Summary
Despite marked improvements in the survival of patients with severe lupus nephritis over the last 50 years the rate of complete clinical remission following immune suppression is less than 50% and renal impairment still occurs in 40% of affected patients. An appreciation of factors leading to the development of chronic kidney disease (CKD) following acute or subacute renal injury in lupus patients is beginning to emerge. Processes that contribute to endstage renal injury include continuing inflammation, activation of intrinsic renal cells, cell stress and hypoxia, metabolic abnormalities, aberrant tissue repair and tissue fibrosis. This understanding is leading to the development of novel or adjunctive therapies that may protect from the secondary non-immune consequences of acute injury. Approaches based on a molecular/proteomic/lipidomic classification of disease should yield new information about the functional basis of disease heterogeneity so that the most effective and least toxic treatment regimens can be formulated for individual patients.
Introduction
Lupus nephritis (LN) affects 30–60% of adults and up to 70% of children with systemic lupus erythematosus (SLE) and is characterized by the glomerular deposition of immune complexes followed by recruitment of an inflammatory response. Glomerular disease is classified by light microscopy into five histologic subtypes of which classes III–V have the potential for long term damage. Further histologic classification uses composite scores for active inflammation and chronic scarring (1–5). Although classification drives therapeutic decision-making, the current scheme does not adequately predict which patients will respond to therapy (3, 6–7), indicating that additional phenotyping based on mechanisms of tissue injury is required. In addition, patients in clinical remission may exhibit progressive inflammation and fibrosis in repeat biopsies suggesting that improved disease monitoring could prevent undertreatment of subclinical disease (8–9).
While advances in immunosuppressive regimens and general medical care have erased most of the differences in long-term outcomes between proliferative and membranous LN (7, 10–11), the rate of complete remission for proliferative disease remains below 50% (12–14), and up to 40% of LN patients still develop some degree of renal impairment (11, 14). This failure of immunosuppressive agents to adequately treat LN, even in the setting of well-monitored clinical trials, reflects an incomplete understanding of disease pathogenesis. Either immune cell proliferation is not the only relevant cause of renal injury, or there is a therapeutic time window after which this type of intervention is no longer effective.
In the first section of this review I will discuss current knowledge of LN pathogenesis including disease initiation by immune complexes, immune activation in the kidney, and the responses of renal parenchymal cells to this insult. In the second part I will discuss approaches to identifying new pathogenic mechanisms, and review alternate ways to classify and monitor disease that include molecular and proteomic analyses. Finally I will address how these concepts may lead to improved therapies.
Mechanisms of renal damage
Initiation of inflammation by immune complexes
LN is initiated in most cases by the glomerular deposition of IgG and complement. Rarely, LN occurs in the absence of immune complexes, presumably due to direct damage by soluble inflammatory mediators (15). Sources of immune complexes include circulating anti-nuclear, anti-C1q, and crossreactive anti-glomerular autoantibodies (16–20), opsonized apoptotic particles, microparticles, and neutrophil NETs (21–22). Particulate DNA such as that within neutrophil NETs can be resistant to digestion by DNAse (22), and downregulation of renal DNAse I can be a late feature of disease (23). Although antibodies eluted from LN kidneys are enriched for anti-DNA activity, not all anti-DNA antibodies are pathogenic. Furthermore, non-DNA binding antibodies, some arising in situ, also contribute to renal disease (24–25). This heterogeneity of renal depositing antibodies limits the ability of serum antibody profiles to predict LN flares (26).
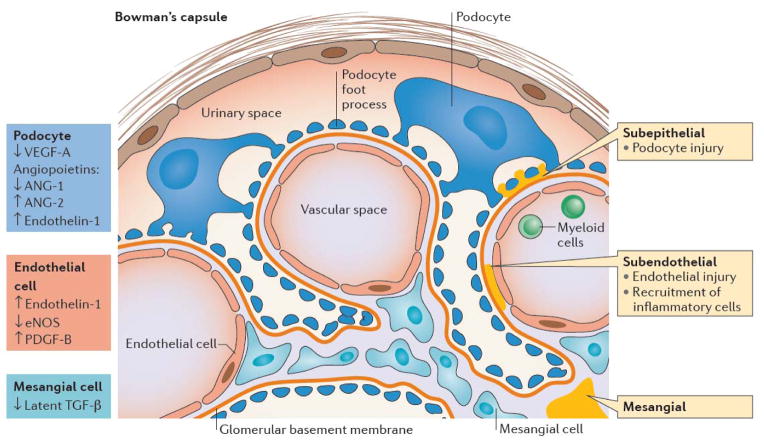
Immune complexes initiate renal damage by several mechanisms (Figure 1). Complexes that deposit in the subendothelium injure endothelial cells and are the hallmark of Class III and IV proliferative disease. These deposits have access to the vascular space and may activate circulating myeloid cells that express Fc receptors, allowing them to infiltrate the renal tissue (27). By contrast, subepithelial deposits, found in Class V disease, injure podocytes but elicit a less severe inflammatory response as they contact only the urinary space. If the glomerular basement membrane ruptures however, these immune complexes can access the whole glomerulus. Immune deposits may initiate the complement cascade, or they may directly activate intrinsic glomerular cells (28–29), inducing the release of inflammatory chemokines and cytokines. Complexes containing nucleic acids additionally activate intracellular TLRs, thereby enhancing the inflammatory response.
Figure 1.
Glomerular injury in LN: View of a glomerular loop containing endothelial cells (yellow) lined by a glycocalyx (orange), mesangial cells (ochre) and podocytes (brown). Podocytes are separated from endothelial cells by the glomerular basement membrane (GBM -red). The glomerulus is contained by Bowman’s capsule (BC) that is lined by parietal epithelial cells. Immune deposits (blue) may be found in the mesangium, subendothelium, or subepithelium. Subendothelial deposits are in contact with the vascular space (V) and can recruit myeloid cells (My) into the renal parenchyma. Subepithelial cells are in contact with podocytes and the urinary space (U). Podocytes and endothelial cells communicate with each other by transfer of soluble growth factors across the GBM, whereas mesangial cells and endothelial cells can interact directly via receptors and their ligands. Alterations of these factors in LN are shown by the arrows and color coded according to cell type.
Immune mechanisms of tissue injury
Soluble inflammatory mediators
Following immune complex deposition, a large variety of inflammatory mediators is produced in LN kidneys with spreading of the response as disease progresses (28, 30). Several locally produced cytokines and chemokines that contribute to inflammatory damage and whose absence or inhibition greatly attenuates disease activity have been identified. Examples include CCL2, a chemokine expressed early in the glomerulonephritis process, and TNF that is expressed at proteinuria onset (31–32). A Type I IFN signature is also a feature of LN kidneys (33–34). IFN has multiple detrimental effects on the kidneys including vascular rarefaction and injury to glomerular parietal cells and podocytes (35–36). Once tissue injury occurs, soluble products released from injured cells amplify the inflammatory response by stimulating extracellular and intracellular innate immune receptors (37–41). Podocytes, for example, are sensitive to TLR-mediated signals and other inflammatory mediators such as NO, which induce foot process effacement and podocyte loss (42). Nevertheless, not all renal inflammatory mediators are necessary for the inflammatory process. For example, IL-17 deficiency alters the course of LN only in some models in which TH17 cells infiltrate the kidneys (43–44). Thus, targeting of single inflammatory mediators may not be sufficient to reverse the established renal inflammatory response that becomes more complex over time.
Cellular infiltrates
Inflammatory cells may infiltrate the kidney through glomerular or interstitial blood vessels. The anatomic organization of these infiltrates is quite variable. They may consist of scattered cells, disorganized aggregates, or rarely, organized structures containing germinal centers (45). There is also evidence for in situ activation of pathogenic adaptive immune responses to degraded or modified renal antigens. Both B cells and T cells from LN kidneys are clonally expanded, and the same T cell expansions have been detected in the peripheral blood (46–47). Multiple T cell cytokines such as IFNγ, IL-21 and IL-17 have also been detected in LN kidneys (48), and T cells appear in the urine of LN patients (49). In a small exploratory study, clonal CD8 T cell infiltrates were found adjacent to epithelial cells in LN biopsies and were associated with more severe disease, suggesting an effector function elicited by local antigens (47).
