Abstract
Early mobilization of critically ill patients with the acute respiratory distress syndrome (ARDS) has emerged as a therapeutic strategy that improves patient outcomes, such as the duration of mechanical ventilation and muscle strength. Despite the apparent efficacy of early mobility programs, their use in clinical practice is limited outside of specialized centers and clinical trials. To evaluate the mechanisms underlying mobility therapy, we exercised acute lung injury (ALI) mice for 2 days after the instillation of lipopolysaccharides into their lungs. We found that a short duration of moderate intensity exercise in ALI mice attenuated muscle ring finger 1 (MuRF1)–mediated atrophy of the limb and respiratory muscles and improved limb muscle force generation. Exercise also limited the influx of neutrophils into the alveolar space through modulation of a coordinated systemic neutrophil chemokine response. Granulocyte colony-stimulating factor (G-CSF) concentrations were systemically reduced by exercise in ALI mice, and in vivo blockade of the G-CSF receptor recapitulated the lung exercise phenotype in ALI mice. Additionally, plasma G-CSF concentrations in humans with acute respiratory failure (ARF) undergoing early mobility therapy showed greater decrements over time compared to control ARF patients. Together, these data provide a mechanism whereby early mobility therapy attenuates muscle wasting and limits ongoing alveolar neutrophilia through modulation of systemic neutrophil chemokines in lung-injured mice and humans.
INTRODUCTION
Early mobilization of critically ill patients has emerged as a promising therapy to improve outcomes of patients with the acute respiratory distress syndrome (ARDS) and other critical illnesses. Clinical research studies suggest that early mobilization of critically ill patients improves a number of meaningful variables, including intensive care unit length of stay (LOS), hospital LOS, delirium, hospital readmissions, and strength (1–5). The mechanisms underlying this therapy are currently unknown, although the improvements in a variety of organ systems (lung, muscle, and brain) suggest that early mobility/exercise may have widespread systemic effects. Despite the apparent efficacy of early mobility in critically ill patients, routine implementation of these programs outside the context of clinical trials remains low (6, 7). There are currently no pharmacologic therapies available to prevent or treat the extensive muscle wasting and functional disability present in survivors of critical illnesses, such as sepsis and ARDS (8). It is therefore important to understand the mechanisms underlying early mobility therapy so that targets can be identified and therapies can be developed to improve the outcomes of patients with these illnesses.
We and others have shown that a mouse model of ARDS [referred to as acute lung injury (ALI) mice] develops profound skeletal muscle atrophy and weakness, which replicate many of the aspects of muscle wasting observed in patients with ARDS (1–5, 9, 10). Initiation of muscle atrophy in this model occurs temporally with lung injury, is associated with nuclear factor κB (NF-κB) muscle activity, and depends on the expression of the muscle-specific E3 ubiquitin ligase muscle ring finger 1 (MuRF1) protein, which ubiquitinates contractile proteins for proteasomal-mediated degradation (6, 7, 9, 10). Although previous animal studies have shown that preconditioning mice with exercise can reduce the response to subsequent lung injury (8, 11), the ability of exercise to therapeutically improve outcomes after lung injury is unknown.
We exercised ALI mice after lung injury to evaluate the effects of therapeutic exercise on lung and muscle injury. Exercise led to marked improvements in lung, limb, and respiratory muscle injury. In this model, exercise altered expression of a systemic cytokine and chemokine response involving neutrophilic bone marrow mobilization and recruitment. Therapeutic exercise reduced neutrophil alveolitis, MuRF1-mediated skeletal muscle atrophy, and muscle weakness. Therapeutic blockade of granulocyte colony-stimulating factor (G-CSF), a known biomarker of poor outcomes in patients with ARDS (12), recapitulated some features of the exercise phenotype in ALI mice. In addition, human patients with acute respiratory failure (ARF) undergoing early mobility therapy showed greater decrements in G-CSF levels over time, compared to non-exercised patients, confirming the relevance of G-CSF as a mediator of the improved outcomes of mobilized/exercised critically ill patients. These data in mice and humans suggest a unifying mechanism underlying the beneficial effects of therapeutic exercise in mice and humans with lung injury.
RESULTS
ALI mice exhibit marked lung inflammation
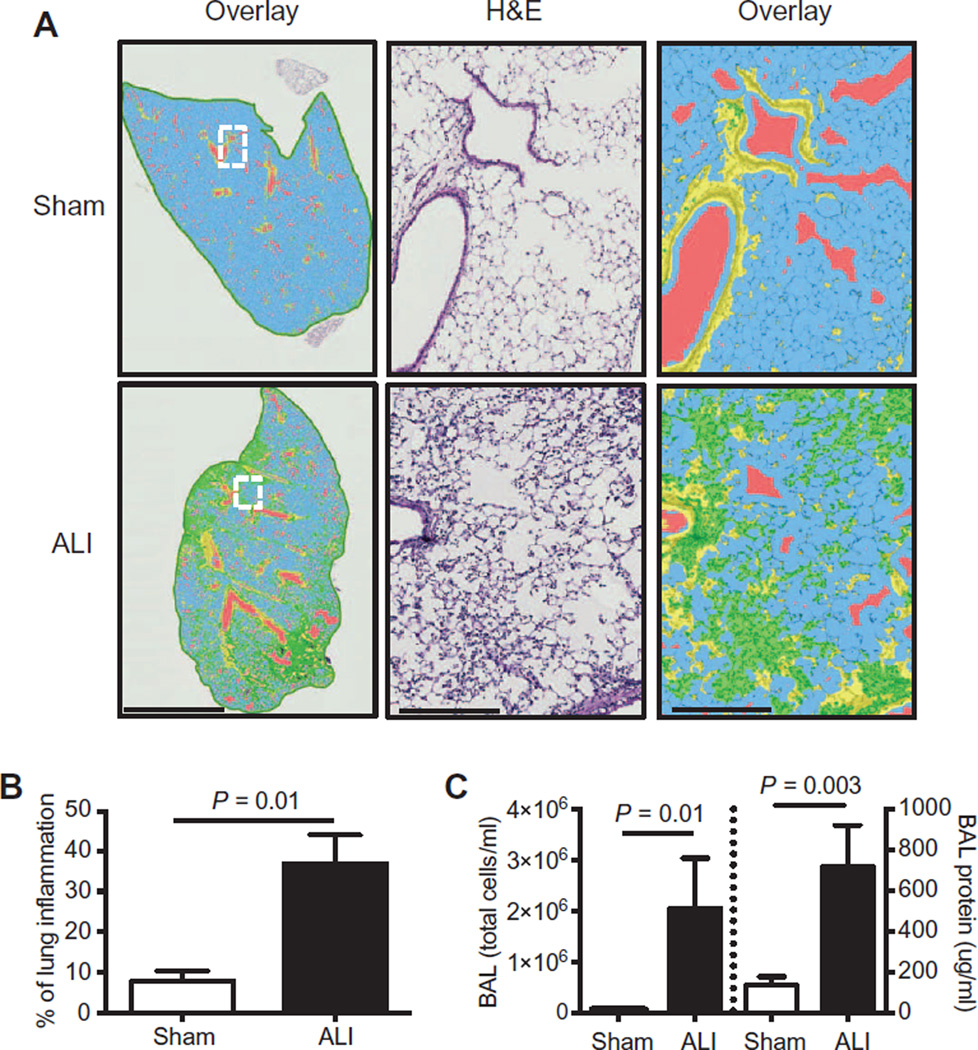
Instillation of lipopolysaccharides (LPS) into the lungs of 2-month-old wild-type (ALI) mice resulted in acute lung inflammation, marked by predominant alveolar and interstitial cellular infiltration and interstitial thickening at day 3, which was quantified by digital morphometry software (Fig. 1, A and B). Day 3 bronchoalveolar lavage (BAL) cells and protein (Fig. 1C) were also similarly increased in ALI mice at this time point of maximal lung injury, on the basis of our previous work with this model (10).
Fig. 1. Intratracheal instillation of LPS causes lung injury in mice.
