Abstract
Since its discovery nearly 30 years ago, the Hedgehog (Hh) signaling pathway has been shown to be pivotal in many developmental and pathophysiological processes in several steroidogenic tissues, including the testis, ovary, adrenal cortex, and placenta. New evidence links the evolutionarily conserved Hh pathway to the steroidogenic organs, demonstrating how Hh signaling can influence their development and homeostasis and can act in concert with steroids to mediate physiological functions. In this review, we highlight the role of the components of the Hh signaling pathway in steroidogenesis of endocrine tissues.
1. HEDGEHOG FAMILY
In the 1980s, analyses of Drosophila melanogaster segmentation led to the identification of the gene encoding Hedgehog (Hh) (1). Shortly thereafter, three vertebrate homologs of the Hh gene were described: Sonic hedgehog (Shh), Desert hedgehog (Dhh), and Indian hedgehog (Ihh) (2–5). Of these, Dhh is most closely related to fly Hh, whereas Ihh and Shh result from a recent gene duplication (6) and are 91% homologous (7). Each of the three mammalian Hh ligands has specialized functions, as well as different spatial and temporal expression patterns. Shh is expressed largely during early embryogenesis in a wide range of tissues, such as the notochord, ventral neural plate, eye, heart, lung, gut-derived organs, smooth muscle, and many others. Dhh is expressed mostly in the gonads and neural sheaths, whereas Ihh is restricted primarily to the primitive endoderm and to prehypertrophic chondrocytes in the growth plates during endochondral bone formation (6, 8–10). Perturbation of Hh signaling has been associated with numerous developmental defects and with the disruption of normal cellular homeostasis in many types of tissues. The global knockout of Shh in mice causes a wide array of developmental abnormalities, including the absence of distal limb structures, spinal column, and ribs; defects in foregut formation and development of midline neural structures; deficiency in ventral neuron specification in the brain; craniofacial anomalies; and holoprosencephaly (11, 12). Additionally, altered Hh signaling is involved in the tumorigenesis of several endodermally derived tissues. For example, overactivation of Hh signaling is observed in a subset of hepatocellular carcinomas, and the degree of Hh derangement correlates with tumor size (13). Hh signaling pathways have also been related to hepatocarcinogenesis due to alcohol abuse and liver fibrosis (14). Increased expression of Shh has been detected in the setting of pancreatic injury and throughout the progression of human and murine pancreatic ductal adenocarcinoma (15, 16). Studies conducted on skin stem cells have found a role for the Shh pathway in the tumor initiation of melanoma (17). Consequently, pharmacological inhibition of the Hh signaling pathway has been employed in numerous clinical trials for novel chemotherapeutic agents for various cancers (18).
1.1. Hedgehog Structure, Production, and Release
Hh ligands are secreted proteins that share a tertiary structure consisting of an N-terminal (Hh-N) biologically active domain and a C-terminal (Hh-C) domain containing autocatalytic activity that is required for the formation of the final Hh protein (19). Hh proteins can act as long-range morphogens able to travel many cell diameters, determining target cell fates on the basis of varying concentration gradients (20). Another distinctive feature of Hh ligands is their dual lipid modification and formation of soluble protein complexes that allow for such long-range signaling (9, 21–23). These lipid modifications typically consist of a C-terminal cholesterol moiety and an N-terminal palmitate. Both of these hydrophobic moieties confer membrane affinity such that the secreted signaling domain becomes tightly associated with Hh-generating cells. In vertebrates, the cellular release of cholesterol-modified Hh ligand is regulated by the 12-transmembrane protein Dispatched (Disp) and by the glycoprotein Scube/You (24). The Hh gradient is mediated by heparan sulfate proteoglycans (25) and by other cell surface molecules that facilitate morphogen movement, signaling, and intracellular trafficking (21). Thus, one biological function of the lipid moieties is to regulate the spatial deployment of Hh morphogens. Lipid modification, however, is not a prerequisite for high-affinity binding of the Hh ligand to receptor complex proteins such as Patched 1 (Ptch1) (22, 26, 27).
The precise role of the C-terminal cholesterol moiety in either facilitating or impeding Hh transport is presently unclear, as several studies have reported contradictory results (9). Earlier analyses in Drosophila imaginal discs show that Hh movement is restricted in the presence of cholesterol. However, when the cholesterol moiety is withheld from Hh, the range of Hh movement is actually spatially more extensive (28, 29). Other studies in the mouse limb conflict with these reports. Indeed, by using Shh-N, which lacks the cholesterol moiety, biological activity is retained but signaling is restricted spatially, suggesting that this C-terminal modification is critical for long-range signaling (27). However, later studies with Shh-N transgenic mice conclude that the C-terminal modification limits Shh diffusion in the anteroposterior axis of the murine limb bud. These seemingly discrepant results have been attributed to the different levels of Shh-N present in the zone of polarizing activity (26). In cells, Mann & Beachy (30) report that Hh release occurs in the absence of the C-terminal cholesterol moiety and the N-terminal palmitate without assistance from Disp. Although the role of cholesterol modification in Hh transport in vivo still needs to be clarified, lipid modifications are important for Hh retention in the membrane and for appropriate diffusion (31).
1.2. Hh Signaling Pathway
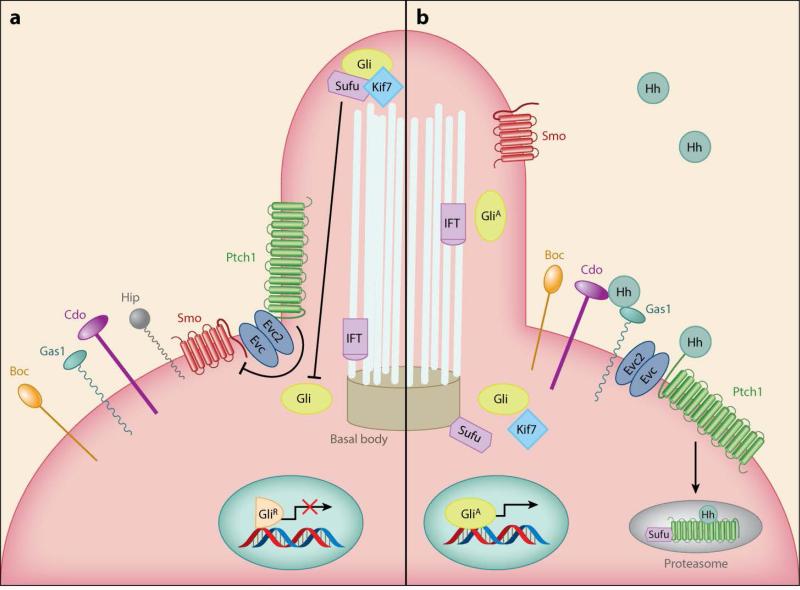
The key components of the Hh signaling pathway, as depicted in Figure 1, are (a) the 12-transmembrane binding protein Ptch1, which functions as the Hh receptor; (b) the recently discovered coreceptors Cdo (cell adhesion molecule–related/downregulated by oncogenes), Boc (brother of Cdo/biregional Cdon-binding protein), and Gas1 (growth arrest specific 1) (32–34); (c) the Hh signal transducer, which is the 7-transmembrane protein related to the G protein–coupled receptor Smoothened (Smo); and (d) the zinc-finger transcription factor named Cubitus interruptus (Ci) in Drosophila and Gli (glioma-associated oncogene) in vertebrates. Vertebrates have three Gli proteins: Gli1, Gli2, and Gli3. Gli2 and Gli3 are the primary mediators of Hh signaling, whereas Gli1 is a target of the Hh pathway, acting via positive feedback to reinforce Gli activity (35, 36).
Figure 1.
The Hh signaling pathway. (a) Ptch1 is located at the base of the primary cilium, where it inhibits Smo. Gli transcription factors are found in the repressor form (GliR) in the cilium. Sufu can therefore complex with Gli proteins and inhibit their nuclear localization and transcriptional activity. (b) Upon Hh ligand binding, Ptch1 releases its repression, and Smo stimulates the accumulation of Gli factors in the active form (GliA), which can promote the transcription of Hh pathway target genes. Abbreviations: Boc, brother of Cdo/biregional Cdon-binding protein; Cdo, Cell adhesion molecule–related/downregulated by oncogenes; Evc2, Ellis van Creveld syndrome protein 2; Gas1, Growth arrest specific 1; Gli, Glioma-associated oncogene; Hh, Hedgehog; Hip, Hedgehog-interacting protein; Kif7, Kinesin family member 7; IFT, intraflagellar transport; Ptch1, Patched 1; Smo, Smoothened; Sufu, Suppressor of Fused.
Critical to Hh signaling are primary cilia present in most organ systems on a subset of cells. In the absence of Hh ligand, Ptch1 is found at the base of the primary cilium, preventing the association of Smo with the cell membrane. The absence of Hh also allows Suppressor of Fused (Sufu), a negative regulator of the Hh pathway, to complex with Gli proteins, which inhibits their nuclear localization and transcriptional activity (37). Gli sequestered by Sufu is phosphorylated and targeted for degradation. Most Gli2 is then proteolytically degraded. However, Gli3 and a small fraction of Gli2 can be cleaved into truncated forms (GliR) that are able to repress transcription of Hh target genes (Figure 1).
The canonical Hh pathway is activated when Hh molecules bind to Ptch1, along with any of the Hh-binding proteins Cdo, Boc, and Gas1, which are part of what is now believed to be a receptor complex, and release the repression exerted by Ptch1 on Smo, leading to its phosphorylation and accumulation in the cilium. Activated phospho-Smo recruits the ciliary proteins Evc and Evc2 (Ellis van Creveld syndrome protein and Ellis van Creveld syndrome protein 2, respectively) to activate Gli proteins by antagonizing Sufu. Consequently, Gli2 and Gli3 escape the proteolytic degradation described above and accumulate in their transcriptionally active forms (Figure 1).
