Abstract
Background
No studies have yet assessed the ability of statin treatment to reduce arterial inflammation and achieve regression of coronary atherosclerosis in HIV-infected patients, a population with elevated risk of myocardial infarction.
Methods
In a randomized, double-blind, placebo-controlled trial, 40 HIV-infected participants with subclinical coronary atherosclerosis, evidence of arterial inflammation in the aorta by fluorodeoxyglucose positron emission tomography (FDG-PET) and low density lipoprotein(LDL)-cholesterol <3·37mmol/L(130mg/dL) were randomized to one year of treatment with atorvastatin (n=19) or placebo (n=21). Randomization was carried out by the MGH Clinical Research Pharmacy using a permuted-block algorithm, stratified by gender with a fixed block size of four, with 1:1 allocation to atorvastatin or identical matching placebo. Study codes were available only to the MGH Research Pharmacy and not to study investigators or participants. The prespecified primary endpoint was arterial inflammation, as assessed by FDG-PET of the aorta. Additional prespecified endpoints included coronary atherosclerotic plaque as assessed by coronary computed tomography angiography. We quantitatively assessed non-calcified and calcified plaque and high risk plaque features. Analysis was performed using intention-to-treat principle, using all available data, without imputation for missing data.
Findings
Thirty seven out of forty (92·5%) subjects completed the study, with equivalent discontinuation rates in both groups. Baseline parameters were similar between groups. After 12 months, change in FDG-PET uptake of the most diseased segment of the aorta was not different between atorvastatin and placebo, but technically adequate results comparing longitudinal changes in identical regions could only be assessed in a subset of patients (atorvastatin Δ −0·03 [95% CI: −0·17, 0·12] vs. placebo Δ −0·06 [−0·25, 0·13], p=0·77, n=21). Change in plaque could be assessed in all subjects completing the study. Atorvastatin reduced noncalcified coronary plaque volume compared to placebo (−19·4%(IQR: −39·2%, 9·3%) vs. +20·4%(−7·1%, 94·4%), p=0·009, n=37). In addition, the number of high risk plaques was significantly reduced by atorvastatin compared to placebo (change in number of low attenuation plaques −0·2[95% CI: −0·6, 0·2] vs. 0·4[0·0, 0·7], p=0·03, n=37 and change in number of positively remodeled plaques −0·2[95% CI −0·4, 0·1] vs. 0·4[−0·1, 0·8], p=0·04, n=37). Direct LDL-cholesterol (−1·00[95% CI −1·38, 0·61] vs. 0·30[0·04, 0·55] mmol/L, p<0·0001) and lipoprotein-associated phospholipase A2 (−52·2[95% CI −70·4, −34·0] vs. −13·3[−32·8, 6·2] ng/mL, p=0·005, n=37) significantly decreased with atorvastatin compared to placebo. Statin therapy was well-tolerated, with low incidence of clinical adverse events.
Interpretation
Compared to placebo, statin therapy reduces noncalcified plaque volume and high risk plaque features in HIV-infected patients with subclinical coronary atherosclerosis. Significant effects of statin therapy on arterial inflammation of the aorta by FDG-PET were not seen. Further studies should assess whether reduction in high risk coronary artery disease translates into effective prevention of cardiovascular events in this at risk population.
INTRODUCTION
Coronary artery disease (CAD) is a major cause of morbidity and mortality for patients living with long term HIV infection1–4. Indeed, epidemiological studies fully adjusting for numerous cardiovascular (CVD) risk factors and investigating validated cardiovascular events demonstrate an increased risk of CVD events in HIV vs. non HIV-infected patients5. There is a critical need to determine efficacious cardiovascular risk reduction interventions in HIV-infected patients.
A number of studies have demonstrated increased prevalence of subclinical atherosclerosis, including a predominant increase in noncalcified plaque in HIV-infected patients compared to non HIV patients, controlling for traditional CAD risk factors6–8. Additional studies in HIV-infected patients have demonstrated evidence of arterial inflammation9 and increased vulnerable plaque morphology on coronary computed tomography angiography (CCTA)10. Greater volumes of noncalcified plaque and vulnerable plaque morphology on CCTA are associated with future major adverse cardiac events11.
Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) have been consistently shown to reduce cardiac events and mortality in the general population. Imaging trials performed in non HIV patients have also shown that intensive statin therapy can slow progression of coronary atherosclerosis and even result in disease regression12–14. Prior studies of statin therapy in HIV-infected patients have demonstrated improvement in lipids and reduction in markers of inflammation, as well as improvement in endothelial function, but no studies have yet prospectively investigated the effects of statins on direct measurements of arterial inflammation and coronary atherosclerosis in this patient population15–18. Thus, we performed a randomized double-blind placebo-controlled trial to investigate the effects of a statin on arterial inflammation and coronary atherosclerosis in HIV-infected patients without known cardiovascular disease or elevated LDL-cholesterol, but who demonstrated subclinical atherosclerosis and arterial inflammation.
We hypothesized that coronary atherosclerosis would progress at a rapid rate in this population of HIV-infected patients, despite a normal baseline LDL-cholesterol and that treatment with a statin would reduce arterial inflammation, deter the progression of coronary atherosclerosis, and reduce noncalcified plaque volume as well as vulnerable plaque morphology, resulting in a decrease in high risk atherosclerotic lesions shown to be prevalent in HIV-infected patients6, 10.
METHODS
Study Design
This study was a randomized, double-blind, placebo-controlled clinical trial with enrollment starting in 2009 and completion of the last study visit in 2013. The study took a number of years to complete as treatment duration was one year and recruitment occurred at a single site. As planned, we recruited 40 men and women with HIV disease, no prior history of cardiovascular disease or cardiac symptoms, and evidence of subclinical coronary atherosclerosis, defined by presence of one or more plaques on CCTA but without significant stenosis, defined as >50% left main stenosis or >70% stenosis in any major vessel. In addition, participants who demonstrated plaque on CCTA underwent subsequent screening with fluorodeoxyglucose positron emission tomography (FDG-PET) to assess arterial inflammation. Subjects were required to have evidence of arterial inflammation, defined as an aortic to venous target to background ratio of > 1·6 using methods as previously described9 in addition to plaque on CCTA to be eligible for the study. Additional inclusion criteria were age 18 to 60 years of age, on stable antiretroviral therapy (ART) with no changes in ART regimen in the preceding six months, and LDL-cholesterol between 1·81mmol/L to 3·37mmol/L (70mg/dL-130mg/dL). Participants did not meet guidelines in place at the time for statin therapy. Exclusion criteria were concurrent use of a statin, contraindication to statins, AST or ALT three times greater than the upper limit of normal and/or treatment for active liver disease, renal disease and/or creatinine >130 μmol/L, acute infectious illness in the preceding three months, contraindication to beta-blocker or nitroglycerin use as these drugs are given as part of the standard cardiac CT protocol, significant radiation exposure within the year prior to the study, e.g. prior nuclear scans or radiation therapy, body weight greater than 300 lbs due to CT scanner table limitations, allergy to iodine-containing contrast media, pregnancy or breastfeeding. Participants were recruited via referral by infectious disease providers, newspaper and broadcast advertisements, and posted flyers in community and health centers.
Patients who met enrollment criteria were randomized in 1:1 ratio to either atorvastatin (starting at dose of 20mg per day and escalating to 40mg per day at three months visit if study drug was well-tolerated) or placebo groups. Pre-specified discontinuation criteria for the study included 1) CPK > 5× upper limit of normal with symptoms of myalgia, myositis or rhabdomyolysis, 2) CPK >10× upper limit of normal, 3) ALT or AST>3× upper limit of normal range. For those patients in whom AST was greater than 2× upper limit of normal range at screening visit, continuation was permitted unless AST exceeded 5× upper limit of normal range. If after starting the 40mg dose, a subject developed symptoms of myalgias or elevation in CPK, AST, or ALT above study stated limits, the dose was reduced from 40mg back to 20mg of atorvastatin or placebo once a day. All subjects received standardized lifestyle counseling based on National Cholesterol Education Program (NCEP) guidelines at baseline, including standardized recommendations on smoking, exercise, and diet. All participants in both the atorvastatin and placebo groups received counseling on the Therapeutic Lifestyle Changes (TLC) diet by MGH bionutritionists. Subjects were encouraged to be compliant with ART, as prescribed by clinical care givers. Subjects were seen at 1 month, 3, 6, 9 and 12 months post baseline. Primary and secondary endpoints were assessed at baseline and end of study. Safety was assessed at interim visits and final visit (1, 3, 6, 9 and 12 months) including assessment of symptoms, interim history, physical examination, AST, ALT and CPK. Endothelial function by reactive hyperemia peripheral arterial tonometry was assessed at baseline and 12 months and is undergoing evaluation and will be reported separately.