Strikingly, a measurable proportion of B cells derived from human LN biopsies recognize vimentin, an intracellular structural protein that is cleaved and extruded from apoptotic cells (46). Serum anti-vimentin antibodies are associated with decreasing GFR and increasing tubulointerstitial damage in other forms of CKD, and are similarly associated with severe interstitial disease in LN (46, 50). Autoantibodies to annexin1 and αenolase have also been detected in LN kidneys (51). These local adaptive immune responses may amplify inflammation independently of systemic autoimmunity.
Macrophages play a central role in both injury and repair; renal infiltration of LN kidneys with macrophages, particularly at the second biopsy, is associated with poor prognosis (52–54). Both infiltrating inflammatory macrophages and resident interstitial macrophages may be present in diseased kidneys (53, 55–56). Macrophages recruited from the peripheral blood are pro-inflammatory during acute inflammation but can then switch to a reparative phenotype (53, 57). Resident interstitial macrophages may have self-renewal properties (58), and in mouse models of chronic LN, they increase in number, become activated, and acquire MMP and cathepsin activity, suggesting that they contribute to aberrant tissue remodeling (30, 55).
It is increasingly recognized that several types of dendritic cells also infiltrate the kidneys during LN (59) potentially propagating local adaptive immune responses (60). Importantly, an increase in both CD141hi and myeloid DCs is observed in human glomerulonephritis biopsies and correlates with fibrosis (61).
Non-immune mechanisms of tissue injury in LN
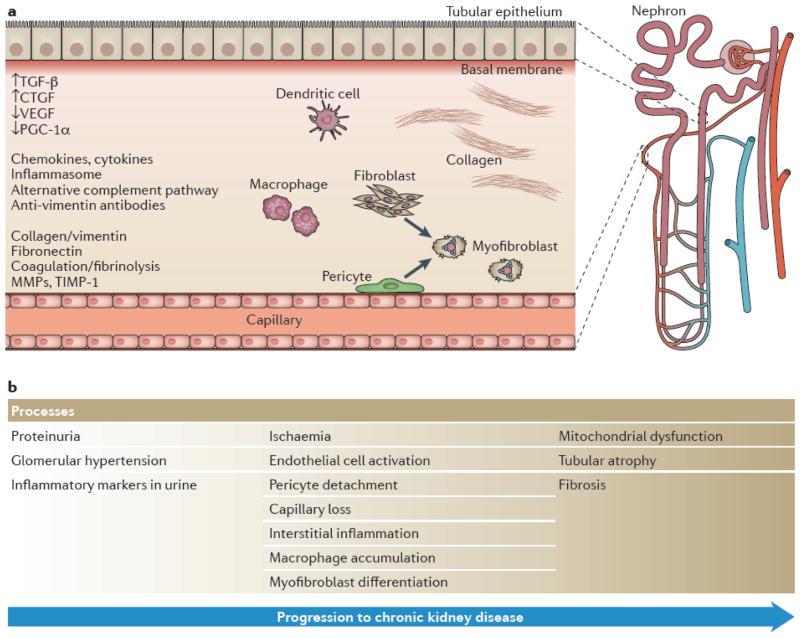
Several lines of evidence suggest that non-immune mechanisms of renal damage need to be therapeutically addressed in LN. First, many patients do not adequately respond to immunologic interventions. Second, remission can take months, during which time damage may continue to accrue. Third, progression may occur even if systemic autoimmunity is controlled. Although the role of each intrinsic renal cell during the stages of injury and repair is still not fully understood, some general mechanisms of CKD should also be applicable to LN (Figure 2).
Figure 2.
Interstitial injury in LN: A single nephron with its tubular and capillary system is shown. Cellular components that contribute to interstitial injury include tubular cells, endothelial cells (Cap), pericytes (P), fibrobasts (F), inflammatory lymphocytes (Ly), infiltrating and resident macrophages (Mac) and infiltrating dendritic cells (DC). Both pericytes and fibrobasts can differentiate into myofibroblasts (Myo). The sequential processes occurring during the progression to CKD are shown on the right and key molecules are shown on the left.
Disruption of cell-cell interactions
Maintenance of the complex structure of the nephron requires cell-cell interactions (62) (Figure 1). In the glomerulus, endothelial cells directly contact mesangial cells (63). Mesangial cells sequester latent TGFβ, preventing TGFβ-mediated damage to endothelial cells (64). Endothelial cells produce PDGF-B whose interaction with PDGF-Rβ on mesangial cells is required for glomerular development. Expression of PDGF isoforms is upregulated in many forms of renal injury, causing mesangial hyperproliferation, matrix production, cytokine and chemokine release, and renal fibrosis (65).
Podocytes and endothelial cells also interact by bidirectional diffusion of cytokines/growth factors through the glomerular basement membrane (63). Podocytes secrete angiopoietin 1 and VEGF-A that support endothelial cell survival; loss of renal VEGF-A characterizes LN both in humans and mouse models and distinguishes LN from other forms of CKD (34). In diseased tissue, both activated glomerular endothelial cells and damaged podocytes release endothelin 1 that amplifies glomerular injury by causing mitochondrial stress (66). Since podocyte regenerative capacity is limited in adults, loss of podocytes eventually leads to glomerulosclerosis. As nephrons are lost, compensatory mechanisms cause a rise in intraglomerular pressure and glomerular stress in the remaining nephrons (67).
A cell that has received much recent attention is the pericyte which contacts and shares a basement membrane with capillary endothelial cells. Pericytes secrete VEGF-A, chemokines and other inflammatory mediators, they help maintain endothelial cell quiescence, they contribute to the basement membrane and they regulate both medullary blood flow and cell traffic through the endothelial barrier during inflammation (68–70). Mesangial cells are the pericytes of the glomerulus and the interstitium has its own pericyte network attached to tubular capillaries. During inflammation, these pericytes rapidly dissociate from tubular capillaries and migrate into the interstitial space where they differentiate into myofibrobasts (71).
Abnormal vascular function and tissue hypoxia
Glomerular endothelial cells are coated with a glycocalyx layer that forms a crucial barrier to protein loss (72); this barrier can be degraded by the enzyme heparanase that is induced by endothelial hypoxic stress (73). As pericytes dissociate from capillaries and VEGF-A production diminishes, the capacity for angiogenesis and capillary repair is lost, leading to capillary rarefaction in both the glomerulus and the interstitium (71, 74). Other disturbances of angiogenesis reported in LN include a decrease in the ratio of pro-angiogenic Ang1/anti-angiogenic Ang2, downregulation of the angiogenic factor FGF-2, an increase in the VEGF inhibitor ADAMTS-1, and alterations in endothelial nitric oxide synthase (68, 75–77). In a mouse model of LN, downregulation of VEGF-A and FGF-2 persists even after induction of complete remission, suggesting that vascular injury is not completely reversible (30).
Some areas of the kidney, especially the medulla, maintain a low pO2 even under normal physiologic conditions and are sensitive to hypoxia. The interstitial blood supply derives from post-glomerular blood vessels, so glomerular hypertension and sclerosis can cause interstitial ischemia. Loss of VEGF-A, oxidative stress due to inflammation, increased energy demands, endothelial cell injury by cytokines, enhanced endothelin release, hypertension and anemia may all contribute to hypoxia (78–80); the relative contribution of each of these mechanisms is not well understood.
Tubular dysfunction and atrophy
Ischemia, hypertension or failed regeneration contribute to atrophy of tubular epithelial cells; such injury promotes both interstitial immune cell infiltrates and fibrosis. Recent studies have shown that injured renal tubular cells have a defect in fatty acid oxidation which causes mitochondrial dysfunction, reprograms them to a pro-fibrotic phenotype, and contributes to their death (30). The defect can be reversed by tubule-specific expression of the mitochondrial biogenesis regulator PGC-1α. Importantly, human fibrotic kidneys have a similar alteration in tubular fatty acid metabolism, suggesting a potential new therapeutic approach (81–82).