2. STEROIDOGENESIS
Steroidogenesis is the multistep process of steroid hormone biosynthesis from cholesterol and, in most tissues, requires the activity of steroidogenic factor 1 (Sf1, also known as NR5A1 or Ad4BP). Sf1 is a phospholipid-binding (R.D. Blind, E.P. Sablin, K. Kuchenbecker, H.J. Chiu, A.M. Deacon, et al., manuscript in final review) nuclear receptor that is necessary to initiate and maintain steroid production in numerous tissues. Sf1 loss-of-function mutations result in significant defects in the adrenal cortex and gonad, emphasizing the importance of this master regulator of steroidogenesis.
Steroid hormones play a crucial role in several developmental and physiological processes in multiple tissues, including the adrenal cortex, testis (Leydig cells), ovary (granulosa and theca cells), and placenta (syncytiotrophoblast cells). Important functions of these various steroid hormones include regulation of the mammalian stress response, electrolyte and fluid homeostasis, and the development and maintenance of both primary and secondary sexual characteristics.
Cholesterol is the common building block of all steroid hormones, and the first step of steroidogenesis is the cellular allocation of cholesterol for this purpose. Numerous sources of cholesterol can be made available to the cell for steroidogenesis. These include cholesterol delivered by serum low-density lipoprotein (LDL) and high-density lipoprotein (HDL) particles, hydrolysis of intracellular cholesterol esters stored in lipid droplets, cholesterol that is internalized from the plasma membrane, and de novo synthesis (38). Here we review the multiple sources of cholesterol available to the steroidogenic cell and describe the complex biochemical pathways responsible for steroid production. Finally, Sf1 is discussed in greater detail, given its importance in the regulation and maintenance of steroid synthesis.
2.1. Cholesterol Biosynthesis
Cells synthesize cholesterol de novo from acetyl-CoA and acetoacetyl-CoA in the endoplasmic reticulum (ER) through stepwise enzymatic reactions. First, acetyl-CoA is transported from the mitochondria to the cytosol, where, in combination with acetoacetyl-CoA, it is converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). It is then converted to mevalonate by hydroxymethylglutaryl-CoA reductase (HMG-CoAR), the rate-limiting enzyme of the pathway. HMG-CoAR is localized to the ER membrane and contains a conserved sterol-sensing domain (SSD) characterized by five membrane-spanning α-helices shared by other proteins involved in sterol regulation (39, 40). The SSD of HMG-CoAR is important for its association with the ER membrane protein Insig and for the regulation of its degradation (41).
The next step of cholesterol synthesis involves the conversion of mevalonate to isopentenyl pyrophosphate and then to squalene following two successive phosphorylations. Squalene is oxidized and undergoes cyclization to yield lanosterol, which then goes through a series of 19 additional reactions to produce cholesterol. The enzyme 7-dehydrocholesterol (7-DHC) reductase, localized to the microsomal membrane and encoded by the Dhcr7 gene, is responsible for the final reaction in cholesterol biosynthesis. Mutations of Dhcr7 cause Smith-Lemli-Opitz syndrome (RSH/SLOS), which is characterized by increased levels of 7-DHC and reduced levels of cholesterol, resulting in multiple developmental malformations and behavioral problems (42).
2.2. Cholesterol Homeostasis and Regulation of Cholesterol Production
Cholesterol homeostasis involves a complex balance of dietary uptake, de novo synthesis, and multipurpose utilization. It can be used as a component of the cell membrane or act as a precursor for synthesis of steroid hormones, vitamin D, and bile acids. Reduced levels of cholesterol in the ER trigger the synthesis and uptake of cholesterol, which are achieved primarily by increasing the transcription of key rate-limiting enzymes and by regulating the degradation of HMG-CoAR. Intracellular cholesterol levels are also maintained by cholesterol acyltransferase (ACAT), an ER membrane enzyme that esterifies cholesterol by using acyl-CoA. Esterified cholesterol is stored in lipid droplets and is accessed by activation of hormone-sensitive lipase. ACAT also regulates plasma cholesterol levels via LDL receptor–mediated uptake and HDL-mediated reverse transport, which allows peripheral cholesterol to be returned to liver in LDLs (43).
HMG-CoAR activity is subject to both short- and long-term control. Short-term control of HMG-CoAR can involve feedback inhibition by cholesterol, ubiquitin-mediated degradation, and phosphorylation-dependent regulation. In contrast, long-term control reflects changes in expression of the HMG-CoAR gene. Each of these mechanisms involves cholesterol, which (a) is a feedback inhibitor of preexisting HMG-CoAR, (b) promotes rapid HMG-CoAR degradation, and (c) regulates the phosphorylation status of HMG-CoAR. HMG-CoAR is most active in the unphosphorylated state and is phosphorylated by AMP-activated protein kinase. Adequate concentrations of LDL suppress HMG-CoAR activity. The activity of HMG-CoAR and the uptake of LDL cholesterol are also promoted by adrenocorticotropic hormone (ACTH), an anterior pituitary–derived hormone that functions in the hypothalamic–pituitary–adrenal axis by stimulating the release of steroid hormones from the adrenal cortex. Moreover, ACTH increases the availability of free cholesterol for steroid hormone synthesis by stimulating hormone-sensitive lipase and by inhibiting ACAT (43).
The transcription factor SREBP (sterol response element binding protein) controls HMG-CoAR expression. SREBPs are basic helix-loop-helix leucine zipper proteins that regulate genes involved in the biosynthesis of cholesterol and fatty acids (44). Mature SREBP is generated in the Golgi complex and translocates to the nucleus to activate genes involved in cholesterol synthesis and uptake. When cholesterol is abundant, SREBP transcriptional activity is suppressed by retention in the ER through its interaction with the sterol-sensing protein SCAP, which in turn binds the ER retention protein Insig. As noted above, Insig also binds to the SSD of HMG-CoAR in a sterol-dependent manner, enhancing its degradation (40, 45).
2.3. Cholesterol Transport
Cellular cholesterol stores include the plasma membrane and lipid droplets. Regardless of the source of cholesterol to be used in steroid synthesis, transport of cholesterol into the cytoplasm is the first necessary step in steroidogenesis. The vast majority of a cell's cholesterol is located in the plasma membrane, which is composed of a phospholipid bilayer also consisting of cardiolipin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin. Excess cholesterol not incorporated into the plasma membrane, also termed active or free cholesterol, travels to intracellular membranes, as mentioned above, to regulate cholesterol synthesis, for storage in lipid droplets, or for conversion to steroids. This free cholesterol moves within the cell from one organelle to another through vesicular or nonvesicular transport. Vesicular transport occurs through the formation of a bud that travels from the intracellular membranes to a vesicle; this bud then fuses with the acceptor membrane and is able to deliver multiple types of cargos, including lipids, cholesterol, and proteins. Nonvesicular transport of cholesterol also occurs and involves the binding of cholesterol to cytosolic carrier proteins or to protein-mediated membrane contact sites (46).
2.4. Steroidogenic Reactions
Steroid hormone synthesis is regulated largely at the initial steps of cholesterol mobilization and transport into the mitochondrial matrix during conversion of cholesterol to pregnenolone. The transport of cholesterol from the cytosol and outer mitochondrial membrane (OMM) is facilitated by steroidogenic acute regulatory protein (StAR), which is located in the OMM and is the rate-limiting step in steroid synthesis. StAR is expressed in most steroid-producing cells, such as the theca and luteal cells in the ovary, Leydig cells in the testis, and certain cells of the adrenal cortex (43). The exact mechanism of StAR-mediated cholesterol transport into the mitochondria is not completely understood. StAR belongs to a larger family of related proteins that share a conserved START (StAR-related transfer) lipid binding domain. StarD4, −D5, and −D6 are members of this START family and are structurally related to StAR. In addition to StAR, these three START proteins appear to play major roles in intracellular cholesterol transport (47). StarD family proteins lack signal sequences that target them to specific subcellular organelles. Therefore, such proteins appear to be confined to the cytoplasm, where they bind insoluble lipids, permitting their transport across aqueous cytosol. StarD4 and StarD5 have been proposed to play a role in the movement of cholesterol from intracellular lipid droplets to the OMM (48). Surprisingly, StarD4 knockout mice display no disruption of steroidogenesis and only minor alterations in lipid metabolism. Compensation by other transport proteins may account for the nearly normal phenotype observed in StarD4-null mice (48). Whereas in most cells StarD4-related proteins are proposed to shuttle cholesterol from either lipid droplets or the ER to the OMM, only in steroidogenic cells does StAR deliver cholesterol from the OMM to the inner mitochondrial membrane (IMM) (49).
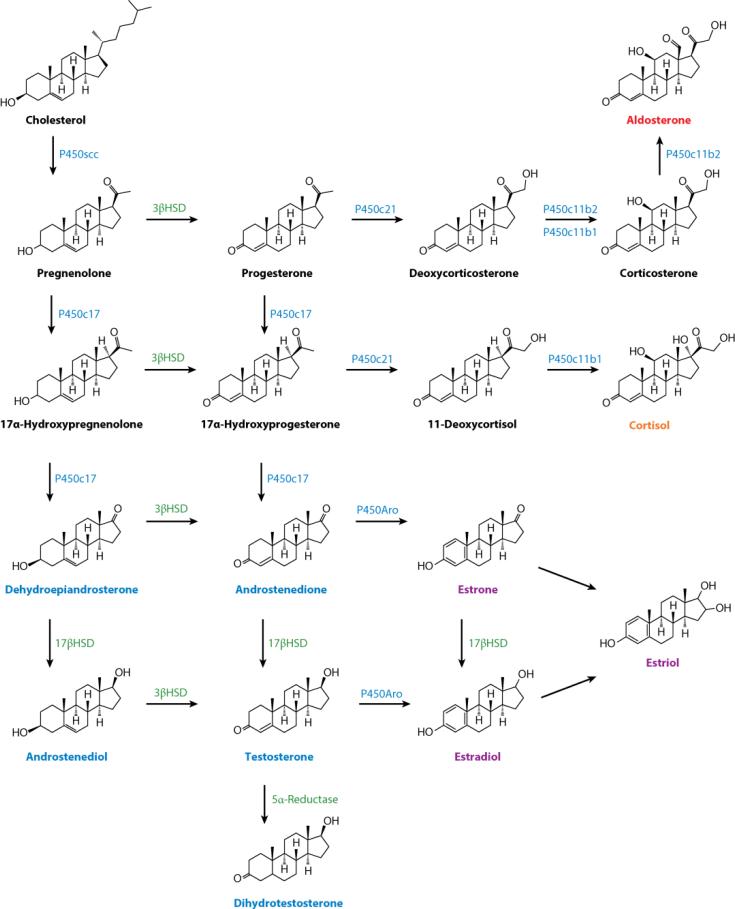
Once cholesterol molecules are successfully transferred into the mitochondria, the first reaction of steroidogenesis is catalyzed by the IMM-associated P450 side-chain cleavage enzyme (P450scc), which is encoded by the CYP11A1 gene. This enzymatic reaction produces pregnenolone. Pregnenolone then travels from the mitochondria to the ER, where it undergoes further enzymatic modification to become cell-specific hormones (Figure 2).