All participants provided written informed consent and the study was approved by the institutional IRB. The trial is registered on ClinicalTrials.gov (NCT00965185). A Data and Safety Monitoring Board consisting of an HIV specialist, a cardiologist, and a biostatistician met every six months for safety monitoring. All potential subjects completed a brief telephone screen followed by two clinical evaluations to determine eligibility for the study. Study visits were performed in the Clinical Research Center at Massachusetts General Hospital(MGH).
Outcomes
The prespecified primary endpoint was arterial inflammation, as assessed by FDG-PET of the aorta. Other prespecified endpoints were coronary atherosclerotic plaque (non-calcified and calcified plaque and high risk plaque features) as assessed by coronary computed tomography angiography, lipids, inflammatory, immunological, and biochemical parameters.
18F-FDG PET Imaging of Aorta
Participants underwent PET imaging after an overnight fast to reduce myocardial FDG uptake. The PET imaging was performed 3 h after administration of 13mCi of 18F-FDG (Siemens ECAT Exact HR+ PET or biograph 64 system, Knoxville, Tennessee)19. At the time the study was initiated, the only option to assess Aortic FDG-PET was via a dedicated PET scanner, as a combined PET/CT scanner was not available for non clinical use.
Image analysis was performed by an experienced cardiologist who was blinded to clinical data and randomization. Co-registration of the PET and CT images was performed using anatomical landmarks including ascending aorta, left atrium, and spine. The ascending aorta was chosen for measurement. The target-to-background ratio (TBR) was calculated by dividing the mean arterial standardized uptake value (SUV) by the mean venous SUV9. The mean arterial SUV is derived from the average of the maximum SUV values in serial axial measurements. All images were assessed for quality prior to unblinding, and were rated as to acceptability based on ability to co-register CT and PET and comparability of registration between serial scans. Derivation of tissue activity was not performed for images that were deemed to be of unacceptable quality.
Coronary CT Angiography (CCTA) Protocol
ECG-gated CCTA with tube current modulation was performed on a Somatom Definition Flash 128-slice dual source CT (Siemens Medical Solutions, Forchheim, Germany) according to the guidelines of the Society of Cardiac Computed Tomography (SCCT)20 at enrollment and one year follow-up. Prior to CCTA, between 0 and 15mg of intravenous metoprolol was administered in 5mg doses to achieve a resting heart rate of <65 beats per minute. 0·6 mg of sublingual nitroglycerin was administered for vasodilation. The CT protocol included a noncontrast CT for calcium scoring, a timing bolus, and the CCTA. Following a 20cc timing bolus, intravenous contrast (Iopamidol 370 g/cm3, Bracco Diagnostics, Inc., Princeton, NJ USA, 60–80 cc at 4·5–5·4cc/s based on subject size) was injected followed by a 40cc normal saline flush. Acquisition parameters were tube voltage 120kV; ECG-dependent tube current modulation with retrospective gating and with reference 320 mAs; adaptive pitch 0·2–0·4 based on heart rate; collimation 128 × 0·6mm; rotation time 280ms; temporal resolution 75ms. All scans were performed in an inspiratory breath hold. Images were reconstructed at 5% intervals between 60 to 75% of the R-R interval and at 10% intervals elsewhere in the cardiac cycle with a slice thickness of 0·75mm and an increment of 0·7mm. The cardiac phase or phases which minimized motion artifact for each of the coronary arteries were identified and used for image analysis.
CCTA Analysis
All image data at baseline and one year follow up were analyzed using an offline workstation (Syngo Multimodality Workplace, Siemens, Forchheim, Germany) by an experienced cardiac radiologist who was blinded to clinical data and randomization. For assessment of total and non-calcified plaque in CCTA data sets, cross sectional and multiplanar reconstructed images were assessed for the presence and qualitative composition of atherosclerotic plaque for each coronary artery segment defined by the SCCT model20. Non-calcified plaque was defined as any discernible structure that could be assigned to the coronary artery wall with a density < 130 HU that could be identified in at least two independent planes21. Luminal stenosis was graded as <25%, 25–50%, 50–69%, or >70% according to the guidelines of SCCT20. Significant progression in coronary artery stenosis was defined as an increase to > 50% stenosis from a baseline stenosis of <50% or an increase to >70% stenosis in those with >50% but <70% stenosis in any coronary artery segment at baseline.
Coronary Plaque Volume
Semi-automated plaque volume measurements were performed on a second analysis package (Aquarius iNtuition, Terarecon, Foster City, CA USA). The software generated curved planar reformats around a centerline through the coronary artery lumen, which was edited as necessary22, 23. The plaque length was established visually with a marker in the proximal and distal plaque limits. Plaque length was kept constant between the enrollment and follow up CT to minimize variability24. The inner and outer borders of the vessel lumen and plaque were established using a semiautomatic tracer and plaque volume was calculated automatically. When the contours deviated from the vessel/plaque border, they were manually corrected. Voxels with attenuation <130 HU were assigned to the noncalcified volume of the plaque. This volumetric plaque measurement technique has excellent intraobserver, interobserver, and interscan reproducibility23, 25–29.
High Risk Plaque Features
Each coronary segment was then further assessed for high-risk plaque features defined as positive remodeling, low attenuation plaque, and spotty calcium. Positive remodeling was defined as plaque segment outer diameter/reference segment outer diameter >1·0530. If low attenuation was visually noted in non-calcified plaque, five region-of-interest measurements (area=1·0 mm2) were placed within the low attenuation portion. Low attenuation plaque was defined when the smallest mean attenuation number in these regions of interest was <40HU30. This threshold was also used in our prior report establishing high prevalence of low attenuation plaque in the HIV population10. Spotty calcium was defined by calcified plaque with a maximum diameter of 3 mm occupying only one side of the vessel wall31. Our group and others have previously described excellent intra and interobserver variability for these high risk features32, 33.
Coronary Artery Calcification
The presence and extent of coronary artery calcification (CAC) was assessed on non-contrast enhanced images and calculated using the Agatston method34. Calcium mass, volume and density were also determined using the non-contrast enhanced images.
Lipid Parameters
Direct LDL was measured by homogeneous enzymatic colorimetric assay (Roche COBAS INTEGRA). Other lipid levels including total cholesterol, high density lipoprotein and triglycerides were determined using standard techniques. Blood was drawn after a 12-hour fast.
Inflammatory, Metabolic, Biochemical, and Immunologic Assessments
Glucose and hemoglobin A1c were determined using standard techniques after 12-hour overnight fast. CRP levels were measured using ELISA. CD4+ T cell counts were assessed by flow cytometry. HIV RNA was measured in real time using clinically available ultrasensitive reverse-transcription polymerase chain reaction assays. From December 2009-November 2011, Ampliprep Taqman V1.5(Roche) was used to measure HIV RNA (linear range 48–10 million copies/mL of plasma). From 2012-current, Ampliprep Taqman 48 V2(Roche) was used (linear range 20–10 million copies/mL). Lipoprotein-associated phospholipase A2 (Lp-PLA2) was measured by ELISA (PLAC Test; diaDexus, Inc., South San Francisco, CA). Assessment of flow cytometry and other immune activation indices was planned. These data were collected but have not yet been fully analyzed. In contrast this manuscript focuses on clinical relevant inflammatory, biochemical, and immunologic assessments. A Veterans Aging Cohort Study (VACS)35 score was calculated for each patient at baseline and end of study.
Body Composition and Dietary Assessment
Weight and anthropometric measurements were determined in the morning, prior to breakfast. Abdominal visceral and abdominal subcutaneous adipose tissue area (VAT and SAT, respectively) were quantified using a cross-sectional abdominal CT scan at the level of the L4 pedicle36, 37. DEXA parameters of bone were obtained, but not included in this manuscript, which focuses on cardiovascular endpoints. Four day food records were completed by the participants and analyzed using Minnesota Nutrition Data System software.
Randomization and Masking
Randomization was carried out by the MGH Clinical Research Pharmacy using a permuted block algorithm, stratified by gender with a fixed block size of four, with 1:1 allocation to atorvastatin or identical matching placebo. Study codes were available only to the MGH Research Pharmacy and not to study investigators or participants. Dose escalations were similarly blinded with matching increases for placebo-treated patients. Investigators performing image analyses related to FDG-PET and CCTA were blinded to clinical data and randomization.