(A) Representative hematoxylin and eosin (H&E)–stained left lung sections of sham and ALI mice are shown and were evaluated for parenchymal inflammation using digital imaging software at low (left panels) or high (middle and right panels) magnification at day 3. In overlay images, normal alveolar space (blue), airways (red), bronchial epithelium (yellow), and injured areas (green) can be identified. Scale bar, 3 mm (left panels); 300 µm (middle and right panels). (B) Quantification of the percentage of lung inflammation based on digital morphometry images. (C) Bronchoalveolar total cell counts (left axis) and protein levels (right axis) in sham and ALI mice on day 3. n = 4 to 6 per group. Data were analyzed using the Student’s two-tailed t test.
ALI mice have reduced physical activity
We have previously shown that ALI mice display profound limb muscle wasting, mediated in part by the muscle expression of MuRF1 protein, which is up-regulated in the limb muscles of ALI mice during days 1 to 4 after LPS instillation (10). Here, we observed that ALI mice exhibited reduced physical activity, quantified by both traveling (Fig. 2A) and rearing activity (Fig. 2B).
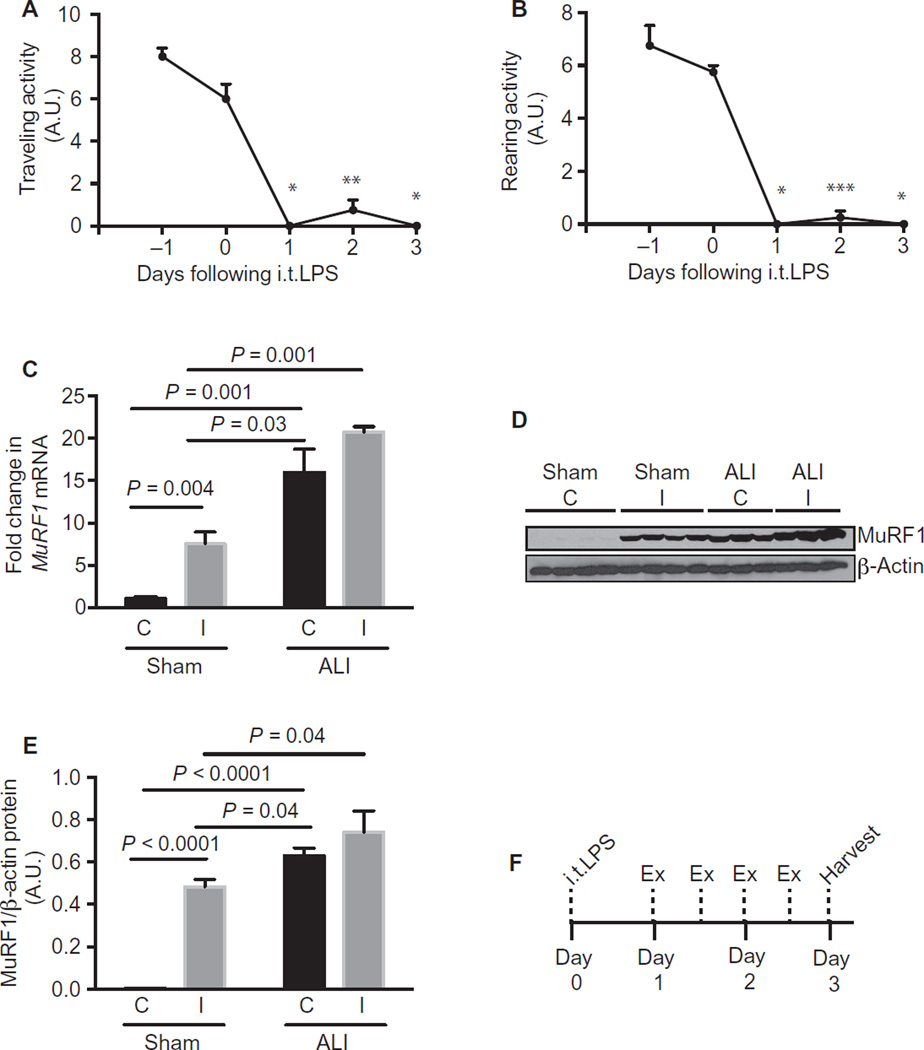
Fig. 2. Physical activity is reduced in ALI mice.
(A and B) Traveling (A) and rearing (B) activity was measured 2 days before and 3 days after instillation of intratracheal LPS (i.t.LPS). A.U., arbitrary unit. (C to E) Sham and ALI mice underwent hindlimb immobilization at day 0 and were harvested at day 3. MuRF1 mRNA (C) and protein (D and E) levels were quantified in the tibialis anterior muscle of sham and ALI control (C) and immobilized (I) muscles. (F) Experimental scheme of most exercise experiments detailed within this article. Deviations from this scheme are noted within the text. Values represent means ± SEM. n = 4 per group (A and B); n =3 to 4 per group (C to E). (A and B) *P = 0.00002, **P = 0.0003, and ***P = 0.00006 with Bonferroni correction at all time points compared to time 0 using the Student’s two-tailed t test.
To determine whether muscle wasting in ALI mice phenotypically differs from that induced by reduced activity or immobility, we adopted a model of hindlimb immobilization in sham and ALI mice. As previously reported, one hindlimb is secured in a flexed position so that the muscles of that limb are completely immobile (13). The contralateral rear hindlimb serves as an internal control. We applied this hindlimb immobilization model to sham and ALI mice at day 0 and harvested the mice at day 3. We found that complete limb immobility increased MuRF1 mRNA (Fig. 2C) and protein levels (Fig. 2, D and E) in the tibialis anterior muscle in sham mice as previously reported (13). However, ALI control conditions were associated with greater MuRF1 levels compared to sham immobilized conditions. ALI + immobilized muscles failed to display further significant MuRF1 up-regulation, showing that most MuRF1 activation in ALI mice can be accounted for by ALI conditions alone and that muscle wasting in ALI mice is phenotypically different from that induced by immobility. We hypothesized that this reduction in physical activity was maladaptive and sought to determine whether increased activity/exercise after instillation of LPS would attenuate the muscle wasting that occurs in ALI mice.
Therapeutic exercise attenuates skeletal muscle atrophy in ALI mice
We designed exercise regimens that would be expected to approximate 40 to 60% maximal oxygen consumption (VO2 max) in a healthy young C57BL/6J mouse (14), ranging from 5 min daily to 35 min twice daily starting 24 hours after the instillation of LPS (day 1). We found that exercise regimens ranging from 5 min daily to 25 min twice daily (table S1) attenuated the loss of muscle mass in ALI mice in individual muscles with a variety of fiber-type compositions. Tibialis anterior (type IIa/x and type IIb fibers), extensor digitorum longus (type IIax and type IIb fibers), and soleus (type IIa/x, type IIb, and type I fibers) muscle mass loss was attenuated at most doses of exercise. At the highest intensity and duration of exercise, 35 min twice daily, exercise failed to attenuate the loss of muscle mass in ALI mice (fig. S1A). Figure 2F demonstrates the ALI and exercise regimen used in the remaining experiments in this article at a dose of 25 min twice daily, hereby referred to ALI + Ex mice. We found a trend toward improved survival in ALI + Ex mice using this dose of exercise, but the difference did not reach statistical significance (97% ALI + Ex versus 90% ALI, P = 0.06) (fig. S1B).
We further examined the myofiber composition of the soleus and diaphragm in sham, ALI, and ALI + Ex mice. Similar to our previous reports, we found that ALI conditions caused type II myofiber atrophy in the limb muscle (soleus), with relative preservation of type I myofibers. In contrast, ALI mice displayed atrophy of both type I and type II myofibers of the diaphragm. Exercise in ALI mice attenuated type II myofiber atrophy in both the soleus and the diaphragm (Fig. 3, A to C). These data show that therapeutic exercise in ALI mice attenuates type II myofiber atrophy.