Renal fibrosis and progression to chronic kidney disease
Renal fibrosis is a poor prognostic feature in LN as in CKD generally. The extent of fibrosis is determined by the balance of matrix production with enzyme-mediated matrix breakdown and turnover (83). Mesangial cells are the classical myofibroblasts in the glomerulus, but parietal epithelial cells and podocytes may also contribute (83). In the interstitium, pericytes and resident fibroblasts are the major sources of myofibroblasts that produce extracellular matrix, MMP inhibitors and collagen (84). Both tissue macrophages and tubular epithelial cells produce growth factors that support myofibroblast differentiation, including TGFβ, PDGF and CTGF (85–86). These factors are balanced by local anti-fibrotic factors such as the TGFβ antagonists BMP-7 and hepatocyte growth factor. In addition, cytokines, chemokines, matrix proteins, procoagulants and remodeling enzymes produced by inflammatory cells, tubular cells and macrophages provide a pro-fibrotic microenvironment (87).
Although it has not been shown unequivocally that an area of fibrosis will damage adjacent healthy nephrons (88), several lines of evidence indicate that fibrosis can amplify renal damage. Fibroblasts may contribute to tissue injury by producing pro-inflammatory mediators (89). Fibrotic tissue may disrupt normal anatomic structures and interfere with oxygen diffusion, thus exacerbating hypoxia (83). In addition, epigenetic changes can reprogram fibroblasts to maintain their profibrotic state even after inflammation has resolved (90–92). One epigenetic change that has received considerable attention is the upregulation of miR-21 that protects against acute renal inflammation and damage but may also exacerbate fibrosis (93–94).
Identifying novel pathways that contribute to initiation and progression of lupus nephritis
Improved therapy for SLE will likely require disease classification based on pathogenic mechanisms. Some of these mechanisms will be common to most patients, some may be stage-specific, and others may apply to smaller patient subgroups or even individuals. Several approaches to identify commonly shared pathways have been explored.
Clinical and Epidemiologic studies
Factors associated with poor outcomes in lupus nephritis are shown in Table 1 (11, 95–96). Long term outcome depends on the degree of treatment response, with resolution of proteinuria being the best surrogate marker of a favourable prognosis (97–98). The severity of renal damage at presentation, manifested by serum creatinine and the degree of interstitial inflammation and fibrosis, strongly influences the response to treatment. These data support the need for improved monitoring of high risk individuals, and suggest that early detection of LN and prompt intervention will improve outcomes. Not surprisingly, non-response to therapy, recurrent flares and residual inflammation or fibrosis at repeat biopsy are associated with a poorer prognosis (54, 99–100). Repeat biopsies are not routinely performed but may be useful to guide tapering regimens and/or the need for changes in maintenance therapies.
Table 1.
| Renal extrinsic |
| Genetic |
| African-American race |
| Male gender |
| Older age |
| Socio-economic |
| Delay in diagnosis |
| Poor compliance |
| Limited access to qualified providers |
| Lack of health insurance |
| Renal intrinsic |
| ISN/RPS histologic classes III, IV and V |
| Increased serum creatinine |
| Interstitial infiltrates |
| Fibrosis |
| Mixed renal intrinsic/extrinsic |
| Anemia |
| Hypertension |
| Inadequate response to therapy |
| Failure to resolve proteinuria by 6–12 months |
| Recurrence |
| Inflammation, macrophage infiltration, fibrosis at second biopsy |
Importantly, recent epidemiologic data from the US has shown an increase in ESRD in uninsured individuals and in geographic areas with poor access to ambulatory care, indicating a significant effect of healthcare policy, healthcare access and patient compliance on LN outcomes (101). Similar results have been shown in other countries (102). Unless this public health issue is addressed it is difficult to envision improved outcomes by medical management alone.
Genetic risk for LN
Genome-wide analyses (GWAS)
A recent meta-analysis of 3 GWAS identified several inherited polymorphisms that predispose to initiation of LN, including the PDGFRA locus, the glucose transporter Slc5A11, and hyaluronan synthase 2 that is involved in extracellular matrix formation (103). Other risk genes include ABIN1, TNFSF4, Stat4, ITGAM, kallikreins and FcγRIIIa low-binding alleles (104–110). These results suggest a link between inflammation and LN as well as a contribution from pathways that regulate the renal response to inflammation and injury. However the relative risk associated with most of these genetic variants is low, and some are relevant only in certain ethnic populations, making it challenging to generate a clinically meaningful genetic risk-prediction test. The function of the various polymorphisms will need to be unravelled in order to understand their clinical significance.
Only a few small studies have addressed the genetic risk for increased disease severity in LN patients. Polymorphisms in APOL1 and MYH9 confer an increased risk of renal failure in other chronic renal diseases, and appear to confer a modest risk for LN progression that may be dependent on ethnicity (111–113). No studies have addressed the genetic basis for responsiveness to therapy.
Local epigenetic changes
The epigenetic landscape of renal injury is just starting to be described, particularly in the interstitium (114–115). Examples relevant to LN include chromatin structure modifications that regulate the production of CCL2 and TNF, DNA demethylation that regulates C3 production and histone acetylation that regulates the production of PDGF and pro-fibrotic genes (115). Histone deacetylase inhibitors alleviate progression in multiple models of renal injury and fibrosis by preventing cytokine release and cell apoptosis and by inducing the protective molecule BMP-7 (116–117). Epigenetic changes can also be mediated by non-coding RNAs. TGFβ induces several miRNAs that enhance pro-fibrotic genes while at the same time inhibiting miRNAs that are protective (93–94). The utility of blood and urine miRNA signatures as biomarkers for LN is starting to be examined (118–119).
Molecular and proteomic signatures of renal disease
Molecular and/or proteomic phenotyping of the kidney or urine may identify the immunologic and/or injury process currently affecting each individual, and could deliver biomarkers to improve patient stratification for clinical trials and a more rational therapeutic approach.
Molecular profiling of LN kidneys
Several studies have shown signatures of immune cell infiltration and activation, extracellular matrix formation and fibrosis, endothelial cell activation, fibrinolysis, mitochondrial dysfunction, and tubular injury (34, 120–121). Because pre-nephritis and repeat biopsies are rarely available from humans, mouse models have been useful for characterizing renal molecular profiles at sequential disease stages. Overlay of these data onto data from human LN biopsies allows identification of pathways that are relevant to human disease. Using this approach in NZB/W lupus-prone mice we identified two dominant gene clusters that are dynamically regulated during disease progression; the first is detected at proteinuria onset and reflects the inflammatory component of nephritis – the second occurs during established proteinuria and reflects the mitochondrial and metabolic signature of chronic disease. Remission induction therapy administered promptly after nephritis onset reverses most of this abnormal gene expression profile but we noted that the mitochondrial and metabolic signature recurred before clinical relapse, perhaps reflecting an increased propensity to tissue hypoxia conferred by the initial tissue insult (30). By following mice from remission to relapse, we further identified podocyte loss, renal tubular dysfunction, endothelial cell activation and tissue remodeling as the functional features associated with the progression to renal failure (30). Some of these processes may be resistant to standard immune suppression (34, 122).
One important observation in mice is that glomerular disease does not always progress to tubular and endothelial dysfunction (123–124). For example, BAFF deficient or BAFF-R-Ig treated lupus-prone mice have autoantibodies, glomerulonephritis and interstitial inflammatory infiltrates but do not die of renal failure (30, 123). Similarly, a genetic model has been generated in which acute glomerular injury occurs, but progression to CKD does not (125). How this transition from renal inflammation to renal failure is regulated is not completely understood (126–128) and is an important knowledge gap that needs to be filled (121–123). In addition, there are several LN models in which renal deposition of immune complexes does not induce the recruitment of effector cells. These include myeloid cell deficiency of Fc receptors, CCL2 deficiency and intense costimulatory blockade (27, 129–130). These studies, in sum, suggest that there are several opportunities for therapeutic intervention that target the effector response in the kidney, regardless of systemic autoimmunity.