Statistical Analysis
Comparisons between the two groups (atorvastatin vs. placebo) were performed using Student t test for normally-distributed continuous variables and Wilcoxon rank sum test if the distribution was non-normal. To assess changes within each group, paired t-test was performed. For comparison between groups of dichotomous variables, Fisher’s exact test was used with cell numbers less than 5, otherwise chi-square test was used. Two-tailed probability values are reported, and statistical significance was assumed when p< ·05. Means and 95% confidence intervals [CI] are reported to describe changes in continuous variables with normal distribution, otherwise, medians and interquartile ranges (IQR) are used. CRP was log transformed to reach normal distribution. All statistical analyses were performed using SAS JMP (SAS Institute). The prespecified primary study endpoint was arterial inflammation as data showing effects on FDG-PET of the carotid in non HIV patients were available at the time of study initiation to provide data for sample size estimation38, whereas no data on effects of statin on coronary plaque volume measured by CCTA were available at time of study initiation. The main endpoint for plaque was noncalcified plaque volume. With 40 subjects and a planned dropout rate of 15%, the study was designed to detect an approximate 15% difference in arterial inflammation between groups and a one SD change in other endpoints at alpha =0·05, 85% power. The dropout rate was less than anticipated, at 7·5%. Intent to treat analysis was performed using all available data. No imputation was made for missing data as attrition was extremely low and equal among groups. A sensitivity analysis was performed among patients who maintained undetectable viremia throughout the study. Sensitivity analyses were also performed, controlling for baseline plaque volume. The relationship between the change in LDL-cholesterol and plaque was assessed by Wilcoxon rank sum test comparing changes in plaque parameters in participants who achieved LDL-cholesterol < 2·59mmol/L or not, and <1·8mmol/L or not. The IRB approved protocol anticipated screening up to 100 patients to enroll 40 subjects into the trial. Initial plans to enroll HIV subjects without plaque into a longitudinal natural history study were also made, but not carried out due to time and expense. Randomization to statins was never planned for these subjects. Randomization was stratified by gender and gender effect was assessed by the Breslow Day test.
Role of the Funding Source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Characteristics of the Participants at Baseline
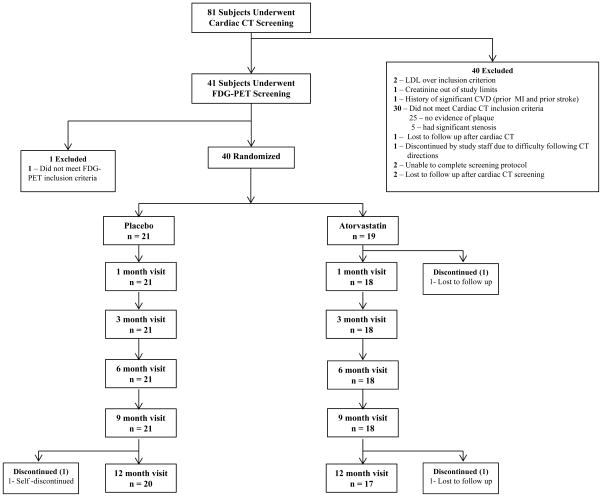
Eighty-one HIV-infected subjects underwent screening for this study. The screening was sequential such that the presence of plaque was first established by CCTA. Subjects with plaque and otherwise qualified for entry then underwent FDG-PET assessment for arterial inflammation. Thirty subjects did not meet coronary CT inclusion criteria (25 without any subclinical disease, five with critical stenosis) and one subject did not meet FDG-PET inclusion criterion. Therefore, the entry criterion of subclinical plaque on CT was demonstrated in approximately 65% of subjects screened. An additional 6% of the subjects demonstrated significant stenoses at screen and were excluded on this basis. In addition, two subjects were excluded for LDL greater than entry criteria; one subject was excluded due to creatinine greater than study entry criteria; one person was excluded due to history of cardiovascular disease (prior MI and stroke). Other subjects were excluded as per Figure 1. As planned, 40 participants were randomized to atorvastatin or placebo (Figure 1). Demographic and clinical characteristics of these 40 study participants are described in Table 1.
Figure 1.
Consort Diagram -- Flow of Participants
Table 1.
Demographic and Clinical Characteristics of Study Participants at Baseline
| Characteristic | Placebo (n=21) | Atorvastatin (n=19) | P-value |
|---|---|---|---|
| Age – yr | 50·0 ± 5·6 | 52·2 ± 3·8 | 0·18 |
| Gender (male) – no. (%) | 17 (81) | 15 (79) | 0·87 |
| Race or Ethnic group – no. (%) | 0·79 | ||
| White | 13 (68) | 13 (68) | |
| Black | 3 (16) | 3 (16) | |
| Asian | 1 (5) | 0 (0) | |
| Hispanic | 1 (5) | 1 (5) | |
| More than 1 race | 1 (5) | 2 (11) | |
| Framingham 10 year risk estimate (%) | 5·4 ± 4·4 | 6·9 ± 4·1 | 0·28 |
| Hypertension – no. (%) | 2 (10) | 4 (21) | 0·31 |
| Diabetes Mellitus – no. (%) | 2 (10) | 2 (11) | 0·92 |
| Current Smoker – no. (%) | 6 (29) | 5 (26) | 0·87 |
| Lipids | |||
| Total cholesterol, mmol/L | 4·97 ± 0·70 | 5·14 ± 0·98 | 0·51 |
| HDL-Cholesterol, mmol/L | 1·31 ± 0·39 | 1·34 ± 0·50 | 0·84 |
| Direct LDL-Cholesterol, mmol/L | 3·23 ± 0·83 | 3·20 ± 0·95 | 0·93 |
| Triglycerides, mmol/L | 1·28 (1·04, 1·53) | 1·36 (1·10, 2·31) | 0·39 |
| HIV Disease Related Parameters | |||
| CD4+ T-lymphocytes | 590 ± 289 | 522 ± 263 | 0·45 |
| HIV RNA Viral Load, copies/mL | <48 (<48, 48) | <48 (<48, <48) | 0·74 |
| Undetectable HIV RNA (< 48 copies/mL), no. (%) | 17 (81) | 16 (84) | 0·79 |
| Duration Since HIV Diagnosis, yr | 15·0 ± 6·9 | 16·8 ± 5·1 | 0·36 |
| Currently on Antiretroviral Therapy, % | 100 | 100 | N/A |
| Duration of Antiretroviral Therapy, yr | 11·4 ± 5·8 | 12·4 ± 3·7 | 0·54 |
| Current Protease Inhibitor (PI) Treatment – no. (%) | 8 (38) | 11 (58) | 0·21 |
| Duration of PI Treatment, yr | 4·9 ± 5·8 | 6·8 ± 5·6 | 0·39 |
| Current NRTI Treatment – no. (%) | 21 (100) | 17 (89) | 0·08 |
| Duration of NRTI Treatment – yr | 11·3 ± 6·1 | 10·8 ± 4·4 | 0·81 |
| Current NNRTI Treatment – no. (%) | 12 (57) | 7 (37) | 0·20 |
| Duration of NNRTI Treatment, yr | 4·6 ± 5·4 | 1·9 ± 3·0 | 0·10 |
| Anthropometric parameters | |||
| Body mass index, kg/m2 | 25·8 ± 4·8 | 25·6 ± 2·9 | 0·87 |
| Visceral adipose tissue area (VAT), cm2 | 142 ± 74 | 166 ± 130 | 0·47 |
| Subcutaneous adipose tissue area (SAT), cm2 | 202 ± 120 | 152 ± 93 | 0·15 |
| Hemodynamic parameters | |||
| Systolic blood pressure, mm Hg | 119 ± 16 | 117 ± 13 | 0·67 |
| Diastolic blood pressure, mm Hg | 76 ± 10 | 73 ± 8 | 0·32 |
| Metabolic parameters | |||
| Fasting glucose, mmol/L | 4·92 ± 0·38 | 4·76 ± 0·66 | 0·36 |
| Hemoglobin A1c, % | 5·5 ± 0·3 | 5·6 ± 0·4 | 0·25 |
| Inflammatory Parameters | |||
| C-reactive protein, median (IQR), mg/L | 1·1 (0·4, 2·4) | 0·8 (0·3, 1·9) | 0·36 |
| Interleukin-6, median (IQR), ng/L | 0·8 (0·5, 1·2) | 0·6 (0·4, 1·6) | 0·75 |
Data reported as mean ± standard deviation (SD) or percentage, except for variables with non-normal distributions, which are reported as median (IQR=interquartile range). NRTI, Nucleoside/Nucleotide Reverse Transcriptase Inhibitors; NNRTI, Non-nucleoside Reverse Transcriptase Inhibitors; HDL, High-Density Lipoprotein; LDL, Low-Density Lipoprotein; IQR, Interquartile Range.