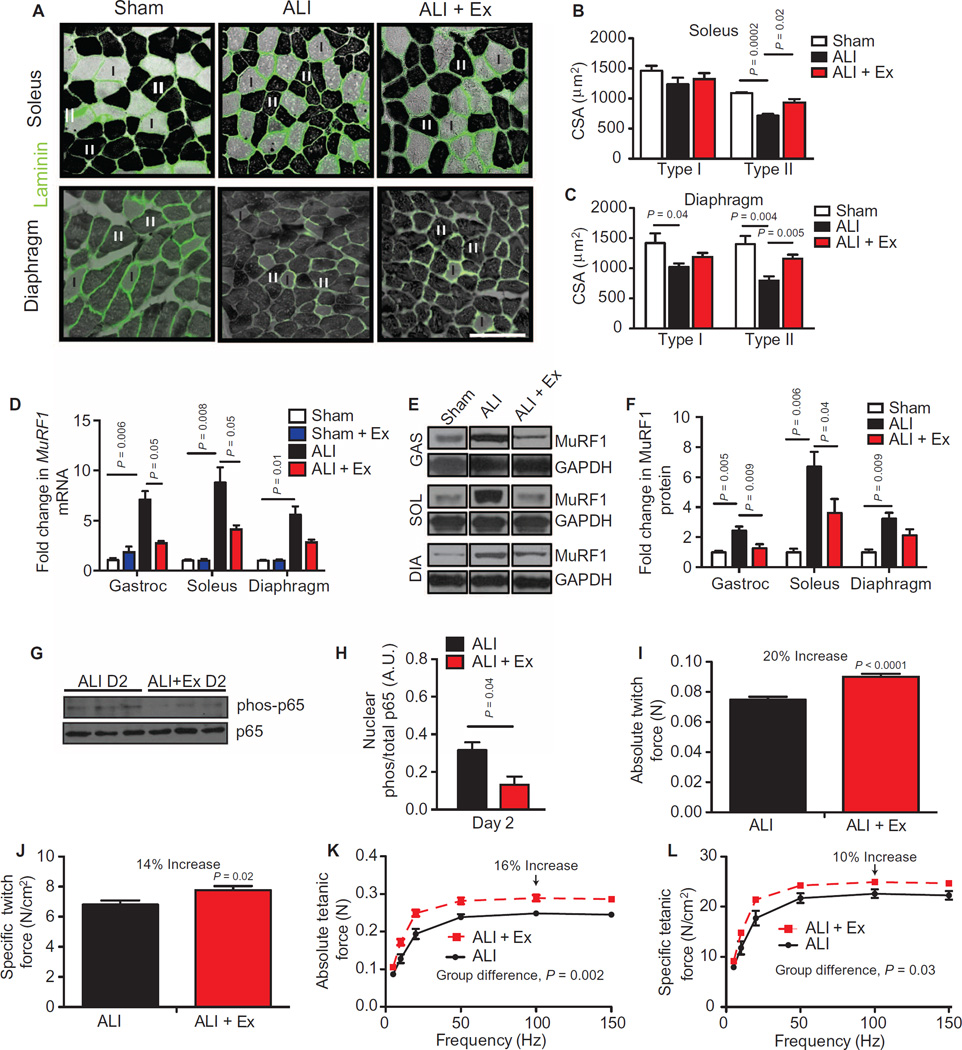
Fig. 3. Therapeutic exercise attenuates ALI-induced muscle atrophy and improves muscle performance.
(A) Type I (light) and II (dark) myofibers of the soleus and the diaphragm were identified by adenosine triphosphatase (ATPase) and laminin (green) staining. Scale bar, 100 µm. (B and C) Muscle fiber cross-sectional area (CSA) was quantified in sham, ALI, and ALI + Ex mice. (D to F) MuRF1 mRNA (D) and protein (E and F) levels normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in gastrocnemius (GAS), diaphragm (DIA), and soleus (SOL) muscles. (G and H) Phosphorylated and total p65 protein from gastrocnemius nuclear extracts. D2 (day 2). (I to L) Ex vivo isolated soleus (I and J) absolute and specific twitch and (K and L) tetanic contractile force measurements. Values represent means ± SEM. All experimental time points are at day 3, other than (G) and (H), which are at day 2. n = 4 to 5 per group (A to D); n = 6 to 8 per group of two combined experiments (F); n = 3 per group (G and H); n = 4 animals and 8 muscles per group (I to L). Data were analyzed using the Student’s two-tailed t test or analysis of variance (ANOVA) for group differences with multiple time points.
Therapeutic exercise in ALI mice attenuates MuRF1-mediated atrophy and improves muscle performance
We have previously shown that up-regulation of MuRF1 protein is necessary for the skeletal muscle atrophy that occurs in this model at days 3 to 4 (10, 15). Here, we confirmed that ALI mice had increased MuRF1 mRNA and protein expression compared to sham mice in all muscles evaluated. Notably, ALI + Ex mice exhibited reduced MuRF1 mRNA and protein expression in both the limb muscles and the diaphragm compared to ALI mice (Fig. 3, D to F).
To determine whether therapeutic exercise alters muscle MuRF1 expression in the absence of ALI, we exercised sham mice (Sham + Ex) with the same exercise protocol used in the ALI + Ex mice. Exercise alone had no effect on MuRF1 mRNA in the absence of ALI (Fig. 3D).
We and others have shown that ALI mice have increased muscle NF-κB activation (9, 10), which is a known upstream mediator of MuRF1 induction (16). Here, we found that ALI + Ex mice had reduced muscle NF-κB activation compared to ALI mice at day 2, measured by reduced phosphorylation of the NF-κB subunit p65 in nuclear preparations (Fig. 3, G and H).
We have previously shown that ALI mice develop limb muscle weakness, with a ~30% decrease in maximal tetanic force compared to sham mice (10). To determine whether exercise in ALI mice preserves muscle function, we isolated the soleus muscle and stimulated the muscle directly via electrical field stimulation according to previously published methods (17) adapted for the soleus muscle. We found a 20 and 14% increase in twitch absolute and specific forces, respectively, in ALI + Ex compared to ALI mice (Fig. 3, I and J). Likewise, absolute and specific tetanic forces were increased at all measured frequencies in ALI + Ex mice. Maximal tetanic absolute and specific forces were increased 16 and 10%, respectively, in ALI + Ex versus ALI mice (Fig. 3, K and L). We found no difference in soleus muscle fatigability between ALI versus ALI + Ex mice (fig. S1C). These data demonstrate that exercise in ALI mice reduces MuRF1-mediated atrophy and improves muscle function in ALI mice.
Exercise in ALI mice attenuates neutrophil alveolitis
Because muscle atrophy and function were improved in ALI + Ex mice, and muscle atrophy is temporally correlated with lung injury in this model (10), we quantified lung injury parameters in the three groups. Surprisingly, we found that exercise in ALI mice limited the influx of neutrophils into the alveolar compartment. BAL total cells were reduced at day 3 in ALI + Ex compared to ALI mice, with reductions in monocytes/macrophages, neutrophils, and lymphocytes at this time point of peak lung inflammation (Fig. 4, A and B). We also found reduced total cell counts in the alveolar space of ALI + Ex mice at most doses of exercise studied (fig. S1D). Neutrophils are the predominant inflammatory cell in the lung at day 3 (Fig. 4B), so the reduction in alveolar cell counts with exercise can primarily be accounted for by reduced alveolar neutrophilia. To evaluate the possibility that exercise increased alveolar neutrophil apoptosis, we labeled BAL neutrophils for the apoptosis markers 7-aminoactinomycin D and annexin V. We found no evidence of increased neutrophil apoptosis in the alveolar space of ALI + Ex mice (fig. S1E). In contrast to the reduced cells found in the alveolar compartment, cell-free BAL total protein or albumin was not reduced in ALI + Ex mice (fig. S1, F and G). These data suggest that therapeutic exercise in ALI mice reduces neutrophil alveolitis, a finding associated with poor outcomes in ARDS (18, 19).