Figure 2.
Steroidogenesis. Scheme of the steroidogenic reactions and intermediate products necessary for steroid synthesis.
The adrenal gland is one of the major sites of steroid hormone synthesis in all mammals. This specialized endocrine organ consists of an inner medulla and an outer cortex. The latter is further subdivided into three distinct zones of hormone synthesis: the zona glomerulosa (zG), the zona fasciculata (zF), and the zona reticularis (zR). Each of these zones produces a distinct steroid hormone profile through a series of biochemical reactions that originate with pregnenolone. The adrenal cortex is unique in that it produces all three major classes of steroid hormones. Therefore, our discussion of steroidogenesis in the adrenal cortex is structured around these three distinct functional zones.
The zG primarily produces the mineralocorticoid aldosterone, which is necessary for the maintenance of fluid and electrolyte homeostasis. Biosynthesis of aldosterone begins with 3β-hydroxysteroid dehydrogenase (3βHSD) conversion of pregnenolone to progesterone. Progesterone is then converted to a new intermediate compound, deoxycorticosterone (DOC), by the P450c21 enzyme. DOC is further metabolized into corticosterone by 11β-hydroxylase (P450c11b1 and P450c11b2) and is finally converted to aldosterone by P450c11b2 (also known as P450c11AS or aldosterone synthase) (50). The conversion of DOC to aldosterone is regulated at the level of P450c11AS transcription, which is promoted by serum potassium and angiotensin II levels and depends on three nuclear receptors: SF1, NURR1 (NR4A2), and NGFIB (NR4A1) (49, 51).
The zF is responsible for the production for glucocorticoids, progesterone, and adrenal androgens. Pregnenolone can be metabolized in the zF to either progesterone or 17α-hydroxypregnenolone (17OHP) by 3βHSD or P450c17, respectively. Again, P450c21 catalyzes the 21-hydroxylation of progesterone to DOC and of 17α-hydroxyprogesterone to 11-deoxycortisol, which are intermediates in the biosynthesis of glucocorticoids in rodents and primates, respectively. P450c11β, located on the IMM, catalyzes the final step of glucocorticoid synthesis (Figure 2). P450c11β, expressed exclusively in the adrenal gland, is encoded by CYP11B1 in humans, whereas three genes for CYP11B have been identified in rats (52). P450c11β activity is reflective of expression of the CYP11B1 gene, which is induced by ACTH in a cAMP-dependent manner, and is repressed by glucocorticoids (53). In primates, the presence of P450c17 in the adrenal cortex allows for the production of cortisol as the final glucocorticoid, whereas in rodents, corticosterone is the final product. Although corticosterone is also an intermediate of aldosterone and has mild mineralocorticoid activity, the lack of P450c17 in the adrenal cortex means that rodents are unable to synthesize cortisol in the adrenal cortex.
In the zR, pregnenolone is hydroxylated by the P450c17 enzyme to yield 17OHP. 17OHP in the zR is converted to dehydroepiandrosterone (DHEA) by the 17, 20-lyase activity of P450c17 and then to androstenedione or sulfated DHEA (DHEA-S). Androstenedione released from the zR is the precursor to the hormones testosterone in males and estradiol in females. P450c17 is expressed in human gonads and the adrenal cortex, where its expression depends on several transcription factors, notably SF1, which is discussed in detail below (54, 55). The 17β-hydroxysteroid dehydrogenases (17βHSDs), also known as 17-oxidoreductases or 17-ketosteroid reductases, promote the interconversion of androstenedione to testosterone, DHEA to androsta-5-ene-3β,17β-diol, estrone to estradiol, androsterone to 5α-androstane-3α, 17β-diol, and 5α-androstanedione to 5α-DHT. All these interconversions can take place in the adrenal cortex. The microsomal aromatase P450aro catalyzes a series of reactions to produce estrogens by the aromatization of androgens (56) (Figure 2). P450aro is encoded by the Cyp19a1 gene and is not expressed in the adrenal cortex but is expressed in the steroidogenic granulosa cells of the ovary and in the placenta, as well as in nonsteroidogenic tissues such as fat and bone. Five unique transcription start sites with individual promoters allow for tissue-specific regulation of Cyp19a1 expression (49).
Steroid synthesis also occurs in other tissues not typically associated with steroid hormone production. For example, local steroid production in the central nervous system yields two neuro-steroids, allopregnanolone and tetrahydrodeoxycorticosterone (49), that are reported to modulate the activity of the ion-gated neurotransmitter GABAA (γ-aminobutyric acid receptor) (49). Neurosteroids also modulate myelinization and are neuroprotective, as well as promoting the growth of axons and dendrites (49).
The rate and type of steroid produced in the adrenal cortex are controlled by cell-specific expression of both receptors activated by tropic hormones and steroidogenic enzymes, as mentioned above. The zF and zR release cortisol or corticosterone after stimulation by ACTH, whereas cells in the zG respond to angiotensin II and potassium to control aldosterone synthesis. At adrenarche in select primates, the zR begins to produce adrenal androgens in response to ACTH, as well as other hormones such as sex steroids, insulin, prolactin, and growth hormone.
2.5. Steroidogenic Factor 1
SF1 is expressed in the adrenal glands, gonads, spleen, ventromedial hypothalamus (VMH), and pituitary gonadotroph cells (57), where it plays a critical role in promoting the expression of steroidogenic genes. Many studies have been performed utilizing the mouse adrenal gland, which serves as an excellent model for understanding the function of SF1 in human adrenal gland development. In mice, Sf1 is first expressed in the adrenogonadal primordium (AGP), which emerges in the urogenital ridges at e9.5. The AGP divides into two distinct primordia, one giving rise to the adrenal cortex and another forming the gonads (58, 59). Sf1 expression in the urogenital region is subsequently restricted to the forming adrenal glands and gonads (58), whereas in postnatal animals, Sf1 can be detected in the adrenal cortex, Leydig and Sertoli cells of the testis, the theca and granulosa cells of the ovaries, the VMH, and pituitary gonadotropes (59, 60).
SF1 is homologous to fushi tarazu factor 1 (ftzf1) in Drosophila and was cloned in 1992 (60, 61). The human SF1 gene is located on chromosome 9q33 (the murine homolog Sf1 is on chromosome 2) (62) and encodes a 461-amino-acid protein characterized by two zinc-finger DNA binding domains, the first of which is a proximal box that confers DNA sequence specificity (63, 64). Additional structural characteristics include an A-box or an Ftz-F1 box, which recognizes nucleotides flanking the AGGTCA nuclear receptor core binding sequence on its 5′ side and allows for binding to DNA (65–68); a hinge region; and a transactivation (AF2) domain. The AF2 domain is necessary but not sufficient for SF1 transactivating activity (69). A mutation in the A-box resulting in sex reversal and adrenal gland failure underscores the importance of this domain in SF1 activity (70).
SF1 regulates the expression of multiple genes, notably those involved in steroidogenesis and cholesterol mobilization, including those encoding StAR, HMG-CoA synthase, several cytochrome P450 steroid hydroxylases, and two key proteins involved in male sex determination and differentiation: SOX9 and anti-Müllerian hormone (AMH) (71). SF1 also mediates expression of the genes coding for the ACTH receptor, MC2R, and 3βHSD, which are important in the differentiation and control of the hypothalamic–pituitary–adrenal axis (65, 72). ACTH and LH/FSH (luteinizing hormone/follicle-stimulating hormone) increase expression of SF1 target genes, via the cAMP/PKA signaling pathway, and mediate both acute and chronic regulation of steroidogenesis (73). Acute regulation of steroidogenesis occurs in minutes and consists of rapid mobilization of cholesterol esters stored in lipid droplets, increased transport of cholesterol to mitochondria, and rapid synthesis of new steroids. The chronic response to ACTH and LH/FSH is characterized by a longer-term transcriptional regulation of steroidogenic enzyme genes resulting in an increase in steroid production (74, 75). The functional importance of these two responses in endocrine physiology is best exemplified by the disorders of steroid production referred to collectively as congenital adrenal hyperplasia, wherein loss-of-function mutations in steroidogenic enzymes result in decreased cortisol production, compensatory increases in ACTH, subsequent hyperplasia of the gland, and increases in other classes of adrenal hormones (e.g., adrenal androgens) (49).
Posttranscriptional modifications of Sf1 play an important role in modulating its transcriptional activity. Phosphorylation at a single serine residue (Ser-203) by mitogen-activated protein kinase (76) is key for Sf1 activation. Reports in the mouse adrenocortical cell line Y1 that Ser-203 is phosphorylated by a cyclin-dependent kinase 7 (CDK7)-mediated process, in contrast to a process mediated by Erk1, raise the question of whether this posttranslational modification plays a role in Sf1-mediated cell proliferation versus Sf1-mediated cell differentiation (77). Acetylation potentiates Sf1 activity (78), whereas inhibiting histone deacetylation increases Sf1 ubiquitination and subsequent degradation, resulting in decreased steroidogenesis (79). Several studies show Sf1 as a target of SUMO (a small ubiquitin-like modulator) (80–82). SUMOylation of Sf1 by UBC9 and E3 SUMO ligases represses Sf1 activity by inducing the translocation of Sf1 to the PML (promyelocytic leukemia bodies) nuclear speckle, which contains a transcriptional repressor (80). Sf1 is SUMOylated at Lys-194, which is located within a synergy control motif. These motifs are conserved in the suppression domains of a variety of transcription factors and are essential for the regulation of synergistic transcription. Mutations of the synergy control motif enhance synergistic transcription from promoters containing multiple Sf1 transcription sites (81). SUMOylation of Sf1 Lys-194 inhibits Sf1 phosphorylation by CDK7 and prevents Sf1 occupancy on a variety of steroidogenic promoters (85, 86). These data support the mounting evidence that SUMOylation represses the transcriptional activity of Sf1, in part by preventing additional activating posttranslational modifications.