Age, gender, race/ethnicity, BMI, and Framingham 10-year risk estimate were similar between both groups at baseline (Table 1). Prevalence of hypertension, diabetes mellitus, and current smoking were similar between groups as were blood pressure, fasting glucose, hemoglobin A1c, total cholesterol, HDL, LDL, and CRP measurements. HIV disease parameters, including treatment parameters of ART duration and type, were also similar between the two groups. Participants were all on antiretroviral therapy and most patients had undetectable viremia with similar immunological and virological indices between groups (Table 1). Four patients in the placebo group and 3 in the atorvastatin group had detectable viremia but the maximum viral load at baseline was 355 copies/mL and all but 3 had viral load < 100 copies. Subjects were all on ART for an average of 11–12 years without differences between the groups. All subjects had started ART more than 2 years prior to initiating the study and all were on a stable regime for at least 6 months prior to study initiation. Baseline VACS scores were not different between the atorvastatin and placebo groups (See Supplementary Table 1). Viral load information was given to referring physicians who were responsible for ongoing clinical care of the patients. At baseline, there were no significant differences in arterial inflammation, plaque volume or other atherosclerosis parameters between the groups (Table 2).
Table 2.
Coronary Plaque Measurements and Characteristics
| Baseline Placebo Group (n=21) | Baseline Atorvastatin Group (n=19) | Between Group P-value | Change in Placebo Group (n=20) | Change in Atorvastatin Group (n=17) | Between Group P-value | |
|---|---|---|---|---|---|---|
| Plaque Volume | ||||||
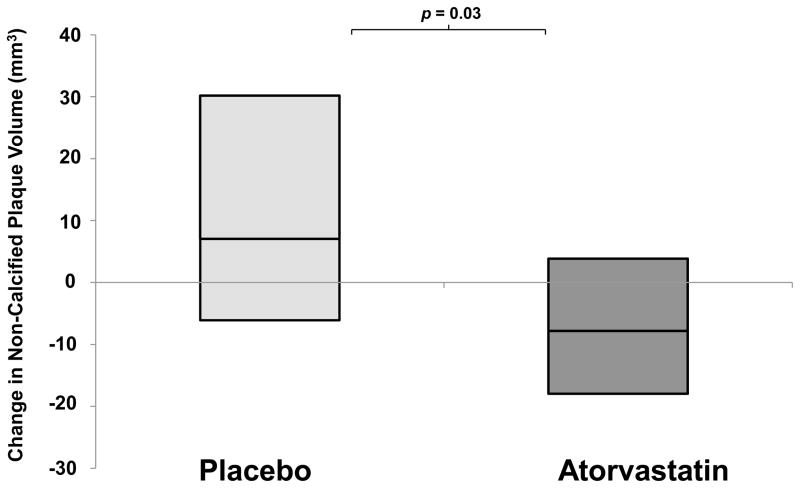
| Noncalcified (<130 HU), median (IQR), mm3 | 66·1 (14·8, 94·8) | 33·7 (19·6, 83·5) | 0·72 | 6·7 (−6·5, 29·8) | −8·2 (−18·3, 3·5) | 0·03 |
| % Change in Noncalcified Plaque Volume, median (IQR), % | N/A | N/A | N/A | 20·4 (−7·1, 94·4) | −19·4 (−39·2, 9·3) | 0·009 |
| Total Plaque Volume, median (IQR), mm3 | 81·1 (30·6, 134·9) | 55·2 (23·0, 153·6) | 0·63 | 12·0 (0·8, 100·4) | −0·8 (−16·8, 14·2) | 0·02 |
| % Change in Total Plaque Volume, (IQR),% | N/A | N/A | N/A | 18·2 (1·5, 59·9) | −4·7 (−25·4, 15·9) | 0·01 |
| Coronary Artery Calcium | ||||||
| Calcium Score, median (IQR), Total Agatson Score | 24·4 (0·0, 46·9) | 10·9 (0·0, 92·6) | 0·98 | 1·7 (0·0, 28·0) | 0·9 (0·0, 18·5) | 0·74 |
| Calcium Mass, median (IQR), mg | 4·1 (0·0, 6·9) | 1·9 (0·0, 12·8) | 1·00 | 0·2 (0·0, 2·9) | 0·7 (0·0, 3·9) | 0·48 |
| Calcium Volume, median (IQR), mm3 | 17·8 (0·0, 37·3) | 10·5 (0·0, 70·4) | 1·00 | 1·2 (0·0, 18·5) | 5·0 (0·0, 25·8) | 0·63 |
| Calcium Density, median (IQR), mg/mm3 | 0·2 (0·2, 0·2) | 0·2 (0·2, 0·2) | 0·90 | 0·0 (0·0, 0·0) | 0·0 (0·0, 0·0) | 0·51 |
| Plaque Vulnerability Features | ||||||
| Low Attenuation Plaque (<40HU), no.† | 0·4 ± 0·6 | 0·8 ± 1·0 | 0·14 | 0·4 [0·0, 0·7] | −0·2 [−0·6, 0·2] | 0·03 |
| Positively Remodeled Plaque (Remodeling Index>1·05), no.† | 2·1 ± 1·8 | 2·0 ± 2·4 | 0·59 | 0·4 [−0·1, 0·8] | −0·2 [−0·4, 0·1] | 0·04 |
| Plaque With Spotty Calcification, no.† | 2·0 ± 2·1 | 2·4 ± 2·9 | 0·76 | −0·2 [−0·4, 0·0] | 0·0 [−0·3, 0·3] | 0·25 |
| % Patients with Progression of Coronary Stenosis Beyond Clinically significant thresholds*, no. (%) | N/A | N/A | N/A | 3/20 (15%) | 0/17 (0%) | 0·23‡ |
| % of Patients with Any Regression of Plaque Volume | ||||||
| Progression of Total Plaque Volume, no. (%) | N/A | N/A | N/A | 16/20 (80·0%) | 6/17 (35·3%) | 0·008‡ |
| Regression of Total Plaque Volume, no. (%) | N/A | N/A | N/A | 4/20 (20·0%) | 11/17 (64·7%) | |
For ease of interpretation, some non-normally distributed data are presented at mean ± SD, but p values by nonparametric comparison
Progression from <50% to >50% stenosis or progression from <70% to >70% stenosis
Fisher’s exact test is used for small cell numbers
Non-normally distributed data are presented as median (IQR). Normally distributed data are presented as mean ± SD. For change columns, normally distributed data are presented as mean [95% C.I.].
After randomization, 1 out of 21 patients in the placebo group and 2 out of 19 in the statin group discontinued, resulting in a similar discontinuation rate between groups (Figure 1). Adherence was determined by pill count and was similar between groups at each visit, see Supplementary Table 2.
Aortic FDG-PET Uptake
After manual co-registration of PET and CT scans, change from baseline to 12 months was assessed. Although manual co-registration was thought at study initiation to be adequate to accomplish the purpose of the study and proved to be adequate for assessing baseline data for screening, manual coregistration proved difficult to assess identical anatomical regions on serial scans. Of the available data pairs, 21 pairs were deemed to be of acceptable image quality, sufficient to permit assessment of change over time in identical regions in serial scans. Among this group, no statistically significant differences between the atorvastatin and placebo groups were detected (Table 3).
Table 3.