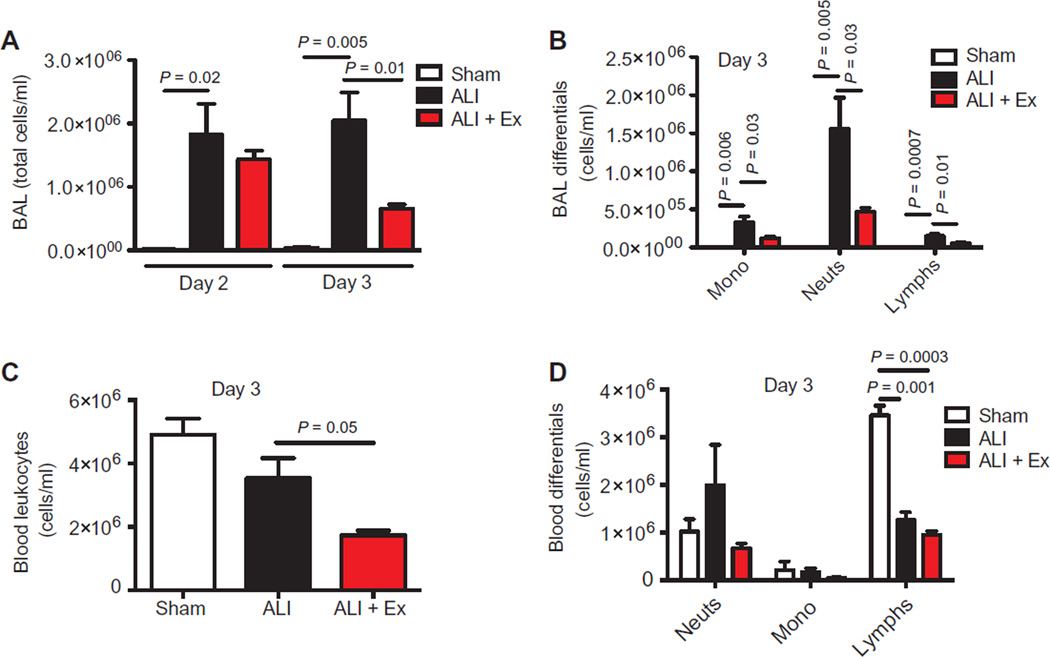
Fig. 4. Therapeutic exercise reduces alveolar neutrophilia.
(A and B) BAL total leukocyte (A) and differential cell counts (B) in sham, ALI, and ALI + Ex mice at specified time points. (C and D) Total blood leukocytes (C) and differential cell counts (D) were measured in sham, ALI, and ALI + Ex mice at day 3. n = 3 to 6 per group. Data were analyzed using the Student’s two-tailed t test.
Exercise in ALI mice alters the systemic neutrophil cytokine/chemokine response
We found that exercise (ALI + Ex) reduced total blood leukocytes compared to ALI mice without exercise (Fig. 4C). ALI conditions shifted blood leukocytes from a predominant lymphocytic (sham mice) to a predominant neutrophilic (ALI mice) profile (Fig. 4D). The reduction in total leukocytes in ALI + Ex mice compared to ALI mice was primarily accounted for by an about twofold reduction in blood neutrophils (Fig. 4D).
To evaluate for potential systemic mediators underlying the improvements in lung and muscle injury seen in ALI + Ex mice, we performed an unbiased inflammatory biomarker array on 144 murine cytokines in ALI and ALI + Ex mice. Table 1 lists the top up- and down-regulated proteins with P ≤ 0.08 in the array. We found that 10 proteins were significantly differentially regulated (P ≤ 0.05) in ALI + Ex versus ALI mice (Table 1). Seven of the 10 significantly differentially regulated proteins (P ≤ 0.05; starred and bolded in Table 1) and 9 of the 15 potentially differentially regulated proteins (P ≤ 0.08; starred in Table 1) have known roles in neutrophil mobilization and recruitment. Data from all 144 proteins can be found in table S2. Notably, therapeutic exercise did not change the levels of tumor necrosis factor–α (TNF-α), interleukin-6 (IL-6), or IL-10 (table S2), which have been implicated in the protective effect of 5 weeks of prophylactic aerobic exercise in lung-injured mice (11). These data suggest that therapeutic exercise in ALI mice has specific immunomodulatory effects, most notably on regulation of cytokines and chemokines involved in neutrophil mobilization and migration. This effect appears mechanistically different from chronic aerobic “preconditioning” exercise.
Table 1.
Exercise in ALI mice induces a systemic neutrophil cytokine/chemokine response.
| Response to exercise | Protein | Fold change | % Change | P |
|---|---|---|---|---|
| Down-regulation | G-CSF* | −2.6 | −160 | 0.02 |
| CXCL15* | −2.4 | −140 | 0.02 | |
| KC* | −2.0 | −100 | 0.08 | |
| Osteoprotegerin | −1.9 | −90 | 0.03 | |
| GAS 6* | −1.7 | −70 | 0.04 | |
| IL-17F* | −1.6 | −60 | 0.05 | |
| VEGFR3 | −1.4 | −40 | 0.02 | |
| IL-17B* | −1.2 | −20 | 0.02 | |
| DKK-1 | −1.2 | −20 | 0.06 | |
| IL-1ra | −1.5 | −50 | 0.06 | |
| CXCL10* | −1.5 | −50 | 0.06 | |
| HGF | −1.3 | −30 | 0.07 | |
| Up-regulation | Galectin-1* | 2.1 | +110 | 0.009 |
| MMP-3 | 2.0 | +100 | 0.03 | |
| MIP-2* | 1.3 | +30 | 0.02 |
An unbiased inflammatory biomarker array of 144 proteins was performed on the plasma of ALI + Ex and ALI mice at day 3. n = 4 per group. Displayed are the proteins with P values ≤0.08 in the ALI + Ex versus ALI mice. Proteins in bold have P values ≤0.05. All proteins can be found in table S2. Starred cytokines (*) have been previously implicated in neutrophil mobilization, activation, and/or migration.
Exercise reduces systemic G-CSF concentrations in ALI mice
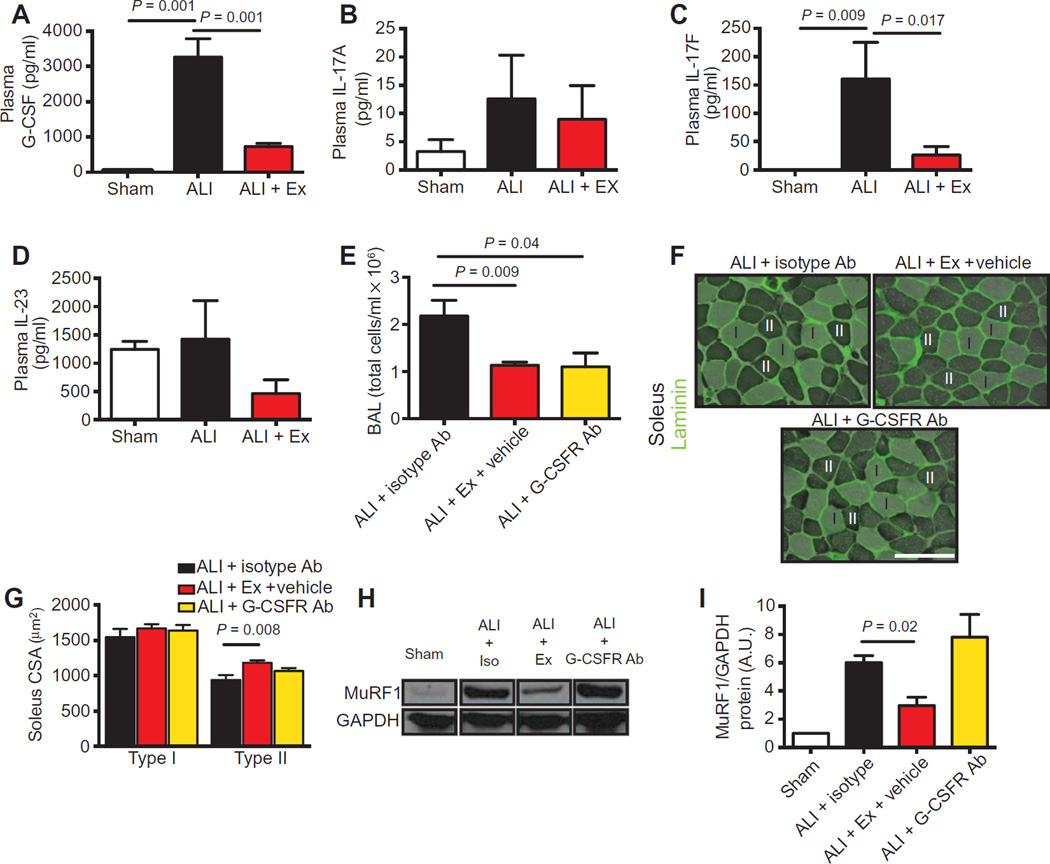
We chose to focus further investigation on the top down-regulated cytokine in our array, G-CSF, because of its well-described mechanisms of action (20) and its known relevance in human ARDS (12, 19). G-CSF protein was markedly up-regulated (~100-fold) in the plasma of ALI compared to sham mice. Exercise profoundly reduced systemic G-CSF levels in ALI mice (Fig. 5A). We also quantified IL-17A, IL-17F, and IL-23, known upstream regulators of G-CSF production. We found similar trends in the pattern of expression of these cytokines, namely, reduction in systemic levels with exercise, although only IL-17F reached statistical significance (Fig. 5, B to D).