A problem with the interpretation of molecular profiles of whole tissues is that the signatures are biased towards the most frequent cell types and towards infiltrating cells that are rare in normal kidneys. The only way to overcome these biases is to study each cell type individually. No studies of this type have as yet been performed in human LN biopsies and even the data from mouse models is still quite limited. Molecular studies in both mice and humans have suggested that macrophages/DCs are key players in the development of LN (34). Comparative molecular profiling of isolated renal macrophages from young and nephritic NZB/W mice and NZW/BXSB mice (34, 55) revealed that several functional pathways are upregulated in nephritic macrophages including the alternate complement pathway, cell adhesion, phagocytosis and efferocytosis, Fc receptor signaling, the nucleic acid sensing pathway and tissue repair. Pathways that are downregulated include fatty acid metabolism and angiogenesis. This profile suggests aberrant resolution of inflammation which may be driven by ongoing immune complex deposition and/or tissue damage. It remains to be determined whether it is possible to harness the reparative function of macrophages for optimal tissue repair without fibrosis.
Proteomic analysis of LN kidneys
Proteomic analysis of kidneys has been challenging due to the limited amount of available tissue and the inability to detect small or low abundance proteins; the feasibility of this approach is increasing with new technical advances. A proteomic analysis of human lupus glomeruli revealed loss of podocyte proteins, activation of the alternate complement pathway, loss of anti-oxidants and downregulation of normal metabolic pathways, consistent with the findings of the molecular studies (131–132). Analyses of proliferative LN biopsies using a targeted set of 500 immune response genes showed that activation of IFN pathways, production of IL-10 and T and B cell signaling pathways are associated with lack of response to therapy (6). These data are consistent with the clinical observation that interstitial infiltrates are associated with poor prognosis. It is also becoming clear that variability in the renal proteome can occur within a WHO Class, adding potential for subclassification based on proteomic analysis (133).
In sum, the preliminary molecular and proteomic studies of LN kidneys have extended mechanistic knowledge beyond the histologic data by identifying two phases of disease characterized by inflammation and metabolic dysfunction, have identified both shared and unique effector signatures and have identified a number of checkpoints along the path to irreversible renal damage that could potentially be targeted by new therapies. The molecular studies have been less successful at identifying the earliest features of LN since only few genes are upregulated in the kidneys prior to the onset of proteinuria (30); a proportion of these is associated with steroid and lipid metabolism. Proteomic and metabolomic studies may be more suitable for detecting such early changes (134–135).
Analysis of urines
Longitudinal monitoring of urine offers a window into the kidney that could be used for treatment decisions. The technical challenges inherent in this approach have recently been reviewed (133). The major clinical use of urine proteomics has so far been to identify biomarkers that either predict an impending renal flare (6, 136) or identify response to therapy. There are several longitudinal studies of single urine biomarkers including the chemokine CCL2, the tubular marker lipocalin2, the acute phase protein hepcidin and the TNF-like molecule TWEAK (reviewed (133, 137)). These studies are still preliminary and have not yet been reliably reproduced or tested in the setting of a clinical trial. Combination panels of these and other potential biomarkers identified in cross-sectional studies may have a higher predictive value than single analytes alone.
It is important to note that cells and microparticles of cells are also shed into the urine during inflammation and this could be exploited for discovery of other protein and RNA-based biomarkers and for early detection of podocyte loss.
Using therapeutics to test the pathogenicity of target molecules
A final approach to identifying key pathways in LN is to identify molecules that are potentially pathogenic based on their known biology and then test their role in pre-clinical models either by genetic depletion or therapeutic targeting. This approach is often successful when the correct preclinical model expressing the protein of interest is used and the treatment is timed either before or at the first presentation of LN (138). Nevertheless this is proving to be a cumbersome and expensive approach in human LN that has been hampered by the primitiveness of disease phenotyping, the confounding effects of standard medications such as glucocorticoids and the absence of a uniformly accepted definition of renal outcome (139).
When choosing such targets, it is important to consider the sometimes opposing effects of pleiotropic immune pathways and cytokines on systemic immunity vs. local effector responses. Genetic deficiencies of TNF, TGFβ, components of the innate pathway such as TLR9 and STING and Nox2, and inflammasome components NLRP3 and ASC all induce or exacerbate autoimmunity in mice (140–142). This may be due to the loss regulatory functions of these proteins or to diminished capacity to fully control infectious insults. On the other hand both TNF and TGF beta are highly expressed in inflamed kidneys and their inhibition at this stage of disease improves disease outcome in mouse models (32, 143). TNF inhibition does indeed improve chronic LN in patients without inducing systemic flares although its clinical utility has been limited by other toxicities (144). Nevertheless, targeting these types of pathways will need to be approached with caution.
Implications for treatment
Targeting systemic autoimmunity
Given the role of systemic autoimmunity in the initiation of LN, a major focus of management should be on prevention. Indeed in mouse models of lupus, LN can be completely prevented by immune interventions that prevent the renal deposition of autoantibodies or by genetic manipulations that prevent tissue responses to immune complex deposition (145). There are however many obstacles to a similar approach in humans including the heterogeneity of immune responses in individual patients, the absence of non-invasive monitoring tools for early detection, the potential toxicities of new therapies in patients with quiescent disease and the expense of time-to-flare studies that need large study cohorts.
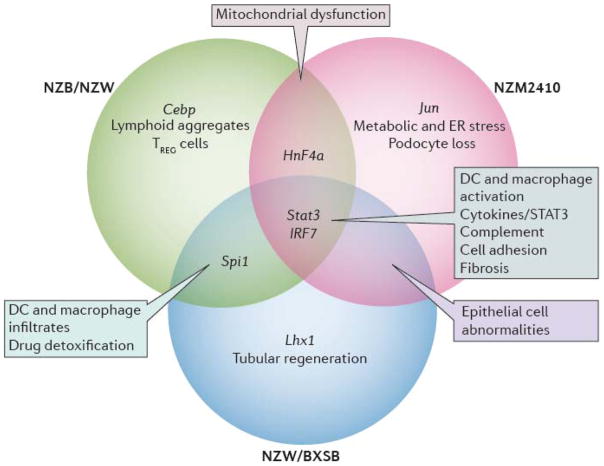
Once disease onset occurs, there may only be a limited time window before disease mechanisms that are insensitive to preventive strategies arise. In mice it is much easier to prevent disease onset than to treat established disease with drugs that target T or B lymphocytes and in some strains drug combinations are needed to induce remission (138, 145). Delay in diagnosis and treatment may result in the recruitment of inflammatory amplification circuits or the onset of metabolic or structural changes in the kidneys that are difficult to reverse with immunotherapy. Variability in treatment responses may also be due to intrinsic differences between patients. Considerable variations in responses to immunotherapy are observed in different mouse models of LN even when intervention is initiated at disease onset (138); this may reflect heterogeneity in the severity of inflammation, the relative involvement of innate vs. adaptive immune pathways, the balance of inflammation vs. metabolic abnormalities and the degree of tissue hypoxia, nephron loss and tubular regenerative capacity (Figure 3).
Figure 3.
Shared pathogenic pathways identified in the kidneys of the three different lupus-prone mouse strains. NZB/W and NZW/BXSB mice develop proliferative glomerulonephritis and NZM2410 mice develop glomerulosclerosis with limited inflammation. Pathways that are shared between all three strains and human LN are shown in green. The major functional pathway that is overrepresented in each of the three individual strains is shown in purple. Transcription factors are shown in blue and other pathways shared by two strains are shown in black. (adapted from (122))
Although biologic agents have not yet been successful in patients with LN (146–147) many new approaches remain to be tested (146, 148). Obvious targets include key cytokines expressed in inflamed kidneys such as IL1, IL6, M-CSF, IFNγ, Type I IFN, BAFF, IL17 and IL21, signaling pathways such as tyrosine kinases, costimulatory molecules such as ICOS and TWEAK (149) and effector molecules such as those in the complement pathway (reviewed (150)). Chemokines are also appealing targets but despite extensive efforts have not yet successfully been translated to human autoimmune disease (151–152). One successful pilot approach has been to deplete plasma cells with a proteasome inhibitor, thus clearing immune complexes and allowing the kidney to heal (153). Nevertheless, the overall experience with immune suppression shows that non-immune based approaches to preventing disease progression (154) also need to be considered in order to optimize the resolution and repair process (Table 2). This field is still in its infancy but new approaches are being tested and developments in this field may also be applicable to LN.