Metabolic, Inflammatory and Body Composition Parameters
| Baseline Placebo (n=21) | Baseline Atorvastatin (n=19) | Between Group P-value | Change Placebo (n=20) | Change Atorvastatin (n=17) | Between Group P-value | |
|---|---|---|---|---|---|---|
| Lipids | ||||||
| Total cholesterol, mmol/L | 4·97 ± 0·70 | 5·14 ± 0·98 | 0·51 | 0·12 [−0·12, 0·37] | −1·23 [−1·53, −0·93] | <0·0001 |
| HDL-Cholesterol, mmol/L | 1·31 ± 0·39 | 1·34 ± 0·50 | 0·84 | −0·04 [−0·17, 0·09] | 0·02 [−0·11, 0·16] | 0·48 |
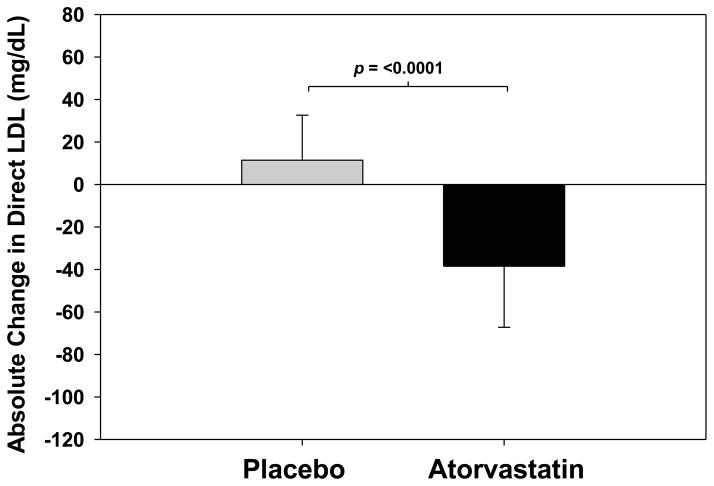
| Direct LDL-Cholesterol, mmol/L | 3·23 ± 0·83 | 3·20 ± 0·95 | 0·93 | 0·30 [0·04, 0·55] | −1.00 [−1·38, 0·61] | <0·0001 |
| Triglycerides, mmol/L | 1·28 (1·04, 1·53) | 1·36 (1·10, 2·31) | 0·39 | 0·08 (−0·46, 0·38) | −0·10 (−0·46, 0·44) | 0·64 |
| HIV Disease Related Parameters | ||||||
| CD4+ T-lymphocytes | 590 ± 289 (n=20) | 522 ± 263 | 0·45 | 4 [−72, 80] (n=19) | 17 [−68, 101] | 0·81 |
| HIV RNA Viral Load, copies/mL | <48 (<48, 48) | <48 (<48, <48) | 0·74 | 0 (0, 0) | 0 (0, 0) | 0·40 |
| Anthropometric parameters | ||||||
| Body mass index, kg/m2 | 26 ± 5 | 26 ± 3 | 0·87 | −0·1 [−0·9, 0·8] | −0·4 [−1·0, 0·3] | 0·60 |
| Visceral adipose tissue area (VAT), cm2 | 142 ± 74 | 166 ± 130 | 0·47 | 8 [−8, 24] | −5 [−26, 17] | 0·31 |
| Subcutaneous adipose tissue area (SAT), cm2 | 202 ± 120 | 152 ± 93 | 0·15 | 2 [−20, 24] | 6 [−8, 19] | 0·76 |
| Hemodynamic parameters | ||||||
| Systolic blood pressure, mm Hg | 119 ± 16 | 117 ± 13 | 0·67 | 3 [−1, 7] | 7 [3, 11] | 0·15 |
| Diastolic blood pressure, mm Hg | 76 ± 10 | 73 ± 8 | 0·32 | 3 [1, 6] | 6 [3, 8] | 0·14 |
| Metabolic parameters | ||||||
| Fasting glucose, mmol/L | 4·92 ± 0·38 | 4·76 ± 0·66 | 0·36 | 1·00 [−0·37, 2·36] | 0·10 [−0·22, 0·41] | 0·21 |
| Hemoglobin A1c, % | 5·5 ± 0·3 (n=19) | 5·6 ± 0·4 (n=17) | 0·25 | 0·3 [0·5, 1·0] | −0·1 [−0·2, 0·1] | 0·36 |
| Inflammatory Parameters | ||||||
| Log C-reactive protein, mg/L | 0·1 ± 0·5 | −0·1 ± 0·6 | 0·54 | 0·1 [−0·2, 0·3] | −0·3 [−0·6, 0·.0] | 0·06 |
| Interleukin-6, median (IQR), ng/L | 0·8 (0·5, 1·2) | 0·6 (0·4, 1·6) | 0·75 | 0·1 (−0·2, 0·4) | 0·2 (−0·8, 0·3) | 0·77 |
| Marker of Vascular Inflammation | ||||||
| Lp-PLA2, ng/mL | 272·5 ± 73·7 | 285·6 ± 75·0 | 0·58 | −13·3 [−32·8, 6·2] | −52·2 [−70·4, −34·0] | 0·005 |
| Mean FDG-PET TBR of the aorta | 2·20 ± 0·37 (n=12) | 2·08 ± 0·32 (n=12) | 0·43 | −0·05 [−0·28, 0·17] (n=11) | 0·04 [−0·08, 0·16] (n=10) | 0·41 |
| Mean FDG-PET TBR of the most diseased segment of the aorta | 2·26 ± 0·37 (n=12) | 2·18 ± 0·33 (n=12) | 0·56 | −0·06 [−0·25, 0·13] (n=11) | −0·03 [−0·17, 0·12] (n=10) | 0·77 |
Non-normally distributed data are presented as median (IQR). Normally distributed data are presented as mean ± SD. For change columns, normally distributed data are presented as mean [95% C.I.].
TBR = target-to-background ratio.
Coronary Atherosclerosis Endpoints
Plaque Volume
Atorvastatin decreased noncalcified coronary plaque volume as compared to placebo over 12 months (Figure 2, atorvastatin Δ −8·2 (IQR: −18·3, 3·5) vs. placebo Δ +6·7 (−6·5, 29·8) mm3, p=0·03) On a percentage basis, subjects receiving atorvastatin had a −19.4% (IQR: −39·2%, 9·3%) decrease in plaque volume vs. an increase in noncalcified plaque volume of 20·4% in the placebo group (−7·1%, 94·4%) (p=0·009)(Table 2). In addition, atorvastatin reduced total coronary plaque volume compared to placebo (Δ −0·8 (IQR: −16·8, 14·2) vs. 12·0 (0·8, 100·4)mm3, p=0·02)(percent changes were −4·7% (IQR: −25·4%, 15·9%) vs. 18·2% (1·5%, 59·9%), p=0·01) over 12 months.
Figure 2. Comparison of the 1-Year Change in Noncalcified Plaque Volume in HIV Patients Randomized to Atorvastatin vs. Placebo.
Horizontal line in center of box denotes median and top of box denotes 75th centile and lower end of box denotes 25th centile.
Plaque Vulnerability Features
The number of segments with low attenuation plaques (<40 HU) and number of positively remodeled plaques (remodeling index >1·05) were significantly reduced by atorvastatin compared to placebo (p = 0.03 and 0.04, respectively, Table 2). Change in number of plaques with spotty calcifications was not significantly different between the two groups (Table 2).
Plaque Progression and Regression
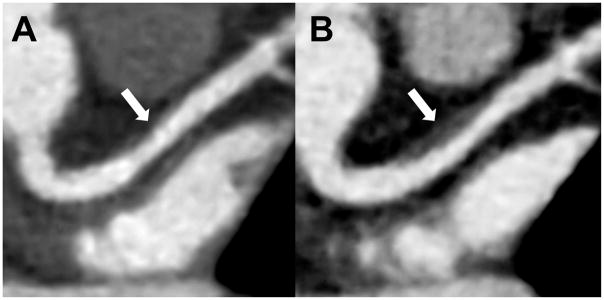
11/17 (64·7%) of patients in the atorvastatin group demonstrated regression of coronary atherosclerosis (based on any reduction in plaque volume) compared to 4/20 (20·0%) in the placebo group (p=0·008) Conversely, 16/20 (80%) of patients in the placebo group demonstrated progression of coronary atherosclerosis (based on any increase in plaque volume) compared to 6/17 (35·3%) of patients in the atorvastatin group (Table 2). No obvious effect of gender on plaque regression or progression was seen by Breslow Day test (p=0.17). An example of progression of coronary plaque in the proximal left anterior descending coronary artery of an HIV-infected participant who was randomized to placebo is shown in Figure 3.
Figure 3. Increasing Noncalcified Plaque in proximal left anterior descending (LAD) coronary artery in a Patient on Placebo.
Progressive coronary plaque in the proximal left anterior descending coronary artery (LAD, arrow) on A) initial and B) 12-month follow-up CCTA in a 58-year-old woman with HIV. Plaque volume increased from 11 to 124 mm3 (arrow). High risk morphology (HRM) features including positive remodeling and low density lipid core developed on follow-up. This participant was randomized to placebo during the study.