Fig. 5. Blockade of G-CSF activity limits lung injury but does not attenuate muscle atrophy.
(A to D) G-CSF, IL-17A, IL-17F, and IL-23 protein quantification in the plasma of sham, ALI, and ALI + Ex mice. (E) BAL cell counts were quantified at day 3 after systemic administration of isotype antibody (ALI + isotype Ab), exercise (ALI + Ex + vehicle), or G-CSFR–blocking antibody (ALI + G-CSFR Ab) 1 day after i.t.LPS administration. (F) Type I (light) and II (dark) myofibers of the soleus were identified by ATPase and laminin (green) staining. (G) Cross-sectional area was quantified in ALI + isotype Ab, ALI + Ex + vehicle, and ALI + G-CSFR Ab mice. Scale bar, 100 µm. (H and I) Soleus muscle lysates were probed for MuRF1 protein and normalized to GAPDH in sham, ALI + isotype Ab, ALI + Ex + vehicle, and ALI + G-CSFR Ab mice (H) and quantified by densitometry (I). n = 3 to 7 per group. Data were analyzed using the Student’s two-tailed t test.
Blockade of the G-CSF receptor in vivo reduces neutrophilic alveolar influx but does not prevent muscle atrophy
To determine the specificity of the role of G-CSF in mediating neutrophilic lung injury and muscle wasting, we systemically administered G-CSF receptor–blocking antibodies to ALI mice (ALI + G-CSFR Ab), starting 24 hours after i.t.LPS, and harvested the mice at day 3. We found that G-CSFR blockade reduced cells in the alveolar space compared to ALI mice receiving an isotype control antibody (ALI + isotype) to a similar degree to that seen in ALI + Ex mice (Fig. 5E). In contrast, blockade of the G-CSFR did not attenuate soleus type II myofiber atrophy as seen in the ALI + Ex mice (Fig. 5, F and G). Likewise, MuRF1 protein levels in ALI + G-CSFR Ab–treated mice were not reduced compared to those in ALI + isotype mice (Fig. 5, H and I) as they were in ALI + Ex mice. We also administered G-CSFR Ab starting at day −1 or 0 and continued daily through day 2 until harvest at day 3. Similarly, we found that G-CSFR blockade reduced cellular alveolitis in ALI mice (fig. S2A) but did not attenuateMuRF1-mediated atrophy (fig. S2, B to D).
These data suggest that G-CSF, potentially driven by IL-17F and/or IL-23, is a key regulator of the improved alveolar neutrophilic injury in exercised ALI mice, although the attenuation in MuRF1-mediated muscle atrophy in ALI + Ex mice occurs through a G-CSF–independent mechanism.
Therapeutic exercise in humans with ARF is associated with reduced plasma G-CSF concentrations over time
To confirm the relevance of our murine studies, we obtained banked plasma from a randomized controlled pilot study of early mobilization (exercise) versus usual care (no exercise) in patients with ARF in the Wake Forest Medical Intensive Care Unit. The details of this study can be found in Supplementary Materials and Methods. Briefly, all patients randomized to exercise received passive range of motion of the extremities three times daily, replaced by active physical therapy as patient interaction improved, which included sitting on the edge of bed, standing, and walking based on a previously described protocol (1). The baseline characteristics of patients at enrollment in each arm can be found in table S3.
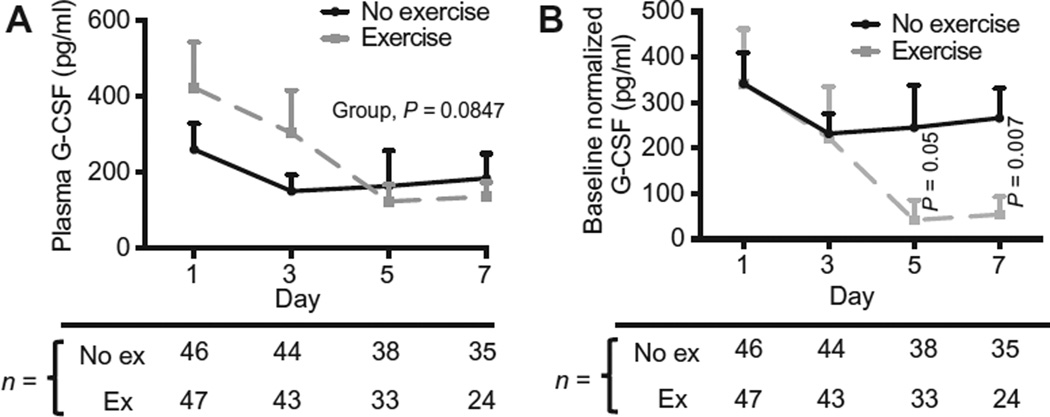
Respiratory failure patients randomized to exercise had a 68% reduction in G-CSF levels from baseline to day 7 versus a 29% reduction in control patients over this time interval (Fig. 6A). Despite randomization, baseline (day 1) levels of G-CSF were higher in the exercise group. We therefore normalized the data to adjust to the mean baseline level of G-CSF for each group (Fig. 6B). After this adjustment, we found that G-CSF was significantly reduced at the day 5 (P = 0.0538) and day 7 (P = 0.0072) time points in the exercise versus no exercise groups. These data add human relevance to the murine findings described above, which show that therapeutic exercise reduces systemic levels of G-CSF in ALI mice.
Fig. 6. Early mobility therapy reduces plasma G-CSF over time in patients with ARF.
(A) G-CSF concentrations measured by enzyme-linked immunosorbent assay (ELISA) at specified time points on plasma samples from patients in a randomized controlled study of early mobility therapy. (B) G-CSF concentrations normalized to the mean baseline concentration of G-CSF of each group at specific time points. Group differences over time were analyzed using the generalized estimating equation (GEE) method, and time point differences in (B) were compared using the Mann-Whitney test. ex, exercise.
DISCUSSION
In these studies, we have identified mechanisms underlying the benefits of early mobility therapy demonstrated by previous clinical investigations (1, 2, 4, 5). We find that therapeutically treating ALI mice with moderate intensity exercise of 2 days’ duration improved both skeletal muscle wasting and neutrophilic alveolar influx. Exercise appears to have specific immunomodulatory effects, most notably on limiting the mobilization and recruitment of neutrophils from the bone marrow to the lung by neutrophil cytokines and chemokines. G-CSF appears to be a key mediator of this process and is reduced in humans and mice with lung injury undergoing therapeutic exercise. The attenuation in muscle atrophy with exercise in ALI mice occurs independently of G-CSF, because inhibition of the G-CSFR did not improve muscle atrophy in ALI mice.
