:4469-76; PMID:11792812 [DOI] [PubMed] [Google Scholar]
- 34.Fölsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol 2001; 152:595-606; PMID:11157985; http://dx.doi.org/ 10.1083/jcb.152.3.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Distel B, Bauer U, Le Borgne R, Hoflack B. Basolateral sorting of the cation-dependent mannose 6-phosphate receptor in Madin-Darby canine kidney cells. Identification of a basolateral determinant unrelated to clathrin-coated pit localization signals. J Biol Chem 1998; 273:186-93; PMID:9417063; http://dx.doi.org/ 10.1074/jbc.273.1.186 [DOI] [PubMed] [Google Scholar]
- 36.Nabi IR, Le Bivic A, Fambrough D, Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol 1991; 115:1573-84; PMID:1757463; http://dx.doi.org/ 10.1083/jcb.115.6.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell 1991; 66:907-20; PMID:1909606; http://dx.doi.org/ 10.1016/0092-8674(91)90437-4 [DOI] [PubMed] [Google Scholar]
- 38.Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Höning S, Evans PR, Owen DJ. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 2010; 141:1220-9; PMID:20603002; http://dx.doi.org/ 10.1016/j.cell.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem 1993; 268:3313-20; PMID:8381425 [PubMed] [Google Scholar]
- 40.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell 2002; 109:523-35; PMID:12086608; http://dx.doi.org/ 10.1016/S0092-8674(02)00735-3 [DOI] [PubMed] [Google Scholar]
- 41.Kirchhausen T. Clathrin adaptors really adapt. Cell 2002; 109:413-6; PMID:12086597; http://dx.doi.org/ 10.1016/S0092-8674(02)00751-1 [DOI] [PubMed] [Google Scholar]
- 42.Canagarajah BJ, Ren X, Bonifacino JS, Hurley JH. The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci: Pub Protein Soc 2013; 22:517-29; PMID:23424177; http://dx.doi.org/ 10.1002/pro.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 2013; 152:755-67; PMID:23415225; http://dx.doi.org/ 10.1016/j.cell.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 2003; 114:299-310; PMID:12914695; http://dx.doi.org/ 10.1016/S0092-8674(03)00603-2 [DOI] [PubMed] [Google Scholar]
- 45.Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci U S A 2004; 101:14108-13; PMID:15377783; http://dx.doi.org/ 10.1073/pnas.0406102101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol 2003; 162:113-24; PMID:12847086; http://dx.doi.org/ 10.1083/jcb.200301006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fields IC, King SM, Shteyn E, Kang RS, Fölsch H. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell 2010; 21:95-105; PMID:19864464; http://dx.doi.org/ 10.1091/mbc.E09-01-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shteyn E, Pigati L, Fölsch H. Arf6 regulates AP-1B-dependent sorting in polarized epithelial cells. J Cell Biol 2011; 194:873-87; PMID:21911479; http://dx.doi.org/ 10.1083/jcb.201106010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasqualato S, Menetrey J, Franco M, Cherfils J. The structural GDP/GTP cycle of human Arf6. EMBO Rep 2001; 2:234-8; PMID:11266366; http://dx.doi.org/ 10.1093/embo-reports/kve043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X, Mattera R, Ren X, Chen Y, Retamal C, González A, Bonifacino JS. The adaptor protein-1 mu1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev Cell 2013; 27:353-66; PMID:24229647; http://dx.doi.org/ 10.1016/j.devcel.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eskelinen EL, Meyer C, Ohno H, von Figura K, Schu P. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep 2002; 3:471-7; PMID:11964383; http://dx.doi.org/ 10.1093/embo-reports/kvf092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiba Y, Takatsu H, Shin HW, Nakayama K. Gamma-adaptin interacts directly with Rabaptin-5 through its ear domain. J Biochem 2002; 131:327-36; PMID:11872161; http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a003107 [DOI] [PubMed] [Google Scholar]
- 53.Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature 2008; 452:719-23; PMID:18401403; http://dx.doi.org/ 10.1038/nature06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Fölsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol 2007; 177:477-88; PMID:17485489; http://dx.doi.org/ 10.1083/jcb.200610047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol 2007; 177:103-14; PMID:17403927; http://dx.doi.org/ 10.1083/jcb.200608126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 1998; 93:731-40; PMID:9630218; http://dx.doi.org/ 10.1016/S0092-8674(00)81435-X [DOI] [PubMed] [Google Scholar]