Coronary Stenosis
Three participants in the placebo group developed clinically significant increases in the degree of stenosis (two participants in the placebo group had stenosis that progressed to beyond 70% at 12 months and one participant in the placebo group developed >50% stenosis at 12 months). Although of clinical relevance, the difference between groups was not statistically significant. None of these patients developed any symptoms of coronary artery disease. In contrast, no participants in the atorvastatin group developed clinically significant increases in degree of stenosis (Table 2).
Supplementary Analysis of Plaque Segments
In a supplementary analysis, the effect of atorvastatin on plaque segments was analyzed. This analysis demonstrated that number of segments with plaque and number of segments with noncalcified plaque both decreased in the group treated with atorvastatin compared to placebo, but segments with calcified plaque or mixed plaque did not change between the groups (Supplementary Table 3).
Coronary Artery Calcium
There were no significant differences in coronary artery calcium score, calcium mass, calcium volume or calcium density at baseline or change over time between the two groups (Table 2).
Changes in Biochemical Measurements
Total cholesterol and direct LDL significantly decreased with atorvastatin compared to placebo (total cholesterol change −1·23[95% CI : −1·53, −0·93] vs. 0·12 [−0·12, 0·37]mmol/L, p<0·0001; direct LDL change −1·00[95% CI : −1·38, 0·61] vs. 0·30[0·04, 0·55] mmol/L, p<0·0001)(Table 3)(Figure 4). There was no statistically significant relationship between change in LDL and plaque (See Supplementary Results). No statistically significant differences in changes in fasting glucose or hemoglobin A1c were detected between the two groups (Table 3).
Figure 4. Comparison of the 1-Year Change in Direct LDL in HIV Patients Randomized to Atorvastatin vs. Placebo.
Bars represent means and error bars represent standard deviations.
Lipoprotein-associated phospholipase A2 significantly decreased with atorvastatin compared to placebo (Δ −52·2 [95% CI : −70·4, −34·0]vs. −13·3[−32.8, 6·2]ng/mL, p=0·005). Log CRP tended to decrease in the atorvastatin group compared to placebo (p = 0.06) (Table 3).
Changes in HIV Disease Related Parameters
Changes in CD4 count and HIV RNA did not differ between the atorvastatin and placebo groups after one year(Table 3). Change in VACS score was not different between groups including individual components such as hemoglobin and FIB-4 index (see Supplementary Table 1).
Changes in Body Composition and Dietary Assessment
No statistically significant differences in changes in BMI, visceral adipose tissue, or subcutaneous adipose tissue areas were detected between the two treatment groups(Table 3). Food records were returned from only a subset of patients and showed intake of total calories, fat, protein or carbohydrates did not differ between the groups at baseline and the changes over the course of the study did not differ significantly between the groups (Supplementary Table 4).
Clinical Adverse Events and Laboratory Abnormalities
Adverse events were distributed fairly evenly between the atorvastatin and placebo treatment groups. Myalgias and liver function tests (LFT) abnormalities occurred in both treatment and placebo groups at similar rates without any differences in the timing of adverse events (Table 4). No adverse event led to discontinuation from the study(Table 4). One participant experienced myalgias and was reduced to 10 mg, which was tolerated for the duration of the trial. After unblinding at the conclusion of the study, this participant was found to have received atorvastatin. A second participant underwent a dose reduction from 40 mg back to 20 mg because of a rise in ALT. Unblinding after the conclusion of the study revealed this participant received placebo.
Table 4.
Adverse Events
| Placebo No. of patients | Atorvastatin No. of patients | |
|---|---|---|
| Any serious adverse event* | 1 | 2 |
| Any adverse event leading to study-drug discontinuation | 0 | 0 |
| Any adverse event leading to study-drug dose decrease** | 1 | 1 |
| Any adverse event leading to non-escalation of dose at 3 months*** | 2 | 0 |
| Loose stools | 1 | 3 |
| Nausea | 2 | 1 |
| Muscle Aches/Cramps | 5 | 6 |
| LFT abnormalities (≥ 3× upper limit of normal) | 2 | 3 |
| CPK elevation (≥ 5× upper limit of normal) | 0 | 0 |
| Elevated fasting blood glucose (>126 mg/dL)**** | 2 | 0 |
| Rash | 1 | 0 |
| Abdominal pain | 1 | 0 |
| Allergic reaction (to study medication) | 0 | 0 |
SAEs include: 1 Subject in placebo group - hepatocellular carcinoma; 1 subject in atorvastatin group – detection of large cyst at routine ultrasound, underwent partial hysterectomy; 1 Subject in atorvastatin group – treated in the emergency department for gastrointestinal virus-induced dehydration.
1 Subject in placebo group – ALT elevation; 1 Subject in atorvastatin group – myalgia
1 Subject in placebo group had myalgia and did not escalate in dose until myalgia resolved at 9 month visit; 1 Subject in placebo group had myalgia temporarily and did not escalate in dose until 6 month visit
Fasting glucose checked at 12 month visit
In the atorvastatin group, initial presentation of myalgias occurred at 15 days, 1 month, 2 months, 3 months, 5 months, and 9 months. In the placebo group, initial presentation of myalgias occurred at 16 days, 1 month, 1 month, 2 months, and 3 months.
Liver function test (LFT) abnormalities occurred at 3 months, 6 months, and 6 months in the atorvastatin group. Liver function test abnormalities occurred at 3 months and 6 months in the placebo group.
CPK = creatine phosphokinase
Sensitivity Analyses
Among participants who maintained undetectable viral loads throughout the study, similar results in plaque and other parameters were seen (See Supplementary Results). Sensitivity analyses controlling for baseline noncalcified or total plaque volume using ANCOVA, in respective analyses, demonstrated similar results, with significant effects of atorvastatin to reduce noncalcified (atorvastatin effect estimate −16·2 mm3 [95% CI: −31·3, −1·0], p=0·04) and total plaque volume (atorvastatin effect estimate −23·0 mm3 [95% CI: −44·6, −1·4], p=0·04).
DISCUSSION
In this randomized double-blind placebo-controlled study of HIV-infected patients with subclinical atherosclerosis, we were unable to show that a statin reduced arterial inflammation in the aorta but found that treatment with a statin deterred overall coronary plaque progression and induced coronary plaque regression among HIV-infected patients, largely through effects on noncalcified plaque volume. Atorvastatin demonstrated a rapid and significant response by reducing coronary noncalcified plaque volume by 19·4% in one year in the patients in our study, compared to an increase in the placebo group of 20·4%. We also demonstrated that atorvastatin reduced high risk plaque features such as low attenuation and positive remodeling, which have been linked to acute coronary events39. These changes occurred in the context of a net change in LDL-cholesterol of 1·30 mmol/L (1·00 mmol/L reduction in atorvastatin group and 0·30mmol/L increase in placebo group) among a group in whom baseline LDL-cholesterol was not elevated by design, e.g. ≤3·37 mmol/L(130mg/dL). The study population, on ART, without a clinical history of cardiovascular disease, but with subclinical plaque and arterial inflammation is representative of HIV-infected subjects at risk for coronary artery disease. The VACS score in our population was comparable to that seen in other HIV populations including subjects described in the ART Cohort Collaboration35.
A primary objective of this study was to assess the effects of statins on specific inflammatory endpoints. Prior studies of statin therapy measured biomarkers of inflammation, immune activation 16, 17, 40 and endothelial function15 among HIV-infected patients but have not assessed direct measures of arterial inflammation. A significant reduction of CRP was not seen in a recent study of rosuvastatin in HIV-infected patients16. In our study, we found a near significant trend towards reduction in log CRP with atorvastatin at 40 mg/day. In addition, we demonstrate a significant reduction in lipoprotein-associated phospholipase A2 (Lp-PLA2) by 18·3%, consistent with findings from others17. Lp-PLA2 is an inflammatory enzyme secreted by monocytes, macrophages, and other inflammatory cells which hydrolyzes phospholipids on lipoproteins including oxidized LDL particles within the arterial intima, and leads to recruitment of monocytes to the intima41, 42. Levels of Lp-PLA2 and its activity are predictive of cardiovascular events in many large epidemiological cohorts43, however, darapladib, a selective oral inhibitor of Lp-PLA2, did not reduce cardiovascular events in a study of non HIV-infected patients44.