Table 2.
| Immune based therapies |
| Innate immune mechanisms, eg inflammasome |
| Adaptive immunity eg costimulatory blockade |
| Effector cells, eg plasma cells, macrophages |
| Effector pathways, eg alternative complement pathway, TWEAK/Fn14 pathway |
| Soluble inflammatory mediators, eg cytokines/chemokines |
| Signaling molecules eg, kinases, mTOR |
| Protection of intrinsic renal cells |
| Renin angiotensin system |
| Endothelin 1 |
| Growth factors, eg PDGF |
| Oxidative stress, eg NADP oxidases |
| Improved microcirculation |
| Phosphodiesterase 5 |
| Anti-fibrotics |
| Growth factors, eg TGFβ, CTGF |
| Growth factor antagonists, eg HGF mimetic, BMP7 mimetic |
| Others |
| Epigenomic reprogramming |
| Mesenchymal stem cells |
Targeting endothelial cell dysfunction
An improved understanding of the mechanisms of endothelial dysfunction and hypoxia may lead to novel therapies. Endothelial glycocalyx deterioration may be prevented by angiotensin 2 inhibition and mycophenolate can inhibit PDGF-B production (155). ACE inhibitors also reduce endothelial activation by increasing NO production independently of their anti-hypertensive effects (76). Podocytes lacking both endothelin receptors are protected from damage in animal models of diabetes and an endothelin antagonist reduced proteinuria in patients with diabetic nephropathy (156). Targeting of VEGF-A (157) or PDGF has proved more challenging because the relationships between angiogenesis, inflammation and fibrosis are still not fully understood. For example, some proangiogenic factors also enhance inflammation or fibrosis (76) and PDGF may be involved in tubular regeneration (85). In addition angiogenesis may be detrimental early in disease, helping to mediate glomerular hypertrophy, before becoming dysfunctional at later stages (76).
Preventing and reversing renal fibrosis
Several strategies for preventing or reversing renal fibrosis are in clinical development and include monoclonal antibodies to TGFβ and CTGF or their downstream signaling pathways, growth factor antagonists such as BMP-7 mimetics, phosphodiesterase inhibitors and NADPH oxidase inhibitors (85, 158). Nevertheless, the wide array of potentially profibrotic factors that arise in inflamed kidneys (87) suggest that broad approaches may be needed. One exciting new therapeutic area is the prevention of tubular epithelial injury based on a better understanding of the mechanisms of tubular stress. Data from mouse models suggests that pharmacologic enhancement of fatty acid oxidation may protect tubules from death and help prevent fibrosis (82).
Reparative macrophages that arise during the healing phase of acute inflammation may act to reverse early fibrosis by endocytosis and degradation of collagen (159) or by secreting enzymes that break down collagen and matrix. These macrophages can also stimulate tubular epithelial cell regeneration through a mechanism involving the Wnt pathway (160). Importantly however, prolonged exposure to reparative macrophages may cause renal fibrosis or excessive remodeling (161). Strategies targeting dysfunctional macrophage repair have included antagonism of the interstitial macrophage receptor CCR1 and of the resident macrophage receptor CX3CR1 (162). More effective targeting of macrophages awaits a better understanding of how their inflammatory, pro-fibrotic and reparative functions are regulated during the various stages of disease.
Conclusions
Progress continues to be made in our understanding of the mechanisms of acute and chronic renal injury as they pertain to LN. Although immunosuppressive agents continue to be the mainstay of treatment, new biologic agents continue to be tested and development of treatments that protect the kidneys against endothelial and tubular injury and fibrosis may help prevent chronic damage. Progress in developing new therapies for LN will require the formation of LN consortia, patient and physician engagement in research protocols, improved clinical trial design, improved definitions of renal outcome that may include second biopsies, testing of molecular and proteomic analyses in clinical trials and, finally, strategies for prevention and treatment based on these results. The most cost effective current approach involves early recognition (163) with prompt intervention and treatment of reversible risk factors. This requires ensuring access both to properly trained health care providers and to medications and is the necessary foundation upon which individualized care can eventually be built.
Review criteria.
Pubmed searches were carried out for lupus nephritis, renal fibrosis, CKD and treatment, AKI to CKD progression, renal interstitial inflammation, renal macrophages. In addition the personal library of the author containing >4000 references was used. The search was confined to articles written in English.
Key points.
Lupus nephritis remains a major cause of morbidity and mortality in SLE with approximately 40% of affected individuals developing some degree of renal impairment.
Long term prognosis is influenced by both extrinsic and intrinsic renal factors and depends on the severity of disease at presentation, the response to immunosuppressive therapy and access to high quality healthcare
Current methods for classifying LN are based solely on light microscopy of kidney biopsies and do not adequately predict response to therapy or long term outcomes. Additional phenotyping based on mechanisms of injury may improve clinical decision making.
Mechanisms of renal injury involve both local immune responses and aberrant responses by the renal parenchyma including disruption of cell-cell interactions that maintain nephron structure, loss of renal microvasculature, tubular atrophy and renal fibrosis. Some of these processes are not reversible with immunosuppressive therapy and may require different approaches based on targeting non-immune mechanisms for disease progression.
Although LN is initiated by immune complex deposition, the response to injury in individual patients is variable and may reflect the balance of innate vs adaptive immunity, inflammation vs metabolic changes, the degree of hypoxia and the capacity for regeneration and fibrosis.
Genetic analyses, molecular profiling and proteomic analyses are being used to discover mechanisms of renal injury and suggest new and more individualized therapeutic approaches.
Studies in mouse models show that progression to CKD is not inevitable even if systemic autoimmunity persists and that several checkpoints in the progression pathway are susceptible to therapeutic intervention.
Because clinical remission and renal histologic remission are not always correlated, improved disease monitoring and/or second biopsies may help identify patients at risk of progression and in need of more intense maintenance therapy
Biography
Biography
Anne Davidson received her MBBS degree from the University of Melbourne, Australia and is a board-certified rheumatologist. She is an Investigator at the Feinstein Institute for Medical Research and Professor of Molecular Medicine at Hofstra North Shore LIJ School of Medicine New York. Dr Davidson’s research focuses on the pathogenesis and therapy of SLE. One interest is to understand how B-cell tolerance is dysregulated in SLE. A second interest is to understand mechanisms of kidney inflammation using both systems biology approaches and functional studies. She is a past recipient of the Dubois Award and the ACR Basic Science Distinguished Investigator Award.