- 57.Reales E, Sharma N, Low SH, Fölsch H, Weimbs T. Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PloS One 2011; 6:e21181; PMID:21698262; http://dx.doi.org/ 10.1371/journal.pone.0021181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukai S, Matern HT, Jagath JR, Scheller RH, Brunger AT. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. EMBO J 2003; 22:3267-78; PMID:12839989; http://dx.doi.org/ 10.1093/emboj/cdg329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mott HR, Nietlispach D, Hopkins LJ, Mirey G, Camonis JH, Owen D. Structure of the GTPase-binding domain of Sec5 and elucidation of its Ral binding site. J Biol Chem 2003; 278:17053-9; PMID:12624092; http://dx.doi.org/ 10.1074/jbc.M300155200 [DOI] [PubMed] [Google Scholar]
- 60.Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J 2005; 24:2064-74; PMID:15920473; http://dx.doi.org/ 10.1038/sj.emboj.7600699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol 2002; 4:66-72; PMID:11740492; http://dx.doi.org/ 10.1038/ncb728 [DOI] [PubMed] [Google Scholar]
- 62.Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem 2003; 278:51743-8; PMID:14525976; http://dx.doi.org/ 10.1074/jbc.M308702200 [DOI] [PubMed] [Google Scholar]
- 63.Ryan S, Verghese S, Cianciola NL, Cotton CU, Carlin CR. Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways. Mol Biol Cell 2010; 21:2732-45; PMID:20519437; http://dx.doi.org/ 10.1091/mbc.E09-12-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type Igamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with micro1B adaptin. J Cell Biol 2007; 176:343-53; PMID:17261850; http://dx.doi.org/ 10.1083/jcb.200606023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PtdIns3K signalling. Nat Rev 2010; 11:329-41; PMID:20379207; http://dx.doi.org/ 10.1038/nrm2882 [DOI] [PubMed] [Google Scholar]
- 66.Ang AL, Fölsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol 2003; 163:339-50; PMID:14581456; http://dx.doi.org/ 10.1083/jcb.200307046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1999; 1:8-13; PMID:10559857; http://dx.doi.org/ 10.1038/8977 [DOI] [PubMed] [Google Scholar]
- 68.Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic (Copenhagen, Denmark) 2001; 2:556-64; PMID:11489213; http://dx.doi.org/ 10.1034/j.1600-0854.2001.20805.x [DOI] [PubMed] [Google Scholar]
- 69.Fullekrug J, Shevchenko A, Shevchenko A, Simons K. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem 2006; 7:8; PMID:16533391; http://dx.doi.org/ 10.1186/1471-2091-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J 2001; 20:2171-9; PMID:11331583; http://dx.doi.org/ 10.1093/emboj/20.9.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DiNitto JP, Delprato A, Gabe Lee MT, Cronin TC, Huang S, Guilherme A, Czech MP, Lambright DG. Structural basis and mechanism of autoregulation in 3-phosphoinositide-dependent Grp1 family Arf GTPase exchange factors. Mol Cell 2007; 28:569-83; PMID:18042453; http://dx.doi.org/ 10.1016/j.molcel.2007.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langille SE, Patki V, Klarlund JK, Buxton JM, Holik JJ, Chawla A, Corvera S, Czech MP. ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1. J Biol Chem 1999; 274:27099-104; PMID:10480924; http://dx.doi.org/ 10.1074/jbc.274.38.27099 [DOI] [PubMed] [Google Scholar]
- 73.Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, Mellman I, Simons K. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic (Copenhagen, Denmark) 2007; 8:47-60; PMID:17132146; http://dx.doi.org/ 10.1111/j.1600-0854.2006.00506.x [DOI] [PubMed] [Google Scholar]
- 74.Nokes RL, Fields IC, Collins RN, Fölsch H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J Cell Biol 2008; 182:845-53; PMID:18779367; http://dx.doi.org/ 10.1083/jcb.200802176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell 2008; 19:2059-68; PMID:18287531; http://dx.doi.org/ 10.1091/mbc.E07-09-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, et al.. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci 2014; 127:422-31; PMID:24213529; http://dx.doi.org/ 10.1242/jcs.136903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al.. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 2007; 448:366-9; PMID:17597763; http://dx.doi.org/ 10.1038/nature05929 [DOI] [PubMed] [Google Scholar]