Our data support the concept that although neutrophils are critical for the early response to inflammation or infection in the lung, ongoing lung neutrophilia promotes lung injury (21). In mouse models of lung injury induced by endotoxin (22) or endotoxin plus oxygen (23), neutrophil depletion is protective. In addition, prolonged alveolar neutrophilia is associated with mortality in patients with ARDS (18, 19, 24). Our data suggest that therapeutic exercise in mice and humans may act as a rheostat to dampen ongoing neutrophil numbers by reducing G-CSF, which may, in turn, be driven by down-regulation of IL-17F and IL-23 (25). G-CSF’s activity in this model is likely occurring through binding to the G-CSFR, which is present on neutrophils, monocytes, and bone marrow hematopoietic stem/progenitor cells (26), as well as myoblasts (27) and endothelial cells (28).
The administration of recombinant G-CSF (rG-CSF) can cause lung injury in rats (29, 30) and has been associated with ARDS in immunocompromised patients (31, 32), although systemic administration of rG-CSF in nonimmunocompromised patients with pneumonia does not appear to lead to an increase in ARDS (33–35). Suratt et al. demonstrated in a large cohort of ARDS patients that endogenous G-CSF levels are positively correlated with duration of mechanical ventilation, organ failure, and mortality (12). Our results in mice suggest that the endogenous G-CSF contributes to alveolar neutrophilic injury and that therapeutic exercise helps reduce G-CSF over time. Collectively, these data suggest that G-CSF is at least a biomarker and may be pathogenic in ARDS. In contrast to this, the lack of ARDS development with rG-CSF administration in pneumonia patients suggests that rG-CSF alone is not sufficient to cause/promote ARDS.
The actions of G-CSF in skeletal muscle have also previously been examined. Hara et al. have shown that the G-CSFR is transiently expressed in cardiotoxin-injured regenerating mouse skeletal muscle: blockade of the G-CSFR is deleterious for muscle regeneration in this model, suggesting that G-CSF is beneficial for repair after muscle injury (27). Although we found no harm to the skeletal muscle after blocking the G-CSFR in our model, this issue and its potential negative implications for muscle regeneration after injury will need to be considered.
In addition to the well-described role of G-CSF in neutrophil mobilization and activation (36), 9 of the 15 proteins in our array with P < 0.08, including CXCL15 (lungkine) (37), keratinocyte chemoattractant (KC) (23), growth arrest specific 6 (GAS 6) (38), IL-17F/B (39),CXCL10 (40), galectin-1 (41), and macrophage inflammatory protein–2 (MIP-2) (23), have been implicated in neutrophil migration. Because these chemokines alter neutrophil migration, our data suggest that exercise induces a coordinated response that reprograms bone marrow mobilization, activation, and migration of neutrophils. Further studies are needed to define the roles of each of these cytokines/chemokines in this model, as well as the other proteins that are differentially regulated in ALI + Ex mice.
Previous work has demonstrated that 4 to 8 weeks of prophylactic exercise reduce lung injury in mouse models of asthma (42, 43) or ALI (11). Gonçalves et al. showed that 4 to 6 weeks of prophylactic aerobic exercise reduced lung neutrophilia and TNF-α and increased IL-6 and IL-10 after LPS injury (11). We found no change in TNF-α, IL-6, or IL-10 with 2 days of therapeutic exercise after lung injury. This suggests that preconditioning and therapeutic exercise both benefit the lung response to LPS-induced injury, although the mechanisms mediating these benefits may differ.
Our study also has implications for the timing and delivery of early mobility therapies in humans with ARDS. Because exercise in our lung-injured mice affected cytokines involved in neutrophil mobilization and migration, and neutrophil influx into the alveolar space is an early event during the time course of ARDS, our findings imply that early mobility therapies in the critically ill should start as early as possible. We also found that the highest intensity of exercise in ALI mice did not attenuate muscle wasting. Intense exercise in physically fit humans has been associated with leukopenia and an increased risk of upper respiratory tract infections after training (44). Exercise doses ranging from 5 to 25 min twice daily generally attenuated the loss of muscle mass in ALI mice; conversely, the highest exercise dose 35 min twice daily either increased or failed to attenuate (depending on the muscle studied) muscle wasting. This response to exercise, in which low to moderate exercise is protective but the highest intensity exercise is detrimental, fits with the previously proposed “J-curve” immune response to exercise observed in humans (45). Future clinical studies should focus on exercise intensity dosing strategies in ARDS patients.
We have previously shown that muscle wasting in ALI mice is associated with increased activity of muscle NF-κB (10), a known promoter of muscle atrophy and upstream mediator of MuRF1 expression (16). Others have shown that exercise can reduce NF-κB lung activation in injured mouse lungs (42), and we also find that therapeutic exercise acutely reduces muscle NF-κB activation, which may drive the down-regulation of MuRF1 expression in ALI + Ex mice. Previous investigators have shown that the NF-κB–mediated effect of exercise occurs through a glucocorticoid receptor–dependent mechanism (46).
Last, and in line with our previous studies, ALI mice developed type II myofiber atrophy after lung injury. We found both type I and type II myofibers in the diaphragm atrophy in ALI mice, consistent with a recent report of diaphragmatic biopsies in critically ill humans (47). Therapeutic exercise in ALI mice attenuated type II myofiber atrophy in the limb muscle and diaphragm but did not improve type I myofiber atrophy in the diaphragm. Because MuRF1 is preferentially expressed in type II myofibers (48), we hypothesize that this is the reason for the discrepancy, in that exercise reduces whole-muscle MuRF1 expression in both the limb and the diaphragm but does not attenuate the type I myofiber atrophy in the diaphragm. It is also noteworthy that exercise also had beneficial effects on limb muscle function that were independent of muscle atrophy, given that most improvement in force production in the soleus was from specific, not absolute, force in ALI + Ex mice.
There are limitations to our study. We have not examined neutrophil function in ALI + Ex mice. The coordinated neutrophil cytokine modulation seen with exercise would suggest changes in neutrophil functionality, including possibly their ability to migrate across endothelial/epithelial barriers. The roles of other differentially regulated cytokines besides G-CSF in ALI + Ex mice were not examined in this study. Future work should examine the role of therapeutic exercise in mice with sepsis, such as the cecal ligation and puncture model. Additionally, the G-CSF plasma measurements in mobilized human ARF patients need to be confirmed in a larger cohort of patients. Future studies are needed to clarify these issues, as well as the mechanism responsible for decreasing G-CSF during exercise.
In summary, therapeutic exercise improves both neutrophilic alveolar lung injury and skeletal muscle wasting in an animal model of ARDS. A short duration of acute exercise in ALI mice reduces neutrophil mobilization and recruitment to the lung and attenuates MuRF1-mediated skeletal atrophy and the loss of specific force. Pharmacologic blockade of neutrophil mobilization and recruitment via G-CSF and other neutrophil chemokines are attractive targets to facilitate resolution of lung injury but may not attenuate the skeletal muscle wasting associated with ARDS. Early mobilization of patients with lung injury is a promising therapy to improve the outcomes of patients with ARDS. Additional studies are needed to further elucidate the mechanisms underlying its benefits, which may reveal new molecular targets for ARDS treatment.
MATERIALS AND METHODS
Study design
The aim of this study was to uncover mechanisms underlying the clinical benefits of early mobility therapy documented in various clinical studies. The study was performed using both a murine lung injury model and plasma from lung-injured humans undergoing early mobility therapy. The mouse studies were carried out by instillation of LPS into the lungs of mice. Subsets of mice underwent limb immobilization or performed therapeutic exercise after lung injury using a treadmill. Mice were randomly assigned to treatment groups, and where possible, the researchers were blinded to the treatment groups until statistical analysis. The predefined study endpoints included various aspects of muscle function and size, including maximal tetanic force, myofiber cross-sectional area, and quantification of muscle-wasting genes and proteins. Lung injury was quantified by alveolar cell counts and protein and histology scores. Systemic mediators of therapeutic exercise were quantified in mouse plasma with an inflammatory biomarker array. Quantification of lung and muscle injury parameters was performed with the following techniques: immunostaining, Western blotting, flow cytometry, ex vivo muscle stimulation, ELISA, and reverse transcription quantitative polymerase chain reaction (RT-qPCR).