We assessed FDG uptake in the wall of the aorta as a key endpoint to try to define potential effects of statins on direct indices of arterial inflammation, among this cohort in whom arterial inflammation is increased9. At the time the study was initiated, however, there was no existing option of performing the study on a combined PET/CT scanner at our institution. The use of a dedicated PET scanner (which lacks integrated CT imaging) adversely impacted the ability to co-register the molecular and structural imaging data, and limited the ability to follow regions of arterial inflammation over time. Thus, we were able to obtain interpretable data in a subset of patients, which limits our ability to obtain meaningful data on the change in FDG uptake within this current study. Further studies with advanced molecular imaging techniques may be useful to assess atherosclerotic inflammation to complement studies on plaque morphology.
Using coronary CT angiography (CCTA), we had previously found a high prevalence of subclinical coronary atherosclerosis even among HIV-infected patients with low Framingham risk estimates and that the increased plaque type in HIV-infected patients is noncalcified plaque rather than calcified plaque6. Noncalcified plaque is lipid-laden, has higher macrophage content, and is considered to be more vulnerable to rupture. Thus, it is meaningful that a statin could decrease noncalcified plaque in HIV patients, suggesting that statin therapy may reduce the necrotic core of these plaques39. We had also previously shown that vulnerable plaque features were common in HIV-infected patients and were more prevalent than in matched controls10. Now, we demonstrate that statin therapy can reduce the number of plaques with these vulnerability features in HIV-infected patients as well as the volume of noncalcified plaque. This study is relevant to the large number of HIV-infected patients with subclinical atherosclerotic disease. In this study, this number represented more than half of the asymptomatic HIV patients screened, consistent with the high prevalence shown in our prior studies. Of note, we did not see a statistically significant relationship of change in LDL to plaque, suggesting other mechanisms may also be operative to achieve these results. Additional studies are important to further determine the relationship of LDL lowering to plaque reduction in this population. Results were similar in terms of plaque endpoints in analyses restricted to those who maintained viremic control.
The rapid progression and nature of coronary plaque in those HIV-infected patients randomized to placebo provide evidence regarding the natural history of atherosclerosis in HIV. Not only did plaque volume increase, but three of 20 patients in the placebo arm demonstrated progression of clinically significant coronary stenosis after one year of follow-up. In contrast, none of the statin-treated group progressed to clinically significant stenosis. Evidence for increased atherosclerotic disease progression in HIV-infected patients may suggest the need for more aggressive treatment with statins as in other high risk populations.
Our study is the first to investigate the effects of a statin on coronary atherosclerosis in the HIV patient population (panel). In the non HIV-infected population, statins at high doses can induce regression of atheroma volume measured by intravascular ultrasound (IVUS)13, 14 in patients with known coronary disease. In the current study, we investigated rates of plaque regression over one year in HIV-infected subjects with subclinical plaque using CCTA and demonstrated a one year plaque regression rate of 64.7% with atorvastatin up to 40mg a day. Prior studies utilizing CCTA to assess treatment effects of statins in the general population were not randomized trials, but also shed light on the potential effects of statins on atherosclerotic lesions. In a recent retrospective observational study in non-HIV patients, statin therapy was shown to reduce the progression of low attenuation plaque and noncalcified plaque measured by CCTA46. In another study of non-HIV patients that was nonrandomized, fluvastatin was found to reduce low attenuation plaque volume by serial cardiac CTA assessment, similar to what we found in HIV-infected patients47. Therefore non-randomized studies in non-HIV infected patients suggest a potential effect of statins on noncalcified plaque. The current study is the first randomized placebo-controlled trial to show an effect of statins on noncalcified plaque and plaque regression in any population by CCTA.
Due the potential concern for statin tolerability in the HIV population, particularly among a group with a significant percentage using protease inhibitors, we employed a dose escalation algorithm to increase the dose from 20 to 40mg after three months in which tolerability was demonstrated in case of potential ART effects on statin metabolism. Newer statins, such as pitavastatin are not known to interact with any ART, but were not available at the time of study initiation. Subjects in the current study were made aware of the potential risks of statin therapy including myositis and other effects in the informed consent document. Using this dose escalation algorithm, we demonstrated that atorvastatin appeared generally safe and well tolerated in the current study. In the current study, 40mg of atorvastatin lowered LDL-cholesterol by 1·00mmol/L over one year in HIV patients with LDL-cholesterol <3·37mmol/L(130mg/dL). Other studies in non HIV patients have shown an adverse effect of statins, including rosuvastatin and atorvastatin, on glucose metabolism48, 49. Moreover, rosuvastin worsened glucose homeostasis in the recent SATURN trial among HIV-infected patients50 whereas pitavastatin did not affect glucose parameters among HIV-infected patients51. In contrast, we did not detect any adverse changes in glucose or hemoglobin A1c in the current study but further larger studies assessing effects of statins on glucose parameters in the HIV population are needed. Myopathies and mylagias were seen in similar numbers of placebo and atorvastatin subjects and no subjects in either group demonstrated CPK >5× upper limit of normal. No specific pattern of timing of appearance of adverse events related to study drug initiation or dose escalation was seen.
The current study utilized state of the art techniques to phenotype changes in coronary plaque, including sensitive volumetric measurements and morphological assessments. Using CCTA, we were able to show in a randomized, placebo-controlled trial, highly significant and clinically relevant changes in coronary plaque, with significant overall regression and reduction in noncalcified plaque as well as improvement in vulnerable plaque morphology. This study therefore shows for the first time that statins successfully target a number of the specific pathophysiological features that may uniquely contribute to increased CVD rates in HIV. Larger and longer trials are now needed to show effects on events related to these changes in plaque, but this was not the purpose of the current study.
This study has strengths and limitations. It is a randomized, double blind placebo-controlled trial with very low attrition and comparable baseline characteristics, including immune function. Given the limitation we faced in assessing identical regions of aortic arterial inflammation in serial scans due to manual co-registration, the study did not end up having adequate power to assess changes in arterial inflammation due to availability of interpretable data in only a subset of participants. Subjects were similar in disease characteristics to subjects on ART in developed countries, the majority of whom do not have clinical cardiovascular disease. In line with our prior studies6 and those of Post et al.7, we found a significant number of such subjects to have subclinical disease on CT angiographic screening, but it is possible that referral bias led to a higher percentage of subjects with plaque than in the general population. VACS scores in these subjects were comparable to those seen in other cohorts of HIV patients on ART35. Although all patients were on ART, a small number had a low level of detectable viremia, but this did not differ between groups and did not influence the results as shown in sensitivity analyses. Our study does not prove that statins will prevent coronary heart disease, and further, larger, longer term studies, investigating hard endpoints, will be needed to determine if the reduction in plaque and improvement in high risk plaque morphology resulting from statins will translate into a protective effect on CVD in this population.
In conclusion, the findings of the current study demonstrate significant effects of statin therapy to reduce coronary plaque in HIV-infected patients with subclinical atherosclerotic disease. The current results show that statin therapy can reduce lipid-laden noncalcified plaque and reduced plaque with high risk morphology in HIV-infected patients. We showed that statins reduced markers of generalized vascular inflammation, but were not able to show an effect on arterial inflammation using FDG-PET. The results of this study on coronary plaque are likely to be relevant to the many HIV-infected patients who have subclinical atherosclerosis and non elevated LDL. The responses seen to statin therapy in this study argue for further study of early statin intervention strategies to prevent cardiovascular events in this population of HIV-infected patients.
Supplementary Material
Panel: Research in Context.
Systematic Review
We performed a systematic review of all clinical trials of statins on cardiovascular endpoints in patients with HIV disease in the last 10 years using PubMed, using the search terms “statin” and “HIV”. In addition, we searched for clinical trials assessing effects of statins on plaque volume by imaging with either intravascular ultrasound (IVUS) or coronary computed tomography angiography (CCTA) among HIV patients. Similar searches on these parameters were performed for studies conducted in the general population to provide a reference for our results. Searches were last performed on November 17th, 2014. Although a few randomized trials in HIV-infected patients have assessed the effects of statins on lipids, inflammatory markers, HIV disease parameters, and safety, no randomized trial has assessed the effects of a statin on direct measures of arterial inflammation using FDG-PET or coronary atherosclerosis, either by IVUS or CCTA, in HIV-infected patients. In the general population, randomized trials investigating effects of statins using IVUS have been performed but randomized studies have not assessed the effects of statins using CCTA. With respect to events, meta-analyses of data from over 170,000 individuals in 27 randomised trials in the general population have demonstrated the benefit of statins in reducing major vascular events,45 but no such data exist for the HIV population.