Footnotes
This work was supported by NIH R01 DK085241-01
Competing Interests
None
References
- 1.Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN. Review: Lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus. 2010;19(5):557–74. doi: 10.1177/0961203309358187. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz GS, D’Agati VD. Classification of lupus nephritis. Curr Opin Nephrol Hypertens. 2009;18(3):220–5. doi: 10.1097/mnh.0b013e328327b379. [DOI] [PubMed] [Google Scholar]
- 3.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MM, Lan SP, Bernstein J, Hill GS, Holley K, Lewis EJ. Irreproducibility of the activity and chronicity indices limits their utility in the management of lupus nephritis. Lupus Nephritis Collaborative Study Group. Am J Kidney Dis. 1993;21(4):374–7. doi: 10.1016/s0272-6386(12)80263-0. [DOI] [PubMed] [Google Scholar]
- 5.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25(4):689–95. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 6.Rovin BH, Parikh SV, Alvarado A. The kidney biopsy in lupus nephritis: is it still relevant? Rheum Dis Clin North Am. 2014;40(3):537–52. ix. doi: 10.1016/j.rdc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandepapeliere J, Aydin S, Cosyns JP, Depresseux G, Jadoul M, Houssiau FA. Prognosis of proliferative lupus nephritis subsets in the Louvain Lupus Nephritis inception Cohort. Lupus. 2014;23(2):159–65. doi: 10.1177/0961203313514623. [DOI] [PubMed] [Google Scholar]
- 8.Alvarado A, Malvar A, Lococo B, Alberton V, Toniolo F, Nagaraja H, et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus. 2014 doi: 10.1177/0961203313518625. [DOI] [PubMed] [Google Scholar]
- 9.Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med. 2014;1(1):e000018. doi: 10.1136/lupus-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois EL. In: Lupus Erythematosus. 2. Dubois EL, editor. Los Angeles: USC Press; 1974. pp. 72–89. [Google Scholar]
- 11.Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La Cuesta C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus. 2005;14(11):890–5. doi: 10.1191/0961203305lu2238oa. [DOI] [PubMed] [Google Scholar]
- 12.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–95. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 13.Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14(1):R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houssiau FA, Lauwerys BR. Current management of lupus nephritis. Best Pract Res Clin Rheumatol. 2013;27(3):319–28. doi: 10.1016/j.berh.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MM. The pathology of lupus nephritis. Semin Nephrol. 2007;27(1):22–34. doi: 10.1016/j.semnephrol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Madaio MP. The role of autoantibodies in the pathogenesis of lupus nephritis. Semin Nephrol. 1999;19(1):48–56. [PubMed] [Google Scholar]
- 17.Kalaaji M, Sturfelt G, Mjelle JE, Nossent H, Rekvig OP. Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 2006;54(3):914–26. doi: 10.1002/art.21622. [DOI] [PubMed] [Google Scholar]
- 18.Hedberg A, Mortensen ES, Rekvig OP. Chromatin as a target antigen in human and murine lupus nephritis. Arthritis Res Ther. 2011;13(2):214. doi: 10.1186/ar3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trouw LA, Groeneveld TW, Seelen MA, Duijs JM, Bajema IM, Prins FA, et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest. 2004;114(5):679–88. doi: 10.1172/JCI21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsirogianni A, Pipi E, Soufleros K. Relevance of anti-C1q autoantibodies to lupus nephritis. Ann N Y Acad Sci. 2009;1173:243–51. doi: 10.1111/j.1749-6632.2009.04750.x. [DOI] [PubMed] [Google Scholar]
- 21.Ullal AJ, Reich CF, 3rd, Clowse M, Criscione-Schreiber LG, Tochacek M, Monestier M, et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J Autoimmun. 2011;36(3–4):173–80. doi: 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seredkina N, Rekvig OP. Acquired loss of renal nuclease activity is restricted to DNaseI and is an organ-selective feature in murine lupus nephritis. Am J Pathol. 2011;179(3):1120–8. doi: 10.1016/j.ajpath.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlahakos DV, Foster MH, Adams S, Katz M, Ucci AA, Barrett KJ, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41(6):1690–700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 25.Liang Z, Xie C, Chen C, Kreska D, Hsu K, Li L, et al. Pathogenic Profiles and Molecular Signatures of Antinuclear Autoantibodies Rescued from NZM2410 Lupus Mice. J Exp Med. 2004;199(3):381–98. doi: 10.1084/jem.20030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroni G, Quaglini S, Radice A, Trezzi B, Raffiotta F, Messa P, et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J Immunol Res. 2015;2015:106904. doi: 10.1155/2015/106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177(10):7287–95. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 28.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12(7):1369–82. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 29.Segerer S, Schlondorff D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol. 2007;27(3):260–74. doi: 10.1016/j.semnephrol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Bethunaickan R, Berthier CC, Zhang W, Eksi R, Li HD, Guan Y, et al. Identification of stage-specific genes associated with lupus nephritis and response to remission induction in (NZB x NZW)F1 and NZM2410 mice. Arthritis Rheumatol. 2014;66(8):2246–58. doi: 10.1002/art.38679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190(12):1813–24. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bethunaickan R, Sahu R, Liu Z, Tang YT, Huang W, Edegbe O, et al. Anti-TNF treatment of IFN induced lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 2012 doi: 10.1002/art.34553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 2006;7(2):156–68. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 34.Berthier CC, Bethunaickan R, Gonzalez-Rivera T, Nair V, Ramanujam M, Zhang W, et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol. 2012;189(2):988–1001. doi: 10.4049/jimmunol.1103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, et al. The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. 2013;183(2):431–40. doi: 10.1016/j.ajpath.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Gurkan S, Cabinian A, Lopez V, Bhaumik M, Chang JM, Rabson AB, et al. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J Pathol. 2013;231(2):248–56. doi: 10.1002/path.4235. [DOI] [PubMed] [Google Scholar]
- 37.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141(9):3050–4. [PubMed] [Google Scholar]
- 38.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Diaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998;7(3):154–8. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Wang H, Dai C, Zhang H, Huang Y, Wang S, et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65(12):3176–85. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahlenberg JM, Kaplan MJ. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol. 2014;26(5):475–81. doi: 10.1097/BOR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, Wang H, Huang Y, Zhang H, Wang S, Gaskin F, et al. Lupus Nephritis: Glycogen Synthase Kinase 3beta Promotion of Renal Damage Through Activation of the NLRP3 Inflammasome in Lupus-Prone Mice. Arthritis Rheumatol. 2015;67(4):1036–44. doi: 10.1002/art.38993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–7. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193(2):540–3. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt T, Paust HJ, Krebs CF, Turner JE, Kaffke A, Bennstein SB, et al. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 2015;67(2):475–87. doi: 10.1002/art.38955. [DOI] [PubMed] [Google Scholar]
- 45.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186(3):1849–60. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinloch AJ, Chang A, Ko K, Henry Dunand CJ, Henderson S, Maienschein-Cline M, et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 2014;66(12):3359–70. doi: 10.1002/art.38888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell beta-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012;64(5):1589–600. doi: 10.1002/art.33488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucci M, Stucci S, Strippoli S, Silvestris F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:457146. doi: 10.1155/2010/457146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enghard P, Rieder C, Kopetschke K, Klocke JR, Undeutsch R, Biesen R, et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann Rheum Dis. 2014;73(1):277–83. doi: 10.1136/annrheumdis-2012-202784. [DOI] [PubMed] [Google Scholar]
- 50.Rose ML. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013;74(11):1459–62. doi: 10.1016/j.humimm.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruschi M, Sinico RA, Moroni G, Pratesi F, Migliorini P, Galetti M, et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin AI. J Am Soc Nephrol. 2014;25(11):2483–98. doi: 10.1681/ASN.2013090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–97. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Duffield JS. Macrophages in kidney repair and regeneration. J Am Soc Nephrol. 2011;22(2):199–201. doi: 10.1681/ASN.2010121301. [DOI] [PubMed] [Google Scholar]