The human mobility study was approved by the Wake Forest Human Subjects Committee and the Institutional Review Board and conducted from July 2007 to July 2009. Plasma was obtained from 93 patients randomized to receive early mobility (exercise) or usual care (no exercise). After performing our murine studies in 2013, we analyzed the banked plasma collected during the human study for quantification of G-CSF.
ALI animal model
All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest School of Medicine. Eight- to 12-week-old male wild-type C57BL/6 mice (The Jackson Laboratory) were anesthetized with an intraperitoneal injection of ketamine (150 mg/kg) and acetylpromazine (13.5 mg/kg), and the trachea was exposed. Escherichia coli LPS (O55:B5 L2880, lot 111M4035V, Sigma-Aldrich) (ARDS mice) at 3 µg/g mouse or an equivalent volume of sterile water (sham mice) was instilled intratracheally using a 20-gauge catheter as previously described (10).
Hindlimb immobilization model
A recently described hindlimb immobilization model using a surgical staple was applied as previously described (13). Sham and ALI mice underwent hindlimb immobilization at the time of i.t.LPS or i.t.H2O conditions by applying a surgical staple to the distal hindlimb in the normal flexion position. The contralateral hindlimb served as a control.
Mouse treadmill
Mice exercised on a six-lane mouse treadmill (Columbus Instruments) at 0° incline at varying intensities and durations, using a graded protocol with durations ranging from 5 min once daily to 35 min twice daily. Exercise regimens began 24 hours after i.t.LPS instillation (day 1) and continued through day 2. Animals were sacrificed on day 3, 12 hours after the final treadmill exercise. Speed was increased every 5 min. Maximal tolerated speed or duration was defined as the lack of willingness to run on the treadmill for more than 2 s despite a 2-mA shock. See table S1 for the dose and duration of each exercise group.
In vivo G-CSFR inhibition
A neutralizing antibody to the G-CSF receptor (G-CSFR/CD114 Ab, MAB6039, R&D Systems) or isotype control (MAB0061, R&D Systems) was administered to ALI mice at 0.5 µg per mouse per day on days 1 and 2 after the administration of i.t.LPS (at day 0). The dose was based on a previous publication (27). For G-CSFR Ab prophylaxis experiments, G-CSFR Ab was administered at day −1 or 0 and continued daily through day 2 until harvest at day 3.
Other experiments
Details of activity and lung injury measurements, muscle contractile and histology assessments, Western blotting, biomarker arrays, ELISAs, RT-qPCR, and the human mobility study can be found in Supplementary Materials and Methods.
Statistics
Data are presented as means ± SEM. Data from each experiment were confirmed by two or more replicative experiments. The data presented are from representative or combined experiments where indicated. Differences between two groups were compared with the Student’s t test or the Mann-Whitney test for nonparametric data. Pairwise comparisons were performed using the Student’s t test. Survival was analyzed using the log-rank (Mantel-Cox) test. These data were analyzed with GraphPad Prism 6.0e (GraphPad Software). Inflammatory biomarker array data were analyzed using t test statistics to get P values for each protein, which are reported as uncorrected values without correction for multiplicity testing. The fold change for each protein was the ratio of the averaged intensities of the two groups. The human G-CSF data differences between groups over time were compared using the GEE implemented in R package GEEPACK (49), where the regression model included the interaction between treatment and time, and we used an unstructured working matrix. Human G-CSF data were normalized by adjusting all the data with the averaged value for each group at time zero. All raw data can be found in table S4.
Supplementary Material
Acknowledgments
We thank P. Gann and R. Deaton at the University of Chicago Research Histology and Tissue Imaging Core for the digital lung imaging and analysis. D.C.F. thanks S. Kritchevsky and R. D. Hite for their mentorship and critical evaluation of the data. We thank the study participants, who made this work possible. Funding: These studies were supported by institutional funds from the Wake Forest School of Medicine, the Department of Medicine (D.C.F.), the Claude D. Pepper Older Americans Independence Center (P30-AG21332) (D.C.F., O.D., and P.E.M.), the Parker B. Francis Foundation (D.C.F.), the American Thoracic Society Foundation (D.C.F.), Wake Forest Health Sciences Translational Science Institute (M.S.), American Heart Association (FTF7280014) (N.R.A.), R00HL103973 (F.R.D.), NIH 1R01NR011186-01 (P.E.M.), NIH/National Institute on Aging R01AG13934 (O.D.), and R01AG15820 (O.D.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Materials and Methods
Fig. S1. Supplemental lung and muscle data from ALI and ALI + Ex mice.
Fig. S2. Prophylactic blockade of the G-CSFR in ALI mice.
Table S1. Exercise protocols in lung-injured mice.
Table S2. Inflammatory biomarker array for ALI + Ex versus ALI mice.
Table S3. Patient characteristics at enrollment of randomized pilot study of early mobility in patients with ARF.
Table S4. Original data (provided as a separate Excel file).
References (50–52)
Author contributions: Conceived and designed the experiments: D.C.F., O.D., N.R.A., and F.R.D. Performed the experiments: C.L., D.C.F., A.P., L.P., Z.-M.W., N.R.A., B.T.G., J.R.M., B.D.S., S.L., A.L.L., X.F., S.P., F.R.D., and M.S. Analyzed the data: D.C.F., J.C., A.P., N.R.A., and O.D. Contributed reagents/materials/analysis tools: D.C.F., M.S., L.S.K., O.D., J.C., R.R.Y., T.Z., and P.E.M. Wrote the manuscript: D.C.F. Provided edits to the manuscript: all authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data from this study have been made available to the journal.