Interpretation
Our findings provide the first evidence of the effects of statin therapy on coronary atherosclerois in a randomized double blind trial of HIV-infected patients with subclinical coronary disease using CCTA to quantify and characterize coronary plaque. Our study is also the first randomized double blind trial to demonstrate reduction in coronary plaque using CCTA. The results of our study, showing a reduction in plaque volume and plaques with vulnerability features by atorvastatin in HIV-infected patients, extend data from studies in the general population suggesting overall reduction in plaque burden and especially reduction of lipid-rich vulnerable plaque by statin therapy.
Acknowledgments
Funding
This work was supported by NIH K23HL092792 (JL), NIH 5T32HL076136 (MTL), NIH 5K24HL113128 (UH), NIH R01HL095123 (SKG), NIH P30 DK040561 (SKG). The project described was also supported by Grant Number 1 UL1 RR025758-04, Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
The authors thank the patients who generously donated their time to participate in this study and the nurses and bionutritionists at the MGH Clinical Research Center of the Harvard CTSA.
Footnotes
Clinical Trial Registration: The trial is registered on http://clinicaltrials.gov registry number: (NCT00965185).
Contributors
Janet Lo: literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical revision of manuscript
Michael Lu: literature search, figures, data collection, data analysis, data interpretation, writing, critical revision of manuscript
Ezinne J. Ihenachor: figures, data collection, data analysis, critical revision of manuscript
Jeffrey Wei: figures, data collection, data analysis, critical revision of manuscript
Sara E. Looby.: data collection, data analysis, critical revision of manuscript
Kathleen V. Fitch.: data collection, data analysis, critical revision of manuscript
Jinhee Oh: data analysis, critical revision of manuscript
Chloe O. Zimmerman: data analysis, figures, writing, critical revision of manuscript
Janice Hwang: data collection, critical revision of manuscript
Suhny Abbara: study design, data collection, critical revision of manuscript
Jorge Plutzky: study design, critical revision of manuscript
Ahmed Tawakol: study design, data analysis, data interpretation, writing, critical revision of manuscript
Gregory Robbins: data collection, critical revision of manuscript
Udo Hoffmann: data analysis, data interpretation, writing, critical revision of manuscript
Steven K. Grinspoon: literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical revision of manuscript
Declaration of Interests
Dr. Grinspoon has consulted with Navidea, AstraZeneca, NovoNordisk, and Theratechnologies, and received grant support from Gilead, Amgen, KOWA Pharmaceuticals, and Theratechnologies, unrelated to this manuscript. Dr. Robbins has received grant support from Gilead Sciences unrelated to this manuscript. Dr. Hoffmann received grant support from Siemens Healthcare, American College of Radiology Imaging Network, and HeartFlow Inc., unrelated to this manuscript. Dr. Tawakol has received grant support from Genentech/Roche, BMS, Takeda, GSK, and VBL and personal fees from Novartis, Genentech/Roche, BMS, Cerenis, Takeda, Actelion, GSK, and Amgen unrelated to this manuscript. All authors report no conflicts of interest.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–31. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 3.Lohse N, Hansen A-BE, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of Persons with and without HIV Infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post WSBM, Kingsley L, Palella FJ, Witt MD, Xiuhong Li, George RT, Brown TT, Jacobson LP. Associations Between HIV Infection and Subclinical Coronary Atherosclerosis. Ann Intern Med. 2014;160(7):458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified Coronary Atherosclerotic Plaque and Immune Activation in HIV-Infected Women. J Infect Dis. 2013;208(11):1737–46. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased Coronary Atherosclerotic Plaque Vulnerability by Coronary Computed Tomography Angiography in HIV-Infected Men. AIDS. 2013;27:1263–72. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen TS, Kofoed KF, Kuhl JT, Nielsen WB, Nielsen MB, Kelbaek H. Prognostic implications of nonobstructive coronary plaques in patients with non-ST-segment elevation myocardial infarction: a multidetector computed tomography study. J Am Coll Cardiol. 2011;58(5):502–9. doi: 10.1016/j.jacc.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 12.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 13.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. Jama. 2006;295(13):1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 15.Stein JH, Merwood MA, Bellehumeur JL, Aeschlimann SE, Korcarz CE, Underbakke GL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147(4):E18. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4):588–95. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–64. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Bechir M, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92(1):110–2. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611–20. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 20.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. Journal of cardiovascular computed tomography. 2009;3(2):122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109(1):14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulou S, Neefjes LA, Garcia-Garcia HM, Flu W, Rossi A, Dharampal AS, et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovascular imaging. 2012;5(3 Suppl):S28–37. doi: 10.1016/j.jcmg.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Oberoi S, Meinel FG, Schoepf UJ, Nance JW, De Cecco CN, Gebregziabher M, et al. Reproducibility of noncalcified coronary artery plaque burden quantification from coronary CT angiography across different image analysis platforms. AJR Am J Roentgenol. 2014;202(1):W43–9. doi: 10.2214/AJR.13.11225. [DOI] [PubMed] [Google Scholar]
- 24.Schlett CL, Ferencik M, Celeng C, Maurovich-Horvat P, Scheffel H, Stolzmann P, et al. How to assess non-calcified plaque in CT angiography: delineation methods affect diagnostic accuracy of low-attenuation plaque by CT for lipid-core plaque in histology. Eur Heart J Cardiovasc Imaging. 2013;14(11):1099–105. doi: 10.1093/ehjci/jet030. [DOI] [PubMed] [Google Scholar]
- 25.Hamirani YS, Zeb I, Pagali SR, Kadakia J, Saraff G, Choi T, et al. Normalization of automatic plaque quantification in cardiac computed tomography (CCT) Int J Cardiol. 2011;146(2):282–90. doi: 10.1016/j.ijcard.2010.10.086. [DOI] [PubMed] [Google Scholar]
- 26.Nakazato R, Shalev A, Doh J, Koo B, Gransar H, Gomez M, et al. Aggregate Plaque Volume by Coronary Computed Tomography Angiography Is Superior and Incremental to Luminal Narrowing for Diagnosis of Ischemic Lesions of Intermediate Stenosis Severity. Journal of the American College of Cardiology. 2013;62(5):460–7. doi: 10.1016/j.jacc.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33(8):1007–16. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 28.Versteylen M, Kietselaer B, Dagnelie P, Joosen I, Dedic A, Raaijmakers R, et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. Journal of the American College of Cardiology. 2013;61(22):2296–305. doi: 10.1016/j.jacc.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 29.Schuhbaeck A, Dey D, Otaki Y, Slomka P, Kral BG, Achenbach S, et al. Interscan reproducibility of quantitative coronary plaque volume and composition from CT coronary angiography using an automated method. Eur Radiol. 2014;24(9):2300–8. doi: 10.1007/s00330-014-3253-3. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2(2):153–60. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed Tomographic Angiography Characteristics of Atherosclerotic Plaques Subsequently Resulting in Acute Coronary Syndrome. Journal of the American College of Cardiology. 2009;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47(8):1655–62. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 33.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. Journal of the American College of Cardiology. 2007;50(4):319–26. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 35.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–72. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36(1):172–7. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 37.Lo J, You SM, Canavan B, Liebau J, Beltrani G, Koutkia P, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA. 2008;300(5):509–19. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–31. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 40.Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–64. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338( Pt 2):479–87. [PMC free article] [PubMed] [Google Scholar]
- 42.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115(21):2715–21. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 43.Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 45.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198–204. doi: 10.1016/j.atherosclerosis.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3(7):691–8. doi: 10.1016/j.jcmg.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dormuth CR. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. doi: 10.1136/bmj.g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McComsey GA, Jiang Y, Erlandson KM, Debanne SM. Rosuvastatin Improves Hip Bone Mineral Density but Worsens Insulin Resistance. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. p. Oral abstract 134. [Google Scholar]
- 51.Aberg J, Sponseller CA, Kryzhanovski VA, Kartman CE, Thompson MA. Neutral effects of pitavastatin 4 mg and pravastatin 40 mg on blood glucose and HbA1c levels over 12 weeks: prespecified safety analysis from INTREPID (HIV-infected patieNts and TREatment with PItavastatin vs pravastatin for Dyslipidemia), a phase 4 trial Endocrine Society Conference; 2013; San Francisco, CA. 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.