- 54.Hill GS, Delahousse M, Nochy D, Remy P, Mignon F, Mery JP, et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59(1):304–16. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 55.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186(8):4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de Lema G, et al. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol. 2004;15(6):1504–13. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- 57.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733–43. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 58.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 59.Sahu R, Bethunaickan R, Singh S, Davidson A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol. 2014;66(6):1596–607. doi: 10.1002/art.38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119(5):1286–97. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassianos AJ, Wang X, Sampangi S, Muczynski K, Healy H, Wilkinson R. Increased tubulointerstitial recruitment of human CD141(hi) CLEC9A(+) and CD1c(+) myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305(10):F1391–401. doi: 10.1152/ajprenal.00318.2013. [DOI] [PubMed] [Google Scholar]
- 62.Salmon AH, Neal CR, Harper SJ. New aspects of glomerular filtration barrier structure and function: five layers (at least) not three. Curr Opin Nephrol Hypertens. 2009;18(3):197–205. doi: 10.1097/MNH.0b013e328329f837. [DOI] [PubMed] [Google Scholar]
- 63.Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308(4):F287–F97. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan S, Lakhe-Reddy S, McCarty JH, Sorenson CM, Sheibani N, Reichardt LF, et al. Mesangial cell integrin alphavbeta8 provides glomerular endothelial cell cytoprotection by sequestering TGF-beta and regulating PECAM-1. Am J Pathol. 2011;178(2):609–20. doi: 10.1016/j.ajpath.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19(1):12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 66.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124(4):1608–21. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67(2):404–19. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 68.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24(4):559–72. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alon R, Nourshargh S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat Immunol. 2013;14(1):14–5. doi: 10.1038/ni.2489. [DOI] [PubMed] [Google Scholar]
- 70.Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol. 2014;34(4):374–83. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51(4):247–58. doi: 10.1159/000365149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–43. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. 2010;53(9):2056–65. doi: 10.1007/s00125-010-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular CrossTalk by Vascular Endothelial Growth Factor A Maintains Peritubular Microvasculature in Kidney. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumpers P, David S, Haubitz M, Hellpap J, Horn R, Brocker V, et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis. 2009;68(10):1638–43. doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 76.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29(3):333–42. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilkeson GS, Mashmoushi AK, Ruiz P, Caza TN, Perl A, Oates JC. Endothelial nitric oxide synthase reduces crescentic and necrotic glomerular lesions, reactive oxygen production, and MCP1 production in murine lupus nephritis. PLoS One. 2013;8(5):e64650. doi: 10.1371/journal.pone.0064650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol. 2010;185(7):4457–69. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahlenberg JM, Yalavarthi S, Zhao W, Hodgin JB, Reed TJ, Tsuji NM, et al. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 2014;66(1):152–62. doi: 10.1002/art.38225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shoji K, Tanaka T, Nangaku M. Role of hypoxia in progressive chronic kidney disease and implications for therapy. Curr Opin Nephrol Hypertens. 2014;23(2):161–8. doi: 10.1097/01.mnh.0000441049.98664.6c. [DOI] [PubMed] [Google Scholar]
- 81.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003–14. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol. 2015;11(4):233–44. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 85.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol. 2014;10(12):700–11. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 86.Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol. 2012;27(7):1041–50. doi: 10.1007/s00467-011-1892-z. [DOI] [PubMed] [Google Scholar]
- 87.Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 2014;4(1):2–8. doi: 10.1038/kisup.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta. 2013;1832(7):931–9. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 89.Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102(2):258–69. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 90.Tampe B, Zeisberg M. Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol Dial Transplant. 2014;29(Suppl 4):iv72–9. doi: 10.1093/ndt/gft025. [DOI] [PubMed] [Google Scholar]
- 91.Dressler GR, Patel SR. Epigenetics in kidney development and renal disease. Transl Res. 2015;165(1):166–76. doi: 10.1016/j.trsl.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16(5):544–50. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lai JY, Luo J, O’Connor C, Jing X, Nair V, Ju W, et al. MicroRNA-21 in Glomerular Injury. J Am Soc Nephrol. 2015;26(4):805–16. doi: 10.1681/ASN.2013121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duffield JS, Grafals M, Portilla D. MicroRNAs are potential therapeutic targets in fibrosing kidney disease: lessons from animal models. Drug Discov Today Dis Models. 2013;10(3):e127–e35. doi: 10.1016/j.ddmod.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69(10):1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 96.Isenberg D, Appel GB, Contreras G, Dooley MA, Ginzler EM, Jayne D, et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology (Oxford) 2010;49(1):128–40. doi: 10.1093/rheumatology/kep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamirou F, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Fiehn C, et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dall’Era M, Cisternas M, Smilek D, Straub L, Houssiau F, Cervera R, et al. Predictors of long-term renal outcome in Lupus Nephritis Trials: Lessons learned from the Euro-Lupus Nephritis Cohort. Arthritis Rheumatol. 2015 doi: 10.1002/art.39026. [DOI] [PubMed] [Google Scholar]
- 99.Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus. 2013;22(14):1446–54. doi: 10.1177/0961203313507986. [DOI] [PubMed] [Google Scholar]
- 100.Esdaile JM, Levinton C, Federgreen W, Hayslett JP, Kashgarian M. The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: a study of 87 patients and review of the literature. Q J Med. 1989;72(269):779–833. [PubMed] [Google Scholar]
- 101.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken) 2014;66(4):617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol. 2014;33(5):649–57. doi: 10.1007/s10067-014-2527-0. [DOI] [PubMed] [Google Scholar]
- 103.Chung SA, Brown EE, Williams AH, Ramos PS, Berthier CC, Bhangale T, et al. Lupus nephritis susceptibility Loci in women with systemic lupus erythematosus. J Am Soc Nephrol. 2014;25(12):2859–70. doi: 10.1681/ASN.2013050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caster DJ, Korte EA, Nanda SK, McLeish KR, Oliver RK, G’Sell RT, et al. ABIN1 dysfunction as a genetic basis for lupus nephritis. J Am Soc Nephrol. 2013;24(11):1743–54. doi: 10.1681/ASN.2013020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez E, Nadig A, Richardson BC, Freedman BI, Kaufman KM, Kelly JA, et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann Rheum Dis. 2011;70(10):1752–7. doi: 10.1136/ard.2011.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bolin K, Sandling JK, Zickert A, Jonsen A, Sjowall C, Svenungsson E, et al. Association of STAT4 polymorphism with severe renal insufficiency in lupus nephritis. PLoS One. 2013;8(12):e84450. doi: 10.1371/journal.pone.0084450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim-Howard X, Maiti AK, Anaya JM, Bruner GR, Brown E, Merrill JT, et al. ITGAM coding variant (rs1143679) influences the risk of renal disease, discoid rash and immunological manifestations in patients with systemic lupus erythematosus with European ancestry. Ann Rheum Dis. 2010;69(7):1329–32. doi: 10.1136/ard.2009.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sanchez E, Kelly JA, et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009;119(4):911–23. doi: 10.1172/JCI36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee YH, Bae SC. Association between the functional ITGAM rs1143679 G/A polymorphism and systemic lupus erythematosus/lupus nephritis or rheumatoid arthritis: an update meta-analysis. Rheumatol Int. 2014 doi: 10.1007/s00296-014-3156-2. [DOI] [PubMed] [Google Scholar]
- 110.Dong C, Ptacek TS, Redden DT, Zhang K, Brown EE, Edberg JC, et al. Fcgamma receptor IIIa single-nucleotide polymorphisms and haplotypes affect human IgG binding and are associated with lupus nephritis in African Americans. Arthritis Rheumatol. 2014;66(5):1291–9. doi: 10.1002/art.38337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66(2):390–6. doi: 10.1002/art.38220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colares VS, Titan SM, Pereira Ada C, Malafronte P, Cardena MM, Santos S, et al. MYH9 and APOL1 Gene Polymorphisms and the Risk of CKD in Patients with Lupus Nephritis from an Admixture Population. PLoS One. 2014;9(3):e87716. doi: 10.1371/journal.pone.0087716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin CP, Adrianto I, Lessard CJ, Kelly JA, Kaufman KM, Guthridge JM, et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes Immun. 2012;13(3):232–8. doi: 10.1038/gene.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung AC, Lan HY. MicroRNAs in renal fibrosis. Front Physiol. 2015;6:50. doi: 10.3389/fphys.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodriguez-Romo R, Berman N, Gomez A, Bobadilla NA. Epigenetic regulation in the acute kidney injury (AKI) to chronic kidney disease transition (CKD) Nephrology (Carlton) 2015 doi: 10.1111/nep.12521. [DOI] [PubMed] [Google Scholar]