SF1 also interacts with numerous coactivators and corepressors to regulate transcription of target genes. Among SF1 coregulators, the corepressor DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, chromosome X, gene 1) has a binding affinity unique for SF1 and colocalizes with SF1 in multiple cell lineages (87). DAX1 is a nuclear receptor homolog that lacks a DNA binding domain; it is thought to function as a dominant negative regulator of the transcription of other nuclear receptors. Notably, Sf1 and Dax1 coexpression is observed in the mouse embryonic testis, ovary, adrenal cortex, hypothalamus, and anterior pituitary. In these tissues, Dax1 inhibits Sf1 activity (88) and therefore negatively regulates the expression of steroidogenic genes (89).
The essential roles of Sf1 in cellular differentiation and organ development were elucidated from in vivo genetic manipulation of Sf1 expression. Mice heterozygous for the Sf1 gene show moderate defects such as hypoplastic adrenal glands, whose functional insufficiency is revealed under stress conditions. In contrast, total Sf1 knockout mice exhibit adrenal and gonadal agenesis by e12.5 (90, 91). Conversely, targeted overexpression of Sf1 in mouse adrenal cortex leads to adrenal hyperplasia (92) and to the development of adrenal cortical tumors (93). These observations demonstrate that maintaining the proper levels of Sf1 is essential throughout development. Similarly, human SF1 mutations have been associated with testicular dysgenesis and male infertility (94–96), premature ovarian failure (97, 98), and adrenal insufficiency (99, 100).
A recent study in mice showing that lineage marks Sf1-expressing cells and, at the same time, deletes Sf1 from these same cells (101) confirmed the role of Sf1 as a regulator of steroidogenesis. Moreover, the marked change in cell morphology and the concomitant loss of steroidogenic identity indicate an essential role of Sf1 in the differentiated state of steroidogenic tissues.
3. ROLE OF HEDGEHOG SIGNALING IN STEROIDOGENIC TISSUES
3.1. Adrenal Gland
The main steroidogenic organs, which consist of the adrenal gland, testis, and ovary, share a common embryonic origin, the AGP. The AGP is characterized by SF1 expression in precursor cells (58, 90) that emerges during the fourth week of gestation in humans (e9.0 in mice). At the eighth week of human gestation (e10.5 in mice), the AGP divides into a distinct adrenal primordium and gonadal primordium. In the developing adrenal primordium, cells that express SF1 are responsible for the establishment of the fetal cortex (also known as the X-zone in mice) (90, 102). SF1 expression is critical for proper adrenal organogenesis and is required for steroidogenic function in both the fetal and the adult cortex. Zubair et al. (103) identified a fetal adrenal enhancer (FAdE) that directs Sf1 expression in the fetal cortex of the mouse. After separation of the adrenal primordium from the AGP, a transcriptional activation complex is recruited to the FAdE to initiate expression of Sf1 within the fetal zone, which is maintained thereafter by autoregulation. During the eighth to ninth week (e11.5–12.5 in mice) of development, mesenchymal cells surround the adrenal primordium to form the capsule, and the adult cortex begins to develop. Cells of the neural crest migrate through the fetal cortex to form the medulla, which produces catecholamines. In mice, the FAdE-mediated expression of Sf1 abates at e14.5. The fetal zone regresses at puberty in male mice and at the onset of the first pregnancy in female mice (102). Lineage-tracing studies in mice show that the adult cortex arises from cells of the fetal cortex, indicating a switch in Sf1 transcriptional regulation during adrenal cortex development. In humans, the fetal zone regresses at birth, leaving the adult cortex.
As mentioned above, the steroid-secreting cortex that surrounds the medulla is derived from cells of the intermediate mesoderm and is organized into three concentric zones enclosed within the capsule: the outer zG, responsible for mineralocorticoid production; the intermediate zF, which secretes glucocorticoids; and the zR, which produces sex steroid precursors in humans and most primates (104). Rat adrenal glands exhibit a discrete zone between the zG and the zF—named the zona undifferentiated (zU), which is formed by cells in a functionally undifferentiated state—but are capable of producing corticosteroid precursors (105). The zU has been proposed to be the site where stem and/or progenitor cells reside in the adult rat adrenal gland (106).
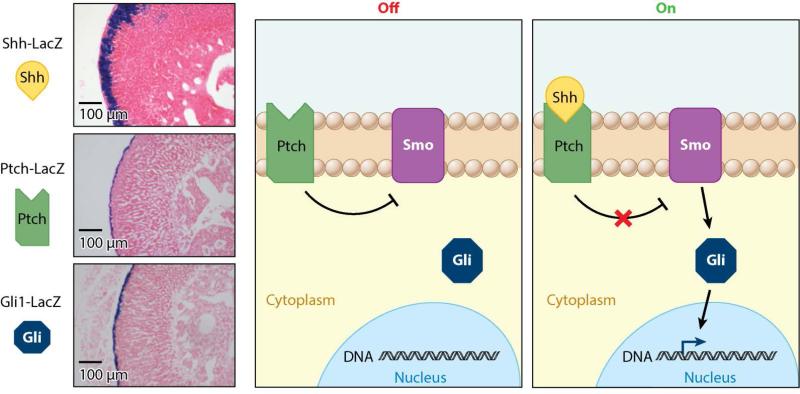
Recent studies have revealed multiple roles for the Hh pathway in adrenal development and function. Shh is the only Hh family member expressed in the mouse adrenal gland. Shh is localized in discrete clusters in the subcapsular region and presumably signals to the capsular region, where Ptch1 and Gli are present (107–109) (Figure 3 and Table 1). In rat adrenal cortices, Shh-expressing cells are organized in a continuous layer immediately under the zG cells and in the outer layer of the zU. These cells do not express Cyp11b1 or Cyp11b2 (110), suggesting that they do not participate in steroid hormone synthesis. Overall, Shh expression is restricted to a pre-steroidogenic cell population that does not express any of the enzymes necessary for the terminal reactions of either mineralocorticoid or glucocorticoid production (111, 112). Interestingly, in human adrenal glands, Gli is not only expressed in the capsule but is also found in all three zones (113), raising questions about species-specific roles and mechanisms of Hh signaling in the adrenal gland.
Figure 3.
Hedgehog pathway expression in the adrenal gland. β-Galactosidase activity staining of reporter mice shows that, in the adrenal gland, Gli1 and Ptch are expressed primarily in the capsule, whereas the predominant ligand, Shh, is found in the subcapsular region of the cortex. In the absence of Shh, Ptch inhibits Smo. Upon Shh binding, Smo is released from Ptch inhibition and activates nuclear translocation of Gli transcription factors. Abbreviations: Gli, Glioma-associated oncogene; Ptch, Patched; Shh, Sonic hedgehog; Smo, Smoothened.
Table 1.
Sites of expression of Hedgehog pathway genes in steroidogenic tissues
| Tissue | Gene | Localization |
|---|---|---|
| Adrenal gland | Shh | Subcapsule |
| Ptch, Gli | Capsule | |
| Testis | Dhh | Sertoli precursor cells |
| Ptch, Gli | Interstitial cells, Leydig cells, and endothelial cells | |
| Ovary | Dhh, Ihh | Granulosa cells of antral follicles |
| Ptch, Gli | Theca cells surrounding follicles; expression lost during ovulation; low expression levels in ovarian stroma |
Abbreviations: Dhh, Desert hedgehog; Gli, Glioma-associated oncogene; Ihh, Indian hedgehog; Ptch, Patched; Shh, Sonic hedgehog.
In mice, concomitant with the onset of expression of Scc (cholesterol side-chain cleavage enzyme)/Cyp11a1, Shh is detected in the adrenal primordium starting at e12.5 and persists throughout adult life. However, its expression appears to be limited to an undifferentiated adrenal lineage that lacks steroidogenic enzymes such as Cyp11b1 or Cyp11b2. During embryonic development and the early postnatal period, Ptch1 and Gli1 are expressed in the mesenchyme surrounding Sf1-positive cells. Notably, Gli1-expressing cells do not express Sf1 (107, 109). Lineage-tracing experiments have identified the capsular Gli1-expressing cells as descendants of fetal adrenal cells (114). Moreover, Gli1-expressing cells can contribute to the steroidogenic lineage of the cortex that expresses both Sf1 and Scc (107, 109). Intriguingly, other lineage-tracing experiments (102, 114) have revealed that the adult adrenal cortex originates from two populations present in the fetal adrenal cortex: Sf1-positive cells and undefined cells that do not express Sf1. Cell-fate mapping determined that Shh-positive cell descendants exist at the periphery of the cortex and, over time, are centripetally displaced into the cortex, expanding into columns traversing the gland. These findings indicate that some progenitor cells reside at the cortical periphery and give rise to the cells of the adrenal cortex (109, 112) and that Shh is expressed in adrenal progenitor cells. Consistent with these data, proliferation studies (115) have identified the peripheral Shh-expressing cells as a relatively quiescent population in mouse adult adrenal cortices, a trait compatible with stem cell/progenitor cell characteristics.