REFERENCES AND NOTES
- 1.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit. Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 2.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, Hermans G, Decramer M, Gosselink R. Early exercise in critically ill patients enhances short-term functional recovery. Crit. Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 3.Morris PE, Griffin L, Berry M, Thompson C, Hite RD, Winkelman C, Hopkins RO, Ross A, Dixon L, Leach S, Haponik E. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am. J. Med. Sci. 2011;341:373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch. Phys. Med. Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr H-J, Kaltwasser A, Mende H, Rothaug O, Schuchhardt D, Schwabbauer N, Needham DM. Early mobilization of mechanically ventilated patients: A 1-day point-prevalence study in Germany. Crit. Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 7.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, Denehy L. Intensive care unit mobility practices in Australia and New Zealand: A point prevalence study. Crit. Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 8.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014;370:1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 9.Langen RC, Haegens A, Vernooy JHJ, Wouters EFM, de Winther MPJ, Carlsen H, Steele C, Shoelson SE, Schols AMWJ. NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am. J. Respir. Cell Mol. Biol. 2012;47:288–297. doi: 10.1165/rcmb.2011-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Files DC, D’Alessio FR, Johnston LF, Kesari P, Aggarwal NR, Garibaldi BT, Mock JR, Simmers JL, DeGorordo A, Murdoch J, Willis MS, Patterson C, Tankersley CG, Messi ML, Liu C, Delbono O, Furlow JD, Bodine SC, Cohn RD, King LS, Crow MT. A critical role for muscle ring finger-1 in acute lung injury–associated skeletal muscle wasting. Am. J. Respir. Crit. Care Med. 2012;185:825–834. doi: 10.1164/rccm.201106-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonçalves CTR, Gonçalves CGR, de Almeida FM, dos Santos Lopes FDTQ, dos Santos Durão ACC, dos Santos FA, da Silva LFF, Marcourakis T, Castro-Faria-Neto HC, de Paula Vieira R, Dolhnikoff M. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit. Care. 2012;16:R199. doi: 10.1186/cc11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suratt BT, Eisner MD, Calfee CS, Allard JB, Whittaker LA, Engelken DT, Petty JM, Trimarchi T, Gauthier L, Parsons PE NHLBI Acute Respiratory Distress Syndrome Network. Plasma granulocyte colony-stimulating factor levels correlate with clinical outcomes in patients with acute lung injury. Crit. Care Med. 2009;37:1322–1328. doi: 10.1097/CCM.0b013e31819c14fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caron AZ, Drouin G, Desrosiers J, Trensz F, Grenier G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. 2009;106:2049–2059. doi: 10.1152/japplphysiol.91505.2008. [DOI] [PubMed] [Google Scholar]
- 14.Schefer V, Talan MI. Oxygen consumption in adult and aged C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996;31:387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 15.Files DC, Xiao K, Zhang T, Liu C, Qian J, Zhao W, Morris PE, Delbono O, Feng X. The posterior cricoarytenoid muscle is spared from MuRF1-mediated muscle atrophy in mice with acute lung injury. PLOS One. 2014;9:e87587. doi: 10.1371/journal.pone.0087587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh B-C, Lidov HGW, Hasselgren P-O, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 17.González E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J. Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 19.Wiedermann FJ, Mayr AJ, Kaneider NC, Fuchs D, Mutz NJ, Schobersberger W. Alveolar granulocyte colony-stimulating factor and α-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest. 2004;125:212–219. doi: 10.1378/chest.125.1.212. [DOI] [PubMed] [Google Scholar]
- 20.Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony-stimulating factor and neutrophils—Forgotten mediators of inflammatory disease. Nat. Clin. Pract. Rheumatol. 2006;2:500–510. doi: 10.1038/ncprheum0291. [DOI] [PubMed] [Google Scholar]
- 21.Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 22.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal NR, D’Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, Fraig MM, Polotsky VY, King LS. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L371–L381. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN. Changes in the inflammatory response of the lung during acute respiratory distress syndrome: Prognostic indicators. Am. J. Respir. Crit. Care Med. 1996;154:76–81. doi: 10.1164/ajrccm.154.1.8680703. [DOI] [PubMed] [Google Scholar]
- 25.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 27.Hara M, Yuasa S, Shimoji K, Onizuka T, Hayashiji N, Ohno Y, Arai T, Hattori F, Kaneda R, Kimura K, Makino S, Sano M, Fukuda K. G-CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. J. Exp. Med. 2011;208:715–727. doi: 10.1084/jem.20101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC, Mantovani A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J. Clin. Invest. 1991;87:986–995. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azoulay E, Attalah H, Yang K, Herigault S, Jouault H, Brun-Buisson C, Brochard L, Harf A, Schlemmer B, Delclaux C. Exacerbation with granulocyte colony-stimulating factor of prior acute lung injury during neutropenia recovery in rats. Crit. Care Med. 2003;31:157–165. doi: 10.1097/00003246-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Hierholzer C, Kelly E, Lyons V, Roedling E, Davies P, Billiar TR, Tweardy DJ. G-CSF instillation into rat lungs mediates neutrophil recruitment, pulmonary edema, and hypoxia. J. Leukoc. Biol. 1998;63:169–174. doi: 10.1002/jlb.63.2.169. [DOI] [PubMed] [Google Scholar]
- 31.Demuynck H, Zachée P, Verhoef GE, Schetz M, Van den Berghe G, Lauwers P, Boogaerts MA. Risks of rhG-CSF treatment in drug-induced agranulocytosis. Ann. Hematol. 1995;70:143–147. doi: 10.1007/BF01682034. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldo JE, Borovetz H. Deterioration of oxygenation and abnormal lung microvascular permeability during resolution of leukopenia in patients with diffuse lung injury. Am. Rev. Respir. Dis. 1985;131:579–583. doi: 10.1164/arrd.1985.131.4.579. [DOI] [PubMed] [Google Scholar]
- 33.Root RK, Lodato RF, Patrick W, Cade JF, Fotheringham N, Milwee S, Vincent J-L, Torres A, Rello J, Nelson S Pneumonia Sepsis Study Group. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit. Care Med. 2003;31:367–373. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]
- 34.Nelson S, Belknap SM, Carlson RW, Dale D, DeBoisblanc B, Farkas S, Fotheringham N, Ho H, Marrie T, Movahhed H, Root R, Wilson J. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. J. Infect. Dis. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- 35.Cheng AC, Stephens DP, Currie BJ. Granulocyte-colony stimulating factor (G-CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults. Cochrane Database Syst. Rev. 2007:CD004400. doi: 10.1002/14651858.CD004400.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2010;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 37.Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, Zlotnik A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J. Immunol. 1999;162:5490–5497. [PubMed] [Google Scholar]
- 38.Giangola MD, Yang W-L, Rajayer SR, Nicastro J, Coppa GF, Wang P. Growth arrest–specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock. 2013;40:485–491. doi: 10.1097/SHK.0b013e3182a588c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H, Sasaki T, Ohteki T, Ranieri VM, dos Santos CC, Kawaoka Y, Akira S, Luster AD, Lu B, Penninger JM, Uhlig S, Slutsky AS, Imai Y. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am. J. Respir. Crit. Care Med. 2013;187:65–77. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auvynet C, Moreno S, Melchy E, Coronado-Martínez I, Montiel JL, Aguilar-Delfin I, Rosenstein Y. Galectin-1 promotes human neutrophil migration. Glycobiology. 2012;23:32–42. doi: 10.1093/glycob/cws128. [DOI] [PubMed] [Google Scholar]
- 42.Pastva A, Estell K, Schoeb TR, Atkinson TP, Schwiebert LM. Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J. Immunol. 2004;172:4520–4526. doi: 10.4049/jimmunol.172.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira RP, Claudino RC, Duarte ACS, Santos ABG, Perini A, Faria Neto HCC, Mauad T, Martins MA, Dolhnikoff M, Carvalho CRF. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am. J. Respir. Crit. Care Med. 2007;176:871–877. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 44.Moreira A, Delgado L, Moreira P, Haahtela T. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 2009;90:111–131. doi: 10.1093/bmb/ldp010. [DOI] [PubMed] [Google Scholar]
- 45.Nieman DC. Exercise, infection, and immunity. Int. J. Sports Med. 1994;15(Suppl. 3):S131–S141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- 46.Pastva A, Estell K, Schoeb TR, Schwiebert LM. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Behav. Immun. 2005;19:413–422. doi: 10.1016/j.bbi.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hooijman PE, Beishuizen A, de Waard MC, de Man FS, Vermeijden JW, Steenvoorde P, Bouwman RA, Lommen W, van Hees HWH, Heunks LMA, Dickhoff C, van der Peet DL, Girbes ARJ, Jasper JR, Malik FI, Stienen GJM, Hartemink KJ, Paul MA, Ottenheijm CAC. Diaphragm fiber strength is reduced in critically ill patients and restored by a troponin activator. Am. J. Respir. Crit. Care Med. 2014;189:863–865. doi: 10.1164/rccm.201312-2260LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moriscot AS, Baptista IL, Bogomolovas J, Witt C, Hirner S, Granzier H, Labeit S. MuRF1 is a muscle fiber-type II associated factor and together with MuRF2 regulates type-II fiber trophicity and maintenance. J. Struct. Biol. 2010;170:344–353. doi: 10.1016/j.jsb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J. Stat. Softw. 2006;15:1–11. [Google Scholar]
- 50.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, Deng J, Ranjan R, Xiao L, Jaffe HA, Corbridge SJ, Kelly EAB, Jarjour NN, Chun J, Prestwich GD, Kaffe E, Ninou I, Aidinis V, Morris AJ, Smyth SS, Ackerman SJ, Natarajan V, Christman JW. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Respir. Crit. Care Med. 2013;188:928–940. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang T, Birbrair A, Wang Z-M, Taylor J, Messi ML, Delbono O. Troponin T nuclear localization and its role in aging skeletal muscle. Age. 2013;35:353–370. doi: 10.1007/s11357-011-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siu PM, Pistilli EE, Murlasits Z, Alway SE. Hindlimb unloading increases muscle content of cytosolic but not nuclear Id2 and p53 proteins in young adult and aged rats. J. Appl. Physiol. 2006;100:907–916. doi: 10.1152/japplphysiol.01012.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.