- 116.Bomsztyk K, Denisenko O. Epigenetic alterations in acute kidney injury. Semin Nephrol. 2013;33(4):327–40. doi: 10.1016/j.semnephrol.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu N, Zhuang S. Treatment of chronic kidney diseases with histone deacetylase inhibitors. Front Physiol. 2015;6:121. doi: 10.3389/fphys.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220–7. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 119.Te JL, Dozmorov IM, Guthridge JM, Nguyen KL, Cavett JW, Kelly JA, et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS One. 2010;5(5):e10344. doi: 10.1371/journal.pone.0010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peterson KS, Huang JF, Zhu J, D’Agati V, Liu X, Miller N, et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest. 2004;113(12):1722–33. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berthier CC, Kretzler M, Davidson A. From the Large Scale Expression Analysis of Lupus Nephritis to Targeted Molecular Medicine. J Data Mining Genomics Proteomics. 2012;3(3) doi: 10.4172/2153-0602.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bethunaickan R, Berthier CC, Zhang W, Kretzler M, Davidson A. Comparative Transcriptional Profiling of 3 Murine Models of SLE Nephritis Reveals Both Unique and Shared Regulatory Networks. PLoS One. 2013;8(10):e77489. doi: 10.1371/journal.pone.0077489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177(4):2671–80. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62(5):1457–68. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ge Y, Jiang C, Sung SS, Bagavant H, Dai C, Wang H, et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J Exp Med. 2013;210(11):2387–401. doi: 10.1084/jem.20130731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Srisawat N, Murugan R, Kellum JA. Repair or progression after AKI: a role for biomarkers? Nephron Clin Pract. 2014;127(1–4):185–9. doi: 10.1159/000363254. [DOI] [PubMed] [Google Scholar]
- 128.Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu261. [DOI] [PubMed] [Google Scholar]
- 129.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171(1):489–97. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 130.Kulkarni O, Eulberg D, Selve N, Zollner S, Allam R, Pawar RD, et al. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J Pharmacol Exp Ther. 2009;328(2):371–7. doi: 10.1124/jpet.108.142711. [DOI] [PubMed] [Google Scholar]
- 131.Satoskar AA, Shapiro JP, Bott CN, Song H, Nadasdy GM, Brodsky SV, et al. Characterization of glomerular diseases using proteomic analysis of laser capture microdissected glomeruli. Mod Pathol. 2012;25(5):709–21. doi: 10.1038/modpathol.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parikh SV, Ayoub I, Rovin BH. The kidney biopsy in lupus nephritis: time to move beyond histology. Nephrol Dial Transplant. 2015;30(1):3–6. doi: 10.1093/ndt/gfu348. [DOI] [PubMed] [Google Scholar]
- 133.Rovin BH, Klein JB. Proteomics and autoimmune kidney disease. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Romick-Rosendale LE, Brunner HI, Bennett MR, Mina R, Nelson S, Petri M, et al. Identification of urinary metabolites that distinguish membranous lupus nephritis from proliferative lupus nephritis and focal segmental glomerulosclerosis. Arthritis Res Ther. 2011;13(6):R199. doi: 10.1186/ar3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nowling TK, Mather AR, Thiyagarajan T, Hernandez-Corbacho MJ, Powers TW, Jones EE, et al. Renal Glycosphingolipid Metabolism Is Dysfunctional in Lupus Nephritis. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reyes-Thomas J, Blanco I, Putterman C. Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol. 2011;40(3):138–50. doi: 10.1007/s12016-010-8197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abulaban KM, Brunner HI. Biomarkers for childhood-onset systemic lupus erythematosus. Curr Rheumatol Rep. 2015;17(1):471. doi: 10.1007/s11926-014-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davidson A, Aranow C. Lupus nephritis: lessons from murine models. Nat Rev Rheumatol. 2010;6(1):13–20. doi: 10.1038/nrrheum.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Corapi KM, Dooley MA, Pendergraft WF., 3rd Comparison and evaluation of lupus nephritis response criteria in lupus activity indices and clinical trials. Arthritis Res Ther. 2015;17(1):110. doi: 10.1186/s13075-015-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lech M, Lorenz G, Kulkarni OP, Grosser MO, Stigrot N, Darisipudi MN, et al. NLRP3 and ASC suppress lupus-like autoimmunity by driving the immunosuppressive effects of TGF-beta receptor signalling. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205496. [DOI] [PubMed] [Google Scholar]
- 141.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4(157):157ra41. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A. 2015;112(7):E710–7. doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saxena V, Lienesch DW, Zhou M, Bommireddy R, Azhar M, Doetschman T, et al. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J Immunol. 2008;180(3):1903–12. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aringer M, Smolen JS. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin Drug Saf. 2008;7(4):411–9. doi: 10.1517/14740338.7.4.411. [DOI] [PubMed] [Google Scholar]
- 145.Liu Z, Davidson A. Taming lupus[mdash]a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–82. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Houssiau FA. Biologic therapy in lupus nephritis. Nephron Clin Pract. 2014;128(3–4):255–60. doi: 10.1159/000368587. [DOI] [PubMed] [Google Scholar]
- 147.Liu Y, Anders HJ. Lupus nephritis: from pathogenesis to targets for biologic treatment. Nephron Clin Pract. 2014;128(3–4):224–31. doi: 10.1159/000368581. [DOI] [PubMed] [Google Scholar]
- 148.Chan TM. Treatment of severe lupus nephritis: the new horizon. Nat Rev Nephrol. 2015;11(1):46–61. doi: 10.1038/nrneph.2014.215. [DOI] [PubMed] [Google Scholar]
- 149.Sanz AB, Izquierdo MC, Sanchez-Nino MD, Ucero AC, Egido J, Ruiz-Ortega M, et al. TWEAK and the progression of renal disease: clinical translation. Nephrol Dial Transplant. 2014;29(Suppl 1):i54–i62. doi: 10.1093/ndt/gft342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rovin BH, Parikh SV. Lupus nephritis: the evolving role of novel therapeutics. Am J Kidney Dis. 2014;63(4):677–90. doi: 10.1053/j.ajkd.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Allegretti M, Cesta MC, Garin A, Proudfoot AE. Current status of chemokine receptor inhibitors in development. Immunol Lett. 2012;145(1–2):68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Asquith DL, Bryce SA, Nibbs RJ. Targeting cell migration in rheumatoid arthritis. Curr Opin Rheumatol. 2015;27(2):204–11. doi: 10.1097/BOR.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 153.Alexander T, Sarfert R, Klotsche J, Kuhl AA, Rubbert-Roth A, Lorenz HM, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cernaro V, Trifiro G, Lorenzano G, Lucisano S, Buemi M, Santoro D. New therapeutic strategies under development to halt the progression of renal failure. Expert Opin Investig Drugs. 2014;23(5):693–709. doi: 10.1517/13543784.2014.899352. [DOI] [PubMed] [Google Scholar]
- 155.Sabuda-Widemann D, Grabensee B, Schwandt C, Blume C. Mycophenolic acid inhibits the autocrine PDGF-B synthesis and PDGF-BB-induced mRNA expression of Egr-1 in rat mesangial cells. Nephrol Dial Transplant. 2009;24(1):52–61. doi: 10.1093/ndt/gfn462. [DOI] [PubMed] [Google Scholar]
- 156.Chandrashekar K, Juncos LA. Endothelin antagonists in diabetic nephropathy: back to basics. J Am Soc Nephrol. 2014;25(5):869–71. doi: 10.1681/ASN.2014020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178(2):911–23. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol. 2014;10(4):226–37. doi: 10.1038/nrneph.2014.14. [DOI] [PubMed] [Google Scholar]
- 159.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202(6):951–66. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229(2):221–31. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 161.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–25. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 162.Vielhauer V, Kulkarni O, Reichel CA, Anders HJ. Targeting the recruitment of monocytes and macrophages in renal disease. Semin Nephrol. 2010;30(3):318–33. doi: 10.1016/j.semnephrol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 163.Ardoin S, Birmingham DJ, Hebert PL, Yu CY, Rovin BH, Hebert LA. An approach to validating criteria for proteinuric flare in systemic lupus erythematosus glomerulonephritis. Arthritis Rheum. 2011;63(7):2031–7. doi: 10.1002/art.30345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parikh SV, Nagaraja HN, Hebert L, Rovin BH. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin J Am Soc Nephrol. 2014;9(2):279–84. doi: 10.2215/CJN.05040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vielhauer V, Anders HJ. Chemokines and chemokine receptors as therapeutic targets in chronic kidney disease. Front Biosci (Schol Ed) 2009;1:1–12. doi: 10.2741/s1. [DOI] [PubMed] [Google Scholar]