Shh is essential for development of the adrenal cortex. Deletion of Shh in Sf1-expressing cells leads to decreased proliferation, resulting in hypoplastic adrenal glands with a thin capsule (107, 109). Residual Gli1 activity in mutant adrenal glands (107, 109) calls into question whether incomplete Cre-mediated recombination (112) disrupts all Hh signaling in the adrenal gland in these conditional deletions of Shh. In this regard, no Gli1 activity is observed in Shh-null adrenal glands. Moreover, adrenal glands in the global Shh knockout are much smaller than those in the conditional Shh knockouts (109). Regardless, conditional ablation of Shh results in adrenocortical hypoplasia and thinning of the capsule beginning at e13.5 and progressing throughout adult life. In contrast, adrenal zonation and differentiation of the medulla are unaffected in these mutant mice (107–109). The ability of these hypoplastic mutant adrenal glands to respond to tropic hormone signaling is impaired, as evidenced by the lack of compensatory growth in response to the elevated ACTH levels observed in adult mice (107). Taken together, these data suggest that Shh expression is essential for proper adrenal gland development, growth, and maintenance of steroidogenic progenitors. Lack of Shh prevents proper expansion of Sf1-expressing cells but does not affect overall cortical differentiation.
Proper posttranslational modification of Sf1 also appears to play a role in regulating Hh signaling and downstream targets (116). Fetal adrenal glands of mutant mice expressing a SUMO-deficient or SUMO-less Sf1 begin to express testis-specific markers, such as Sox9, and exhibit increased and broader expression of the Shh, Gli1, and Cyp17a1 genes, suggesting a role of SUMOylation of Sf1 in restricting Shh gene expression in the adrenal cortex. Furthermore, aged SUMO-less Sf1 mutant mice exhibit adrenocortical hypoplasia and retention of the fetal zone. Reducing the Shh dosage only partially restores the altered steroid profiles observed in SUMO-less Sf1 mutant mice. In vitro studies in murine immortalized hypothalamic mHypoE-40 cells showed that Shh is induced after expression of mutant Sf1 lacking its SUMOylation site (116). Thus, Shh expression is sensitive to the SUMOylation status of Sf1. Similarly, inhibition of SUMOylation with tannic acid in livers of mice expressing human liver receptor homolog 1 triggers ectopic activation of Shh (K.A. Ramos, M. Suzawa, D.A. Miranda, E.J. Faivre, K.K.-H. Ang, et al., manuscript in review).
More evidence relating adult adrenal gland homeostasis and Hh signaling is emerging. Guasti and colleagues (117) showed that Delta-like homolog 1 (Dlk1), an activator of Gli transcription, and Shh are expressed in the outer zU of rat adrenal glands and are coordinately regulated by modulation of the renin-angiotensin system, suggesting a role of Dlk1 in controlling adrenal gland differentiation, homeostasis, and metabolic processes.
The importance of Shh signaling in influencing adrenal cell fate is shown in a recent mouse model that deletes Sf1 in the zG (118). Freedman et al. (118) found that these mutant adrenal glands exhibit morphological changes, including a decrease in Cyp11b2 and P450scc expression, that are consistent with lack of zG dedifferentiation. They also report decreased Shh expression and increased Gli1 expression that indicates a complex signaling and lineage relationship between the capsular Gli1 cells, cortical Shh cells, and the differentiated cells of the zG and zF. Additional tracing studies revealed that the differentiated zG cell population undergo direct lineage conversion and migrate to become morphologically distinct zF cells.
3.2. Testis
In the embryonic testes, Sertoli cells initiate testis morphogenesis and induce the appearance of Sf1-positive/Sry-negative fetal Leydig cells and other somatic cell lineages, including peritubular myoid cells, which reside outside the seminiferous tubules. Two types of Leydig cells are present in the testis: (a) fetal Leydig cells located in the interstitium, which are present in the embryonic testes from e12.5 until shortly after birth, and (b) adult Leydig cells, which emerge at puberty. At approximately e12.0–13.0, the fetal Leydig cells begin to produce testosterone, the levels of which peak before birth (119). Leydig cells are crucial for testicular descent, masculinization of the external genitalia, sexual dimorphism of the brain, and androgen production (58, 104).
In contrast to the developing adrenal glands, Dhh is the primary Hh ligand expressed in the testes. Sertoli cells express Dhh (120) throughout embryonic development until the adult stage (121). The Hh receptor Ptch1 is expressed in interstitial cells, including peritubular myoid cells, Leydig cells, and endothelial cells (120, 122). Gli1 and Gli2 are expressed in Leydig cells and appear to have both redundant and differentiated roles in fetal Leydig cells and in male sexual differentiation (123). In adult testis, Gli1, Gli2, and Gli3 are also expressed in spermatogonia and spermatids (124) (Table 1).
Dhh signaling is critical for normal testes development. Although the phenotypes associated with Dhh-null mice are strain dependent, loss of Dhh signaling significantly impairs testes development and function. Loss of Dhh in a mixed background disrupts organization of the embryonic testes, whereas loss of Dhh in a 129/Sv background results in infertility due to defects in spermatogenesis (122, 125). Dhh-deficient mice also display behavioral changes that are proposed to reflect the lower testosterone levels. Interestingly, DHH mutations are also found in human patients with gonadal dysgenesis (126, 127). The role of Hh signaling in testes development is also supported by pharmacological studies using the SMO analog KAAD-cyclopamine Hh inhibitor (125).
The relationship between Hh signaling and Sf1 in sexual development has been explored in mice. Sf1 haploinsufficiency impairs testis development in both humans and mice. In humans, partial loss of SF1 is associated with impaired Leydig cell function (128, 129), whereas in mice, expression of Leydig and Sertoli cell markers is delayed (130). Deletion of Dhh in Sf1-haploinsufficient mice resulted in poor masculinization and virilization of the Wolffian derivatives that is likely caused by low testosterone due to the lack of differentiated Leydig cells (130). These findings suggest that Dhh is necessary for normal male testis development. Posttranslational modification of Sf1 also appears to play a role in regulating Hh signaling and sexual development. Loss of Sf1 SUMOylation results in abnormal testis cord morphology by e13.5 and in strong ectopic Shh expression in the interstitial compartment that is accompanied by decreased Dhh and an increased number of Leydig cells (116). The interplay between Sf1 and Hh signaling in the testes as well as in the adrenal glands is further supported by the identification of conserved Hh-responsive elements (HRE, TGGGTGGTC) in the mouse Sf1 promoter (121).
In addition to testis development, studies by Barsoum and colleagues (131) support the notion that Hh signaling also plays an important role in sex determination. Not only is Hh signaling sufficient for Leydig cell specification, but ectopic activation of the Hh pathway in ovarian cells that express Sf1 leads to the formation of functional Leydig cells, to the masculinization of female embryos, and to female pseudohermaphroditism.
3.3. Ovary
Hh signaling directs cell fate in the Drosophila ovary (132, 133) but also influences ovary development and function in mammals. Although Dhh was not found initially in mouse fetal ovary (120), it was described to exist in the marsupial Macropus eugenii (commonly known as the tammar wallaby) in granulosa cells throughout follicular development and in the oocyte cytoplasm in primordial follicles (134). In XX mouse embryos, Ptch1 or Gli1 expression is not detected in the ovary, likely due to a fate switch and transformation of ovarian cells into functional Leydig cells (131). However, Huang & Yao report, as unpublished data, the detection of Gli2 expression in the fetal ovary at e14.5 in a subset of somatic cells, possibly endothelial cells surrounding the germ cell nest (121).
Consistent with the lack of Dhh in the ovary, a global knockout or targeted deletion of Dhh in Sf1-positive cells in mice does not alter ovarian development or function (120, 121). However, ectopic activation of Hh signaling in ovarian somatic cells by overexpression of a dominant active allele of Smo leads to anovulatory ovaries, accompanied by defects in vascular development. These observations demonstrate that, although loss of Hh signaling is not detrimental to normal ovarian development, excessive Hh signaling impedes ovarian function. Further profiling of these ovaries reveals increased expression of Shh mRNA and adrenal gland–specific genes, including Cyp21a, Cyp11b1, and Cyp17a, as well as of proteins encoded by these genes (135, 136). Such findings suggest that activating Hh in the ovary elicits differentiation of ovarian cells into adrenal cortex–like cells. In the mouse adult ovary, granulosa cells of antral follicles produce Dhh and Ihh ligands, whereas their targets, Ptch1, Smo, Gli1, Gli2, and Gli3, reside in theca cells, granulosa cells, and the ovarian stroma (137, 138). Interestingly, expression of these Hh signaling components is actually lost during ovulation (138) (Table 1).
Other studies have found correlations between Hh signaling and ovarian steroidogenesis. Inhibition of the Hh signaling pathway by cyclopamine elevates progesterone production in cultured granulosa cells (137). Androgen-producing theca cells located in the intrafollicular space are visible only after the follicle matures into a secondary follicle. Although the differentiation of theca cells is independent of tropic hormones, these cells become LH responsive at postnatal day 5 (139). Androgens produced by the theca cells are converted to estrone and 17β-estradiol by a P450 aromatase in the granulosa cells of the sex cord. Bovine theca cells treated with recombinant Shh exhibit increased cell proliferation and androstenedione production (140), whereas treatment of mouse granulosa cells and follicles with Shh in vitro increases proliferation and growth, respectively.
3.4. Placenta
The placenta is a membranous organ that develops in female mammals following the implantation of the blastocyst and connects the developing embryo to the uterine wall to provide nutrition, waste removal, gas exchange, and endocrine and immune support. The placenta is capable of synthesizing pregnenolone and progesterone, as well as estrogens from fetal adrenal–derived steroids. The placenta is a unique steroidogenic tissue because it does not express P450c17 and, therefore, cannot convert pregnenolone to sex steroids. Sf1 and StAR gene expression is sparse or undetected in the placenta (141). The placenta is able to move cholesterol into placental mitochondria in a StAR-independent manner (49). 3βHSD1, rather than adrenal or gonadal 3βHSD2, is utilized in placental steroidogenesis (49). Sf1 appears to be dispensable for both placental development and placental steroid synthesis (142) and may have been replaced by activating protein 2, which can induce placental P450scc activity (143).