- 78.Shi A, Liu O, Koenig S, Banerjee R, Chen CC, Eimer S, Grant BD. RAB-10-GTPase-mediated regulation of endosomal phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci U S A 2012; 109:E2306-15; PMID:22869721; http://dx.doi.org/ 10.1073/pnas.1205278109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett 1999; 449:215-20; PMID:10338135; http://dx.doi.org/ 10.1016/S0014-5793(99)00432-9 [DOI] [PubMed] [Google Scholar]
- 80.Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci U S A 2009; 106:11143-8; PMID:19549835; http://dx.doi.org/ 10.1073/pnas.0811227106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schreiner R, Frindt G, Diaz F, Carvajal-Gonzalez JM, Perez Bay AE, Palmer LG, Marshansky V, Brown D, Philp NJ, Rodriguez-Boulan E. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int 2010; 78:382-8; PMID:20531453; http://dx.doi.org/ 10.1038/ki.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martensson S, Brunmark C, Ohlsson L, Bak-Jensen E, Butkowski R, Boketoft A, Wieslander J. Heterogeneity of renal carcinoma. Nephrol Dial Transplant 1995; 10:1637-43; PMID:8559482 [PubMed] [Google Scholar]
- 83.Gu FL, Cai SL, Cai BJ, Wu CP. Cellular origin of renal cell carcinoma–an immunohistological study on monoclonal antibodies. Scand J Urol Nephrol Suppl 1991; 138:203-6; PMID:1785005 [PubMed] [Google Scholar]
- 84.Holthofer H, Miettinen A, Paasivuo R, Lehto VP, Linder E, Alfthan O, Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest 1983; 49:317-26; PMID:6193332 [PubMed] [Google Scholar]
- 85.Mimura M, Masuda A, Nishiumi S, Kawakami K, Fujishima Y, Yoshie T, Mizuno S, Miki I, Ohno H, Hase K, et al.. AP1B plays an important role in intestinal tumorigenesis with the truncating mutation of an APC gene. Int J Cancer 2011; 130:1011-20; PMID:21484796; http://dx.doi.org/ 10.1002/ijc.26131 [DOI] [PubMed] [Google Scholar]
- 86.Takahashi D, Hase K, Kimura S, Nakatsu F, Ohmae M, Mandai Y, Sato T, Date Y, Ebisawa M, Kato T, et al.. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 2011; 141:621-32; PMID:21669204; http://dx.doi.org/ 10.1053/j.gastro.2011.04.056 [DOI] [PubMed] [Google Scholar]
- 87.Hase K, Nakatsu F, Ohmae M, Sugihara K, Shioda N, Takahashi D, Obata Y, Furusawa Y, Fujimura Y, Yamashita T, et al.. AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology 2013; 145:625-35; PMID:23684748; http://dx.doi.org/ 10.1053/j.gastro.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Jongeward GD, Sternberg PW. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev 1994; 8:60-73; PMID:8288128; http://dx.doi.org/ 10.1101/gad.8.1.60 [DOI] [PubMed] [Google Scholar]
- 89.Shim J, Sternberg PW, Lee J. Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol Biol Cell 2000; 11:2743-56; PMID:10930467; http://dx.doi.org/ 10.1091/mbc.11.8.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gariano G, Guarienti M, Bresciani R, Borsani G, Carola G, Monti E, Giuliani R, Rezzani R, Bonomini F, Preti A, et al.. Analysis of three mu1-AP1 subunits during zebrafish development. Dev Dyn 2014; 243:299-314; PMID:24123392; http://dx.doi.org/ 10.1002/dvdy.24071 [DOI] [PubMed] [Google Scholar]
- 91.Zizioli D, Forlanelli E, Guarienti M, Nicoli S, Fanzani A, Bresciani R, Borsani G, Preti A, Cotelli F, Schu P. Characterization of the AP-1 mu1A and mu1B adaptins in zebrafish (Danio rerio). Dev Dyn 2010; 239:2404-12; PMID:20652956; http://dx.doi.org/ 10.1002/dvdy.22372 [DOI] [PubMed] [Google Scholar]
- 92.Benhra N, Lallet S, Cotton M, Le Bras S, Dussert A, Le Borgne R. AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr Biol 2011; 21:87-95; PMID:21194948; http://dx.doi.org/ 10.1016/j.cub.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 93.Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, Boulianne GL, Chang HC, Le Borgne R, Krämer H, et al.. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell 2011; 22:2094-105; PMID:21490149; http://dx.doi.org/ 10.1091/mbc.E11-01-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng YH, Yang WK, Lin WH, Lai TT, Chien CT. Nak regulates Dlg basal localization in Drosophila salivary gland cells. Biochem Biophys Res Commun 2009; 382:108-13; PMID:19258011; http://dx.doi.org/ 10.1016/j.bbrc.2009.02.139 [DOI] [PubMed] [Google Scholar]