Progesterone is synthesized during pregnancy starting at midgestation and is important for the continuation of the pregnancy by preventing uterine contractility (144). Progesterone induces Ihh in the murine uterus (145, 146), where its expression is restricted to the uterine epithelium. Ptch1 and chicken ovalbumin upstream promoter transcription factor II (COUP-TFII), which is important for vascular development, are expressed in the endometrial stroma. Deletion of Ihh in cells expressing the progesterone receptor reveals that Ihh is an essential mediator of the communication between the uterine epithelium and stroma required for embryo implantation (147). Female mice deficient in Ihh are infertile due to failure of the blastocyst to attach to the uterine epithelium and the absence of progesterone-mediated proliferation, angiogenesis, and decidualization in the uterus. Conversely, mice with constitutively active Smo and increased Hh signaling in progesterone receptor–expressing cells are infertile due to defects in uterine morphology (148). These studies reveal a pivotal role of the Hh pathway in blastocyst implantation, and thus subsequent formation of the placenta, as well as in uterine function.
4. HEDGEHOG SIGNALING AND DISEASE
Alterations at different levels in the Hh pathway are often related to pathological processes. As mentioned above, defects in cholesterol biosynthesis can cause RSH/SLOS, which is characterized in part by adrenal insufficiency (149). RSH/SLOS has been linked to Shh (149). Mutations of human SHH have been associated with classical autosomal dominant holoprosencephaly (150), leading to the hypothesis that the holoprosencephaly found in some patients with RSH/SLOS may be caused by inadequate autoproteolysis of the SHH ligand. The consequent deficiency of cholesterol-modified SHH signaling may thus be due to low cholesterol levels, a distinctive feature of RSH/SLOS patients (149). However, Cooper and colleagues (151) observed that low total sterol levels in Dhcr7 mutant mouse embryonic fibroblasts reduce Shh signaling by blocking Smo in the responding cells. These findings suggest that increased 7-DHC as seen in RSH/SLOS does not directly affect the posttranslational cholesterol modification of Shh. A direct mechanism of interaction between the N terminus of 7-DHC and Smo that may be diminished due to mutations in Dhcr7 has also been proposed as an alternative mechanism to explain impaired Shh signaling in this scenario (152, 153). Metabolites of 7-DHC may also modulate Hh signaling. The products of cholesterol oxidation, oxysterols, can bind to Smo and activate the Hh signaling pathway, whereas Ptch-mediated transport of provitamin D3, a metabolic product of 7-DHC, modulates Smo function (153, 154).
Impaired Hh signaling is also linked to the autosomal dominant Pallister-Hall syndrome (PHS), a developmental disorder with a wide spectrum of anomalies, such as polydactyly, asymptomatic bifid epiglottis, and hypothalamic hamartoblastoma. Adrenal gland hypoplasia or aplasia and hypopituitarism have been observed in cases of PHS, which is caused by a frameshift mutation leading to constitutive repressor activity of Gli3 (155). Hypopituitarism in PHS is unlikely to contribute to adrenal gland hypoplasia or aplasia, as adrenal gland development does not depend on pituitary-synthesized ACTH (156). In 2002 Böse and colleagues (157) studied a murine model carrying a lethal Gli3 mutation that mimics the truncated Gli3 protein found in PHS patients, and these researchers observed adrenal gland aplasia in embryos. However, a recent study reported the presence of adrenal glands in the same mouse model bred in a different genetic background, with no difference in gross morphology between mutant and control adrenal glands (112). The authors justified this different finding by the displacement of the adrenal glands or by the different genetic backgrounds of the mice. Nonetheless, they concluded that frameshift mutations of Gli3 do not impair adrenal gland development.
Meckel-Gruber syndrome and Bardet-Biedl syndrome are genetic ciliopathies characterized by many malformations of many organs, including the gonads and adrenal glands. Primary ciliary transport and intraflagellar transport are important components for the proper function of the Hh signaling pathway, and many studies have demonstrated that, in the absence of intraflagellar transport, Gli proteins have neither activator nor inhibitor activity, resulting in a nonresponsive signaling pathway (158). Although ciliopathies do not completely phenocopy Hh mutations, it is plausible to hypothesize that altered Hh signaling contributes to the malformations observed in steroidogenic organs in Meckel-Gruber and Bardet-Biedl syndromes.
Hh signaling also plays an important pathophysiological role in many types of human cancer. Approximately 30% of human cancers—including melanoma; brain tumors; and prostate, lung, ovarian, and breast cancer—have demonstrated derangements in Hh signaling. These diseases are characterized by activated Hh signaling, mostly due to ligand-dependent mechanisms or noncanonical Hh signaling activation (159). Microarray data from our lab and others regarding adrenocortical carcinomas and adenomas find no significant linkage with aberrant Hh signaling (160–163). However, in the ovary, Hh signaling contributes to the tumorigenic process. Whereas epithelial ovarian tumors that arise from the surface epithelium of the ovary do not express Hh pathway components, neoplastic ovarian lesions expressed higher levels of genes such as Shh, Dhh, Ptch1, and Gli1; there is also a correlation between Ptch1 and Gli1 overexpression and poor patient survival (164). Gli1 overexpression in an ovarian cancer cell line enhances proliferation, mobility, and invasiveness, whereas treatment with the Hh inhibitor cyclopamine promotes de-differentiation, apoptosis, and cytostasis and represses cell motility and invasiveness (164). A recent clinical trial targeting Hh signaling in maintenance therapy of ovarian cancer (165) did not result in a meaningful improvement in progression-free survival, perhaps because of the unexpectedly low expression of Hh ligands. Nonetheless, more studies are being conducted, and targeting the Hh signaling pathway in ovarian cancer appears to be a promising therapy for patients with this disease.
5. CONCLUDING REMARKS AND PERSPECTIVES
The milestone discovery of Hh has opened new horizons in many disciplines, particularly in developmental biology, physiology, pathology, and pharmacology. The Hh pathway is well conserved among species, and many animal models have been developed to help elucidate its many functions. In recent years, more molecules playing key roles in the pathway have been discovered, and more mechanisms of action have been elucidated, but still many questions about the role of Hh signaling remain unanswered. Improper Hh signaling can result in the onset of several disorders, and overactive Hh signaling has been linked to tumor development and/or aggressiveness. Current efforts are focused on the development and testing of drugs designed to inhibit the Hh pathway, and promising results are emerging from clinical trials for treatment of patients with skin cancer. Data relating to drug resistance are driving research toward the design of new molecules.
Recently, the link between steroidogenic organs and Hh signaling has emerged, as evidenced, for example, by the observation that deletion of Hh in murine adrenocortical cells impairs normal adrenal gland development and function. Intriguingly, different ligands are found in tissues that share the same embryonic origin, and the expression pattern of the Hh components varies depending on the physiological status of the organ, as seen in the ovary. Hh ligands are tissue specific and signal to cells residing primarily in the mesenchyme to control processes such as proliferation and expansion of the steroidogenic population. However, the underlying molecular mechanisms are still unclear. Hh signaling pathways are required for proper embryonic development of steroidogenic organs but have also been found in adult tissues, suggesting that Hh signaling has a role in homeostasis. Future studies are needed to determine how Hh signaling differs between embryonic and adult tissues. An exciting implication of these studies relates to the advancement of regenerative medicine; better knowledge of the role of Hh signaling could be leveraged for restoring organs and their functions after pharmacological therapies or pathologies.
SUMMARY POINTS.
Hh ligands are secreted morphogens with a pivotal role in many developmental, physiological, and pathological processes.
Steroidogenesis is a complex series of reactions to produce steroid hormones, which have important functions in development and physiology.
Both steroidogenesis and the production of Hh molecules depend on cholesterol biosynthesis and availability.
Hh ligands are expressed in a tissue-specific manner; the ovary, testis, adrenal gland, and placenta produce different Hh molecules.
Hh signaling plays a role in testis, pituitary, and adrenal gland development, as well as in the maintenance of tissues, cell proliferation, and hormone production.
Aberrant Hh signaling results in malformation and tumor formation and progression and is being targeted for drug therapy.
Acknowledgments
We thank Joanne Heaton for her critical reading of the manuscript and for her precious suggestions
Footnotes
disclosure statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1•.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 2•.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. doi: 10.1016/0092-8674(93)90627-3. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 3•.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–44. doi: 10.1016/0092-8674(93)90628-4. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 4•.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–16. doi: 10.1016/0092-8674(93)90626-2. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 5•.Chang DT, Lopez A, von Kessler DP, Chiang C, Simandl BK, et al. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120:3339–53. doi: 10.1242/dev.120.11.3339. [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 6•.Cohen MM., Jr The hedgehog signaling network. Am. J. Med. Genet. A. 2003;123A:5–28. doi: 10.1002/ajmg.a.20495. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 7•.Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001;106:107–17. doi: 10.1016/s0925-4773(01)00427-0. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 8•.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 9•.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 10•.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 11•.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 12•.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 13•.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–57. doi: 10.1093/carcin/bgi292. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 14•.Chan IS, Guy CD, Machado MV, Wank A, Kadiyala V, et al. Alcohol activates the hedgehog pathway and induces related procarcinogenic processes in the alcohol-preferring rat model of hepatocarcinogenesis. Alcohol Clin. Exp. Res. 2014;38(3):787–800. doi: 10.1111/acer.12279. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. PNAS. 2014;111(30):E3091–100. doi: 10.1073/pnas.1411679111. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Dosch JS, Pasca di Magliano M, Simeone DM. Pancreatic cancer and hedgehog pathway signaling: new insights. Pancreatology. 2010;10(2–3):151–57. doi: 10.1159/000225923. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Santini R, Vinci MC, Pandolfi S, Penachioni JY, Montagnani V, et al. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30(9):1808–18. doi: 10.1002/stem.1160. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 18•.Hadden MK. Hedgehog pathway inhibitors: a patent review (2009–present). Expert Opin. Ther. Pat. 2013;23:345–61. doi: 10.1517/13543776.2013.757304. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 19•.Burglin TR. The Hedgehog protein family. Genome Biol. 2008;9:241. doi: 10.1186/gb-2008-9-11-241. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–49. doi: 10.4161/cc.6.21.4822. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 21•.Guerrero I, Chiang C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol. 2007;17:1–5. doi: 10.1016/j.tcb.2006.11.002. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 22•.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–20. doi: 10.1038/35079648. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 23•.Chen Y, Jiang J. Decoding the phosphorylation code in Hedgehog signal transduction. Cell Res. 2013;23:186–200. doi: 10.1038/cr.2013.10. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 2012;26:1312–25. doi: 10.1101/gad.191866.112. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb. Perspect. Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [CrossRef] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Li Y, Zhang H, Litingtung Y, Chiang C. Cholesterol modification restricts the spread of Shh gradient in the limb bud. PNAS. 2006;103:6548–53. doi: 10.1073/pnas.0600124103. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 28•.Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–15. doi: 10.1016/s0092-8674(00)81677-3. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 29•.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–59. doi: 10.1126/science.274.5285.255. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 30•.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [Abstract] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 31•.Therond PP. Release and transportation of Hedgehog molecules. Curr. Opin. Cell Biol. 24(2):2012, 173–80. doi: 10.1016/j.ceb.2012.02.001. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 32•.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–57. doi: 10.1101/gad.1543607. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, et al. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev. Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, et al. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev. Cell. 2011;20:775–87. doi: 10.1016/j.devcel.2011.04.018. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 36•.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–16. doi: 10.1016/j.cell.2004.07.023. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 37•.Yang C, Chen W, Chen Y, Jiang J. Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell Res. 2012;22:1593–604. doi: 10.1038/cr.2012.134. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010;7:47. doi: 10.1186/1743-7075-7-47. [CrossRef] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol. Cell. 2004;15:259–68. doi: 10.1016/j.molcel.2004.06.019. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 40•.Ikonen E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiol. Rev. 2006;86:1237–61. doi: 10.1152/physrev.00022.2005. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 41•.Sever N, Yang T, Brown MS, Goldstein JL, DeBose-Boyd RA. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell. 2003;11:25–33. doi: 10.1016/s1097-2765(02)00822-5. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 42•.Jira PE, Waterham HR, Wanders RJ, Smeitink JA, Sengers RC, Wevers RA. Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann. Hum. Genet. 2003;67:269–80. doi: 10.1046/j.1469-1809.2003.00034.x. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 43•.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J. Lipid Res. 2011;52:2111–35. doi: 10.1194/jlr.R016675. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002;109:1125–31. doi: 10.1172/JCI15593. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 46•.Brown MS, Kovanen PT, Goldstein JL. Receptor-mediated uptake of lipoproteincholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog. Horm. Res. 1979;35:215–57. doi: 10.1016/b978-0-12-571135-7.50009-6. [Medline] [DOI] [PubMed] [Google Scholar]
- 47•.Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 2003;278:22183–86. doi: 10.1074/jbc.R300003200. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 48•.Riegelhaupt JJ, Waase MP, Garbarino J, Cruz DE, Breslow JL. Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J. Lipid Res. 2010;51:1134–43. doi: 10.1194/jlr.M003095. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Fardella CE, Rodriguez H, Hum DW, Mellon SH, Miller WL. Artificial mutations in P450c11AS (aldosterone synthase) can increase enzymatic activity: a model for low-renin hypertension? J. Clin. Endocrinol. Metab. 1995;80:1040–43. doi: 10.1210/jcem.80.3.7883820. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 51•.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, et al. Adrenocortical zonation in humans under normal and pathological conditions. J. Clin. Endocrinol. Metab. 2010;95:2296–305. doi: 10.1210/jc.2009-2010. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 52•.Mellon SH, Bair SR, Monis H. P450c11B3 mRNA, transcribed from a third P450c11 gene, is expressed in a tissue-specific, developmentally, and hormonally regulated fashion in the rodent adrenal and encodes a protein with both 11-hydroxylase and 18-hydroxylase activities. J. Biol. Chem. 1995;270:1643–49. doi: 10.1074/jbc.270.4.1643. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 53•.Mornet E, Dupont J, Vitek A, White PC. Characterization of two genes encoding human steroid 11 β-hydroxylase (P-45011β). J. Biol. Chem. 1989;264:20961–67. [Medline] [Web of Science ®] [PubMed] [Google Scholar]
- 54•.Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. Cyclic AMP–stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase θ facilitates induction of CYP17. Mol. Cell. Biol. 2007;27:6669–85. doi: 10.1128/MCB.00355-07. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54nrb/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology. 2002;143:1280–90. doi: 10.1210/endo.143.4.8748. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 56•.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, et al. Aromatase—a brief overview. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [Abstract] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 57•.Ozisik G, Achermann JC, Meeks JJ, Jameson JL. SF1 in the development of the adrenal gland and gonads. Horm. Res. 2003;59(Suppl. 1):94–98. doi: 10.1159/000067831. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 58•.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–71. doi: 10.1046/j.1365-2443.1996.00254.x. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 59•.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994;8:654–62. doi: 10.1210/mend.8.5.8058073. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 60•.Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J. Biol. Chem. 1992;267:17913–19. [Medline] [Web of Science ®] [PubMed] [Google Scholar]
- 61•.Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 1992;6:1249–58. doi: 10.1210/mend.6.8.1406703. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 62•.Taketo M, Parker KL, Howard TA, Tsukiyama T, Wong M, et al. Homologs of Drosophila fushi-tarazu factor 1 map to mouse chromosome 2 and human chromosome 9q33. Genomics. 1995;25:565–67. doi: 10.1016/0888-7543(95)80059-u. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 63•.Mader S, Kumar V, de Verneuil H, Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature. 1989;338:271–74. doi: 10.1038/338271a0. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 64•.Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139–46. doi: 10.1016/0092-8674(89)90051-2. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 65•.Val P, Lefrançois-Martinez AM, Veyssière G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl. Recept. 2003;1:8. doi: 10.1186/1478-1336-1-8. [CrossRef] [Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non–zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992;256:107–10. doi: 10.1126/science.1314418. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 67•.Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL. Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J. Mol. Endocrinol. 1996;17:139–47. doi: 10.1677/jme.0.0170139. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 68•.Ueda H, Sun GC, Murata T, Hirose S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol. Cell. Biol. 1992;12:5667–72. doi: 10.1128/mcb.12.12.5667. [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Li LA, Chiang EF, Chen JC, Hsu NC, Chen YJ, Chung BC. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol. Endocrinol. 1999;13:1588–98. doi: 10.1210/mend.13.9.0349. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 70•.Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J. Clin. Endocrinol. Metab. 2002;87:1829–33. doi: 10.1210/jcem.87.4.8376. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 71•.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–34. doi: 10.1038/nature06944. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 72•.Wang CY, Chen WY, Lai PY, Chung BC. Distinct functions of steroidogenic factor-1 (NR5A1) in the nucleus and the centrosome. Mol. Cell. Endocrinol. 2013;371:148–53. doi: 10.1016/j.mce.2012.11.019. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 73•.Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol. Endocrinol. 2010;24:1322–37. doi: 10.1210/me.2009-0519. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Moore CC, Miller WL. The role of transcriptional regulation in steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1991;40:517–25. doi: 10.1016/0960-0760(91)90271-6. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 75•.Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol. Reprod. 1995;52:217–25. doi: 10.1095/biolreprod52.2.217. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 76•.Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell. 1999;3:521–26. doi: 10.1016/s1097-2765(00)80480-3. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 77•.Lewis AE, Rusten M, Hoivik EA, Vikse EL, Hansson ML, et al. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol. Endocrinol. 2008;22:91–104. doi: 10.1210/me.2006-0478. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.Chen WY, Juan LJ, Chung BC. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol. Cell. Biol. 2005;25:10442–53. doi: 10.1128/MCB.25.23.10442-10453.2005. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Chen WY, Weng JH, Huang CC, Chung BC. Histone deacetylase inhibitors reduce steroido-genesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1). Mol. Cell. Biol. 2007;27:7284–90. doi: 10.1128/MCB.00476-07. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J. Biol. Chem. 2004;279(37):38730–35. doi: 10.1074/jbc.M405006200. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 81•.Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, et al. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 2004;18(10):2451–62. doi: 10.1210/me.2004-0173. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 82•.Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 2005;25(5):1879–90. doi: 10.1128/MCB.25.5.1879-1890.2005. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Deleted in proof [Google Scholar]
- 84.Deleted in proof [Google Scholar]
- 85•.Campbell LA, Faivre EJ, Show MD, Ingraham JG, Flinders J, et al. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1). Mol. Cell. Biol. 2008;28:7476–86. doi: 10.1128/MCB.00103-08. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Yang WH, Heaton JH, Brevig H, Mukherjee S, Iniguez-Lluhi JA, Hammer GD. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol. Cell. Biol. 2009;29:613–25. doi: 10.1128/MCB.00295-08. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, et al. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol. Endocrinol. 1996;10:1261–72. doi: 10.1210/mend.10.10.9121493. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 88•.Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, et al. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology. 2002;143:665–73. doi: 10.1210/endo.143.2.8658. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 89•.Lalli E, Melner MH, Stocco DM, Sassone-Corsi P. DAX-1 blocks steroid production at multiple levels. Endocrinology. 1998;139:4237–43. doi: 10.1210/endo.139.10.6217. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 90•.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 91•.Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. PNAS. 2000;97:14488–93. doi: 10.1073/pnas.97.26.14488. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol. Endocrinol. 2009;23:1657–67. doi: 10.1210/me.2009-0055. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 2007;21:2968–87. doi: 10.1210/me.2007-0120. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 94•.Bashamboo A, McElreavey K. Gene mutations associated with anomalies of human gonad formation. Sex. Dev. 2013;7:126–46. doi: 10.1159/000342188. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 95•.Röpke A, Tewes AC, Gromoll J, Kliesch S, Wieacker P, Tüttelmann F. Comprehensive sequence analysis of the NR5A1 gene encoding steroidogenic factor 1 in a large group of infertile males. Eur. J. Hum. Genet. 2013;21:1012–15. doi: 10.1038/ejhg.2012.290. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Köhler B, Achermann JC. Update—steroidogenic factor 1 (SF-1, NR5A1). Minerva Endocrinol. 2010;35(2):73–86. [Medline] [Web of Science ®] [PubMed] [Google Scholar]
- 97•.Lourenco D, Brauner R, Lin L, De Perdigo A, Weryha G, et al. Mutations in NR5A1 associated with ovarian insufficiency. N. Engl. J. Med. 2009;360:1200–10. doi: 10.1056/NEJMoa0806228. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98•.Philibert P, Paris F, Lakhal B, Audran F, Gaspari L, et al. NR5A1 (SF-1) gene variants in a group of 26 young women with XX primary ovarian insufficiency. Fertil. Steril. 2012;99(2):484–89. doi: 10.1016/j.fertnstert.2012.10.026. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 99•.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 1999;22:125–26. doi: 10.1038/9629. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 100•.Malikova J, Camats N, Fernández-Cancio M, Heath K, González I, et al. Human NR5A1/SF-1 mutations show decreased activity on BDNF (brain-derived neurotrophic factor), an important regulator of energy balance: testing impact of novel SF-1 mutations beyond steroidogenesis. PLOS ONE. 2014;9(8):e104838. doi: 10.1371/journal.pone.0104838. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101•.Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–70. doi: 10.1242/dev.087247. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102•.Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol. Cell. Biol. 2008;28:7030–40. doi: 10.1128/MCB.00900-08. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103•.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 2006;26:4111–21. doi: 10.1128/MCB.00222-06. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104•.Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol. Cell. Endocrinol. 2011;336:206–12. doi: 10.1016/j.mce.2010.11.012. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994;135:431–38. doi: 10.1210/endo.135.1.8013381. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 106•.Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim. Biophys. Acta. 2003;1619:317–24. doi: 10.1016/s0304-4165(02)00490-7. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 107•.Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–28. doi: 10.1210/en.2009-0814. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108•.Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47:628–37. doi: 10.1002/dvg.20532. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 109•.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. PNAS. 2009;106:21185–90. doi: 10.1073/pnas.0909471106. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol. Cell. Endocrinol. 2011;336:117–22. doi: 10.1016/j.mce.2010.11.010. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111•.Paul A, Laufer E. Endogenous biotin as a marker of adrenocortical cells with steroidogenic potential. Mol. Cell. Endocrinol. 2011;336:133–40. doi: 10.1016/j.mce.2011.01.015. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112•.Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol. Cell. Endocrinol. 2012;351:19–27. doi: 10.1016/j.mce.2011.10.002. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Werminghaus P, Haase M, Hornsby PJ, Schinner S, Schott M, et al. Hedgehog-signaling is upregulated in non-producing human adrenal adenomas and antagonism of hedgehog-signaling inhibits proliferation of NCI-H295R cells and an immortalized primary human adrenal cell line. J. Steroid Biochem. Mol. Biol. 2014;139:7–15. doi: 10.1016/j.jsbmb.2013.09.007. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 114•.Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–32. doi: 10.1242/dev.092775. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Walczak EM, Kuick R, Finco I, Bohin N, Hrycaj SM, et al. Wnt-signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 2014;28(9):1471–86. doi: 10.1210/me.2014-1060. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116•.Lee FY, Faivre EJ, Suzawa M, Lontok E, Ebert D, et al. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev. Cell. 2011;21:315–27. doi: 10.1016/j.devcel.2011.06.028. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117•.Guasti L, Cavlan D, Cogger K, Banu Z, Shakur A, et al. Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of β1 integrin and ERK1/2. Endocrinology. 2013;154:4675–84. doi: 10.1210/en.2013-1211. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 118•.Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell. 2013;26:666–73. doi: 10.1016/j.devcel.2013.07.016. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119•.Gondos B, Rao A, Ramachandran J. Effects of antiserum to luteinizing hormone on the structure and function of rat Leydig cells. J. Endocrinol. 1980;87:265–70. doi: 10.1677/joe.0.0870265. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 120•.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 121•.Huang CC, Yao HH. Diverse functions of Hedgehog signaling in formation and physiology of steroidogenic organs. Mol. Reprod. Dev. 2010;77:489–96. doi: 10.1002/mrd.21174. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122•.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 2000;63:1825–38. doi: 10.1095/biolreprod63.6.1825. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 123•.Barsoum I, Yao HH. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biol. Reprod. 2011;84:894–99. doi: 10.1095/biolreprod.110.088997. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124•.Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signalling components in adult mouse testis. Dev. Dyn. 2006;235:3063–70. doi: 10.1002/dvdy.20931. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 125•.Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev. Biol. 2002;246:356–65. doi: 10.1006/dbio.2002.0663. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126•.Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, et al. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 2000;67:1302–5. doi: 10.1016/s0002-9297(07)62958-9. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127•.Umehara F, Mishima K, Egashira N, Ogata A, Iwasaki K, Fujiwara M. Elevated anxiety-like and depressive behavior in Desert hedgehog knockout male mice. Behav. Brain Res. 2006;174:167–73. doi: 10.1016/j.bbr.2006.07.022. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 128•.Jameson JL. Of mice and men: the tale of steroidogenic factor-1. J. Clin. Endocrinol. Metab. 2004;89:5927–29. doi: 10.1210/jc.2004-2047. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 129•.Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M, Morel Y. Gonadal dysgenesis without adrenal insufficiency in a 46,XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J. Clin. Endocrinol. Metab. 2004;89:4829–32. doi: 10.1210/jc.2004-0670. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 130•.Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, et al. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132:2415–23. doi: 10.1242/dev.01826. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 131•.Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev. Biol. 2009;329:96–103. doi: 10.1016/j.ydbio.2009.02.025. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132•.Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996;122:3283–94. doi: 10.1242/dev.122.10.3283. [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 133•.Zhang Y, Kalderon D. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development. 2000;127:2165–76. doi: 10.1242/dev.127.10.2165. [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 134•.O'Hara WA, Azar WJ, Behringer RR, Renfree MB, Pask AJ. Desert hedgehog is a mammal-specific gene expressed during testicular and ovarian development in a marsupial. BMC Dev. Biol. 2011;11:72. doi: 10.1186/1471-213X-11-72. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ren Y, Cowan RG, Harman RM, Quirk SM. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol. Endocrinol. 2009;23:711–23. doi: 10.1210/me.2008-0391. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136•.Ren Y, Cowan RG, Migone FF, Quirk SM. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol. Reprod. 2012;86:174. doi: 10.1095/biolreprod.112.099176. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137•.Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM. The hedgehog signaling pathway in the mouse ovary. Biol. Reprod. 2007;77:226–36. doi: 10.1095/biolreprod.106.053629. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 138•.Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology. 2005;146:3558–66. doi: 10.1210/en.2005-0311. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 139•.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 140•.Spicer LJ, Sudo S, Aad PY, Wang LS, Chun SY, et al. The hedgehog-patched signaling pathway and function in the mammalian ovary: a novel role for hedgehog proteins in stimulating proliferation and steroidogenesis of theca cells. Reproduction. 2009;138:329–39. doi: 10.1530/REP-08-0317. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 141•.Sugawara T, Holt JA, Driscoll D, Strauss JF, 3rd, Lin D, et al. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. PNAS. 1995;92:4778–82. doi: 10.1073/pnas.92.11.4778. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142•.Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. PNAS. 1995;92:10939–43. doi: 10.1073/pnas.92.24.10939. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143•.Ben-Zimra M, Koler M, Orly J. Transcription of cholesterol side-chain cleavage cytochrome P450 in the placenta: Activating protein-2 assumes the role of steroidogenic factor-1 by binding to an overlapping promoter element. Mol. Endocrinol. 2002;16:1864–80. doi: 10.1210/me.2002-0056. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 144•.Huang N, Miller WL. Cloning of factors related to HIV-inducible LBP proteins that regulate steroidogenic factor-1–independent human placental transcription of the cholesterol side-chain cleavage enzyme, P450scc. J. Biol. Chem. 2000;275:2852–58. doi: 10.1074/jbc.275.4.2852. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 145•.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 2002;245:280–90. doi: 10.1006/dbio.2002.0645. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 146•.Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol. Endocrinol. 2002;16:2338–48. doi: 10.1210/me.2001-0154. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 147•.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat. Genet. 2006;38:1204–9. doi: 10.1038/ng1874. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 148•.Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, et al. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol. Reprod. 2010;82:991–99. doi: 10.1095/biolreprod.109.081513. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149•.Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, et al. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: Does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am. J. Med. Genet. 1996;66:478–84. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 150•.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet. 1996;14:357–60. doi: 10.1038/ng1196-357. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 151•.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 2003;33:508–13. doi: 10.1038/ng1134. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 152•.Koide T, Hayata T, Cho KW. Negative regulation of Hedgehog signaling by the cholesterogenic enzyme 7-dehydrocholesterol reductase. Development. 2006;133:2395–405. doi: 10.1242/dev.02393. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 153•.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154•.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLOS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155•.Kang S, Graham JM, Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat. Genet. 1997;15:266–68. doi: 10.1038/ng0397-266. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 156.Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology. 2005;146:2555–62. doi: 10.1210/en.2004-1290. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 157•.Böse J, Grotewold L, Ruther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum. Mol. Genet. 2002;11:1129–35. doi: 10.1093/hmg/11.9.1129. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 158•.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLOS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159•.Szkandera J, Kiesslich T, Haybaeck J, Gerger A, Pichler M. Hedgehog signaling pathway in ovarian cancer. Int. J. Mol. Sci. 2013;14:1179–96. doi: 10.3390/ijms14011179. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160•.Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003;162:521–31. doi: 10.1016/S0002-9440(10)63846-1. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161•.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009;15:668–76. doi: 10.1158/1078-0432.CCR-08-1067. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162•.Ragazzon B, Libe R, Gaujoux S, Assie G, Fratticci A, et al. Transcriptome analysis reveals that p53 and β-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 2010;70:8276–81. doi: 10.1158/0008-5472.CAN-10-2014. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 163•.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014;46:607–12. doi: 10.1038/ng.2953. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]
- 164•.Liao X, Siu MK, Au CW, Wong ES, Chan HY, et al. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–40. doi: 10.1093/carcin/bgn230. [CrossRef] [Medline] [Web of Science ®] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165•.Kaye SB, Fehrenbacher L, Holloway R, Amit A, Karlan B, et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin. Cancer Res. 2012;18:6509–18. doi: 10.1158/1078-0432.CCR-12-1796. [CrossRef] [Medline] [Web of Science ®] [DOI] [PubMed] [Google Scholar]